Abstract
A reagent-controlled highly stereoselective reaction between (S)-difluoromethyl phenyl sulfoximine 1 and imines is reported, and this synthetic method provides a variety of enantiomerically enriched α-difluoromethyl amines. The main pros of this approach include high efficiency, high stereoselectivity, and a broad substrate scope, which is probably achieved through a non-chelating transition state.
1. Introduction
The difluoromethyl group (CF2H) is one of privileged fluoroalkyl groups which has attracted increasing interest due to its unique biochemical properties [1,2,3,4]. For example, it has strong lipophilicity and has been proven to be an isostere of OH and SH. At the same time, the hydrogen atom in the CF2H group can serve as a hydrogen bond donor, thus difluoromethyl analogs of the biologically active molecules have the potential to be much more effective drugs compared to its parent molecules. Especially, difluoromethyl compounds have better biological activity than their corresponding trifluoromethyl compounds in some cases [5,6]. Given the above-mentioned properties, α-difluoromethyl amines have been successfully used in the antagonist or inhibitor molecular design. For instance, an NPY antagonist with high Y1 activity and high selectivity for subtype receptors [7] and a drug candidate as a thrombin inhibitor [8] were shown in the Figure 1A.
Figure 1.
Examples of biologically active α-difluoromethyl amines and state-of-the-art methods: (A) representative bioactive molecules containing α-difluoromethyl amine; (B) representative synthetic strategies; (C) substrate- and reagent-controlled strategies.
It is of high importance to develop synthetic methods of chiral α-difluoromethyl amines for the pharmaceutical and biological chemistry, given the fact that the potential dangers of racemic drugs have been documented [9]. Thus, it has attracted many efforts to attain the chiral α-difluoromethyl amines, and they can be divided into three aspects according to the reaction type: (a) chemical resolution [10], (b) asymmetric hydrogenation [11,12], and (c) stereoselective Mannich addition reaction [13] (shown in Figure 1B). After the comparison of the above methods, it was found that the stereoselective addition reaction based on the imine starting material has several advantages: it can not only obtain higher stereoselectivity, but also has a wide range of substrate scope, which can be applicable to both α-monosubstituted difluoromethyl amine and α,α-disubstituted difluoromethyl amine. For instance, Hu group reported the stereoselective addition reaction between tert-butyl sulfinyl protected imines and phenyl difluoromethyl sulfone or TMSCF2H to generate the enantiomerically enriched α-difluoromethyl amine [14,15] (shown in Figure 1C(i)), which served as an example of substrate-controlled Mannich addition. Recently, we have been devoted to the development of nucleophilic fluoroalkylation by using fluoroalkyl sulfoximine reagents [16,17,18,19,20]. In this context, we were interested in developing a reagent-controlled stereoselective Mannich reaction instead of a substrate-controlled version [21,22,23]. Herein, we report the first (S)-phenyl difluoromethyl sulfoximine (1)-enabled highly stereoselective difluoromethylation of imines, affording synthetically valuable chiral α-difluoromethyl amines (shown in Figure 1C(ii)).
2. Results
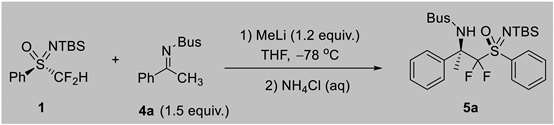
We started our study by examining the (S)-phenyl difluoromethyl sulfoximine 1 and imine 4a as reaction partners. After a careful variation of reaction parameters, we identified the suitable reaction conditions in which a mixture of sulfoximine 1 (1.0 equiv.), imine 4a (1.5 equiv.), and methyl lithium (1.2 equiv.) in THF (0.05 M) afforded 5a in 38% yield with 99/1 dr (Entry 1, Table 1). Further screening revealed that n-butyl lithium is also feasible, which afforded 5a in 35% yield with 99/1 dr (Entry 2, Table 1). However, sodium bis(trimethylsilyl)amide was not suitable for this reaction (Entry 3, Table 1). Various solvents were evaluated, and THF was found to be an optimal solvent (Entries 4–6, Table 1). However, when HMPA was added, the yield was significantly reduced but with 99/1 dr (Entry 7, Table 1). When the ratio of 1/4a/MeLi was changed to 1/2/2.8, the yield could be increased to 77% and dr 99/1 (Entry 8, Table 1). However, when N-Ts and N-SPh-4a were used, the yield decreased (Entries 9 and 10, Table 1). Further optimization showed that when the concentration and temperature decreased, it could afford the desired product in 90% yield and 99/1 dr (Entries 11 and 12, Table 1).

Table 1.
The optimization of reaction conditions a.
Then, we examined the substrate scope of the reaction (Scheme 1). Reactions with various imines can afford 5a–m in high yields (64–99%) and high diastereoselectivity (dr 95/5–99/1). The halo-substituted substrates were tested, and it can afford 5c (72% yield, dr 97/3) and 5d (84% yield, dr 95/5). The substituents such as methyl and isopropyl could be tolerated and 5e (93% yield, dr 99/1) and 5f (97% yield, dr 99/1) were obtained. This reaction is not sensitive to the position of the substituent on the aromatic ring, and 5g (99% yield, dr 99/1), 5h (90% yield, dr 99/1) and 5i (88% yield, dr 99/1) were afforded. 3,4-Disubstituted aryl ketimine was also tolerated and 5j (97% yield, dr 99/1) was obtained. When an aryl ethyl ketimine was used, 5k (95% yield, dr 99/1) was generated. The cyclic imine 4l was also tolerated with the reaction. In addition, the heteroaromatic ring such as the one in the furyl group can afford the desired product 5m (81% yield, dr 99/1).
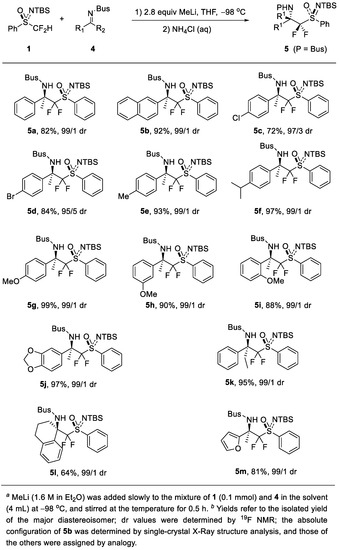
Scheme 1.
Substrate scope of the stereoselective difluoromethylation reaction a,b.
Although 5i, 5j and 5k were parallel with the previous preliminary results [20], the process for the corresponding HCF2-products is vague. To obtain the difluoromethylation products, 5b could undergo deprotection of the silyl group with aqueous acid to yield NH-6b in full conversion. The absolute configuration of 6b was reported by our group [20], and those of the others were assigned by analog. The process of a reductive alkyl C-S bond cleavage with magnesium and an N-S bond cleavage with triflic acid could afford 7b in 69% overall yield (Scheme 2), which implied the products could be modified diversely, and provided the possibility of accessing chiral amine derivatives, especially those molecules with bioactivities.
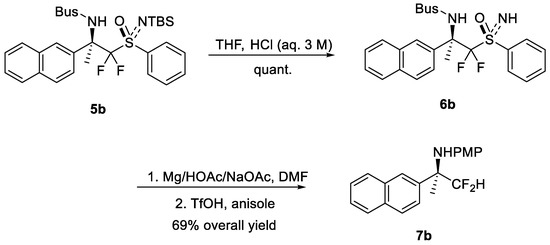
Scheme 2.
Further transformations of the product.
On this basis, we are highly interested in what the rationalization of the high diastereoselectivity is. Due to the addition of HMPA not influencing the diastereoselectivity of the difluoromethylation of 4a with (S)-1 (Entry 6, Table 1), we proposed that the cation might not participate in the transition state. In addition, it is worth noting that it is different from the reactions of lithiated phenyl monofluoromethyl sulfoximine and imines. Two possible non-chelating transition states TS-1 and TS-2 were envisaged in Scheme 3a. Since the repulsive interactions of Ph-Ph in TS-2 are much stronger than those of Ph-CH3 in TS-1, TS-1 is the more favorable transition state. In addition, the possible kinetic interpretation of the reaction with enamidation substrates was proposed [24,25]. The nucleophilic addition of monofluoromethyl phenyl sulfoximine to ketimines requires the preproduction of PhSO(NTBS)CHF− [20,26], while the version of difluoromethyl phenyl sulfoximine was achieved in high yield and stereoselectivity by in situ production of PhSO(NTBS)CF2− in the presence of strong bases. We analyzed the possible reaction process in the system, and it was summarized in Scheme 3b. The production rate of PhSO(NTBS)CF2− and its nucleophilic addition rate to ketimine, namely k1 and k2, are rather critical. When k1 and k2 are much larger than k3 and k4, the enamidation of ketimine and the side reaction of methyllithium addition to ketimine could be avoided, which can ensure the high efficiency between difluoromethyl phenyl sulfoximine and ketimines.
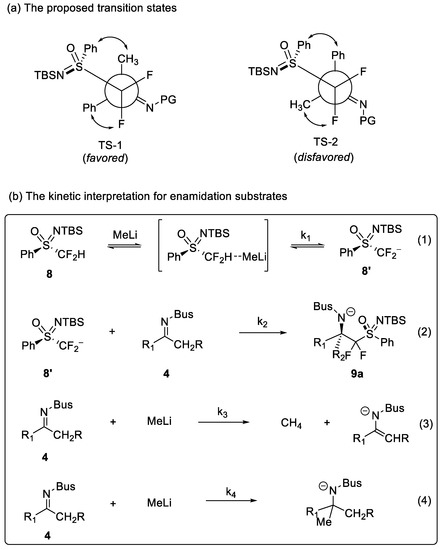
Scheme 3.
(a) The proposed transition states and (b) the kinetic interpretation of the reaction.
3. Materials and Methods
3.1. General Information
Unless otherwise mentioned, solvents and reagents were purchased from commercial sources and used as received. The solvents CH2Cl2, CH3CN, DMF, and HMPA were distilled from CaH2; THF, PhCH3, and Et2O was distilled over sodium before being used. 1H, 13C and 19F NMR spectra were recorded on a 500 MHz, 400 MHz or 300 MHz NMR spectrometer. 1H NMR chemical shifts were determined relative to internal (CH3)4Si (TMS) at δ 0.0 or to the signal of the residual solvent peak: CHCl3 in CDCl3: δ 7.26. 13C NMR chemical shifts were determined relative to internal TMS at δ 0.0. For the isolated compounds, 19F NMR chemical shifts were determined relative to CFCl3 at δ 0.0. Data for 1H, 13C and 19F NMR were recorded as follows: chemical shift (δ, ppm), multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, q = quartet, br = broad). Coupling constants are reported in hertz (Hz). MS (EI) was obtained on a HP5973N mass spectrometer. HRMS (EI) were recorded on a SATURN 2000 mass spectrometer, HRMS (DART) were obtained on an AGILENT1100 mass spectrometer (Shanghai, China), and HRMS (DART-LTQ FTICR) were recorded on a FTMS-7 mass spectrometer (Shanghai, China).
3.2. General Procedure
Under N2 atmosphere, to a solution sulfoximine (S)-1 (0.2 mmol, 1.0 equiv.) and 4 (0.4 mmol, 2.0 equiv.) in THF (8.0 mL), MeLi was added (1.6 M in Et2O, 0.56 mmol, 2.8 equiv.) slowly at −98 °C. After 30 min, the reaction was quenched with aqueous saturated ammonium chloride (4 mL), followed by extraction with ethyl acetate (3 × 10 mL). The organic phase was washed with brine and then dried over anhydrous MgSO4. After the solution was filtered and the solvent was evaporated under vacuum, the residue was subjected to silica gel chromatography to give the major diastereoisomer 5 using petroleum ether/ethyl acetate as eluent.
4. Conclusions
In conclusion, we reported the unprecedented stereoselective nucleophilic difluoromethylation of ketimines using chiral difluoromethyl phenyl sulfoximine. The reagent-controlled highly stereoselective reaction features high efficiency and a broad substrate scope. The reductive cleavage of alkyl C-S bond proved that it could serve as a good access to α-difluoromethyl amines. The possible transition states and kinetic interpretation of the reaction were also demonstrated. Not only does our work provide a valuable synthetic tool and new insights into the intriguing reactivity of sulfoximines, but it also serves as a basis for the further development of chiral fluorinated amines.
Author Contributions
Methodology, software, validation and formal analysis, Q.L. and T.K.; writing–original draft preparation, Q.L.; writing–review and editing, C.N. and J.H.; supervision, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2021YFF0701700), National Natural Science Foundation of China (21632009), the Key Programs of the Chinese Academy of Sciences (KGZD-EW-T08), and the Key Research Program of Frontier Sciences of CAS (QYZDJ-SSW-SLH049).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Xiao Shen for helpful discussion.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.A.; McLoughlin, J.I. Hydrogen Bond Donor Properties of the Difluoromethyl Group. J. Org. Chem. 1995, 60, 1626–1631. [Google Scholar] [CrossRef]
- Narjes, F.; Koehler, K.F.; Koch, U.; Gerlach, B.; Colarusso, S.; Steinkühler, C.; Brunetti, M.; Altamura, S.; De Francesco, R.; Matassa, V.G. A designed P1 cysteine mimetic for covalent and non-covalent inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2002, 12, 701–704. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Abdellatif, K.R.A.; Dong, Y.; Das, D.; Suresh, M.R.; Knaus, E.E. Synthesis of Celecoxib Analogues Possessing a N-Difluoromethyl-1,2-dihydropyrid-2-one 5-Lipoxygenase Pharmacophore: Biological Evaluation as Dual Inhibitors of Cyclooxygenases and 5-Lipoxygenase with Anti-Inflammatory Activity. J. Med. Chem. 2009, 52, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Graneto, M.J.; Philips, W.J. 3-Difluoromethylpyrazolecarboxamide Fungicides, Compositions and Use. U.S. Patent 5,093,347, 3 March 1992. [Google Scholar]
- Gauthier, J.Y.; Belley, M.; Deschênes, D.; Fournier, J.-F.; Gagné, S.; Gareau, Y.; Hamel, M.; Hénault, M.; Hyjazie, H.; Kargman, S.; et al. The identification of 4,7-disubstituted naphthoic acid derivatives as UDP-competitive antagonists of P2Y14. Bioorg. Med. Chem. Lett. 2011, 21, 2836–2839. [Google Scholar] [CrossRef]
- Cho, K.; Ando, M.; Kobayashi, K.; Miyazoe, H.; Tsujino, T.; Ito, S.; Suzuki, T.; Tanaka, T.; Tokita, S.; Sato, N. Design, synthesis and evaluation of a novel cyclohexanamine class of neuropeptide Y Y1 receptor antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 4781–4785. [Google Scholar] [CrossRef]
- Selnick, H.G.; Barrow, J.C.; Nantermet, P.G.; Williams, P.D.; Stauffer, K.J.; Sanderson, P.E.; Rittle, K.E.; Morrissette, M.M.; Wiscount, C.M.; Tran, L.O.; et al. Benzylamine Derivatives and Their Use as Thrombin Inhibitors. WO Patent 2002050056A1, 27 June 2002. [Google Scholar]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The Significance of Chirality in Drug Design and Development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Kanai, M.; Ueda, K.; Yasumoto, M.; Kuriyama, Y.; Inomiya, K.; Ootsuka, T.; Katsuhara, Y.; Higashiyama, K.; Ishii, A. Asymmetric synthesis of α-monofluoromethyl- and α-difluoromethylbenzylamines through regioselective hydrogenolysis. J. Fluorine Chem. 2005, 126, 377–383. [Google Scholar] [CrossRef]
- Abe, H.; Amii, H.; Uneyama, K. Pd-Catalyzed Asymmetric Hydrogenation of α-Fluorinated Iminoesters in Fluorinated Alcohol: A New and Catalytic Enantioselective Synthesis of Fluoro α-Amino Acid Derivatives. Org. Lett. 2001, 3, 313–315. [Google Scholar] [CrossRef]
- Chen, M.-W.; Duan, Y.; Chen, Q.-A.; Wang, D.-S.; Yu, C.-B.; Zhou, Y.-G. Enantioselective Pd-Catalyzed Hydrogenation of Fluorinated Imines: Facile Access to Chiral Fluorinated Amines. Org. Lett. 2010, 12, 5075–5077. [Google Scholar] [CrossRef]
- Funabiki, K.; Nagamori, M.; Goushi, S.; Matsui, M. First catalytic asymmetric synthesis of β-amino-β-polyfluoroalkyl ketones via proline-catalysed direct asymmetric carbon–carbon bond formation reaction of polyfluoroalkylated aldimines. Chem. Commun. 2004, 40, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, J. Highly Diastereoselective Synthesis of α-Difluoromethyl Amines from N-tert-Butylsulfinyl Ketimines and Difluoromethyl Phenyl Sulfone. Chem. Eur. J. 2010, 16, 11443–11454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, W.; Zheng, J.; Hu, J. Efficient and Direct Nucleophilic Difluoromethylation of Carbonyl Compounds and Imines with Me3SiCF2H at Ambient or Low Temperature. Org. Lett. 2011, 13, 5342–5345. [Google Scholar] [CrossRef]
- Shen, X.; Hu, J. Fluorinated Sulfoximines: Preparation, Reactions and Applications. Eur. J. Org. Chem. 2014, 2014, 4437–4451. [Google Scholar] [CrossRef]
- Liu, Q.; Ni, C.; Hu, J. China’s flourishing synthetic organofluorine chemistry: Innovations in the new millennium. Natl. Sci. Rev. 2017, 4, 303–325. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, X.; Ni, C.; Hu, J. Stereoselective Carbonyl Olefination with Fluorosulfoximines: Facile Access to Z or E Terminal Monofluoroalkenes. Angew. Chem. Int. Ed. 2017, 56, 619–623. [Google Scholar] [CrossRef]
- Liu, Q.; Ni, C.; Wang, Q.; Meng, D.; Hu, J. Difluoromethyl 2-Pyridyl Sulfoximine: A Stereoselective Nucleophilic Reagent for Difluoro(aminosulfinyl)methylation and Difluoro(aminosulfonyl)methylation. CCS Chem. 2022, 1–12. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, T.; Ni, C.; Hu, J. Dynamic Kinetic Resolution-Enabled Highly Stereoselective Nucleophilic Fluoroalkylation to Access Chiral β-Fluoro Amines. Org. Lett. 2022, 24, 5982–5987. [Google Scholar] [CrossRef]
- Zhu, G.; Negishi, E.-I. Fully Reagent-Controlled Asymmetric Synthesis of (−)-Spongidepsin via the Zr-Catalyzed Asymmetric Carboalumination of Alkenes (ZACA Reaction). Org. Lett. 2007, 9, 2771–2774. [Google Scholar] [CrossRef]
- You, Y.; Zhang, L.; Luo, S. Reagent-controlled enantioselectivity switch for the asymmetric fluorination of β-ketocarbonyls by chiral primary amine catalysis. Chem. Sci. 2017, 8, 621–626. [Google Scholar] [CrossRef]
- Cui, L.; You, Y.; Mi, X.; Luo, S. Asymmetric Fluorination of α-Branched Aldehydes by Chiral Primary Amine Catalysis: Reagent-Controlled Enantioselectivity Switch. J. Org. Chem. 2018, 83, 4250–4256. [Google Scholar] [CrossRef]
- Schreiber, B.; Martinek, H.; Wolschann, P.; Schuster, P. Kinetic studies on the nucleophilic addition to double bonds. 1. Addition of amines to electrophilic carbon-carbon double bonds. J. Am. Chem. Soc. 1979, 101, 4708–4713. [Google Scholar] [CrossRef]
- Henderson, W.M.; Shelver, W.H. Kinetic Study of the Nucleophilic Addition of Diethyl Phosphonate to Benzylideneanilines. J. Pharm. Sci. 1969, 58, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Miao, W.; Ni, C.; Hu, J. Stereoselective Nucleophilic Fluoromethylation of Aryl Ketones: Dynamic Kinetic Resolution of Chiral α-Fluoro Carbanions. Angew. Chem. Int. Ed. 2014, 53, 775–779. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).