Anti-HIV Ermiasolides from Croton megalocarpus
Abstract
1. Introduction
2. Results and Discussion
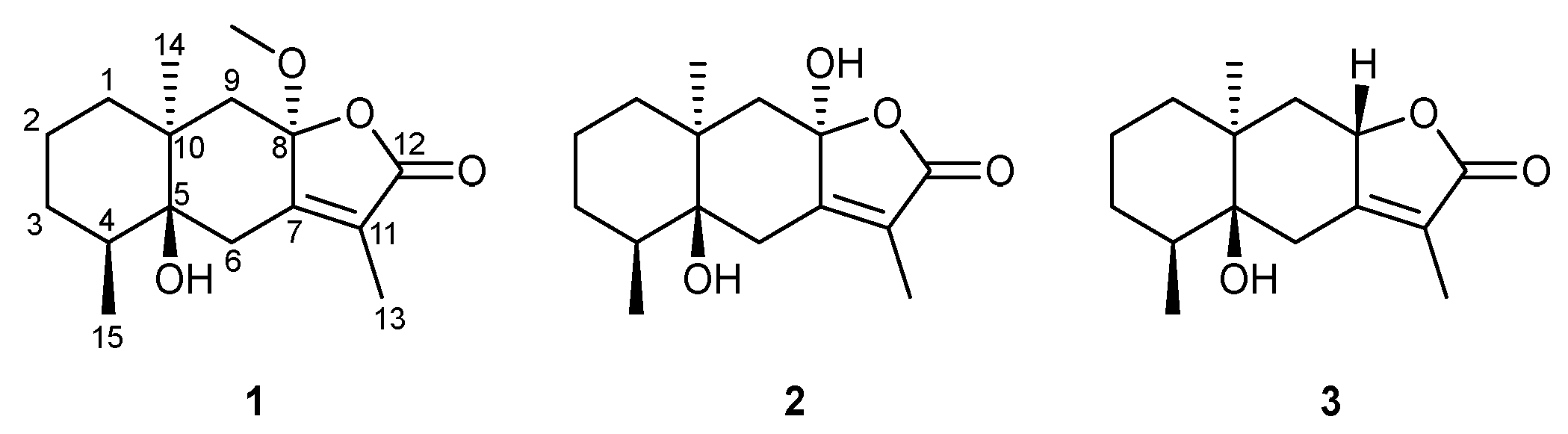
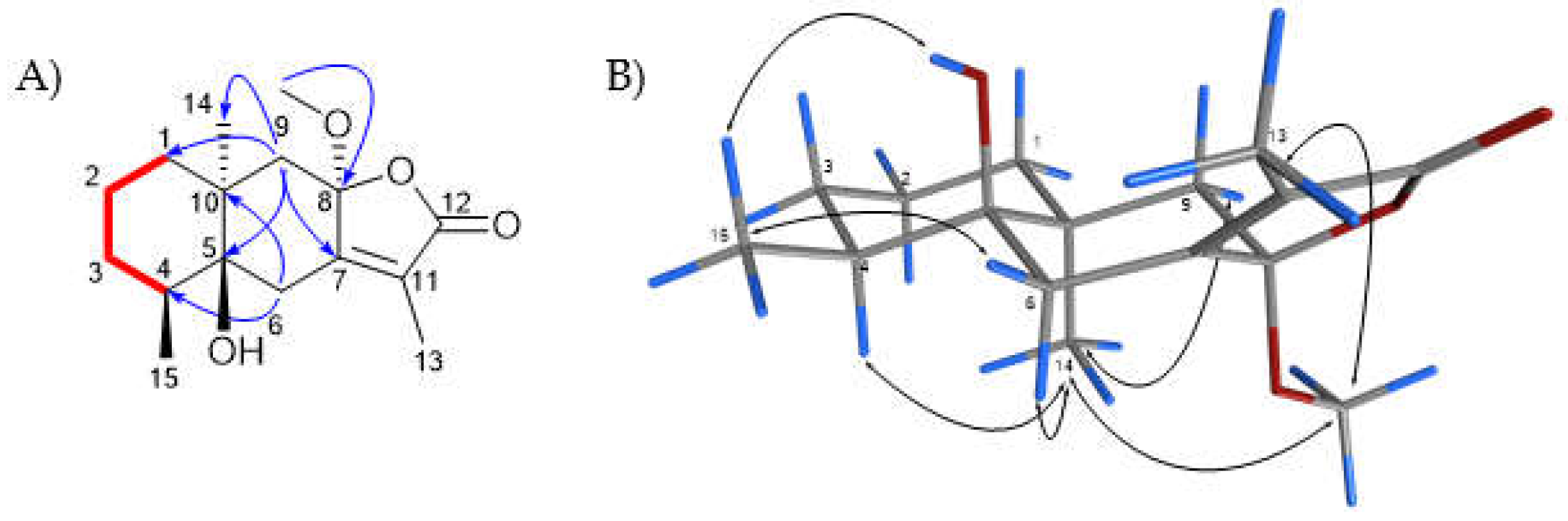
2.1. Characterization and Structural Elucidation
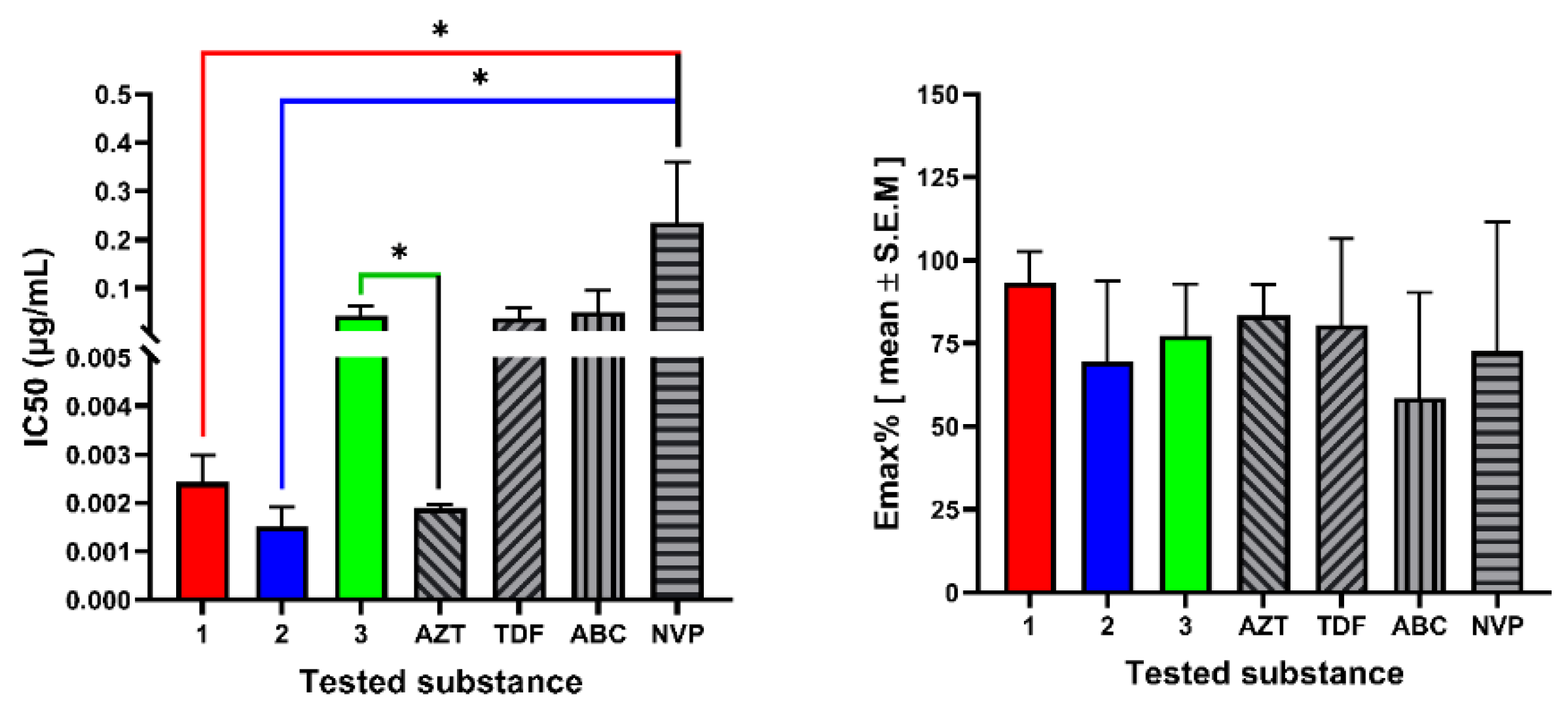
2.2. In Vitro Cytotoxicity and Anti-HIV Assay
2.3. Assay of Anti-HIV Activity In Silico
3. Experimental Section
3.1. Plant Material
3.2. Isolation of Compounds 1–3
3.3. Structural Elucidation of Isolated Compounds
3.4. Cell Culture, Maintenance and Viability Test
3.5. Cytotoxicity Testing
3.6. Viral Culture
3.7. Anti HIV Activity Testing
3.8. Docking Experiments
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Terefe, E.; Okalebo, F.; Derese, S.; Muriuki, J.; Rotich, W.; Mas-Claret, E.; Sadgrove, N.; Padilla-González, G.; Prescott, T.A.K.; Siddique, H.; et al. Constituents of Croton Megalocarpus with Potential Anti-HIV Activity. J. Nat. Prod. 2022, 85, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Terefe, E.M.; Okalebo, F.A.; Derese, S.; Langat, M.K.; Mas-Claret, E.; Aljarba, N.H.; Alkahtani, S.; Batiha, G.E.-S.; Ghosh, A.; El-Masry, E.A.; et al. In Vitro Anti-HIV and Cytotoxic Effects of Pure Compounds Isolated from Croton Macrostachyus Hochst. Ex Delile. BMC Complement. Med. Ther. 2022, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M. A Preliminary Report on the Ethnobotany of the Suei Dorobo in Northern Kenya. African Study Monogr. Suppl. 1987, 7, 1–52. [Google Scholar] [CrossRef]
- Johns, T.; Mhoro, E.B.; Sanaya, P.; Kimanani, E.K. Herbal Remedies of the Batemi of Ngorongoro District, Tanzania: A Quantitative Appraisal. Econ. Bot. 1994, 48, 90–95. [Google Scholar] [CrossRef]
- Nanyingi, M.O.; Mbaria, J.M.; Lanyasunya, A.L.; Wagate, C.G.; Koros, K.B.; Kaburia, H.F.; Munenge, R.W.; Ogara, W.O. Ethnopharmacological Survey of Samburu District, Kenya. J. Ethnobiol. Ethnomed. 2008, 4, 14. [Google Scholar] [CrossRef]
- Kamau, L.N.; Mbaabu, P.M.; Mbaria, J.M.; Gathumbi, P.K.; Kiama, S.G. Ethnobotanical Survey and Threats to Medicinal Plants Traditionally Used for the Management of Human Diseases in Nyeri County, Kenya. Humanit. Med. 2016, 6, 21.1–21.15. [Google Scholar] [CrossRef][Green Version]
- Kipkore, W.; Wanjohi, B.; Rono, H.; Kigen, G. A Study of the Medicinal Plants Used by the Marakwet Community in Kenya. J. Ethnobiol. Ethnomed. 2014, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Kiringe, J. A Survey of Traditional Health Remedies Used by the Maasai of Southern Kaijiado District, Kenya. Ethnobot. Res. Appl. 2006, 4, 061. [Google Scholar] [CrossRef]
- Bussmann, R.W. Ethnobotany of the Samburu of Mt. Nyiru, South Turkana, Kenya. J. Ethnobiol. Ethnomed. 2006, 2, 35. [Google Scholar] [CrossRef]
- Njoroge, N.G.; Bussmann, R.W. Traditional Management of Ear, Nose and Throat (ENT) Diseases in Central Kenya. J. Ethnobiol. Ethnomed. 2006, 2, 54. [Google Scholar] [CrossRef]
- Cyrus, W.G.; Daniel, G.W.; Nanyingi, M.O.; Njonge, F.K.; Mbaria, J.M. Antibacterial and Cytotoxic Activity of Kenyan Medicinal Plants. Mem. Inst. Oswaldo Cruz 2008, 103, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Fratkin, E. Traditional Medicine and Concepts of Healing among Samburu Pastoralists of Kenya. J. Ethnobiol. 1996, 16, 63–98. [Google Scholar]
- Tlankka, N.S.; Mbega, E.R.; Ndakidemi, P.A. Potential of Indigenous Pesticidal Plants in the Control of Field and Post-Harvest Arthropod Pests in Bambara Groundnuts (Vigna subterranea (L.) Verdc.) in Africa: A Review. Am. J. Plant Sci. 2020, 11, 745–772. [Google Scholar] [CrossRef]
- Addae-Mensah, I.; Achenbach, H.; Thoithi, G.N.; Waibel, R.; Mwangi, J.W. Epoxychiromodine and Other Constituents of Croton Megalocarpus. Phytochemistry 1992, 31, 2055–2058. [Google Scholar] [CrossRef]
- Langat, M.K.; Djuidje, E.F.K.; Ndunda, B.M.; Isyaka, S.M.; Dolan, N.S.; Ettridge, G.D.; Whitmore, H.; Lopez, I.; Alqahtani, A.M.; Atiku, I.; et al. The Phytochemical Investigation of Five African Croton Species: Croton Oligandrus, Croton Megalocarpus, Croton Menyharthii, Croton Rivularis and Croton Megalobotrys. Phytochem. Lett. 2020, 40, 148–155. [Google Scholar] [CrossRef]
- Danyaal, M. The Isolation and Purification of Chemical Constituents of Croton Megalocarpus Hutch Husks. Master’s Thesis, Kingston University, London, UK, 2020. [Google Scholar]
- Langat, M.K.; Crouch, N.R.; Smith, P.J.; Mulholland, D.A. Cembranolides from the Leaves of Croton Gratissimus. J. Nat. Prod. 2011, 74, 2349–2355. [Google Scholar] [CrossRef]
- Terefe, E.M. In Vitro and In Silico Pharmacologic Evaluation of the Antiretreoviral Activity of Croton Species; University of Nairobi: Nairobi, Kenya, 2021. [Google Scholar]
- Tuasha, N.; Escobar, Z.; Seifu, D.; Gadisa, E.; Petros, B.; Sterner, O.; Oredsson, S. Cytotoxic and Other Bioactivities of a Novel and Known Sesquiterpene Lactones Isolated from Vernonia Leopoldi (Sch. Bip. Ex Walp.) Vatke in Breast Cancer Cell Lines. Toxicol. Rep. 2022, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Matejić, J.; Šarac, Z.; Ranđelović, V. Pharmacological Activity of Sesquiterpene Lactones. Biotechnol. Biotechnol. Equip. 2014, 24, 95–100. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.X.; Zhao, Y.; Huang, G. Du Unusual Sesquiterpene Lactones with a New Carbon Skeleton and New Acetylenes from Ajania Przewalskii. Food Chem. 2010, 118, 228–238. [Google Scholar] [CrossRef]
- Radulović, N.; Mananjarasoa, E.; Harinantenaina, L.; Yoshinori, A. Essential Oil Composition of Four Croton Species from Madagascar and Their Chemotaxonomy. Biochem. Syst. Ecol. 2006, 34, 648–653. [Google Scholar] [CrossRef]
- Lopes, E.L.; Neto, M.A.; Silveira, E.R.; Pessoa, O.D.L.; Braz-Filho, R. Flavonoides e Sesquiterpenos de Croton Pedicellatus Kunth. Quim. Nova 2012, 35, 2169–2172. [Google Scholar] [CrossRef]
- Ghosh, P.; Mandal, A.; Rasul, M.G. A New Bioactive Ursane-Type Triterpenoid from Croton Bonplandianum Bail. J. Chem. Sci. 2013, 125, 359–364. [Google Scholar] [CrossRef]
- Qiu, M.; Jin, J.; Zhou, L.; Zhou, W.; Liu, Y.; Tan, Q.; Cao, D.; Zhao, Z. Diterpenoids from Croton Crassifolius Include a Novel Skeleton Possibly Generated via an Intramolecular [2+2]-Photocycloaddition Reaction. Phytochemistry 2018, 145, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.L.; Li, C.X.; Shang, X.Y.; Hou, X.W.; Zhang, Y.; Li, L.Z.; Huang, X.X.; Song, S.J. Sesquiterpenoids from the Roots of Croton Crassifolius. J. Asian Nat. Prod. Res. 2019, 21, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef]
- Langat, M.K.; Crouch, N.R.; Nuzillard, J.M.; Mulholland, D.A. Pseudopulchellol: A Unique Sesquiterpene-Monoterpene Derived C-25 Terpenoid from the Leaves of Croton Pseudopulchellus Pax (Euphorbiaceae). Phytochem. Lett. 2018, 23, 38–40. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Langat, M.K.; Crouch, N.R.; Coley, H.M.; Mutambi, E.M.; Nuzillard, J.M. Cembranolides from the Stem Bark of the Southern African Medicinal Plant, Croton Gratissimus (Euphorbiaceae). Phytochemistry 2010, 71, 1381–1386. [Google Scholar] [CrossRef]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Farah, M.A.; Al-Anazi, K.M. An Updated Review of Computer-Aided Drug Design and Its Application to COVID-19. Biomed. Res. Int. 2021, 2021, 8853056. [Google Scholar] [CrossRef]
- Larder, B.A.; Darby, G.; Richman, D.D. HIV with Reduced Sensitivity to Zidovudine (AZT) Isolated during Prolonged Therapy. Science 1989, 243, 1731–1734. [Google Scholar] [CrossRef]
- Pauwels, R.; De Clercq, E.; Desmyter, J.; Balzarini, J.; Goubau, P.; Herdewijn, P.; Vanderhaeghe, H.; Vandeputte, M. Sensitive and Rapid Assay on MT-4 Cells for Detection of Antiviral Compounds against the AIDS Virus. J. Virol. Methods 1987, 16, 171–185. [Google Scholar] [CrossRef]
- Gao, Y.; Nankya, I.; Abraha, A.; Troyer, R.M.; Nelson, K.N.; Rubio, A.; Arts, E.J. Calculating HIV-1 Infectious Titre Using a Virtual TCID50 Method. Methods Mol. Biol. 2009, 485, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, I.; Yoshimoto, S.; Fujishita, M.; Taguchi, H.; Kubonishi, I.; Niiya, K.; Minezawa, M. Natural Adult T-Cell Leukaemia Virus Infection in Japanese Monkeys. Lancet 1982, 320, 658. [Google Scholar] [CrossRef]
- Szucs, G.; Melnick, J.L.; Hollinger, F.B. A Simple Assay Based on HIV Infection Preventing the Reclustering of MT-4 Cells. Bull. World Health Organ. 1988, 66, 729–737. [Google Scholar] [PubMed]
- ACTG Laboratory Technologist Committee TCID50 (50% Tissue Culture Infectious Dose) Determination Quantitation of Viable HIV-1 Virions in Culture Supernatants. 2004.
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Weislow, O.S.; Kiser, R.; Fine, D.L.; Bader, J.; Shoemaker, R.H.; Boyd, M.R. New Soluble-Formazan Assay for HIV-1 Cytopathic Effects: Application to High-Flux Screening of Synthetic and Natural Products for AIDS-Antiviral Activity. J. Natl. Cancer Inst. 1989, 81, 577–586. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT Assay to Evaluate the Cytotoxic Potential of a Drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Popovic, M.; Read-Connole, E.; Gallo, R.C. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet 1984, 324, 1472–1473. [Google Scholar] [CrossRef]
- Harada, S.; Koyanagi, Y.; Yamamoto, N. Infection of HTLV-III/LAV in HTLV-I-Carrying Cells MT-2 and MT-4 and Application in a Plaque Assay. Science 1985, 229, 563–566. [Google Scholar] [CrossRef]
- Uğur, D.; Güneş, H.; Gülneş, F.; Mammadov, R. Cytotoxic Activities of Certain Medicinal Plants on Different Cancer Cell Lines. Turk. J. Pharm. Sci. 2017, 14, 222–230. [Google Scholar] [CrossRef] [PubMed]
- CDC US Department of Health and Human Services. Biosafety in Microbiological and Biomedical Laboratories, 5th ed.; US Government Printing Office, Public Health Service: Washington, DC, USA, 2009; p. 438.
- Gustafson, K.R.; McKee, T.C.; Bokesch, H.R. Anti-HIV Cyclotides. Curr. Protein Pept. Sci. 2004, 5, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyter, J.; De Clercq, E. Rapid and Automated Tetrazolium-Based Calorimetric Assay for the Detection of Anti-HIV Compounds. J. Virol. Methods 1988, 20, 309–321. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.A.; Morales, G.E.; Forero, J.E.; Roldan, J. Cytotoxic and Antiviral Activities of Colombian Medicinal Plant Extracts of the Euphorbia Genus. Mem. Inst. Oswaldo Cruz 2002, 97, 541–546. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| No. | 13C NMR | 1H NMR | 13C NMR | 1H NMR | 13C NMR | 1H NMR |
| 1α | 34.3 | 1.52 | 34.2 | 1.55 m | 36.9 | 1.50 m |
| 1β | 1.14 | 1.14 m | 1.34 m | |||
| 2α | 20.0 | 1.67 m | 20.0 | 1.68 m | 21.2 | 1.67 m |
| 2β | 1.59 m | 1.49 m | 1.59 m | |||
| 3α | 30.1 | 1.48 m | 30.0 | 1.48 m | 30.2 | 1.50 m |
| 3β | 1.30 m | 1.27 m | 1.23 m | |||
| 4 | 34.1 | 1.96 m | 34.1 | 1.94 m | 36.2 | 1.81 m |
| 5 | 77.8 | - | 78.0 | - | 76.5 | - |
| 6α | 32.0 | 2.71 d (13.9) | 31.8 | 2.71 d (13.9) | 35.4 | 2.55 m |
| 6β | 2.27 dd (1.4, 13.9) | 2.43 d (13.9) | 2.55 m | |||
| 7 | 156.8 | - | 158.9 | - | 161.4 | - |
| 8 | 106.5 | - | 104.1 | - | 78.0 | 5.44 m |
| 9α | 45.8 | 1.96 d (13.7) | 47.0 | 1.90 m | 43.2 | 2.24 dd (13.6, 11.2) |
| 9β | 1.82 d (13.7) | 1.88 m | 1.39 dd (5.7, 13.6) | |||
| 10 | 38.9 | - | 38.9 | - | 38.5 | - |
| 11 | 127.7 | - | 125.3 | - | 121.5 | - |
| 12 | 172.0 | - | 173.0 | - | 175.4 | - |
| 13 | 8.6 | 1.85 d (1.3) | 8.4 | 1.78 s | 8.6 | 1.80 s |
| 14 | 21.0 | 1.24 s | 21.2 | 1.29 s | 23.9 | 0.82 s |
| 15 | 15.3 | 0.90 d (6.7) | 15.2 | 0.89 d (6.7) | 14.8 | 0.93 d (6.7) |
| OCH3 | 50.4 | 3.16 s | ||||
| Materials | Cytotoxicity | Antiviral Activity | SI | |||
|---|---|---|---|---|---|---|
| MNTC (µg/mL) | CC50 (µg/mL) | EmaxC (%) | IC50 (µg/mL) | EmaxAV (%) | ||
| FDA approved antiretroviral drugs | ||||||
| AZT | 0.38 ± 0.19 | 0.53 ± 0.29 | 36.28 ± 0.83 | 0.002 ± 0.00 | 83.5 ± 0.57 | 279.4 |
| TDF | 4.92 ± 0.71 | 6.73 ± 0.24 | 13.17 ± 0.43 | 0.04 ± 0.01 | 80.55 ± 0.46 | 176.5 |
| ABC | 0.18 ± 0.03 | 0.26 ± 0.00 | 17.83 ± 0.57 | 0.05 ± 0.031 | 58.67 ± 0.43 | 5.0 |
| NVP | 0.57 ± 0.0 | 0.82 ± 0.0 | 39.13 ± 0.65 | 0.24 ± 0.09 | 72.53 ± 0.47 | 3.5 |
| Ermiasolides isolated from C. megalocarpus | ||||||
| 1 | 41.84 ± 0.11 | 96.77 ± 0.44 | 62.21 ± 0.67 | 0.002 ± 0.00 | 93.4 ± 0.60 | 39,872.3 |
| 2 | 4.973 ± 0.25 | 38.44 ± 0.63 | 81.59 ± 0.41 | 0.002 ± 0.00 | 69.51 ± 0.26 | 25,339.5 |
| 3 | 64.93 ± 0.26 | 148.1 ± 0.05 | 65.00 ± 0.01 | 0.044 ± 0.01 | 77.46 ± 0.93 | 3384.4 |
| Code | Name of Compounds | Free Energy of Binding (ΔG) kcal/mol |
|---|---|---|
| NVP | Nevirapine | −7.679 |
| 1 | 5β-Hydroxy-8α-methoxy eudesm-7(11)-en-12,8-olide | −2.317 |
| 2 | 5β,8α-Dihydroxy eudesm-7(11)-en-12,8-olide | −2.839 |
| 3 | 5β-Hydroxy-8H-β-eudesm-7(11)-en-12,8-olide | −3.555 |
| Code | Name of Compounds | Free Energy of Binding (ΔG) kcal/mol |
|---|---|---|
| ATV | Atazanavir | −10.437 |
| 1 | 5β-Hydroxy-8α-methoxy eudesm-7(11)-en-12,8-olide | −6.067 |
| 2 | 5β,8α-Dihydroxy eudesm-7(11)-en-12,8-olide | −5.994 |
| 3 | 5β-Hydroxy-8H-β-eudesm-7(11)-en-12,8-olide | −5.850 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terefe, E.M.; Okalebo, F.A.; Derese, S.; Langat, M.K.; Mas-Claret, E.; Qureshi, K.A.; Jaremko, M.; Muriuki, J. Anti-HIV Ermiasolides from Croton megalocarpus. Molecules 2022, 27, 7040. https://doi.org/10.3390/molecules27207040
Terefe EM, Okalebo FA, Derese S, Langat MK, Mas-Claret E, Qureshi KA, Jaremko M, Muriuki J. Anti-HIV Ermiasolides from Croton megalocarpus. Molecules. 2022; 27(20):7040. https://doi.org/10.3390/molecules27207040
Chicago/Turabian StyleTerefe, Ermias Mergia, Faith Apolot Okalebo, Solomon Derese, Moses K. Langat, Eduard Mas-Claret, Kamal Ahmad Qureshi, Mariusz Jaremko, and Joseph Muriuki. 2022. "Anti-HIV Ermiasolides from Croton megalocarpus" Molecules 27, no. 20: 7040. https://doi.org/10.3390/molecules27207040
APA StyleTerefe, E. M., Okalebo, F. A., Derese, S., Langat, M. K., Mas-Claret, E., Qureshi, K. A., Jaremko, M., & Muriuki, J. (2022). Anti-HIV Ermiasolides from Croton megalocarpus. Molecules, 27(20), 7040. https://doi.org/10.3390/molecules27207040








