First-Principles-Based Optimized Design of Fluoride Electrolytes for Sodium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
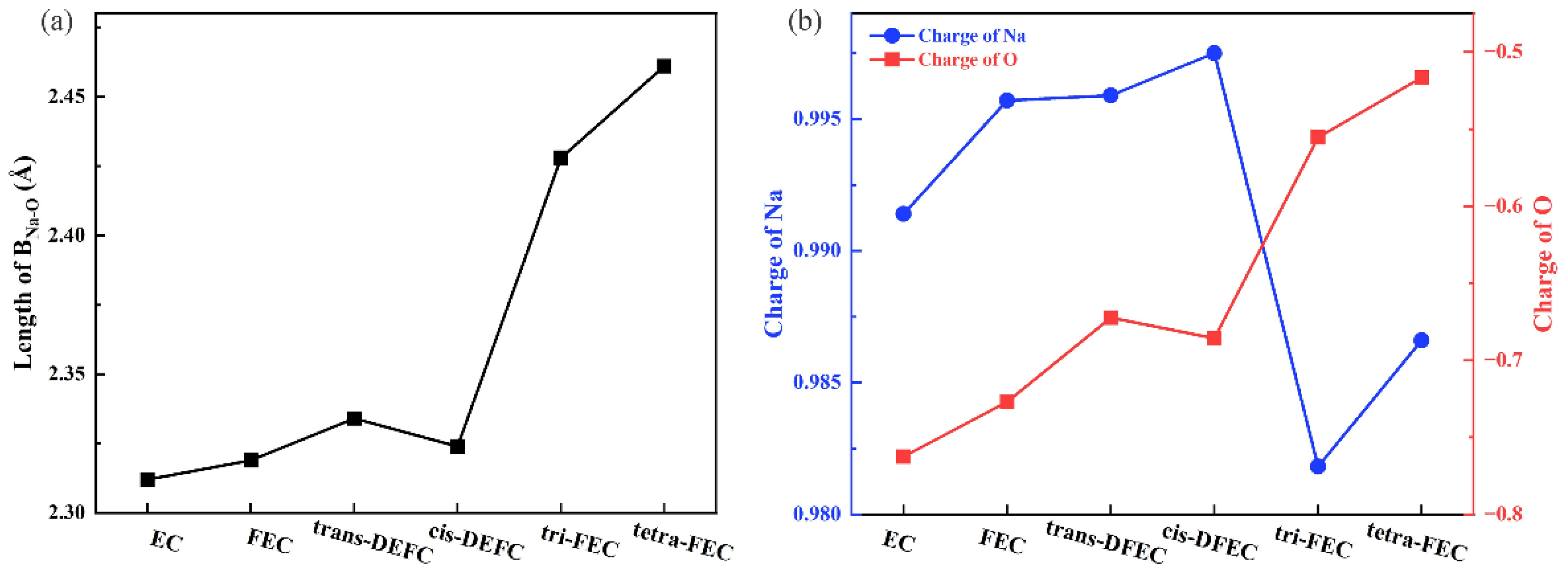
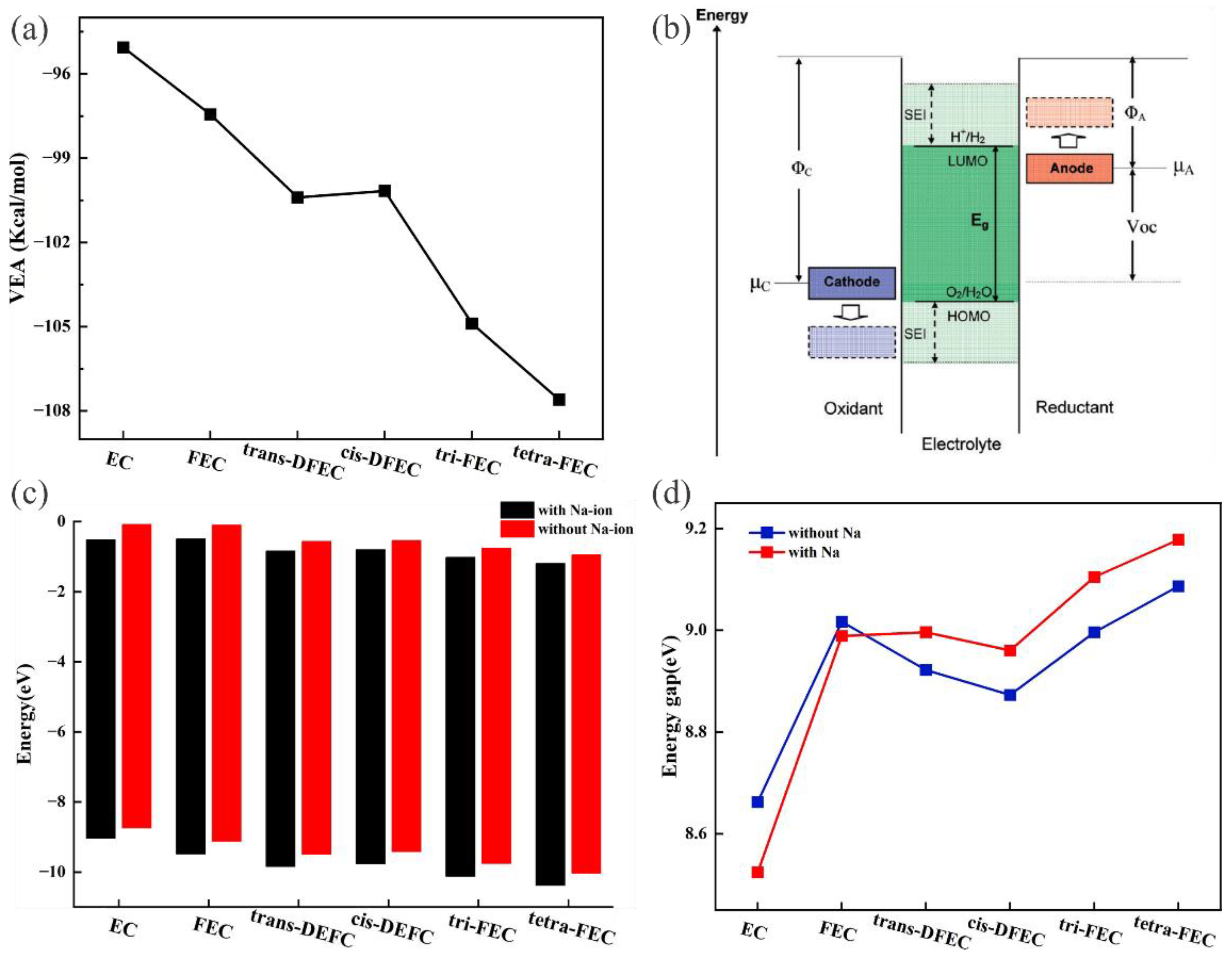
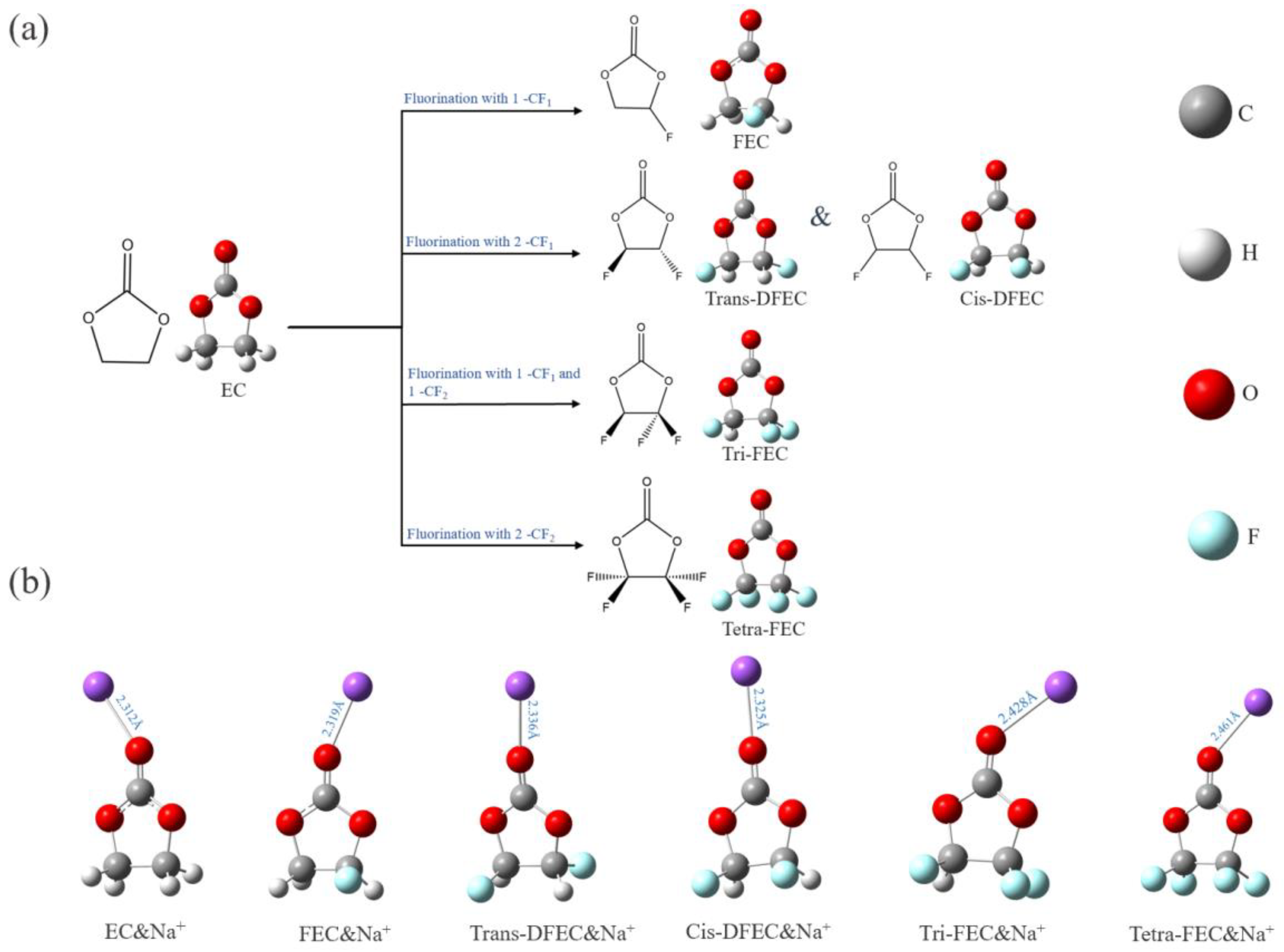
2.1. Fluorination of EC
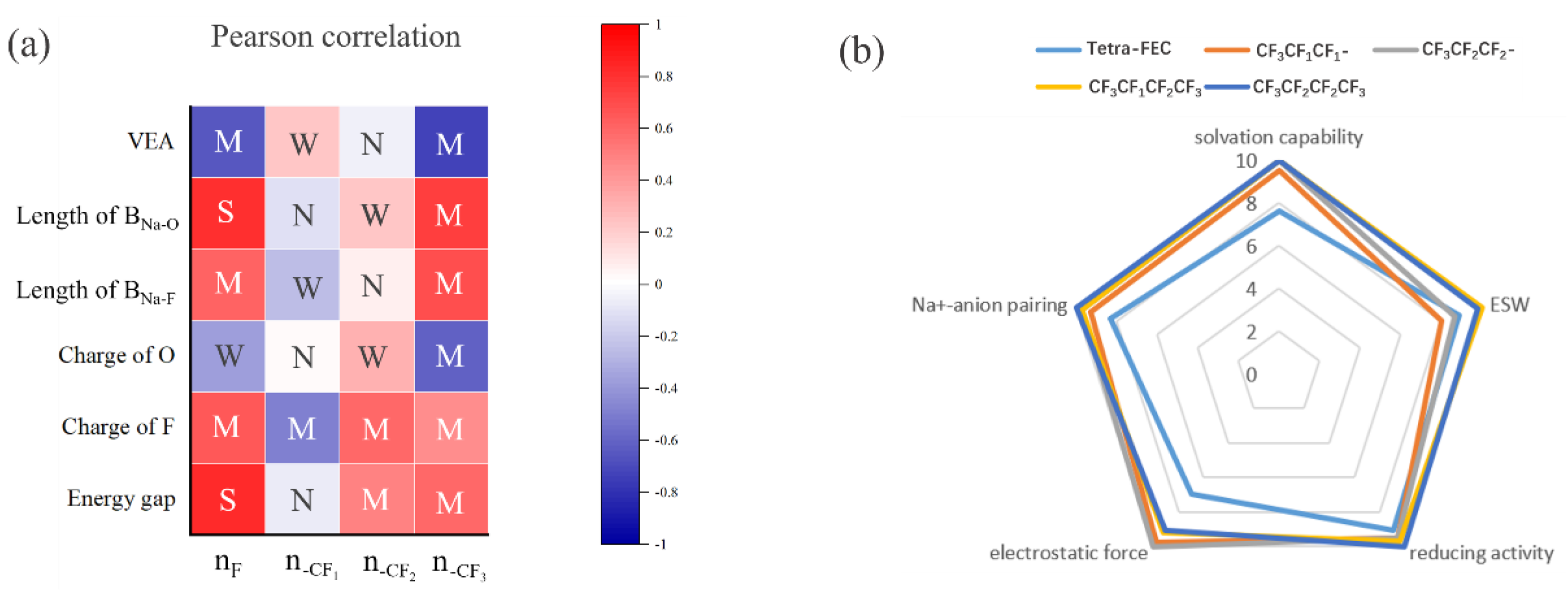
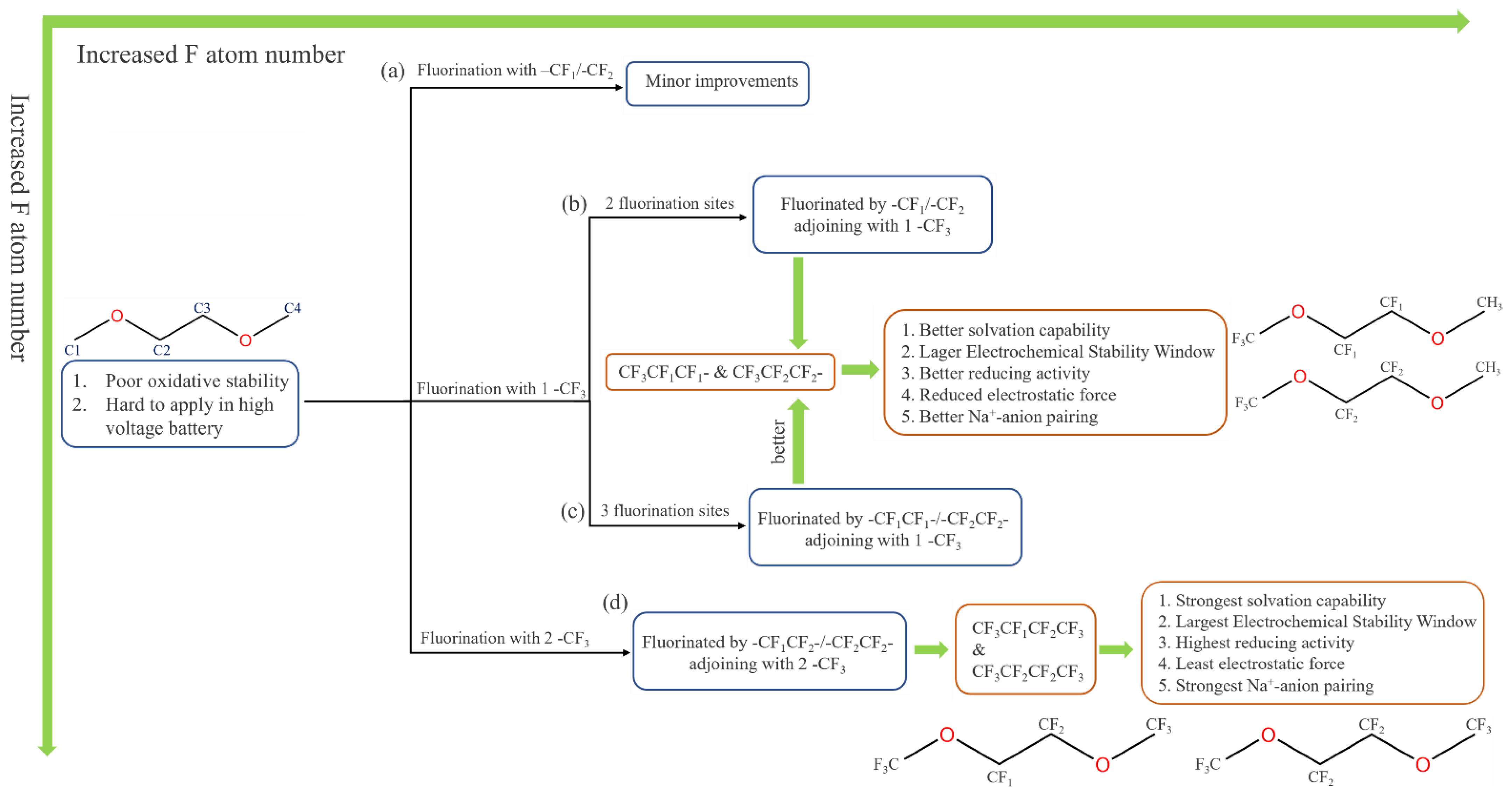
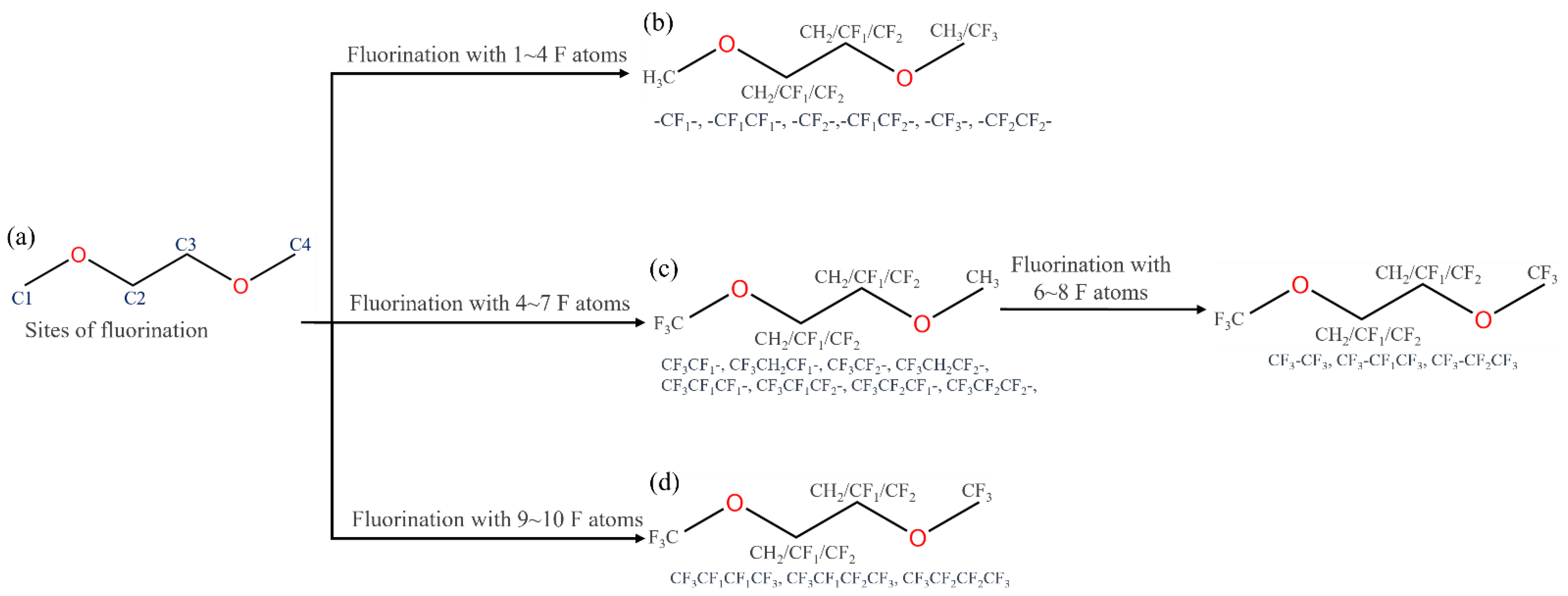
2.2. 1,2-Dimethoxylethane Fluorination
- Compared with carbonate ester solvents, sodium–ether complexes exhibit high LUMO, low solvation energy, and good stability in electrochemical reactions.
- Compared with carbonate ester solvents, ether-based solvents exhibit high reductive stability, which can inhibit dissolution during discharge and form a thin SEI layer. SEI formation typically occurs during the first few charge/discharge cycles of the battery, with sodium salts decomposing preferentially into the inorganic components of the inner layer and ether solvents decomposing into the organic components of the outer layer. An appropriately designed electrolyte facilitates the formation of NaF during the decomposition process and effectively protects the SEI.
- Ether solvents are more compatible with electrodes because of saturated bonds, which reduces the occurrence of side reactions and enhances cycling performance.
3. Materials and Methods
3.1. Design Logic Flow of Fluorinated EC
3.2. Design Logic Flow of Fluorinated DME
3.3. Computational Setups
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SIB | Sodium-ion Battery |
| LIB | Lithium-ion Battery |
| SEI | Solid Electrolyte Interphase |
| EC | Ethylene Carbonate |
| FEC | Fluorinated Ethylene Carbonate |
| Trans-DFEC | Trans-Difluoroethylene Carbonates |
| Cis-DFEC | Cis-Difluoroethylene Carbonate |
| Tri-FEC | Trifluoroethylene Carbonate |
| Tetra-FEC | Tetrafluoroethylene Carbonate |
| FDMB | Fluorinated 1,4-Dimethoxybutane |
| DME | 1,2-Dimethoxylethane |
| CE | Coulombic Efficiency |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| RESP | Restrained Electrostatic Potential |
| VEA | Vertical Electron Affinity |
| ESW | Electrochemical Stability Window |
| NMR | Nuclear Magnetic Resonance |
| PC | Pearson Correlation |
| DFT | Density Function Theory |
| PCM | Polarizable Continuum Model |
References
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Sawicki, M.; Shaw, L.L. Advances and challenges of sodium ion batteries as post lithium ion batteries. RSC Adv. 2015, 5, 53129–53154. [Google Scholar] [CrossRef]
- Ponrouch, A.; Dedryvère, R.; Monti, D.; Demet, A.E.; Mba, J.M.A.; Croguennec, L.; Masquelier, C.; Johansson, P.; Palacín, M.R. Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ. Sci. 2013, 6, 2361–2369. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Li, Y.; Chen, G.; Pei, A.; Nix, O.; Li, Y.; Cui, Y. Solubility-mediated sustained release enabling nitrate additive in carbonate electrolytes for stable lithium metal anode. Nat. Commun. 2018, 9, 3656. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.; Liou, S.-C. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 2018, 13, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ji, X.; Chen, L.; Chen, J.; Deng, T.; Han, F.; Yue, J.; Piao, N.; Wang, R.; Zhou, X. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 2019, 4, 882–890. [Google Scholar] [CrossRef]
- Yamada, Y.; Wang, J.; Ko, S.; Watanabe, E.; Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 2019, 4, 269–280. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J.; Xu, K. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 2018, 4, 174–185. [Google Scholar] [CrossRef]
- Qi, S.; Liu, J.; He, J.; Wang, H.; Wu, M.; Wu, D.; Huang, J.; Li, F.; Li, X.; Ren, Y. Structurally tunable characteristics of ionic liquids for optimizing lithium plating/stripping via electrolyte engineering. J. Energy Chem. 2021, 63, 270–277. [Google Scholar] [CrossRef]
- Shiraishi, S.; Kanamura, K.; Takehara, Z. Surface Condition Changes in Lithium Metal Deposited in Nonaqueous Electrolyte Containing HF by Dissolution-Deposition Cycles. J. Electrochem. Soc. 1999, 146, 1633. [Google Scholar] [CrossRef]
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.-G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2017, 2, 17012. [Google Scholar] [CrossRef]
- He, J.; Wang, H.; Zhou, Q.; Qi, S.; Wu, M.; Li, F.; Hu, W.; Ma, J. Unveiling the role of Li+ solvation structures with commercial carbonates in the formation of solid electrolyte interphase for lithium metal batteries. Small Methods 2021, 5, 2100441. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.J.; Gu, Z.Y.; Zhao, X.X.; Guo, J.Z.; Yang, J.L.; Li, W.H.; Li, B.; Liu, Z.M.; Li, W.L.; Wu, X.L. Ether-Based Electrolyte Chemistry Towards High-Voltage and Long-Life Na-Ion Full Batteries. Angew. Chem. Int. Ed. 2021, 60, 26837–26846. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, S.; Lee, H.; Mei, D.; Engelhard, M.H.; Burton, S.D.; Zhao, W.; Zheng, J.; Li, Q.; Ding, M.S. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 2018, 4, 1877–1892. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Mei, D.; Han, K.S.; Engelhard, M.H.; Zhao, W.; Xu, W.; Liu, J.; Zhang, J.G. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 2018, 30, 1706102. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Chen, X.; Zhang, J.; Engelhard, M.H.; Zhang, Y.; Johnson, B.R.; Crum, J.V.; Blake, T.A.; Liu, X. Effects of carbonate solvents and lithium salts on morphology and coulombic efficiency of lithium electrode. J. Electrochem. Soc. 2013, 160, A1894. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Kong, X.; Kim, S.C.; Boyle, D.T.; Qin, J.; Bao, Z.; Cui, Y. Liquid electrolyte: The nexus of practical lithium metal batteries. Joule 2022, 6, 588–616. [Google Scholar] [CrossRef]
- Von Aspern, N.; Röschenthaler, G.V.; Winter, M.; Cekic-Laskovic, I. Fluorine and lithium: Ideal partners for high-performance rechargeable battery electrolytes. Angew. Chem. Int. Ed. 2019, 58, 15978–16000. [Google Scholar] [CrossRef]
- Yu, Z.; Rudnicki, P.E.; Zhang, Z.; Huang, Z.; Celik, H.; Oyakhire, S.T.; Chen, Y.; Kong, X.; Kim, S.C.; Xiao, X. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 2022, 7, 94–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Viswanathan, V. Not All fluorination is the same: Unique effects of fluorine functionalization of ethylene carbonate for tuning solid-electrolyte interphase in Li metal Batteries. Langmuir 2020, 36, 11450–11466. [Google Scholar] [CrossRef]
- Vogt, L.O.; El Kazzi, M.; Jämstorp Berg, E.; Pérez Villar, S.A.; Novak, P.; Villevieille, C. Understanding the interaction of the carbonates and binder in Na-ion batteries: A combined bulk and surface study. Chem. Mater. 2015, 27, 1210–1216. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, W.; Chen, Y.; Mondonico, L.; Xiao, X.; Zheng, Y.; Liu, F.; Hung, S.T.; Cui, Y.; Bao, Z. Tuning Fluorination of Linear Carbonate for Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 040555. [Google Scholar] [CrossRef]
- Han, J.-G.; Lee, J.B.; Cha, A.; Lee, T.K.; Cho, W.; Chae, S.; Kang, S.J.; Kwak, S.K.; Cho, J.; Hong, S.Y. Unsymmetrical fluorinated malonatoborate as an amphoteric additive for high-energy-density lithium-ion batteries. Energy Environ. Sci. 2018, 11, 1552–1562. [Google Scholar] [CrossRef]
- Liang, H.-J.; Gu, Z.-Y.; Zhao, X.-X.; Guo, J.-Z.; Yang, J.-L.; Li, W.-H.; Li, B.; Liu, Z.-M.; Sun, Z.-H.; Zhang, J.-P. Advanced flame-retardant electrolyte for highly stabilized K-ion storage in graphite anode. Sci. Bull. 2022, 67, 1581–1588. [Google Scholar] [CrossRef]
- Bolloli, M.; Alloin, F.; Kalhoff, J.; Bresser, D.; Passerini, S.; Judeinstein, P.; Leprêtre, J.-C.; Sanchez, J.-Y. Effect of carbonates fluorination on the properties of LiTFSI-based electrolytes for Li-ion batteries. Electrochim. Acta 2015, 161, 159–170. [Google Scholar] [CrossRef]
- He, M.; Su, C.-C.; Peebles, C.; Zhang, Z. The impact of different substituents in fluorinated cyclic carbonates in the performance of high voltage lithium-ion battery electrolyte. J. Electrochem. Soc. 2021, 168, 010505. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, Z.; Liu, X.; Wang, Z.; Wen, J.; Wu, X.; Luo, W.; Huang, Y. Bridging the immiscibility of an all-fluoride fire extinguishant with highly-fluorinated electrolytes toward safe sodium metal batteries. Energy Environ. Sci. 2020, 13, 1788–1798. [Google Scholar] [CrossRef]
- Wu, S.; Su, B.; Ni, K.; Pan, F.; Wang, C.; Zhang, K.; Yu, D.Y.; Zhu, Y.; Zhang, W. Fluorinated Carbonate Electrolyte with Superior Oxidative Stability Enables Long-Term Cycle Stability of Na2/3Ni1/3Mn2/3O2 Cathodes in Sodium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2002737. [Google Scholar] [CrossRef]
- Cresce, A.V.; Russell, S.M.; Borodin, O.; Allen, J.A.; Schroeder, M.A.; Dai, M.; Peng, J.; Gobet, M.P.; Greenbaum, S.G.; Rogers, R.E. Solvation behavior of carbonate-based electrolytes in sodium ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, X.; Dai, Y.; Wu, W.; Huang, Y.; Fu, H.; Huang, Y.; Luo, W. Fluoride-Rich Solid-Electrolyte-Interface Enabling Stable Sodium Metal Batteries in High-Safe Electrolytes. Adv. Funct. Mater. 2021, 31, 2103522. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Z.; Wahyudi, W.; Liu, G.; Park, G.-T.; Cavallo, L.; Anthopoulos, T.D.; Wang, L.; Sun, Y.-K.; Alshareef, H.N. Unraveling the new role of an ethylene carbonate solvation shell in rechargeable metal ion batteries. ACS Energy Lett. 2020, 6, 69–78. [Google Scholar] [CrossRef]
- Chen, X.; Bai, Y.K.; Zhao, C.Z.; Shen, X.; Zhang, Q. Lithium bonds in lithium batteries. Angew. Chem. 2020, 132, 11288–11291. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Zhao, X.; Wang, L.; Yin, Z. Lithium Bond-Enhanced Capacity of Dipyridyl Polysulfides for LSBs. ACS Appl. Energy Mater. 2021, 4, 3495–3501. [Google Scholar] [CrossRef]
- Shakourian-Fard, M.; Kamath, G.; Smith, K.; Xiong, H.; Sankaranarayanan, S.K. Trends in Na-ion solvation with alkyl-carbonate electrolytes for sodium-ion batteries: Insights from first-principles calculations. J. Phys. Chem. C 2015, 119, 22747–22759. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, J. Dual strategy with Li-ion solvation and solid electrolyte interphase for high Coulombic efficiency of lithium metal anode. Energy Storage Mater. 2022, 44, 48–56. [Google Scholar] [CrossRef]
- Ren, X.; Gao, P.; Zou, L.; Jiao, S.; Cao, X.; Zhang, X.; Jia, H.; Engelhard, M.H.; Matthews, B.E.; Wu, H. Role of inner solvation sheath within salt–solvent complexes in tailoring electrode/electrolyte interphases for lithium metal batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 28603–28613. [Google Scholar] [CrossRef]
- Chen, X.; Shen, X.; Li, B.; Peng, H.J.; Cheng, X.B.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. Ion–solvent complexes promote gas evolution from electrolytes on a sodium metal anode. Angew. Chem. Int. Ed. 2018, 57, 734–737. [Google Scholar] [CrossRef]
- Wang, E.; Niu, Y.; Yin, Y.-X.; Guo, Y.-G. Manipulating electrode/electrolyte interphases of sodium-ion batteries: Strategies and perspectives. ACS Mater. Lett. 2020, 3, 18–41. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Li, Y.; Liu, M.; Feng, X.; Bai, Y.; Wu, C. Ether-based electrolytes for sodium ion batteries. Chem. Soc. Rev. 2022, 51, 4484–4536. [Google Scholar] [CrossRef]
- Xing, L.; Zheng, X.; Schroeder, M.; Alvarado, J.; von Wald Cresce, A.; Xu, K.; Li, Q.; Li, W. Deciphering the ethylene carbonate–propylene carbonate mystery in Li-ion batteries. Acc. Chem. Res. 2018, 51, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated ethylene carbonate as electrolyte additive for rechargeable Na batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef]
- Pham, T.A.; Kweon, K.E.; Samanta, A.; Lordi, V.; Pask, J.E. Solvation and dynamics of sodium and potassium in ethylene carbonate from ab initio molecular dynamics simulations. J. Phys. Chem. C 2017, 121, 21913–21920. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Ihsan-Ul-Haq, M.; Mubarak, N.; Liu, Z.; Wu, J.; Luo, Z.; Kim, J.K. NaF-rich solid electrolyte interphase for dendrite-free sodium metal batteries. Energy Storage Mater. 2022, 44, 477–486. [Google Scholar] [CrossRef]
- Liao, Q.; Mu, M.; Zhao, S.; Zhang, L.; Jiang, T.; Ye, J.; Shen, X.; Zhou, G. Performance assessment and classification of retired lithium ion battery from electric vehicles for energy storage. Int. J. Hydrogen Energy 2017, 42, 18817–18823. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, T.; Ashirov, T.; Kazzi, M.E.; Cancellieri, C.; Jeurgens, L.P.; Choi, J.W.; Coskun, A. Fluorinated ether electrolyte with controlled solvation structure for high voltage lithium metal batteries. Nat. Commun. 2022, 13, 2575. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
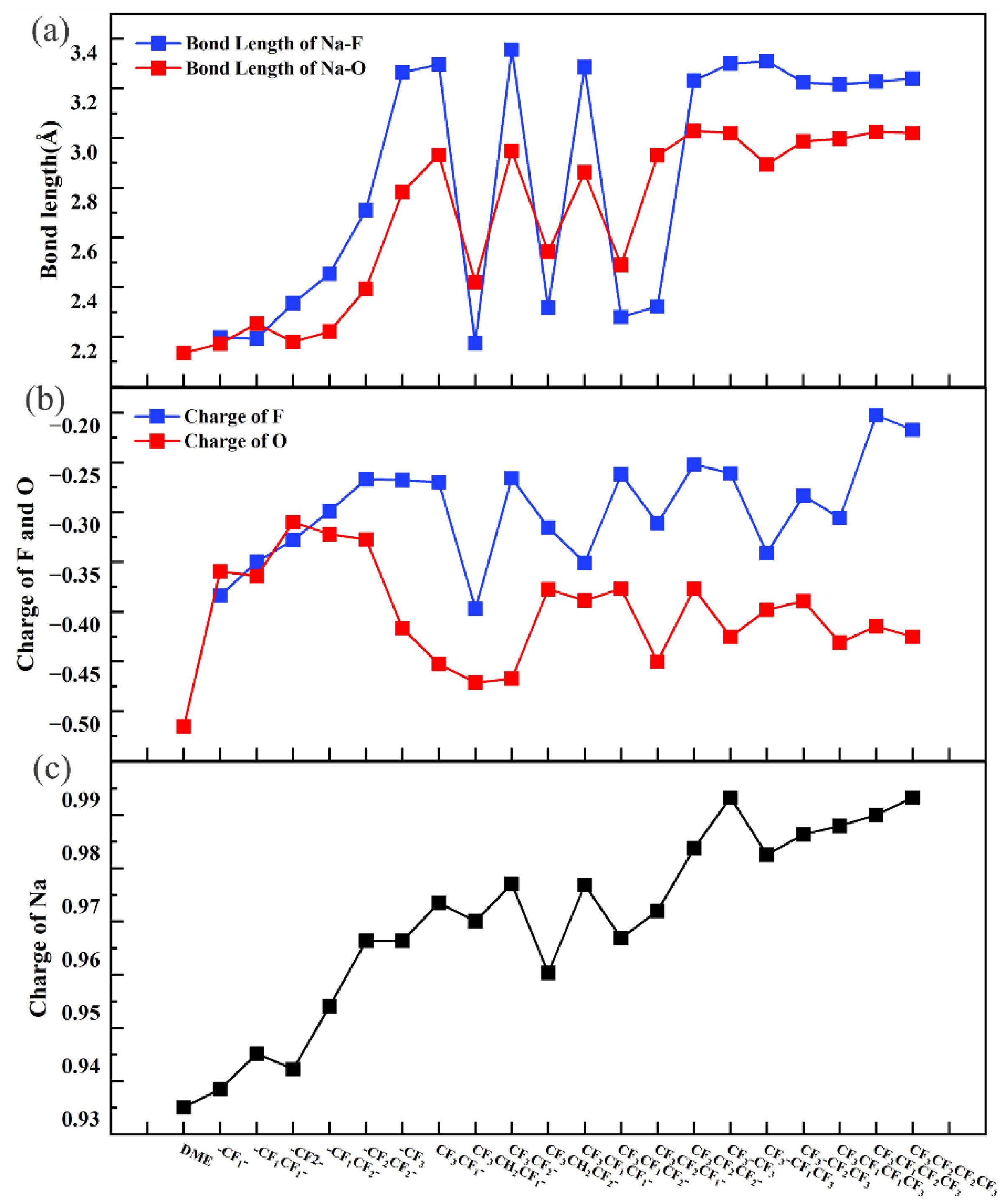


| Bond Length BNa-O(Å) | Bond Length BNa-F(Å) | Bond Length BC-F(Å) | Bond Angle αO-Na-F(deg) | |
|---|---|---|---|---|
| -CF1 | 2.372 | 2.398 | 1.449 | 68.85 |
| -CF1CF1 | 2.455 | 2.393 | 1.435 | 65.953 |
| -CF2 | 2.38 | 2.536~3.593 | 1.380~1.403 | 66.086 |
| -CF1CF2 | 2.421 | 2.654~3.650 | 1.376~1.398 | 63.719 |
| -CF2CF2- | 2.594 | 2.911~3.570 | 1.367~1.381 | 58.812 |
| -CF3 | 2.984 | 3.465 | 1.340~1.356 | 38.426 |
| CF3CF1- | 3.133 | 3.497 | 1.334~1.349 | 37.692 |
| CF3CH2CF1- | 2.621 | 2.375~3.440 | 1.336~1.443 | 64.541 |
| CF3CF2- | 3.149 | 3.556 | 1.333~1.358 | 37.087 |
| CF3CH2CF2- | 2.742 | 2.518~3.469 | 1.334~1.400 | 60.688 |
| CF3CF1CF1- | 3.063 | 3.487~3.491 | 1.332~1.411 | 48.166 |
| CF3CF1CF2- | 2.690 | 2.480~3.713 | 1.328~1.394 | 61.661 |
| CF3CF2CF1- | 3.131 | 2.523~3.823 | 1.330~1.421 | 55.358 |
| CF3CF2CF2- | 3.230 | 3.432~3.512 | 1.329~1.371 | 47.691 |
| CF3-CF3 | 3.082 | 3.501 | 1.355~1.337 | 37.789 |
| CF3-CF1CF3 | 3.094 | 3.511~3.553 | 1.331~1.352 | 48.943 |
| CF3-CF2CF3 | 3.187 | 3.425~3.524 | 1.330~1.356 | 48.926 |
| CF3CF1CF1CF3 | 3.197 | 3.417~3.511 | 1.329~1.375 | 49.056 |
| CF3CF1CF2CF3 | 3.225 | 3.428~3.524 | 1.327~1.351 | 48.489 |
| CF3CF2CF2CF3 | 3.220 | 3.440~3.536 | 1.326~1.347 | 48.710 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Wang, B.; Zhang, P.; Jiang, X.; Zhao, X.; Wang, L.; Yin, Z.; Wu, J. First-Principles-Based Optimized Design of Fluoride Electrolytes for Sodium-Ion Batteries. Molecules 2022, 27, 6949. https://doi.org/10.3390/molecules27206949
Lu S, Wang B, Zhang P, Jiang X, Zhao X, Wang L, Yin Z, Wu J. First-Principles-Based Optimized Design of Fluoride Electrolytes for Sodium-Ion Batteries. Molecules. 2022; 27(20):6949. https://doi.org/10.3390/molecules27206949
Chicago/Turabian StyleLu, Shuhan, Bingqian Wang, Panyu Zhang, Xiaoli Jiang, Xinxin Zhao, Lili Wang, Zhixiang Yin, and Jianbao Wu. 2022. "First-Principles-Based Optimized Design of Fluoride Electrolytes for Sodium-Ion Batteries" Molecules 27, no. 20: 6949. https://doi.org/10.3390/molecules27206949
APA StyleLu, S., Wang, B., Zhang, P., Jiang, X., Zhao, X., Wang, L., Yin, Z., & Wu, J. (2022). First-Principles-Based Optimized Design of Fluoride Electrolytes for Sodium-Ion Batteries. Molecules, 27(20), 6949. https://doi.org/10.3390/molecules27206949






