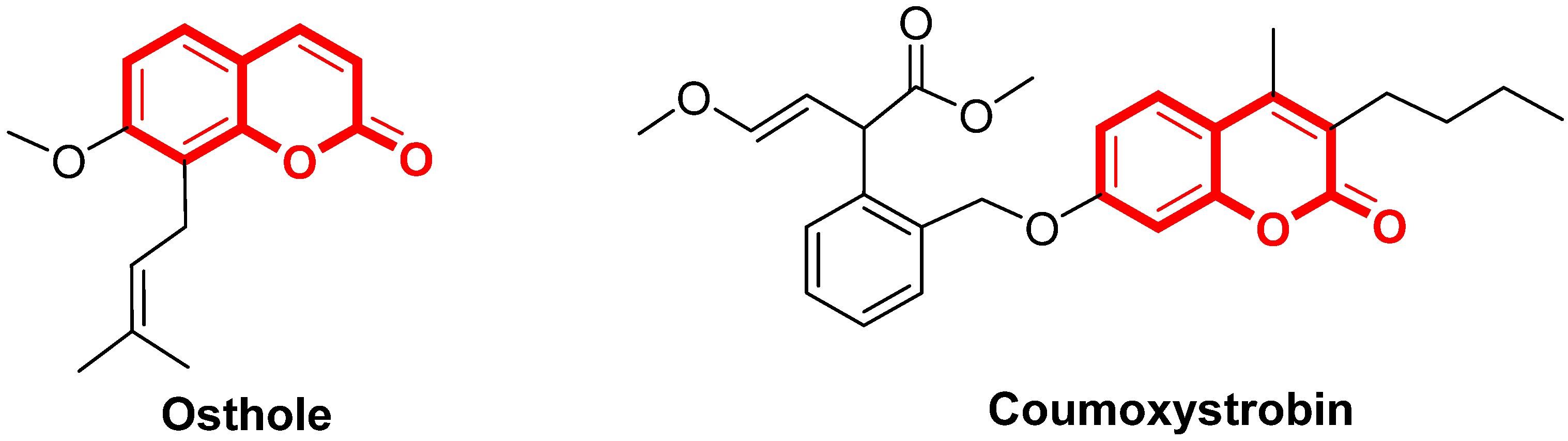
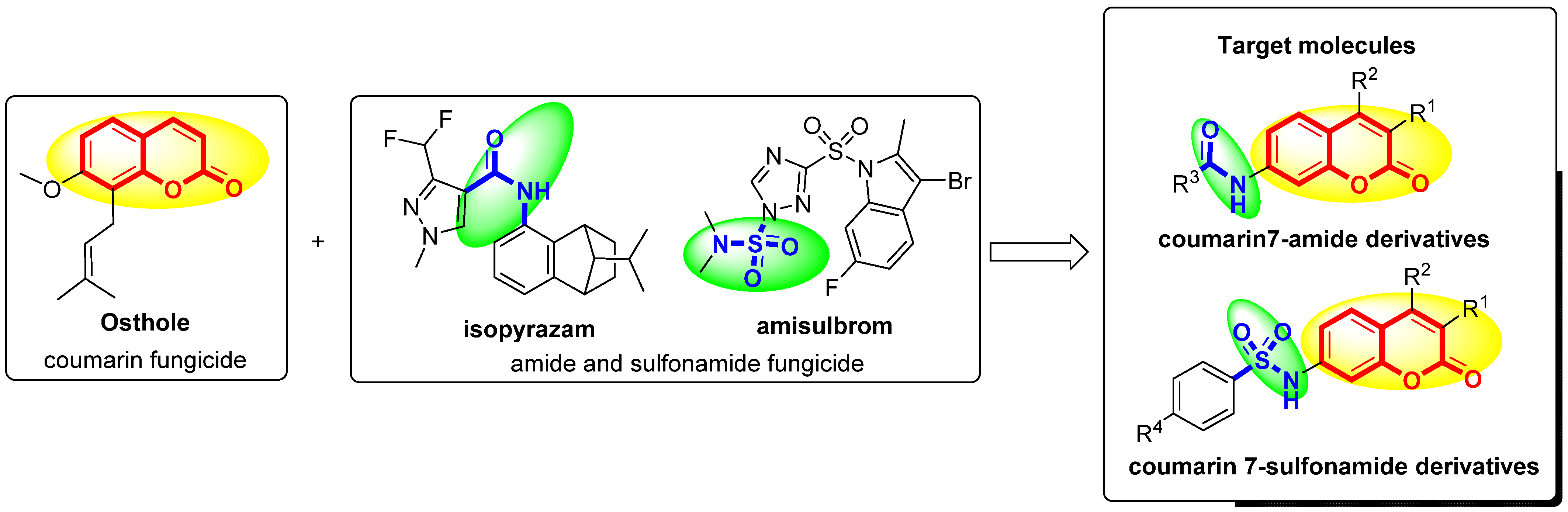
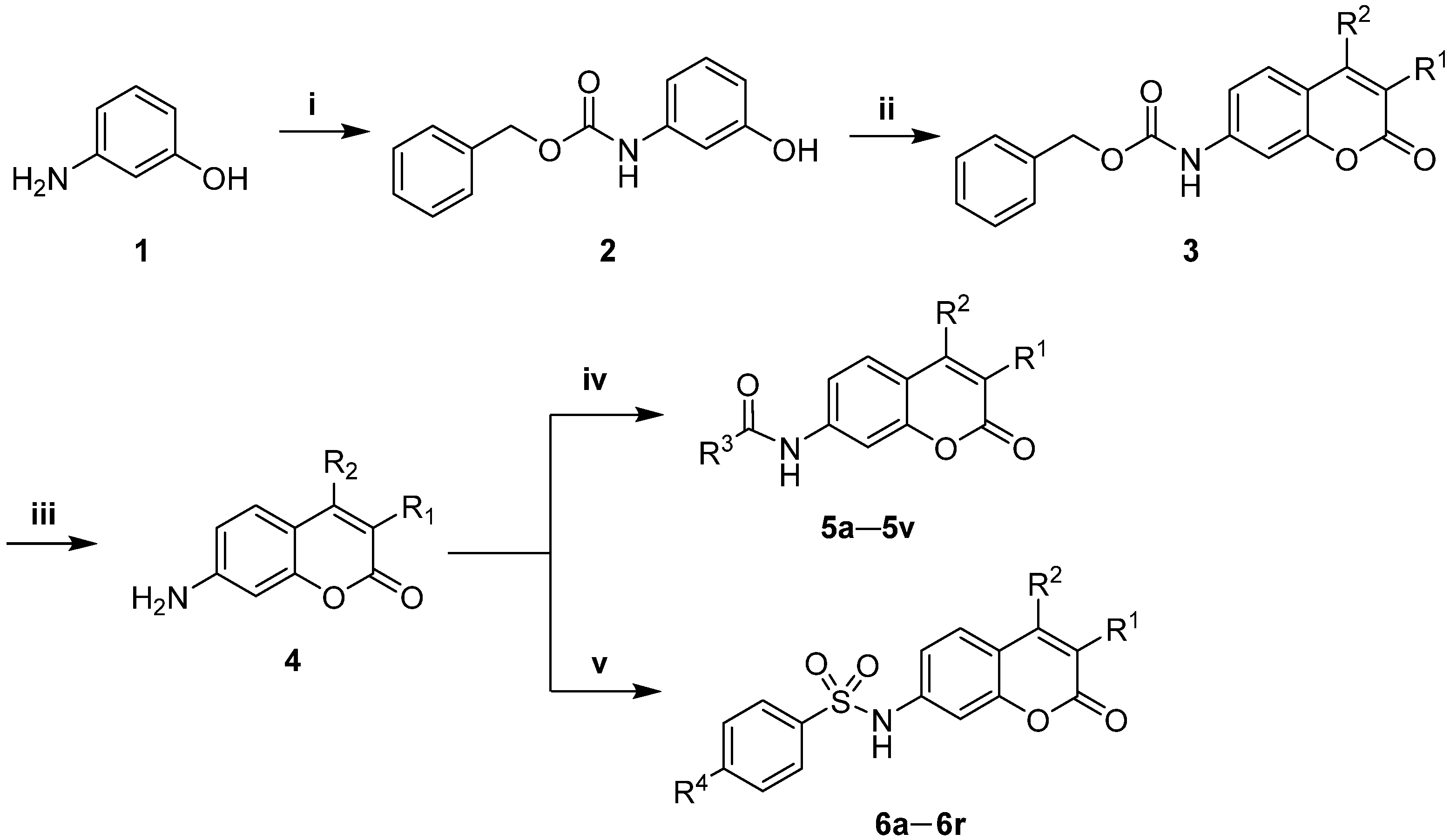
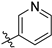
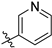
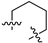
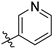
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of the Intermediates 4
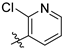
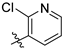
7-Aminocoumarins were synthesized through procedures reported in [
16,
25].
3.2.2. General Procedure for the Synthesis of the Intermediates 5a–5j
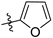
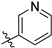
Compound 4 (2.5 mmol) was dissolved in dichloromethane (20.0 mL), and then triethylamine (20.0 mmol) was added to the solution and cooled to 0 °C. To the mixture, 5.0 mmol of aroyl chloride in 20 mL of dichloromethane solution was slowly added and stirred at room temperature for 2 h. After the reaction was completed, the reaction mixture was washed with water (100 mL × 3), and the organic phase was dried over with Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography using dichloromethane/methanol (Vdichloromethane/Vmethanol = 98:2→95:5) as the eluent to give compounds 5a–5j.
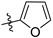
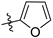
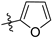
N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)furan-2-carboxamide (5a): a gray solid; mp: 270.1–271.6 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H), 7.98 (d, J = 0.8 Hz, 1H), 7.85 (s, 1H), 7.71 (s, 2H), 7.39 (d, J = 3.4 Hz, 1H), 6.73 (q, J = 3.4, 1.7 Hz, 1H), 2.34 (s, 3H), 2.06 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 161.5, 156.8, 152.2, 147.6, 146.9, 146.6, 141.3, 125.8, 119.8, 116.5, 116.3, 116.0, 112.8, 106.8, 15.2, 13.5; HR-MS (ESI): m/z calcd for C16H14NO4+ ([M + H]+) 284.0917, found 284.0924.
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)furan-2-carboxamide (5b): a gray solid; mp: 239.2–240.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.52 (s, 1H), 7.98 (d, J = 0.9 Hz, 1H), 7.87 (s, 1H), 7.73 (s, 2H), 7.40 (d, J = 3.4 Hz, 1H), 6.74 (dd, J = 3.5, 1.7 Hz, 1H), 2.56 (q, J = 7.4 Hz, 2H), 2.38 (s, 3H), 1.05 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 161.1, 156.9, 152.4, 147.6, 146.6, 146.6, 141.4, 126.0, 125.6, 116.5, 116.4, 116.0, 112.8, 106.8, 20.8, 14.7, 13.4; HR-MS (ESI): m/z calcd for C17H16NO4+ ([M + H]+) 298.1074, found 298.1080.
N-(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3-yl)furan-2-carboxamide (5c): a yellow solid; mp: 287.5–289.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.53 (s, 1H), 7.98 (s, 1H), 7.88 (s, 1H), 7.73 (d, J = 8.3 Hz, 1H), 7.67 (d, J = 8.7 Hz, 1H), 7.40 (d, J = 3.1 Hz, 1H), 6.74 (s, 1H), 2.77 (s, 2H), 2.41 (s, 2H), 1.87–1.52 (m, 4H); 13C NMR (126 MHz, DMSO-d6) δ 161.2, 157.0, 152.4, 147.9, 147.6, 146.4, 141.3, 124.5, 121.6, 116.7, 116.1, 115.8, 112.7, 107.3, 25.1, 24.2, 21.7, 21.4; HR-MS (ESI): m/z calcd for C18H16NO4+ ([M + H]+) 310.1074, found 310.1074.
N-(3-fluoro-4-methyl-2-oxo-2H-chromen-7-yl)furan-2-carboxamide (5d): a brown solid; mp: 268.2–270.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.56 (s, 1H), 7.98 (s, 1H), 7.93 (d, J = 1.5 Hz, 1H), 7.79 (dd, J = 8.8, 1.3 Hz, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.40 (d, J = 3.4 Hz, 1H), 6.74 (dd, J = 3.2, 1.5 Hz, 1H), 2.35 (d, J = 2.6 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 156.9, 155.0 (d, J = 30.0 Hz), 150.6 (d, J = 2.0 Hz), 147.5, 146.7, 141.7, 141.5 (d, J = 2.0 Hz), 131.8 (d, J = 14.0 Hz), 126.3 (d, J = 6.0 Hz), 117.2, 116.1, 115.1 (d, J = 3.0 Hz), 112.8, 107.1, 10.3 (d, J = 3.0 Hz); HR-MS (ESI): m/z calcd for C15H11FNO4+ ([M + H]+) 288.0667, found 288.0669.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)furan-2-carboxamide (5e): a yellow solid; mp: 300.0–301.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 7.99 (s, 1H), 7.93 (s, 1H), 7.82–7.75 (m, 2H), 7.42 (d, J = 3.3 Hz, 1H), 6.74 (d, J = 1.6 Hz, 1H), 2.52 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 156.9, 156.8, 151.8, 148.9, 147.4, 146.8, 142.6, 126.7, 118.0, 117.0, 116.2, 115.4, 112.9, 106.8, 16.4; HR-MS (ESI): m/z calcd for C15H10ClNO4+ ([M + H]+) 304.0371, found 304.0378.
N-(2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl)furan-2-carboxamide (5f): a yellow solid; mp: 240.6–242.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.72 (s, 1H), 8.03 (d, J = 1.8 Hz, 1H), 8.00 (d, J = 0.6 Hz, 1H), 7.83 (dd, J = 8.9, 1.8 Hz, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.44 (d, J = 3.3 Hz, 1H), 6.90 (s, 1H), 6.75 (q, J = 3.4, 1.6 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 159.6, 157.6, 155.5, 147.9, 147.5, 144.1, 140.2 (q, J = 32.1 Hz), 126.2, 122.8 (q, J = 276.1 Hz), 117.8, 117.0, 115.5 (d, J = 5.4 Hz), 113.4, 109.5, 108.1; HR-MS (ESI): m/z calcd for C15H9F3NO4+ ([M + H]+) 324.0478, found 324.0480.
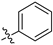
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)benzamide (5g): a yellow solid; mp: 243.3–244.9 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 7.98 (d, J = 7.3 Hz, 2H), 7.93 (d, J = 1.2 Hz, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.76–7.72 (m, 1H), 7.65–7.60 (m, 1H), 7.59–7.53 (m, 2H), 2.58 (q, J = 7.4 Hz, 2H), 2.41 (s, 3H), 1.06 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 166.5, 161.2, 152.4, 146.6, 142.0, 135.0, 132.4, 128.9, 128.3, 126.0, 125.5, 116.5, 116.4, 106.8, 20.8, 14.7, 13.4; HR-MS (ESI): m/z calcd for C19H18NO3+ ([M + H]+) 308.1281, found 308.1285.
N-(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3-yl)benzamide (5h): a yellow solid; mp: 276.8–277.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.58 (s, 1H), 7.97 (d, J = 7.2 Hz, 2H), 7.91 (d, J = 1.8 Hz, 1H), 7.72 (dd, J = 8.7, 1.8 Hz, 1H), 7.64 (dd, J = 16.6, 8.0 Hz, 2H), 7.56 (t, J = 7.4 Hz, 2H), 2.84–2.70 (m, 2H), 2.46–2.35 (m, 2H), 1.82–1.68 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 166.4, 161.2, 152.2, 147.6, 141.8, 135.0, 132.4, 128.9, 128.2, 124.6, 121.2, 116.5, 115.8, 106.8, 25.0, 24.1, 21.6, 21.2; HR-MS (ESI): m/z calcd for C20H18NO3+ ([M + H]+) 320.1281, found 320.1290.
N-(3-fluoro-4-methyl-2-oxo-2H-chromen-7-yl)benzamide (5i): a yellow solid; mp: 278.8–280.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.59 (s, 1H), 7.97 (d, J = 8.1 Hz, 3H), 7.78 (d, J = 8.6 Hz, 1H), 7.69 (d, J = 8.7 Hz, 1H), 7.64–7.59 (m, 1H), 7.55 (t, J = 7.4 Hz, 2H), 2.32 (d, J = 2.2 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 166.4, 154.9 (d, J = 28.0 Hz), 150.6, 144.1, 142.1, 141.65, 134.83, 132.39, 131.72 (d, J = 13.0 Hz), 128.88, 128.24, 126.07 (d, J = 6.0 Hz), 117.08, 114.94, 106.89, 10.22 (d, J = 3.0 Hz); HR-MS (ESI): m/z calcd for C17H13FNO3+ ([M + H]+) 298.0874, found 298.0880.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)benzamide (5j): a gray solid; mp: 276.0–278.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.71 (s, 1H), 8.00 (d, J = 1.9 Hz, 2H), 7.98 (s, 1H), 7.87 (d, J = 8.8 Hz, 1H), 7.81 (dd, J = 8.8, 1.8 Hz, 1H), 7.64 (t, J = 7.3 Hz, 1H), 7.57 (t, J = 7.4 Hz, 2H), 2.56 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 166.6, 156.8, 151.9, 148.9, 143.1, 134.8, 132.5, 128.9, 128.3, 126.7, 118.0, 117.0, 115.3, 106.8, 16.4; HR-MS (ESI): m/z calcd for C17H13ClNO3+ ([M + H]+) 314.0579, found 314.0583.
3.2.3. General Procedure for the Synthesis of the Intermediates 5k–5t
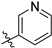
Compound 4 (2.5 mmol) was dissolved in tetrahydrofuran (20.0 mL), and then sodium bicarbonate (10.0 mmol) was added to the solution and cooled to 0 °C. To the mixture, 5.0 mmol of aroyl chloride in 20 mL of tetrahydrofuran solution was slowly added and stirred at room temperature for 2 h. After the reaction was completed, the reaction mixture was washed with water (100 mL × 3), and the organic phase was dried over with Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography using dichloromethane/methanol (Vdichloromethane/Vmethanol = 98:2→95:5) as the eluent to obtain compounds 5k–5t.
N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)nicotinamide (5k): a yellow solid; mp: 270.3–271.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.78 (s, 1H), 9.13 (d, J = 1.8 Hz, 1H), 8.79 (dd, J = 4.8, 1.5 Hz, 1H), 8.39–8.23 (m, 1H), 7.91 (d, J = 2.0 Hz, 1H), 7.72 (dd, J = 8.8, 2.0 Hz, 1H), 7.60 (dd, J = 7.5, 4.8 Hz, 1H), 2.39 (s, 3H), 2.10 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 164.9, 161.5, 152.9, 152.3, 149.2, 146.9, 141.5, 136.1, 130.7, 125.9, 124.0, 119.9, 116.5, 116.5, 106.9, 15.2, 13.5; HR-MS (ESI): m/z calcd for C17H14N2O3Na+ ([M + Na]+) 317.0897, found 317.0914.
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)nicotinamide (5l): a yellow solid; mp: 275.0–276.9 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.77 (s, 1H), 9.13 (d, J = 1.7 Hz, 1H), 8.79 (dd, J = 4.7, 1.3 Hz, 1H), 8.41–8.20 (m, 1H), 7.89 (d, J = 1.8 Hz, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.71 (dd, J = 8.8, 1.9 Hz, 1H), 7.60 (dd, J = 7.9, 4.8 Hz, 1H), 2.57 (q, J = 7.4 Hz, 2H), 2.40 (s, 3H), 1.06 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 164.9, 161.1, 152.8, 152.4, 149.2, 146.5, 141.6, 136.0, 130.6, 126.1, 125.7, 124.0, 116.6, 116.5, 106.9, 20.8, 14.7, 13.4; HR-MS (ESI): m/z calcd for C18H17N2O3+ ([M + H]+) 309.1234, found 309.1244.
N-(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3-yl)nicotinamide (5m): a gray solid; mp: 284.7–285.4 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.76 (s, 1H), 9.12 (d, J = 1.7 Hz, 1H), 8.79 (dd, J = 4.8, 1.4 Hz, 1H), 8.39–8.19 (m, 1H), 7.89 (s, 1H), 7.75–7.65 (m, 2H), 7.60 (dd, J = 7.8, 4.8 Hz, 1H), 2.94–2.61 (m, 2H), 2.37 (d, J = 32.1 Hz, 2H), 2.04–1.52 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 164.9, 161.2, 152.8, 152.1, 149.2, 147.5, 141.4, 136.0, 130.7, 124.6, 124.0, 121.4, 116.4, 116.0, 106.9, 25.0, 24.1, 21.6, 21.2; HR-MS (ESI): m/z calcd for C19H17N2O3+ ([M + H]+) 321.1234, found 321.1252.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)nicotinamide (5n): a brown solid; mp: 243.7–245.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.79 (s, 1H), 9.11 (s, 1H), 8.78 (d, J = 3.4 Hz, 1H), 8.29 (d, J = 7.5 Hz, 1H), 7.97–7.87 (m, 1H), 7.77 (d, J = 8.6 Hz, 1H), 7.71 (d, J = 8.6 Hz, 1H), 7.62–7.52 (m, 1H), 2.49 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 165.0, 156.7, 152.9, 151.8, 149.2, 148.8, 142.7, 136.1, 130.4, 126.7, 124.0, 118.1, 116.9, 115.5, 106.8, 16.4; HR-MS (ESI): m/z calcd for C16H12ClN2O3+ ([M + H]+) 337.0350, found 337.0363.
N-(2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl)nicotinamide (5o): a yellow solid; mp: 232.3–234.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.91 (s, 1H), 9.12 (s, 1H), 8.79 (d, J = 4.4 Hz, 1H), 8.31 (d, J = 7.7 Hz, 1H), 8.02 (s, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.67 (d, J = 8.4 Hz, 1H), 7.63–7.52 (m, 1H), 6.89 (s, 1H); 13C NMR (101 MHz, CF3COOD) δ 164.0, 162.1, 154.2, 146.2, 144.2 (q, J = 34.0 Hz), 143.7, 141.6, 140.5, 133.9, 128.0, 126.6, 122.2, 119.5, 118.7, 111.9, 109.7; HR-MS (ESI): m/z calcd for C16H10F3N2O3+ ([M + H]+) 335.0638, found 335.0656.
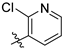
2-chloro-N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)nicotinamide (5p): a brown solid; mp: 259.7–260.4 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.04 (s, 1H), 8.57 (d, J = 3.3 Hz, 1H), 8.16 (dd, J = 18.6, 12.3 Hz, 1H), 7.79 (s, 1H), 7.74 (d, J = 8.7 Hz, 1H), 7.67–7.58 (m, 1H), 7.55 (t, J = 10.2 Hz, 1H), 2.35 (s, 3H), 2.08 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 164.4, 161.3, 152.2, 151.2, 146.9, 146.6, 141.0, 138.8, 133.2, 125.9, 123.6, 120.0, 116.5, 115.8, 106.3, 15.1, 13.4; HR-MS (ESI): m/z calcd for C17H13ClN2O3Na+ ([M + Na]+) 351.0507, found 351.0522.
2-chloro-N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)nicotinamide (5q): a white solid; mp: 237.5–239.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.04 (s, 1H), 8.58 (dd, J = 4.8, 1.8 Hz, 1H), 8.15 (dd, J = 7.5, 1.8 Hz, 1H), 7.80 (d, J = 1.9 Hz, 1H), 7.75 (d, J = 8.7 Hz, 1H), 7.61 (dd, J = 6.7, 4.0 Hz, 1H), 7.60–7.53 (m, 1H), 2.57 (q, J = 7.3 Hz, 2H), 2.39 (s, 3H), 1.06 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 164.4, 161.0, 152.4, 151.3, 146.9, 146.4, 141.2, 138.8, 133.2, 126.2, 125.8, 123.7, 116.7, 115.9, 106.3, 20.8, 14.6, 13.3; HR-MS (ESI): m/z calcd for C18H16ClN2O3+ ([M + H]+) 343.0844, found 343.0874.
2-chloro-N-(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3-yl)nicotinamide (5r): a yellow solid; mp: 275.5–274.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.03 (s, 1H), 8.58 (dd, J = 4.8, 1.8 Hz, 1H), 8.15 (dd, J = 7.5, 1.8 Hz, 1H), 7.78 (d, J = 1.6 Hz, 1H), 7.69–7.57 (m, 2H), 7.54 (dd, J = 8.7, 1.6 Hz, 1H), 2.72 (s, 2H), 2.39 (s, 2H), 1.74 (dd, J = 11.3, 5.9 Hz, 4H); 13C NMR (101 MHz, DMSO-d6) δ 164.4, 161.0, 152.2, 151.3, 147.3, 146.9, 140.9, 138.8, 133.2, 124.7, 123.7, 121.5, 116.1, 115.8, 106.3, 24.9, 24.0, 21.5, 21.2; HR-MS (ESI): m/z calcd for C19H15ClN2O3Na+ ([M + Na]+) 377.0663, found 377.0683.
2-chloro-N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)nicotinamide (5s): a brown solid; mp: 259.7–263.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.14 (s, 1H), 8.58 (dd, J = 4.7, 1.6 Hz, 1H), 8.16 (dd, J = 7.5, 1.6 Hz, 1H), 7.88–7.78 (m, 2H), 7.65–7.57 (m, 2H), 2.53 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 164.5, 156.6, 151.9, 151.4, 148.7, 146.9, 142.3, 138.8, 133.0, 126.9, 123.7, 118.3, 116.4, 115.7, 106.4, 16.4; HR-MS (ESI): m/z calcd for C16H11Cl2N2O3+ ([M + H]+) 349.0141, found 349.0145.
2-chloro-N-(2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl)nicotinamide (5t): a yellow solid; mp: 240.5–242.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.59 (dd, J = 4.8, 1.7 Hz, 1H), 8.16 (dd, J = 7.5, 1.6 Hz, 1H), 7.95 (d, J = 1.3 Hz, 1H), 7.72 (d, J = 8.4 Hz, 1H), 7.66 (dd, J = 8.9, 1.5 Hz, 1H), 7.62 (dd, J = 7.5, 4.9 Hz, 1H), 6.93 (s, 1H); 13C NMR (101 MHz, DMSO-d6) δ 164.7, 158.9, 155.0, 151.4, 147.0, 143.2, 139.6 (q, J = 32.4 Hz), 138.8, 132.9, 125.9, 123.6, 122.0 (q, J = 276.64 Hz), 116.7, 115.1 (d, J = 5.3 Hz), 109.3, 107.1; HR-MS (ESI): m/z calcd for C16H9ClF3N2O3+ ([M + H]+) 369.0248, found 369.0257.
3.2.4. General Procedure for the Synthesis of the Intermediates 6a–6r
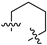
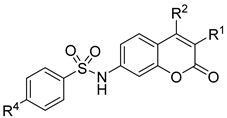
Compound 4 (2.5 mmol) was dissolved in tetrahydrofuran (20.0 mL), and then DMAP (0.25 mmol) was added to the solution and cooled to 0 °C. To the mixture, 5.0 mmol of aryl sulfonyl chloride in 20 mL of pyridine solution was slowly added and stirred at room temperature for 2 h. After the reaction was completed, the reaction mixture was washed with water (100 mL × 3), and the organic phase was dried over with Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography using petroleum ether/ethyl acetate (Vpetroleum ether/Vethyl acetate = 5:1→2:1) as the eluent to produce compounds 6a–6r.
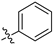
N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)benzenesulfonamide (6a): a yellow solid; mp: 257.0–258.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H), 7.85 (d, J = 7.4 Hz, 2H), 7.68–7.48 (m, 4H), 7.08 (dd, J = 8.7, 1.9 Hz, 1H), 7.02 (d, J = 1.9 Hz, 1H), 2.28 (s, 3H), 2.03 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 161.2, 152.4, 146.6, 140.5, 139.6, 133.7, 129.9, 127.2, 126.5, 119.9, 116.3, 115.2, 105.6, 15.1, 13.4; HR-MS (ESI): m/z calcd for C17H16SNO4+ ([M + H]+) 330.0795, found 330.0818.
N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)-4-methoxybenzenesulfonamide (6b): a white solid; mp: 207.2–208.6 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H), 7.78 (d, J = 8.9 Hz, 2H), 7.62 (d, J = 8.7 Hz, 1H), 7.12–7.04 (m, 3H), 7.01 (d, J = 1.9 Hz, 1H), 3.79 (s, 3H), 2.28 (s, 3H), 2.03 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 163.1, 161.2, 152.4, 146.6, 140.7, 131.1, 129.4, 126.5, 119.8, 116.1, 115.1, 115.0, 105.3, 56.1, 15.1, 13.4; HR-MS (ESI): m/z calcd for C18H17SNO5Na+ ([M + Na]+) 382.0720, found 382.0743.
N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)-4-fluorobenzenesulfonamide (6c): a yellow solid; mp: 238.5–240.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.92 (s, 1H), 7.91 (dd, J = 8.6, 5.1 Hz, 2H), 7.50 (d, J = 8.7 Hz, 1H), 7.39 (t, J = 8.7 Hz, 2H), 7.03 (d, J = 8.7 Hz, 1H), 6.99 (s, 1H), 2.15 (s, 3H), 1.93 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 166.2, 163.7, 161.1, 152.4, 146.4, 140.2, 136.0 (d, J = 3.0 Hz), 130.3 (d, J = 10.0 Hz), 126.5, 120.0, 117.1 (d, J = 23.0 Hz), 115.9 (d, J = 107.53 Hz), 105.8, 15.0, 13.3; HR-MS (ESI): m/z calcd for C17H14SFNO4Na+ ([M + Na]+) 370.0520, found 370.0539.
N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)-4-chlorobenzenesulfonamide (6d): a white solid; mp: 230.7–231.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.97 (s, 1H), 7.84 (d, J = 8.5 Hz, 2H), 7.64 (t, J = 8.9 Hz, 3H), 7.16–6.93 (m, 2H), 2.27 (s, 3H), 2.02 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 161.2, 152.4, 146.7, 140.2, 138.7, 138.4, 130.2, 129.1, 126.7, 120.1, 116.6, 115.5, 105.9, 15.2, 13.4; HR-MS (ESI): m/z calcd for C17H15SClNO4+ ([M + H]+) 364.0405, found 364.0429.
N-(3,4-dimethyl-2-oxo-2H-chromen-7-yl)-4-bromobenzenesulfonamide (6e): a yellow solid; mp: 238.4–240.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.95 (s, 1H), 7.80 (d, J = 8.7 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.7 Hz, 1H), 7.07 (dd, J = 8.7, 2.1 Hz, 1H), 7.03 (d, J = 2.0 Hz, 1H), 2.29 (s, 3H), 2.04 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 161.2, 152.4, 146.6, 140.1, 138.8, 133.1, 129.2, 127.7, 126.7, 120.1, 116.6, 115.4, 105.9, 15.1, 13.4; HR-MS (ESI): m/z calcd for C17H14SBrNO4Na+ ([M + Na]+) 429.9719, found 429.9743.
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)benzenesulfonamide (6f): a gray solid; mp: 199.9–200.4 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.92 (s, 1H), 7.89–7.81 (m, 2H), 7.64–7.62 (m, 2H), 7.60–7.56 (m, 2H), 7.08 (dd, J = 8.7, 2.2 Hz, 1H), 7.04 (dd, J = 8.8, 2.2 Hz, 1H), 2.52–2.50 (m, 2H), 2.30 (s, 3H), 0.99 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 160.8, 152.5, 146.5, 140.6, 139.6, 133.8, 130.0, 127.2, 126.9, 125.7, 116.5, 115.3, 105.6, 20.7, 14.6, 13.3; HR-MS (ESI): m/z calcd for C18H17SNO4Na+ ([M + Na]+) 366.0771, found 366.0794.
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)-4-methoxybenzenesulfonamide (6g): a white solid; mp: 204.4–205.6 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.77 (s, 1H), 7.78 (d, J = 8.8 Hz, 2H), 7.63 (d, J = 8.7 Hz, 1H), 7.12–7.05 (m, 3H), 7.02 (t, J = 5.3 Hz, 1H), 3.78 (s, 3H), 2.51 (q, J = 6.0 Hz, 2H), 2.30 (s, 3H), 1.00 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 163.1, 160.8, 152.5, 146.4, 140.8, 131.1, 129.4, 126.7, 125.6, 116.2, 115.1, 115.0, 105.3, 56.1, 20.7, 14.6, 13.3; HR-MS (ESI): m/z calcd for C19H19SNO5Na+ ([M + Na]+) 396.0876, found 396.0907.
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)-4-fluorobenzenesulfonamide (6h): a gray solid; mp: 209.8–211.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.93 (s, 1H), 7.98–7.79 (m, 2H), 7.65 (d, J = 8.7 Hz, 1H), 7.42 (t, J = 8.8 Hz, 2H), 7.07 (dd, J = 8.7, 2.1 Hz, 1H), 7.03 (d, J = 2.1 Hz, 1H), 2.52 (d, J = 7.6 Hz, 2H), 2.31 (s, 3H), 1.00 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 166.2, 163.7, 160.7, 152.5, 146.3, 140.4, 135.9 (d, J = 3.0Hz), 130.4 (d, J = 10.0Hz), 126.3 (d, J = 97.0Hz), 117.2 (d, J = 23.0Hz), 116.5, 115.4, 105.8, 20.7, 14.5, 13.2; HR-MS (ESI): m/z calcd for C18H16SFNO4Na+ ([M + Na]+) 384.0676, found 384.0709.
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)-4-chlorobenzenesulfonamide (6i): a white solid; mp: 214.6–216.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.93 (s, 1H), 7.98–7.79 (m, 2H), 7.65 (d, J = 8.7 Hz, 1H), 7.42 (t, J = 8.8 Hz, 2H), 7.07 (dd, J = 8.7, 2.1 Hz, 1H), 7.03 (d, J = 2.1 Hz, 1H), 2.52 (d, J = 7.6 Hz, 2H), 2.31 (s, 3H), 1.00 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 160.7, 152.5, 146.3, 140.2, 138.7, 138.4, 130.1, 129.1, 126.8, 125.9, 116.7, 115.5, 105.9, 20.7, 14.6, 13.2; HR-MS (ESI): m/z calcd for C18H17SClNO4+ ([M + H]+) 378.0561, found 378.0592.
N-(3-ethyl-4-methyl-2-oxo-2H-chromen-7-yl)-4-bromobenzenesulfonamide (6j): an orange solid; mp: 215.7–217.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.98 (s, 1H), 7.85–7.69 (m, 4H), 7.60 (d, J = 8.7 Hz, 1H), 7.06 (dd, J = 8.7, 1.9 Hz, 1H), 7.02 (d, J = 1.8 Hz, 1H), 2.52–2.41 (m, 2H), 2.26 (s, 3H), 0.97 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 160.7, 152.5, 146.3, 140.2, 138.8, 133.1, 129.2, 127.7, 126.9, 125.9, 116.7, 115.4, 105.9, 20.7, 14.6, 13.3; HR-MS (ESI): m/z calcd for C20H20SBrNO4+ ([M + H]+) 422.0056, found 422.0053.
N-(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3-yl)-4-methoxybenzenesulfonamide (6k): a white solid; mp: 218.5–220.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.72 (s, 1H), 7.77 (d, J = 8.9 Hz, 2H), 7.56 (d, J = 8.6 Hz, 1H), 7.07 (dd, J = 10.9, 5.3 Hz, 3H), 7.02 (d, J = 1.9 Hz, 1H), 3.79 (s, 3H), 2.68 (d, J = 5.5 Hz, 2H), 2.37 (s, 2H), 1.73–1.69 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 163.1, 160.8, 152.3, 147.4, 140.6, 131.1, 129.4, 125.3, 121.3, 115.7, 115.1, 115.0, 105.5, 56.1, 24.9, 24.0, 21.5, 21.1; HR-MS (ESI): m/z calcd for C20H20SNO5+ ([M + H]+) 386.1056, found 386.1052.
N-(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3-yl)-4-fluorobenzenesulfonamide (6l): a yellow solid; mp: 215.8–217.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H), 7.89 (ddd, J = 8.1, 5.1, 2.5 Hz, 2H), 7.58 (d, J = 8.6 Hz, 1H), 7.42 (dd, J = 12.3, 5.4 Hz, 2H), 7.07 (dd, J = 8.6, 2.1 Hz, 1H), 7.04 (d, J = 2.1 Hz, 1H), 2.69 (s, 2H), 2.37 (s, 2H), 1.83–1.54 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 166.2, 163.7, 160.8, 152.3, 147.4, 140.1, 135.9 (d, J = 3.0 Hz), 130.3 (d, J = 9.0 Hz), 125.4, 121.6, 117.2 (d, J = 23.0 Hz), 115.7 (d, J = 58.0 Hz), 105.9, 24.9, 24.0, 21.5, 21.1; HR-MS (ESI): m/z calcd for C19H17SFNO4+ ([M + H]+) 374.0856, found 374.0855.
N-(6-oxo-7,8,9,10-tetrahydro-6H-benzo[c]chromen-3-yl)-4-bromobenzenesulfonamide (6m): a pink solid; mp: 217.7–219.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.95 (s, 1H), 7.86–7.66 (m, 4H), 7.48 (d, J = 8.5 Hz, 1H), 7.10–6.97 (m, 2H), 2.60 (s, 2H), 2.32 (s, 2H), 1.66 (d, J = 4.3 Hz, 4H); 13C NMR (101 MHz, DMSO-d6) δ 160.8, 152.3, 147.4, 140.0, 138.8, 133.1, 129.2, 127.7, 125.5, 121.7, 116.1, 115.5, 106.0, 24.9, 24.0, 21.5, 21.1; HR-MS (ESI): m/z calcd for C19H17SBrNO4+ ([M + H]+) 434.0056, found 434.0052.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)benzenesulfonamide (6n): a yellow solid; mp: 260.2–261.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.09 (s, 1H), 7.94–7.80 (m, 2H), 7.67–7.61 (m, 2H), 7.61–7.54 (m, 2H), 7.12 (dt, J = 15.1, 7.6 Hz, 1H), 7.06 (d, J = 2.1 Hz, 1H), 2.40 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 157.0, 152.5, 149.2, 142.2, 140.0, 134.4, 130.5, 128.0, 127.7, 118.6, 116.0, 115.9, 105.8, 16.8; HR-MS (ESI): m/z calcd for C16H13SClNO4+ ([M + H]+) 350.0248, found 350.0246.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)-4-methoxybenzenesulfonamide (6o): a yellow solid; mp: 242.9–244.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.93 (s, 1H), 7.80 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 8.8 Hz, 1H), 7.16–7.12 (m, 1H), 7.10 (d, J = 8.9 Hz, 1H), 7.07 (d, J = 2.0 Hz, 1H), 3.80 (s, 1H), 2.47 (s, 1H); 13C NMR (101 MHz, DMSO-d6) δ 163.2, 156.5, 152.1, 148.7, 142.0, 131.0, 129.5, 127.4, 118.0, 115.3, 115.2, 115.1, 105.1, 56.1, 16.3; HR-MS (ESI): m/z calcd for C17H15SClNO5+ ([M + H]+) 380.0354, found 380.0360.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)-4-fluorobenzenesulfonamide (6p): a yellow solid; mp: 234.4–235.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.09 (s, 1H), 7.97–7.88 (m, 2H), 7.65 (d, J = 8.7 Hz, 1H), 7.41 (t, J = 8.8 Hz, 2H), 7.11 (dd, J = 8.7, 2.0 Hz, 1H), 7.05 (d, J = 2.0 Hz, 1H), 2.39 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 166.3, 163.8, 156.4, 152.0, 148.6, 141.5, 135.9 (d, J = 3.0 Hz), 130.3 (d, J = 9.0 Hz), 127.4, 118.2, 117.2 (d, J = 23.0 Hz), 115.5 (d, J = 10.0 Hz), 105.5, 16.3; HR-MS (ESI): m/z calcd for C16H12SFClNO4+ ([M + H]+) 368.0154, found 368.0153.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)-4-chlorobenzenesulfonamide (6q): a yellow solid; mp: 220.1–221.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.13 (s, 1H), 7.87 (d, J = 8.6 Hz, 2H), 7.75 (d, J = 8.7 Hz, 1H), 7.67 (d, J = 8.6 Hz, 2H), 7.14 (dd, J = 8.7, 2.1 Hz, 1H), 7.09 (d, J = 2.0 Hz, 1H), 2.48 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 156.5, 152.1, 148.7, 141.4, 138.8, 138.3, 130.2, 129.1, 127.6, 118.3, 115.7, 115.7, 105.7, 16.4; HR-MS (ESI): m/z calcd for C16H12SCl2NO4+ ([M + H]+) 383.9858, found 383.9842.
N-(3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)-4-bromobenzenesulfonamide (6r): a yellow solid; mp: 207.8–209.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.13 (s, 1H), 7.82 (d, J = 8.8 Hz, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.74 (d, J = 8.7 Hz, 1H), 7.14 (dd, J = 8.7, 1.9 Hz, 1H), 7.09 (d, J = 1.9 Hz, 1H), 2.47 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 156.5, 152.1, 148.7, 141.4, 138.7, 133.2, 129.2, 127.9, 127.7, 118.3, 115.7, 105.6, 16.4; HR-MS (ESI): m/z calcd for C16H12SClBrNO4+ ([M + H]+) 429.9331, found 429.9325.