2D Leaf-Like Structured ZIF-L Embedded Electrochemically Reduced Graphene Oxide Composite as an Electrochemical Sensing Platform for Sensitively Detecting Benomyl
Abstract
1. Introduction
2. Results and Discussion
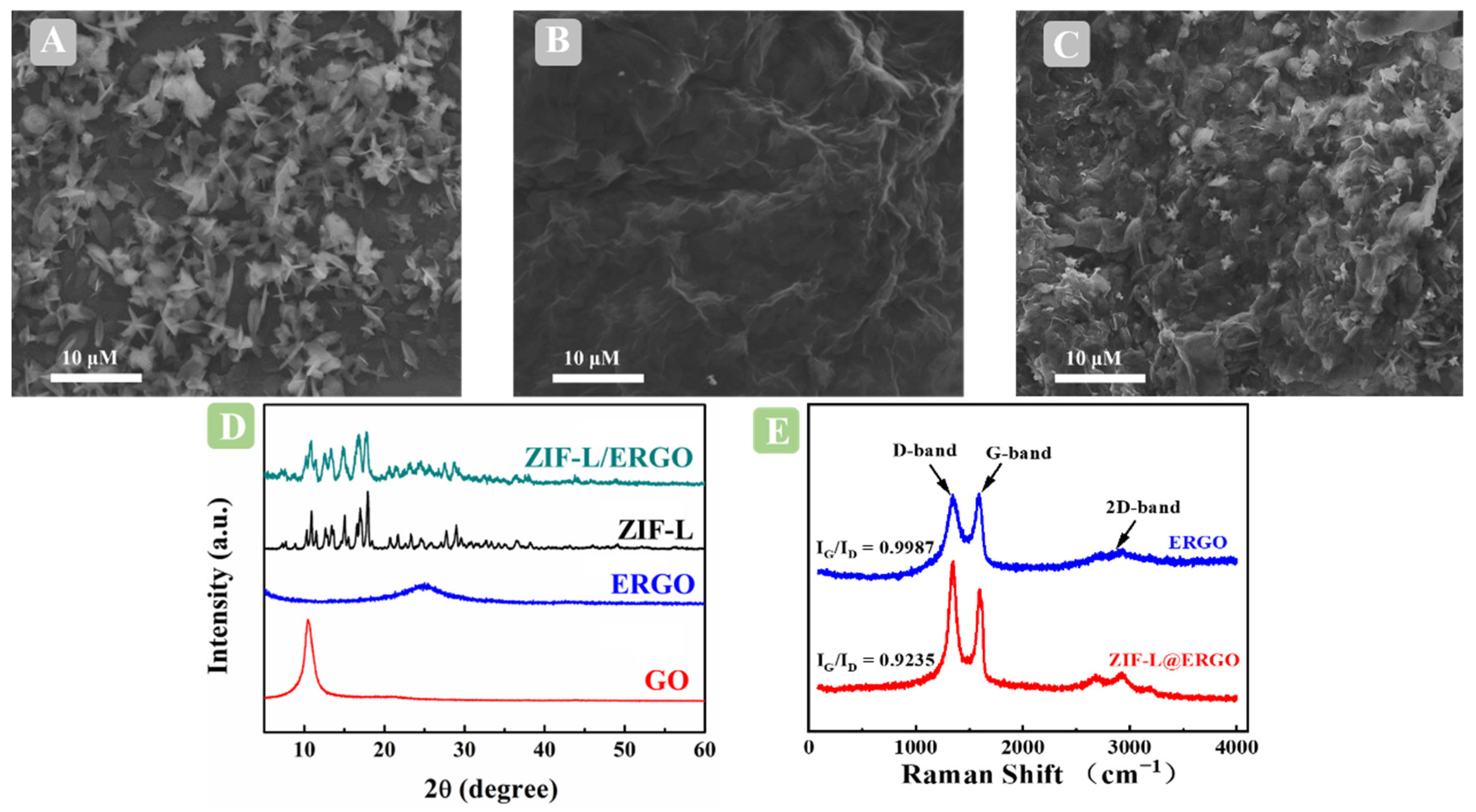
2.1. Structural Characterization
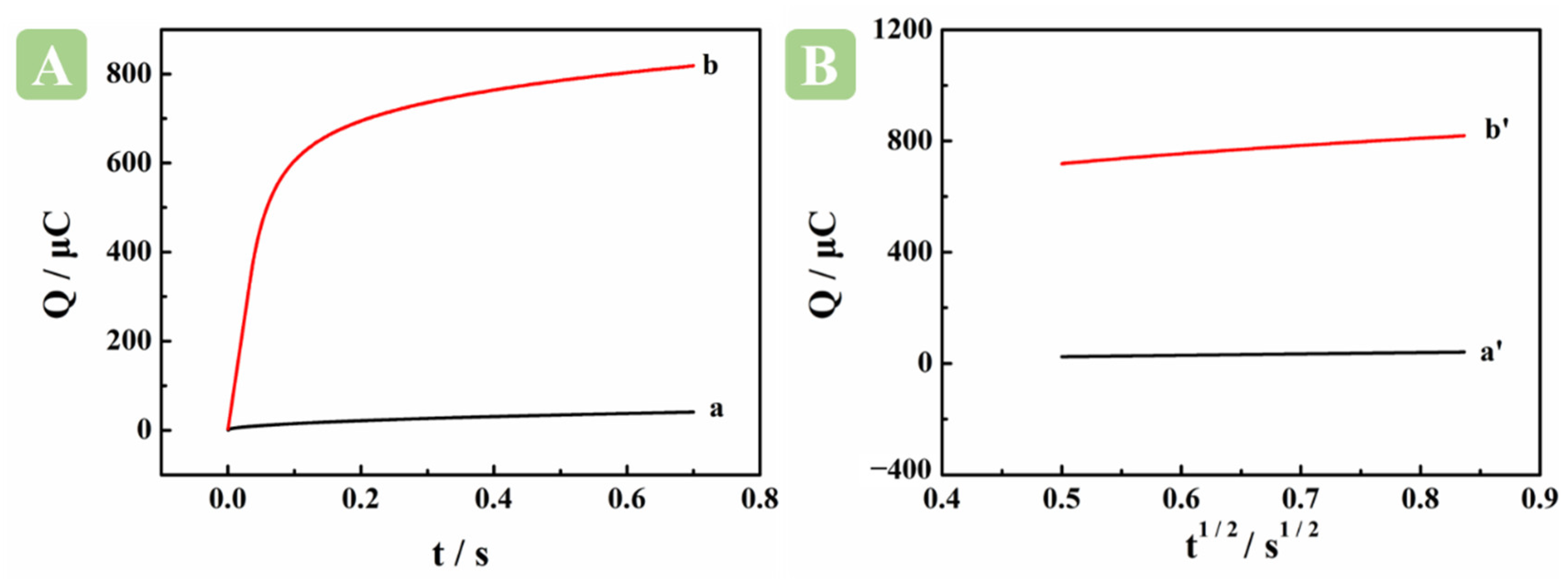
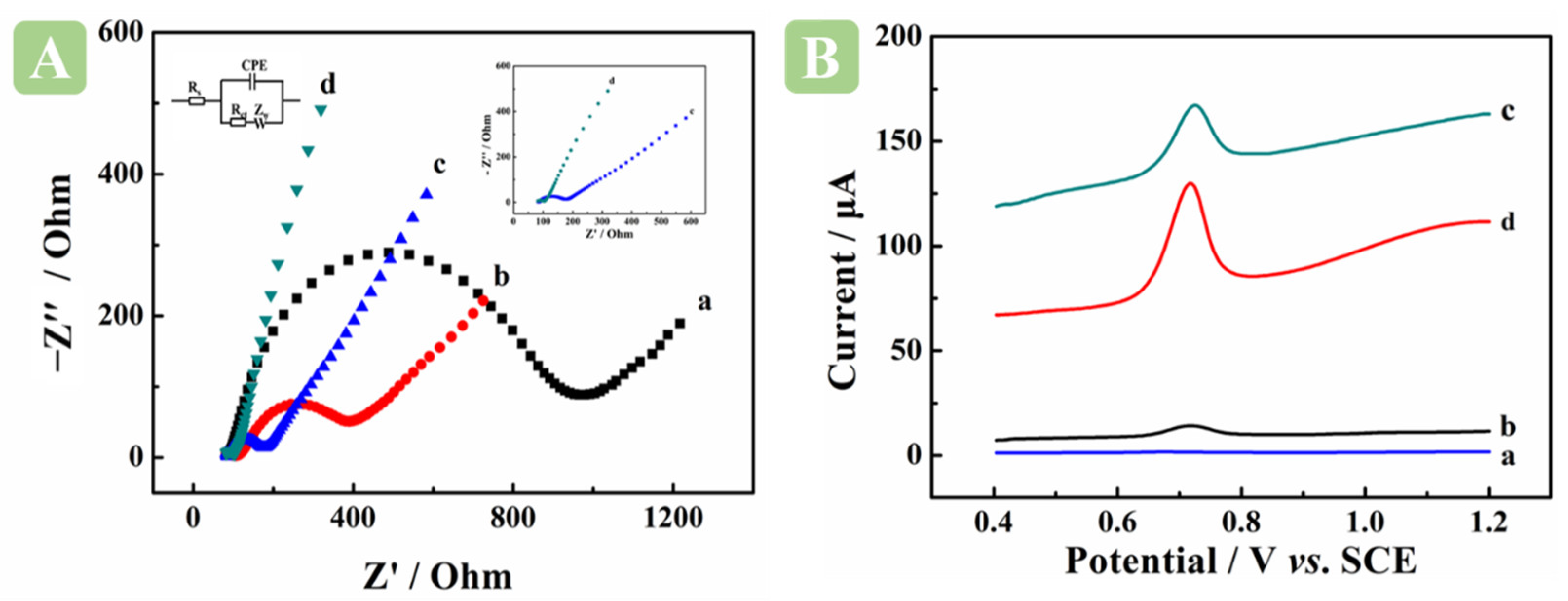
2.2. Electrochemical Characterization
2.3. Electrochemical Behavior of BM
2.4. Optimization of Analytical Parameters
2.4.1. Impact of the GO@ZIF-L Volume
2.4.2. Impact of Accumulation Time
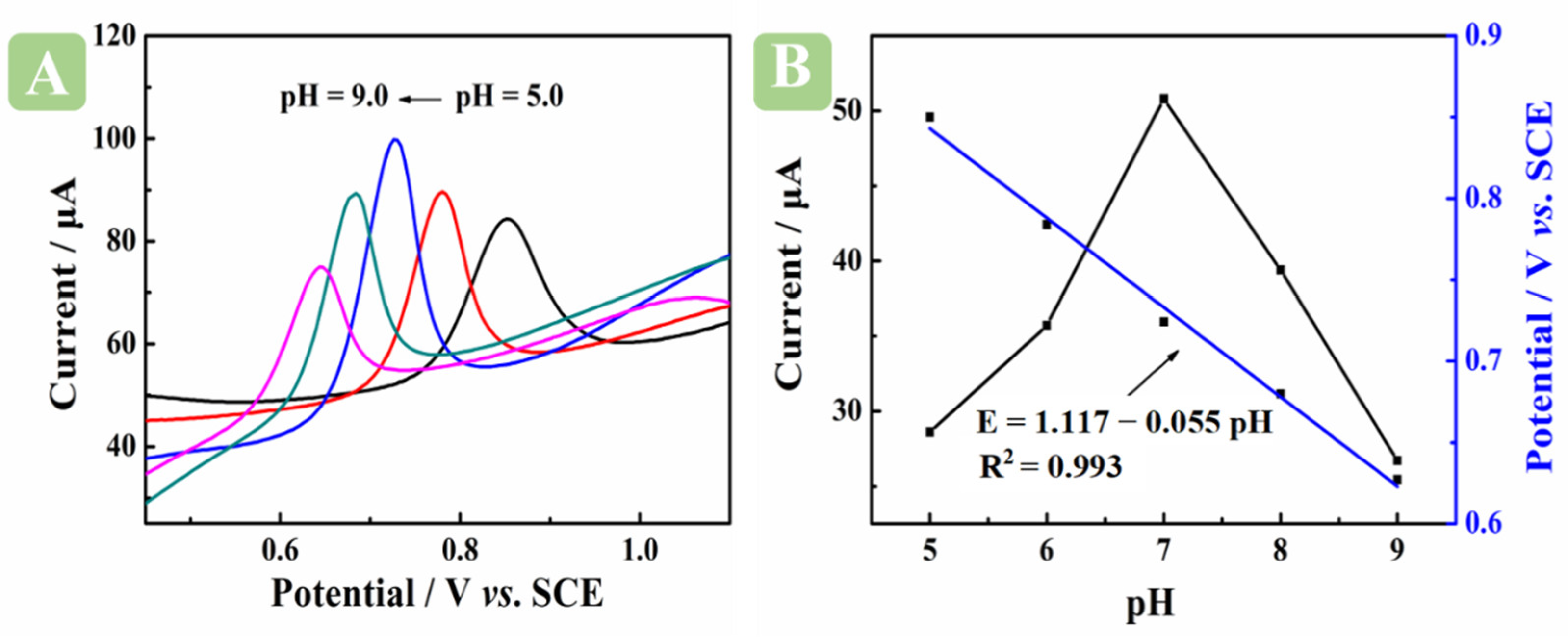
2.4.3. Impact of Buffer Solution pH
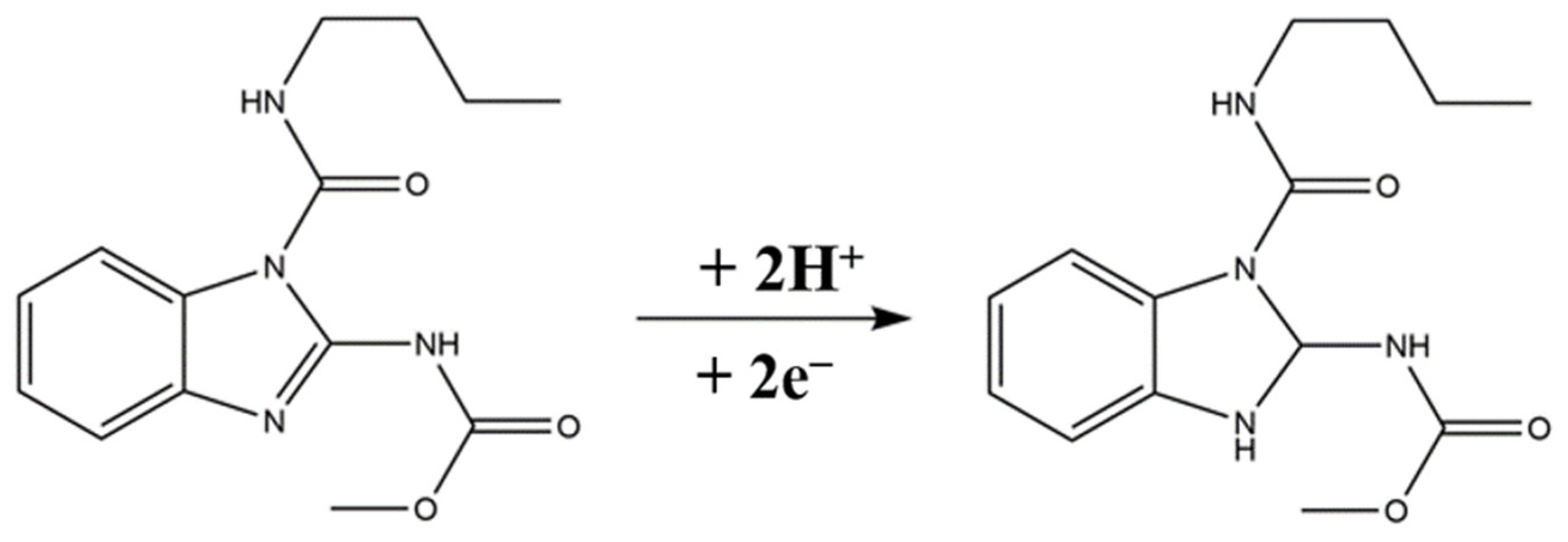
2.5. Electrocatalytic Mechanism
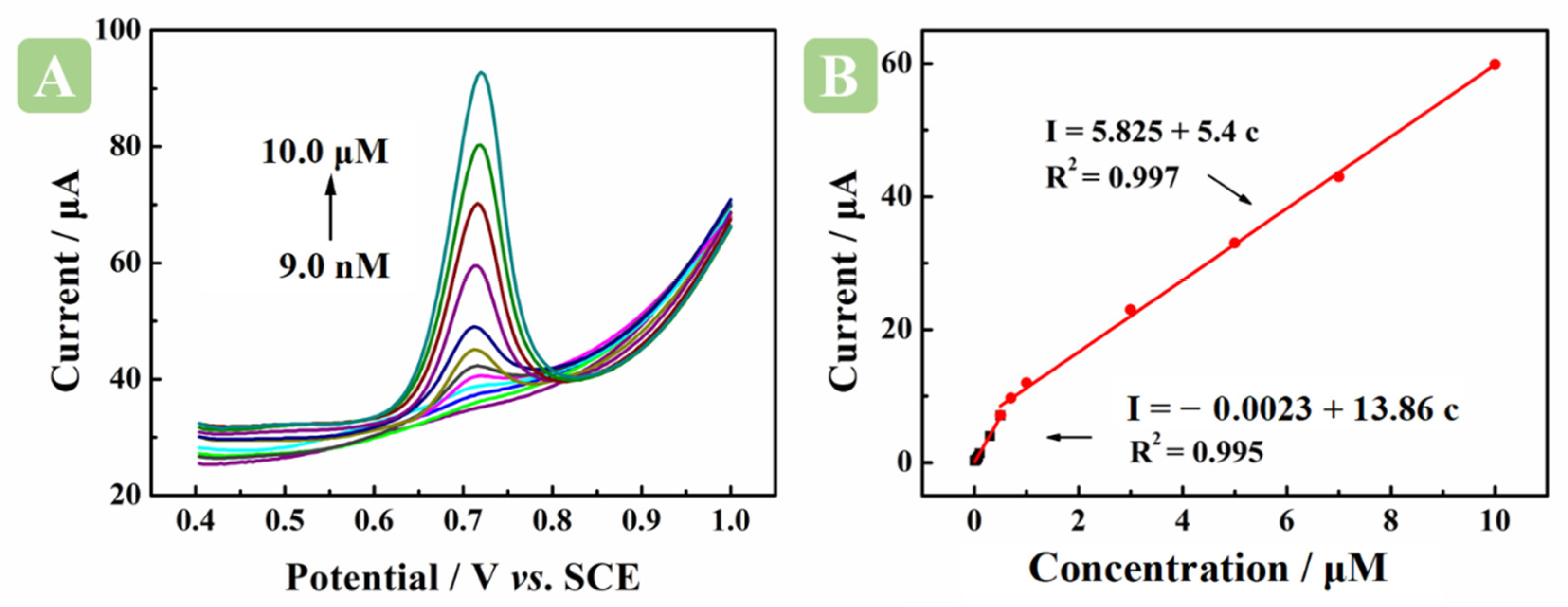
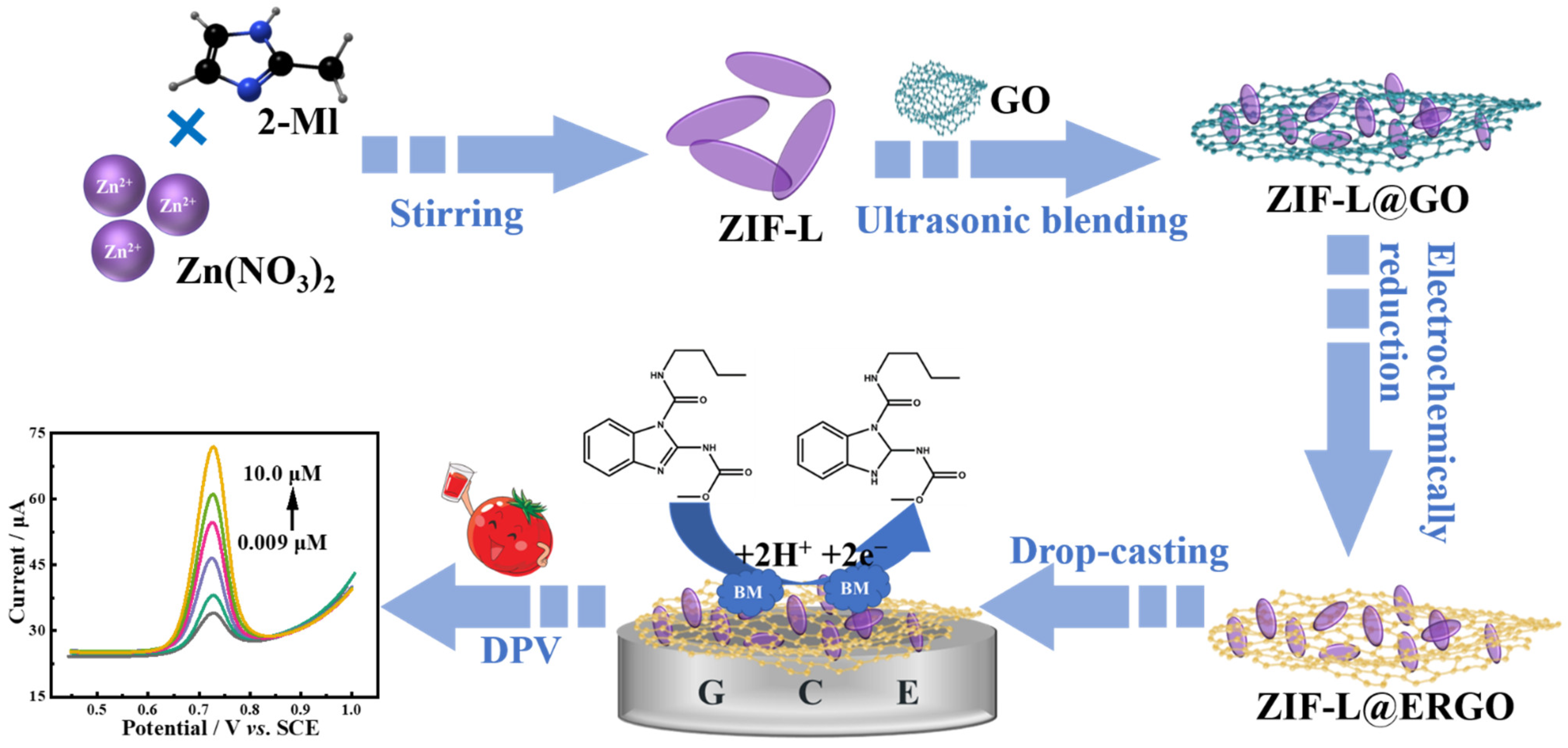
2.6. Determination of BM
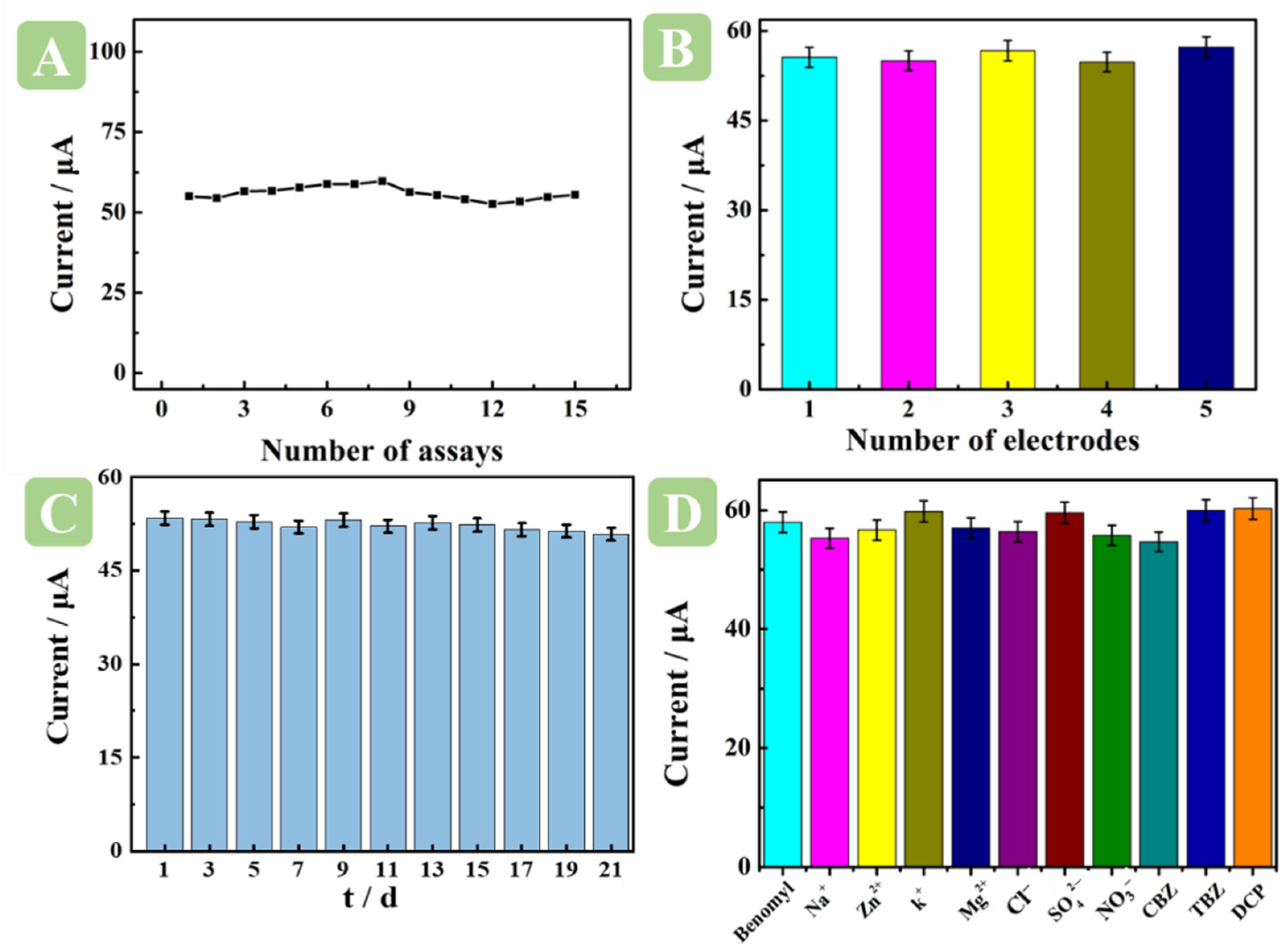
2.7. Repeatability, Reproducibility, Stability, and Anti-Interference
2.8. Real Sample Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Apparatuses
3.3. Preparation of GO@ZIF-L
3.4. Preparation of Modified Electrodes
3.5. Preparation of Practical Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, Y.; Xu, J.; Zhu, Y.; Duan, Y.; Zhou, M. Mechanism of Action of the Benzimidazole Fungicide on Fusarium graminearum: Interfering with Polymerization of Monomeric Tubulin but not Polymerized Microtubule. Phytopathology 2016, 106, 807–813. [Google Scholar] [CrossRef]
- He, X.; Cui, Y.; Yang, C. Thiol–Yne Click Postsynthesis of a Sulfonate Group-Enriched Magnetic Microporous Organic Network for Efficient Extraction of Benzimidazole Fungicides. ACS Appl. Mater. Interfaces 2021, 13, 39905–39914. [Google Scholar] [CrossRef] [PubMed]
- Rathinasamy, K.; Panda, D. Suppression of microtubule dynamics by benomyl decreases tension across kinetochore pairs and induces apoptosis in cancer cells. FEBS J. 2006, 273, 4114–4128. [Google Scholar] [CrossRef] [PubMed]
- Urani, C.; Chiesara, E.; Galvani, P.; Marabini, L.; Santagostino, A.; Camatini, M. Benomyl affects the microtubule cytoskeleton and the glutathione level of mammalian primary cultured hepatocytes. Toxicol. Lett. 1995, 76, 135–144. [Google Scholar] [CrossRef]
- Reyes, J.F.G.; Barrales, P.O.; Díaz, A.M. Gel-surface enhanced fluorescence sensing system coupled to a continuous-flow assembly for simultaneous monitoring of benomyl and carbendazim. Anal. Chim. Acta 2003, 493, 35–45. [Google Scholar] [CrossRef]
- Navickiene, S.; Possidonio De Amarante Junior, O.; Brito, N.M.; Graciolli, L.A.; Ribeiro, M.L. Determination of Benomyl Residues in Shiitake Mushrooms (Lentinula edodes) by Liquid Chromatography with UV Detection. J. Chromatogr. Sci. 2007, 45, 340–344. [Google Scholar] [CrossRef]
- Sánchez, F.G.; Díaz, A.N.; Torijas, M.C. Selective determination of carbaryl and benomyl by fluorescence polarization. Anal. Chim. Acta 2000, 414, 25–32. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Dong, Y.; Zhang, L. One-Step Fabrication of a Multifunctional Magnetic Nickel Ferrite/Multi-walled Carbon Nanotubes Nanohybrid-Modified Electrode for the Determination of Benomyl in Food. J. Agric. Food Chem. 2015, 63, 4746–4753. [Google Scholar] [CrossRef]
- Zhong, W.; Zou, J.; Xie, Y.; Yang, J.; Li, M.; Liu, S.; Gao, Y.; Wang, X.; Lu, L. Three-dimensional nano-CuxO-MWCNTs-COOH/MXene heterostructure: An efficient electrochemical platform for highly sensitive and selective sensing of benomyl in fruit samples. J. Electroanal. Chem. 2022, 920, 116586–116595. [Google Scholar] [CrossRef]
- Zhong, W.; Zou, J.; Yu, Q.; Gao, Y.; Qu, F.; Liu, S.; Zhou, H.; Lu, L. Ultrasensitive indirect electrochemical sensing of thiabendazole in fruit and water by the anodic stripping voltammetry of Cu2+ with hierarchical Ti3C2Tx-TiO2 for signal amplification. Food Chem. 2023, 402, 134379–134387. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, F.; Zou, J.; Liu, L.; Li, M.; Gao, Y.; Liu, S.; Li, M.; Lu, L. MXene@Ag-based ratiometric electrochemical sensing strategy for effective detection of carbendazim in vegetable samples. Food Chem. 2021, 360, 130006–130012. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, J.; Pang, H. Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coord. Chem. Rev. 2020, 410, 213222–213261. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Q.; Wen, Z.; Zhu, W.; Khan, D.; Hu, C.; Huang, T.; Ding, W.; Zou, J. Nano Fe and Mg2Ni derived from TMA-TM (TM = Fe, Ni) MOFs as synergetic catalysts for hydrogen storage in MgH2. Sustain. Energy Fuels 2020, 4, 2192–2200. [Google Scholar] [CrossRef]
- Rostami, S.; Nakhaei Pour, A.; Salimi, A.; Abolghasempour, A. Hydrogen adsorption in metal- organic frameworks (MOFs): Effects of adsorbent architecture. Int. J. Hydrogen. Energ. 2018, 43, 7072–7080. [Google Scholar] [CrossRef]
- Cardenal, A.; Park, H.; Chalker, C.; Ortiz, K.; Powers, D. Cis-decalin oxidation as a stereochemical probe of in-MOF versus on-MOF catalysis. Chem. Commun. 2017, 53, 7377–7380. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Gao, F.; Ma, X.; Zou, J.; Yu, Y.; Li, M.; Qu, F.; Huang, X.; Lu, L. Mxene/carbon nanohorn/β-cyclodextrin-Metal-organic frameworks as high-performance electrochemical sensing platform for sensitive detection of carbendazim pesticide. J. Hazard. Mater. 2020, 396, 122776–122784. [Google Scholar] [CrossRef]
- Gao, F.; Yan, Z.; Cai, Y.; Yang, J.; Zhong, W.; Gao, Y.; Liu, S.; Li, M.; Lu, L. 2D leaf-like ZIF-L decorated with multi-walled carbon nanotubes as electrochemical sensing platform for sensitively detecting thiabendazole pesticide residues in fruit samples. Anal. Bioanal. Chem. 2021, 413, 7485–7494. [Google Scholar] [CrossRef]
- Wang, L.; Meng, T.; Fan, Y.; Chen, C.; Guo, Z.; Wang, H.; Zhang, Y. Electrochemical study of acetaminophen oxidation by gold nanoparticles supported on a leaf-like zeolitic imidazolate framework. J. Colloid Interface Sci. 2018, 524, 1–7. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, Z.; Cao, K.; Nair, S.; Cheng, X.; Zhao, J.; Gomaa, H.; Wu, H.; Pan, F. Pervaporation performance comparison of hybrid membranes filled with two-dimensional ZIF-L nanosheets and zero-dimensional ZIF-8 nanoparticles. J. Membr. Sci. 2017, 523, 185–196. [Google Scholar] [CrossRef]
- Xiao, H.; Zhou, H.; Feng, S.; Gore, D.B.; Zhong, Z.; Xing, W. In situ growth of two-dimensional ZIF-L nanoflakes on ceramic membrane for efficient removal of iodine. J. Membr. Sci. 2021, 619, 118782–118790. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, M.; Jiang, H.; Chen, R. Controllable Synthesis of Pd-ZIF-L-GO: The Role of Drying Temperature. Ind. Eng. Chem. Res. 2021, 60, 4847–4859. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Xiao, K.; Cheng, S.; Zhang, T.; Qian, G.; Zhang, Q.; Feng, Y. N-Doped hierarchically porous carbon derived from heterogeneous core–shell ZIF-L(Zn)@ZIF-67 for supercapacitor application. New J. Chem. 2018, 42, 6719–6726. [Google Scholar] [CrossRef]
- Sakthivel, R.; Lin, L.; Duann, Y.; Chen, H.; Su, C.; Liu, X.; He, J.; Chung, R. MOF-Derived Cu-BTC Nanowire-Embedded 2D Leaf-like Structured ZIF Composite-Based Aptamer Sensors for Real-Time In Vivo Insulin Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 28639–28650. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, X.; Huo, D.; Liu, H.; Yang, M.; Hou, C. Simultaneous detection of Cd2+ and Pb2+ in food based on sensing electrode prepared by conductive carbon paper, rGO and CoZn·MOF (CP-rGO-CoZn·MOF). Anal. Chim. Acta 2022, 1220, 339812–339821. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Z.; Yang, L.; Shi, H.; Fang, D.; Wang, M.; Shao, P.; Luo, X. Electrochemical approach toward reduced graphene oxide-based electrodes for environmental applications: A review. Sci. Total Environ. 2021, 778, 146301–146315. [Google Scholar] [CrossRef] [PubMed]
- Alayande, A.B.; Park, H.D.; Vrouwenvelder, J.S.; Kim, I.S. Implications of Chemical Reduction Using Hydriodic Acid on the Antimicrobial Properties of Graphene Oxide and Reduced Graphene Oxide Membranes. Small 2019, 15, 1901023–1901031. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xu, W.; Jin, H.; Zhu, M.; Yuan, W.; Hao, X. Purified dispersions of graphene in a nonpolar solvent via solvothermal reduction of graphene oxide. Chem. Commun. 2015, 51, 3824–3827. [Google Scholar] [CrossRef]
- Morawski, A.W.; Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Wróbel, R.J.; Ohtani, B.; Aksienionek, M.; Lipińska, L. Photocatalytic degradation of acetic acid in the presence of visible light-active TiO2-reduced graphene oxide photocatalysts. Catal. Today 2017, 280, 108–113. [Google Scholar] [CrossRef]
- Cai, L.; Yu, G. Recent Advances in Growth and Modification of Graphene-Based Energy Materials: From Chemical Vapor Deposition to Reduction of Graphene Oxide. Small Methods 2019, 3, 1900071. [Google Scholar] [CrossRef]
- Ma, B.; Guo, H.; Wang, M.; Li, L.; Jia, X.; Chen, H.; Xue, R.; Yang, W. Electrocatalysis of Cu−MOF/Graphene Composite and its Sensing Application for Electrochemical Simultaneous Determination of Dopamine and Paracetamol. Electroanalysis 2019, 31, 1002–1008. [Google Scholar] [CrossRef]
- Tu, X.; Xie, Y.; Ma, X.; Gao, F.; Gong, L.; Wang, D.; Lu, L.; Liu, G.; Yu, Y.; Huang, X. Highly stable reduced graphene oxide-encapsulated Ce-MOF composite as sensing material for electrochemically detecting dichlorophen. J. Electroanal. Chem. 2019, 848, 113268–113276. [Google Scholar] [CrossRef]
- Li, Z.; Gou, M.; Yue, X.; Tian, Q.; Yang, D.; Qiu, F.; Zhang, T. Facile fabrication of bifunctional ZIF-L/cellulose composite membrane for efficient removal of tellurium and antibacterial effects. J. Hazard. Mater. 2021, 416, 125888–125898. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, R.; Zou, J.; Zhang, T.; Zhang, S.; Zhang, Z.; Hu, X.; Yan, Y.; Ling, X. MNPs@anionic MOFs/ERGO with the size selectivity for the electrochemical determination of H2O2 released from living cells. Biosens. Bioelectron. 2018, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tabassian, R.; Nguyen, V.H.; Umrao, S.; Mahato, M.; Kim, J.; Porfiri, M.; Oh, I.K. Graphene Mesh for Self-Sensing Ionic Soft Actuator Inspired from Mechanoreceptors in Human Body. Adv. Sci. 2019, 6, 1901711–1901723. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S.; Anju Jamatia, T.; Kuritka, I.; Vilcakova, J.; Skoda, D.; Urbánek, P.; Machovský, M.; Masař, M.; Urbánek, M.; Kalina, L.; et al. Superparamagnetic ZnFe2O4 Nanoparticles-Reduced Graphene Oxide-Polyurethane Resin Based Nanocomposites for Electromagnetic Interference Shielding Application. Nanomaterials 2021, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, J.; He, J.; Wang, B.; He, Y.; Luo, L.; Wang, L.; Chen, C.; Shen, F.; Zhang, Y. Synthesis of a superhydrophilic coral-like reduced graphene oxide aerogel and its application to pollutant capture in wastewater treatment. Chem. Eng. Sci. 2022, 260, 117860–117871. [Google Scholar] [CrossRef]
- Ciprian, M.; Ruiz, K.H.; Kassymova, M.; Wang, T.; Zhuiykov, S.; Chaemchuen, S.; Tu, R.; Verpoort, F. 3D derived N-doped carbon matrix from 2D ZIF-L as an enhanced stable catalyst for chemical fixation. Micropor. Mesopor. Mater. 2019, 285, 80–88. [Google Scholar] [CrossRef]
- Apetrei, C.; Alessio, P.; Constantino, C.J.L.; de Saja, J.A.; Rodriguez-Mendez, M.L.; Pavinatto, F.J.; Fernandes, E.G.R.; Zucolotto, V.; Oliveira, O.N. Biomimetic biosensor based on lipidic layers containing tyrosinase and lutetium bisphthalocyanine for the detection of antioxidants. Biosens. Bioelectron. 2011, 26, 2513–2519. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, H.; Yan, L.; Cao, W.; Yan, T.; Wei, Q.; Du, B. Copper-doped titanium dioxide nanoparticles as dual-functional labels for fabrication of electrochemical immunosensors. Biosens. Bioelectron. 2014, 59, 335–341. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, F.; Tu, X.; Ma, X.; Dai, R.; Peng, G.; Yu, Y.; Lu, L. Flake-like neodymium molybdate wrapped with multi-walled carbon nanotubes as an effective electrode material for sensitive electrochemical detection of carbendazim. J. Electroanal. Chem. 2019, 855, 113468–113476. [Google Scholar] [CrossRef]
- Sun, B.; Ni, X.; Cao, Y.; Cao, G. Electrochemical sensor based on magnetic molecularly imprinted nanoparticles modified magnetic electrode for determination of Hb. Biosens. Bioelectron. 2017, 91, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Sarıgül, T.; İnam, R.; Demir, E.; Aboul-Enein, H.Y. Electro-Oxidation and Determination of Benomyl by Square-Wave Adsorptive Stripping Voltammetry. J. AOAC Int. 2014, 97, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P.; García, S.; Hernández, L. Voltammetric use of a graphite electrode modified with OV-17 silicone for the determination of organic compounds. Anal. Chim. Acta 1992, 259, 325–331. [Google Scholar] [CrossRef]



| Method | Detection Limit (nM) | Linear Range (μM) | Reference |
|---|---|---|---|
| Bare GCE | 82.66 | 0.27–5.17 | [42] |
| OV-17 silicon a/CPE b | 237.66 | 0.34–13.77 | [43] |
| NiFe2O4 c/MWCNTs/GCE | 24.8 | 0.09–9.98 | [8] |
| Nano d-CuxO-MWCNTs-COOH /GCE | 3.0 | 0.01–10.00 | [9] |
| ERGO@ZIF-L/GCE | 3.0 | 0.009–10.00 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tomato | 0.00 | 0.00 | - | - |
| 0.50 | 0.97 ± 0.01 | 97.00 | 1.57 | |
| 3.00 | 2.86 ± 0.22 | 95.33 | 3.78 | |
| 5.00 | 5.11 ± 0.64 | 104.8 | 2.76 | |
| 10.00 | 10.18 ± 0.43 | 103.6 | 4.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Peng, G.; Xue, S.; Xu, J.; Gao, Y.; Liu, S.; Duan, X.; Lu, L. 2D Leaf-Like Structured ZIF-L Embedded Electrochemically Reduced Graphene Oxide Composite as an Electrochemical Sensing Platform for Sensitively Detecting Benomyl. Molecules 2022, 27, 6857. https://doi.org/10.3390/molecules27206857
Shi M, Peng G, Xue S, Xu J, Gao Y, Liu S, Duan X, Lu L. 2D Leaf-Like Structured ZIF-L Embedded Electrochemically Reduced Graphene Oxide Composite as an Electrochemical Sensing Platform for Sensitively Detecting Benomyl. Molecules. 2022; 27(20):6857. https://doi.org/10.3390/molecules27206857
Chicago/Turabian StyleShi, Min, Guanwei Peng, Shuya Xue, Jingkun Xu, Yansha Gao, Shuwu Liu, Xuemin Duan, and Limin Lu. 2022. "2D Leaf-Like Structured ZIF-L Embedded Electrochemically Reduced Graphene Oxide Composite as an Electrochemical Sensing Platform for Sensitively Detecting Benomyl" Molecules 27, no. 20: 6857. https://doi.org/10.3390/molecules27206857
APA StyleShi, M., Peng, G., Xue, S., Xu, J., Gao, Y., Liu, S., Duan, X., & Lu, L. (2022). 2D Leaf-Like Structured ZIF-L Embedded Electrochemically Reduced Graphene Oxide Composite as an Electrochemical Sensing Platform for Sensitively Detecting Benomyl. Molecules, 27(20), 6857. https://doi.org/10.3390/molecules27206857






