Nardoguaianone L Isolated from Nardostachys jatamansi Improved the Effect of Gemcitabine Chemotherapy via Regulating AGE Signaling Pathway in SW1990 Cells
Abstract
1. Introduction
2. Results
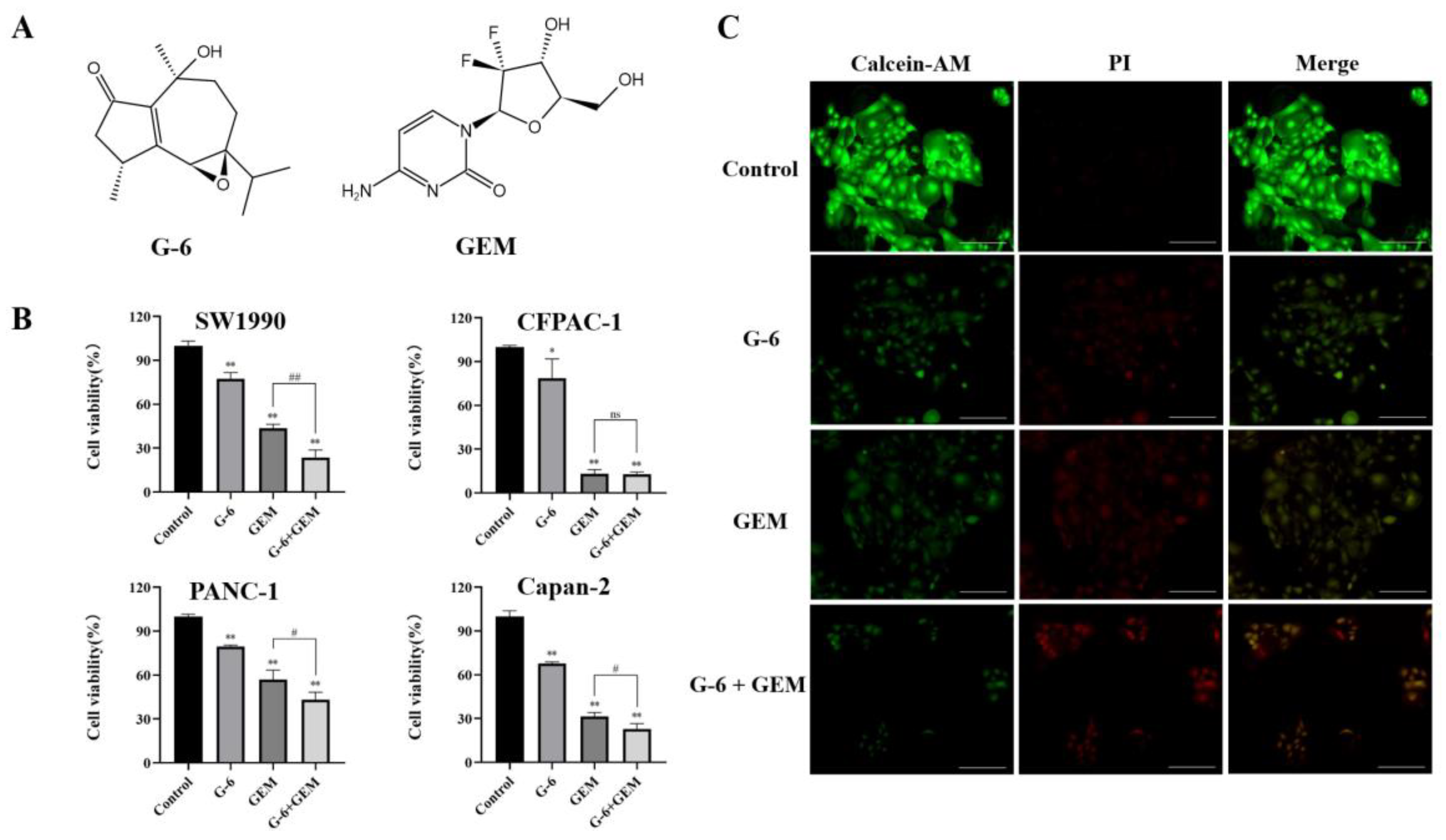
2.1. Toxicity of G-6 Combined with GEM on Four Pancreatic Cancer Cell Lines
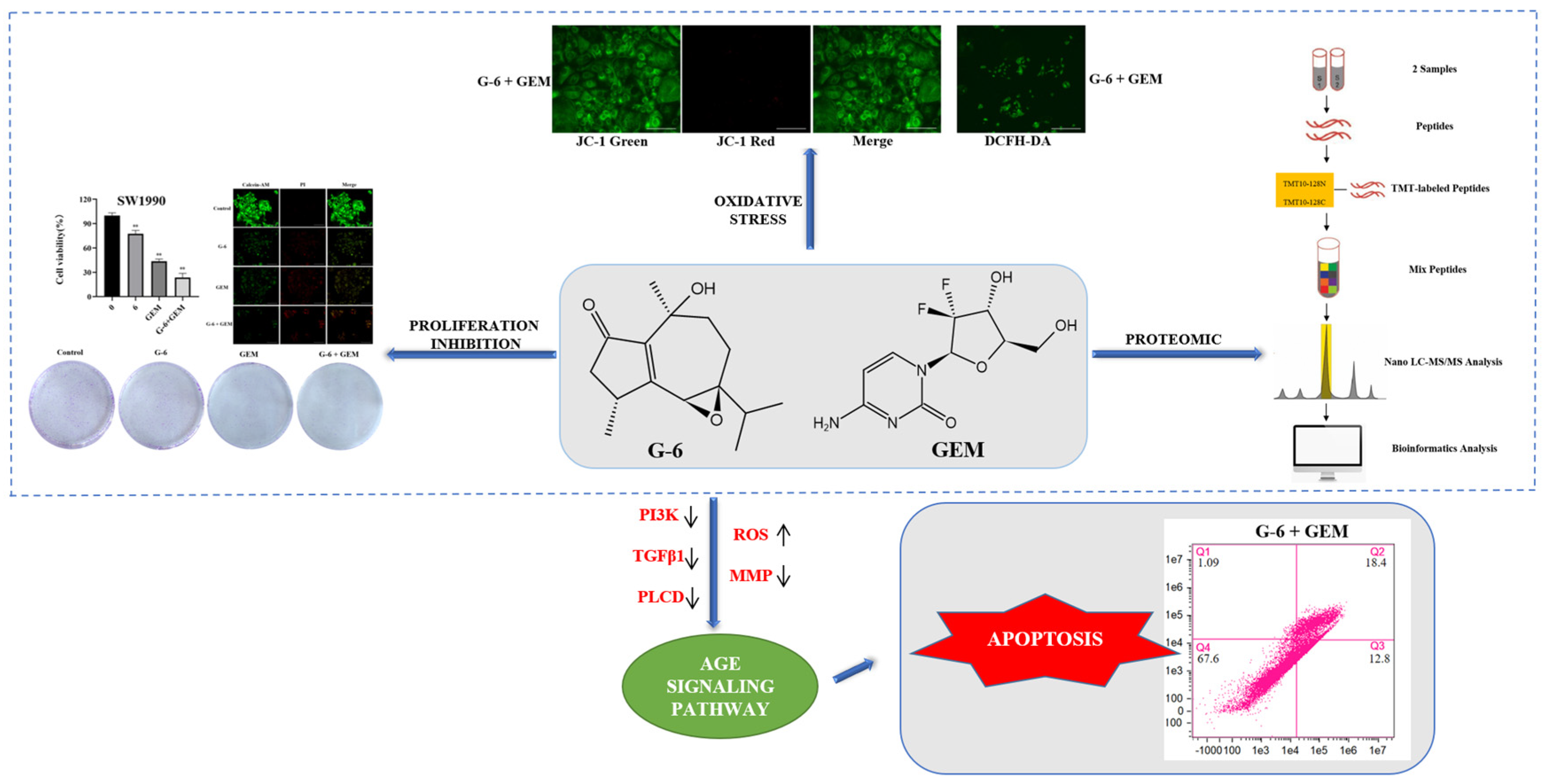
2.2. G-6 Combined with GEM Inhibited the Growth of SW1990 Cells
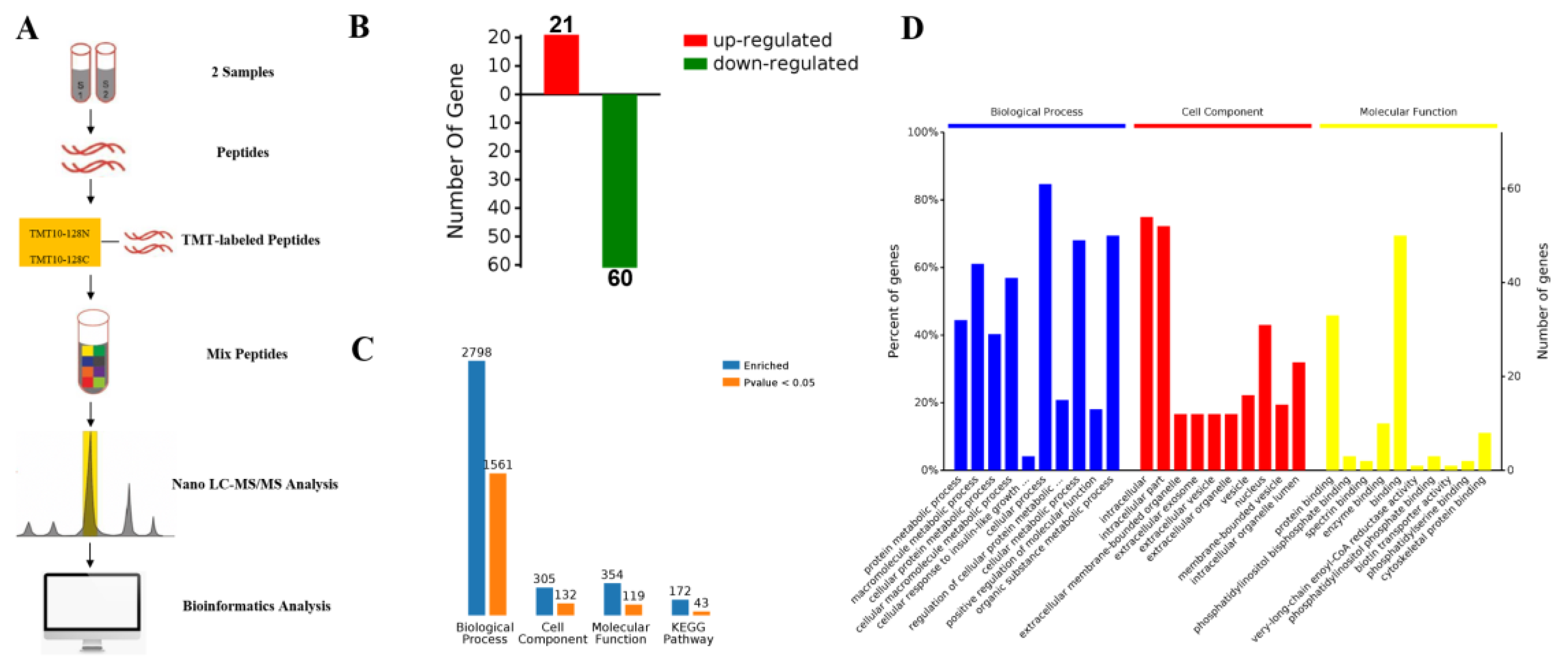
2.3. Proteomic Comparison of G-6 Combined with GEM in SW1990 Cells
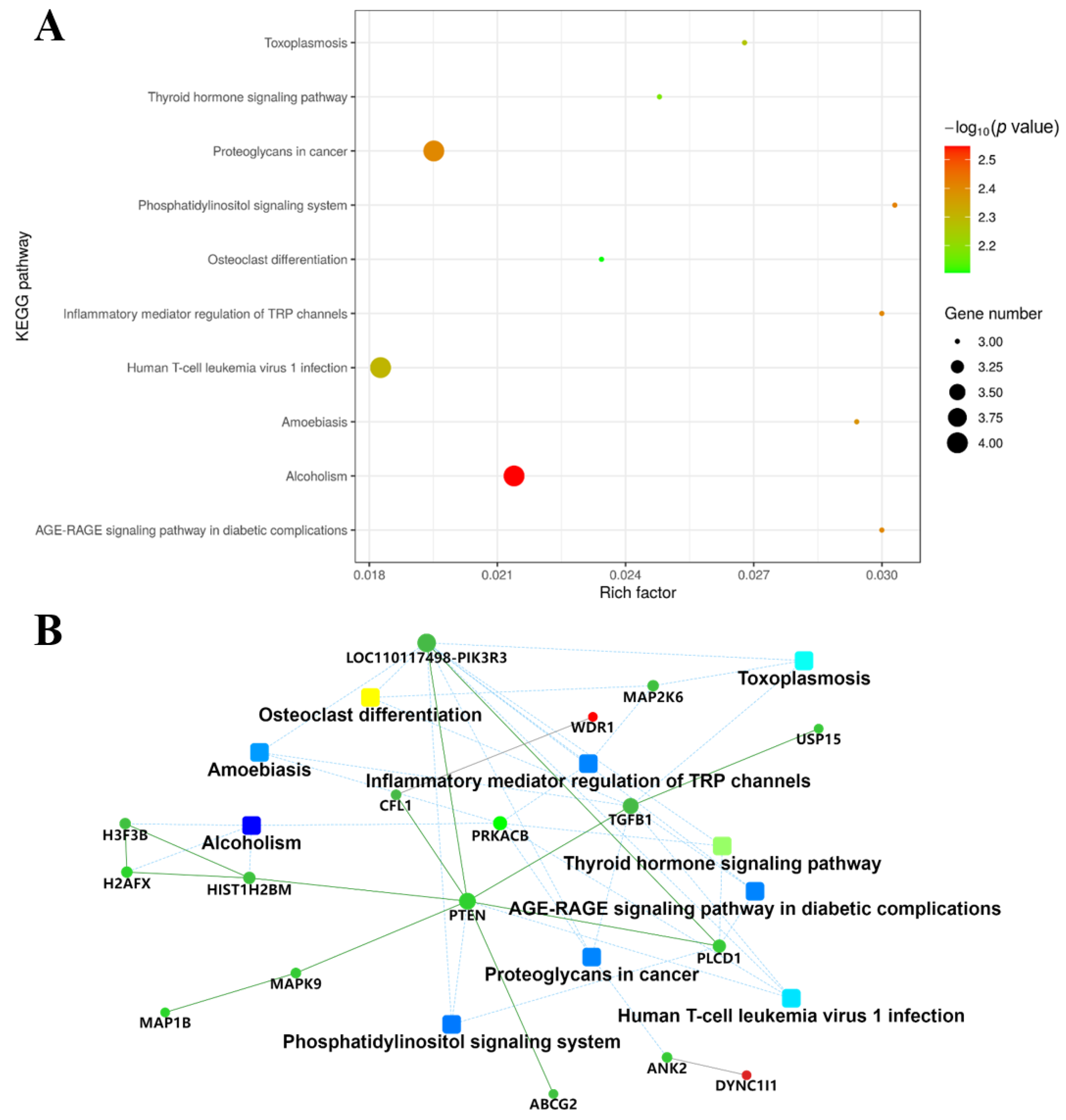
2.4. KEGG Pathway and Protein-Protein Interaction Network (PPI) Analysis
2.5. Evaluation of TMT Results Using ROS Assay and MMP Assay
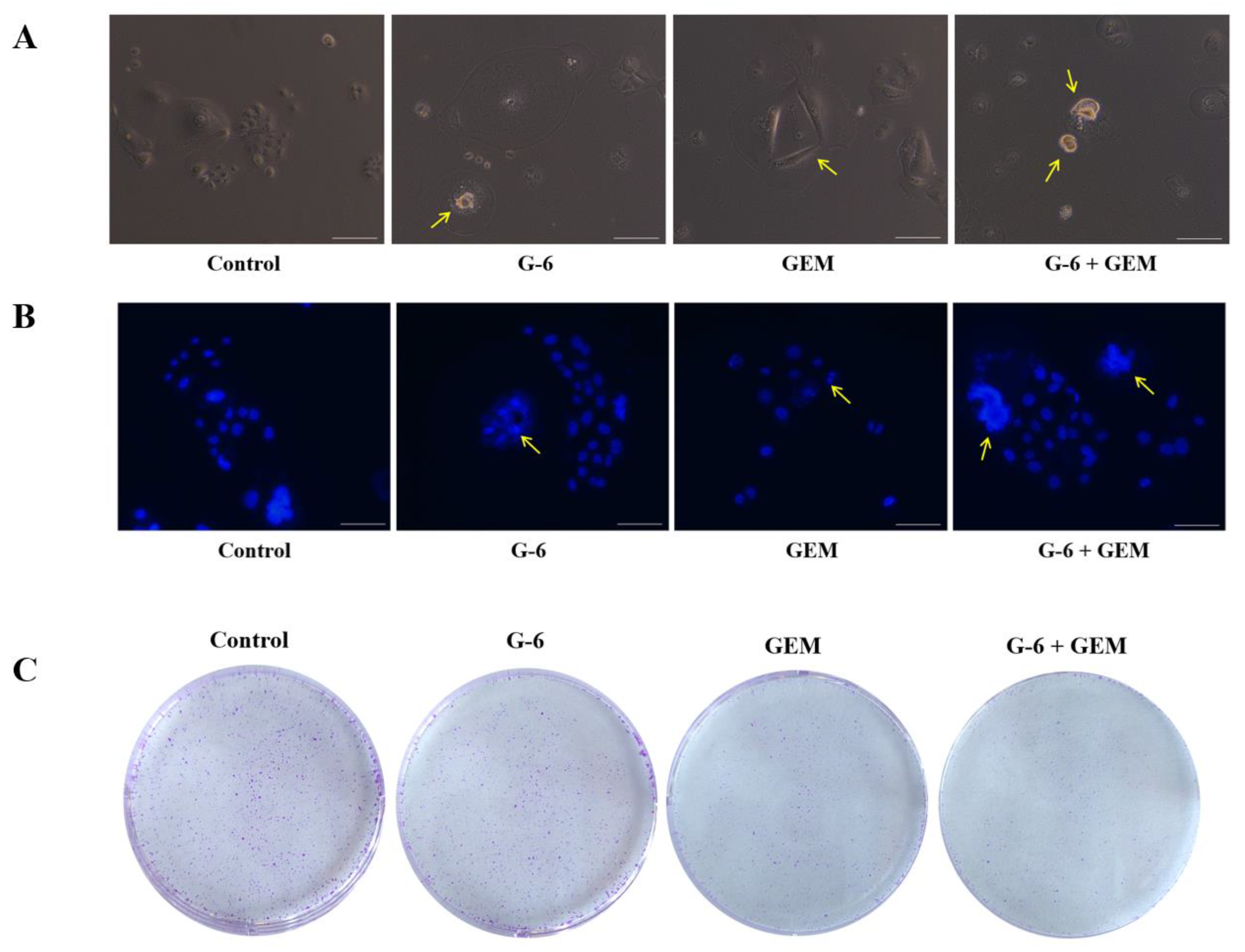
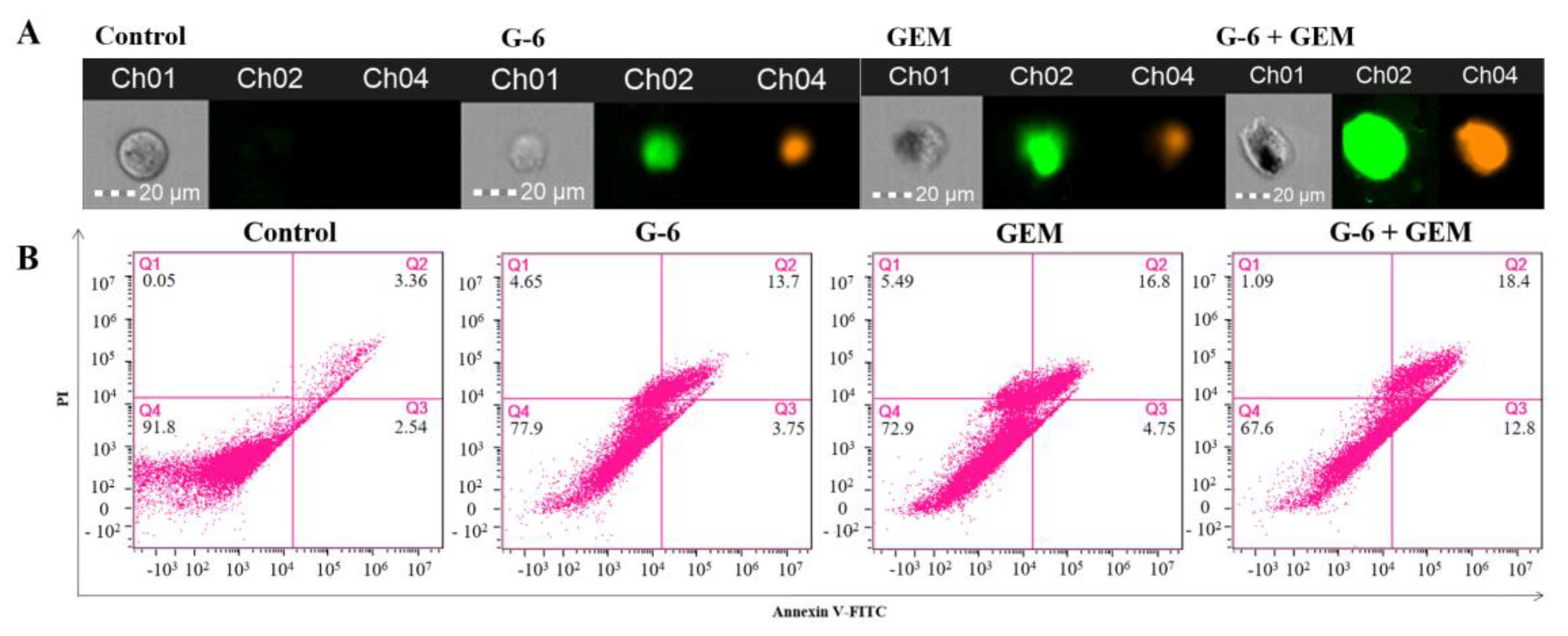
2.6. G-6 Combined with GEM Induced Apoptosis in SW1990 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Grouping
4.3. Cell Viability Assay [38]
4.4. Cytotoxicity Assay
4.5. Colony Formation Assay [39]
4.6. MMP Detection
4.7. Intracellular ROS Detection Analysis
4.8. Nucleus Staining
4.9. Apoptosis Assay [39]
4.10. Protein Preparation and TMT Labeling [40]
4.11. Nano LC-MS/MS Analysis [40]
4.12. Bioinformatics Analysis and Data Analysis [40]
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Michl, P.; Gress, T.M. Current concepts and novel targets in advanced pancreatic cancer. Gut 2013, 62, 317–326. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Kindler, H.L.; Niedzwiecki, D.; Hollis, D.; Sutherland, S.; Schrag, D.; Hurwitz, H.; Innocenti, F.; Mulcahy, M.F.; O’Reilly, E.; Wozniak, T.F.; et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the cancer and leukemia group B (CALGB 80303). J. Clin. Oncol. 2010, 28, 3617–3622. [Google Scholar] [CrossRef]
- Gu, Z.; Du, Y.; Zhao, X.; Wang, C.F. Tumor microenvironment and metabolic remodeling in gemcitabine—Based chemoresistance of pancreatic cancer. Cancer Lett. 2021, 521, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Clinical practice guidelines for the interventional treatment of advanced pancreatic cancer (5th edition). J. Inter. Med. 2021, 4, 159–171. [Google Scholar] [CrossRef]
- Hani, U.; Osmani, R.A.M.; Siddiqua, A.; Wahab, S.; Batool, S.; Ather, H.; Sheraba, N.; Alqahtani, A. A systematic study of novel drug delivery mechanisms and treatment strategies for pancreatic cancer. J. Drug Deliv. Sci. Technol. 2021, 63, 102539. [Google Scholar] [CrossRef]
- Pak, P.J.; Lee, D.G.; Sung, J.H.; Jung, S.H.; Han, T.Y.; Park, S.H.; Chung, N. Synergistic effect of the herbal mixture C5E on gemcitabine treatment in PANC-1 cells. Mol. Med. Rep. 2021, 23, 315. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Wang, Y.; Xie, Z.; Liu, Z.Q.; Zhang, C.F.; Zhao, Q. LY294002 enhances inhibitory effect of gemcitabine on proliferation of human pancreatic carcinoma PANC-1 cells. J. Huazhong Univ. Sci. Med. 2013, 33, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Gao, G.; Zou, G.; Yu, H.; Zheng, X. Natural products as adjunctive treatment for pancreatic cancer: Recent trends and advancements. BioMed Res. Int. 2017, 2017, 8412508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, C.; Gao, F.; Fu, Q.; Fu, C.; He, Y. A systematic review on the rhizome of Ligusticum chuanxiong Hort. (Chuanxiong). Food Chem. Toxicol. 2018, 119, 309–325. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, X.; Dai, K.; Li, R.; Zhang, J.; Sheng, D.; Ming-Yuen Lee, S.; Pak-Heng, L.G.; Zhou, G.C.; Li, J.J. The new andrographolide derivative AGS-30 induces apoptosis in human colon cancer cells by activating a ROS-dependent JNK signalling pathway. Phytomedicine 2022, 94, 153824. [Google Scholar] [CrossRef]
- Li, W.; Cao, L.; Chen, X.; Chen, X.; Lei, J.; Ma, Q. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol. Rep. 2016, 35, 1718–1726. [Google Scholar] [CrossRef]
- Kaur, H.; Lekhak, M.M.; Chahal, S.; Goutam, U.; Jha, P.; Naidoo, D.; Ochattg, S.J.; Kumar, V. Nardostachys jatamansi (D.Don) DC.: An invaluable and constantly dwindling resource of the Himalayas. S. Afr. J. Bot. 2020, 135, 252–267. [Google Scholar] [CrossRef]
- Dhiman, N.; Bhattacharya, A. Nardostachys jatamansi (D.Don) DC.-Challenges and opportunities of harnessing the untapped medicinal plant from the Himalayas. J. Ethnopharmacol. 2020, 246, 112211. [Google Scholar] [CrossRef]
- Ma, L.M.; Wang, K.; Meng, X.; Zheng, Y.D.; Wang, C.B.; Chai, T.; Naghavi, M.R.; Sang, C.Y.; Yang, J.L. Terpenoids from Nardostachys jatamansi and their cytotoxic activity against human pancreatic cancer cell lines. Phytochemistry 2022, 200, 113228. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, H.; Siddiqui, Z.; Khan, M.Y.; Rehman, S.; Shahab, U.; Godovikova, T.; Silnikov, V.; Moinuddine. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin. Cancer. Biol. 2018, 49, 44–55. [Google Scholar] [CrossRef]
- Krisanits, B.A.; Woods, P.; Nogueira, L.M.; Woolfork, D.D.; Lloyd, C.E.; Baldwin, A.; Frye, C.C.; Peterson, K.D.; Cosh, S.D.; Guo, Q.J.; et al. Non-enzymatic glycoxidation linked with nutrition enhances the tumorigenic capacity of prostate cancer epithelia through AGE mediated activation of RAGE in cancer associated fibroblasts. Transl. Oncol. 2022, 17, 101350. [Google Scholar] [CrossRef]
- Shahab, U.; Ahmad, M.K.; Mahdi, A.A.; Mahdi, A.A.; Waseem, M.; Arif, B.; Moinuddin; Ahmad, S. The receptor for advanced glycation end products: A fuel to pancreatic cancer. Semin. Cancer Biol. 2018, 49, 37–43. [Google Scholar] [CrossRef]
- Tong, L.; Chuang, C.; Wu, S.; Zuo, L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015, 367, 18–25. [Google Scholar] [CrossRef]
- Zou, P.; Zhang, J.; Xia, Y.; Kanchana, K.; Guo, G.; Chen, W.; Huang, Y.; Wang, Z.; Yang, S.L.; Liang, G. ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer. Oncotarget 2015, 6, 5860–5876. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, T.; Shen, J.; Zhang, L.; Li, L.; Chan, R.L.; Li, M.X.; Wu, W.K.; Cho, C.H. Miltirone induced mitochondrial dysfunction and ROS-dependent apoptosis in colon cancer cells. Life Sci. 2016, 151, 224–234. [Google Scholar] [CrossRef]
- Song, N.; Ma, J.Y.; Hu, W.; Guo, Y.W.; Hui, L.; Aamer, M.; Ma, J. Lappaconitine hydrochloride inhibits proliferation and induces apoptosis in human colon cancer HCT-116 cells via mitochondrial and MAPK pathway. ACTA Histochem. 2021, 123, 151736. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ozleyen, A.; Tumer, B.T.; Adetunji, C.; Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, C.P.; Liu, H.C.; Ren, Y.M.; Ke, C.Q.; Yao, S.; Cai, Y.Y.; Zhang, N.X.; Ye, Y. Tetramerized sesquiterpenoid ainsliatetramers A and B from ainsliaea f ragrans and their cytotoxic activities. Org. Lett. 2019, 21, 8211–8214. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeyer, M.T.; Hrenn, A.; Kern, C.; Castro, V.; Murillo, R.; Mu¨ller, S.; Laufer, S.; Schulte-Mönting, J.; Siedlea, B.; Merfort, I. Sesquiterpene lactones as inhibitors of IL-8 expression in HeLa cells. Bioorg. Med. Chem. 2006, 14, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Zhang, X.T.; Ma, Y.B.; Geng, C.G.; Huang, X.Y.; Hu, J.; Li, T.Z.; Tang, S.; Shen, C.; Gao, Z.; et al. New guaiane-type sesquiterpenoid dimers from Artemisia atrovirens and their antihepatoma activity. Acta Pharm. Sin. B 2021, 11, 1648–1666. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, W.; Chen, T. Synergistic induction of apoptosis in A549 cells by dihydroartemisinin and gemcitabine. Apoptosis 2014, 19, 668–681. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.; Shen, N.; Wang, G.; Tang, Z.; Chen, X. Dihydroartemisinin increases gemcitabine therapeutic efficacy in ovarian cancer by inducing reactive oxygen species. J. Cell Biochem. 2019, 120, 634–644. [Google Scholar] [CrossRef]
- Pellegrini, E.; Multari, G.; Gallo, F.R.; Vecchiotti, D.; Zazzeroni, F.; Condello, M.; Meschini, S. A natural product, voacamine, sensitizes paclitaxel-resistant human ovarian cancer cells. Toxicol. Appl. Pharmacol. 2022, 434, 115816. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, A.E.; Schmidt, E.V.; Sorger, P.K.; Palmer, A.C. Drug independence and the curability of cancer by combination chemotherapy. Trends Cancer 2022. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Snovida, S.; Liu, Y.; Rogers, J.C.; Li, L. Capillary electrophoresis-electrospray ionization-mass spectrometry for quantitative analysis of glycans labeled with multiplex carbonyl-reactive tandem mass tags. Anal. Chem. 2015, 87, 6527–6534. [Google Scholar] [CrossRef]
- Wrzeszczynski, K.O.; Varadan, V.; Kamalakaran, S.; Levine, D.A.; Dimitrova, N.; Lucito, R. Integrative prediction of gene function and platinum-free survival from genomic and epigenetic features in ovarian cancer. Methods Mol. Biol. 2013, 1049, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Liu, C.; Gong, K.; Mao, X.; Li, W. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int. J. Cancer 2011, 129, 1519–1531. [Google Scholar] [CrossRef]
- Brennand, K.; Savas, J.N.; Kim, Y.; Tran, N.; Simone, A.; Hashimoto-Torii, K.; Beaumont, K.G.; Kim, H.J.; Topol, A.; Ladran, I.; et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol. Psychiatry. 2015, 20, 361–368. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6, 29858338. [Google Scholar] [CrossRef]
- Chen, H.; Pan, H.; Qian, Y.; Zhou, W.B.; Liu, X.A. MiR-25-3p promotes the proliferation of triple negative breast cancer by targeting BTG2. Mol. Cancer 2018, 17, 4–14. [Google Scholar] [CrossRef]
- Zheng, Y.D.; Zhang, Y.; Ma, J.Y.; Sang, C.Y.; Yang, J.L. A Carabrane-Type Sesquiterpenolide Carabrone from Carpesium cernuum Inhibits SW1990 Pancreatic Cancer Cells by Inducing Ferroptosis. Molecules 2022, 27, 5841. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.-D.; Ma, L.-M.; Lu, J.-J.; Chai, T.; Naghavi, M.R.; Ma, J.-Y.; Sang, C.-Y.; Yang, J.-L. Nardoguaianone L Isolated from Nardostachys jatamansi Improved the Effect of Gemcitabine Chemotherapy via Regulating AGE Signaling Pathway in SW1990 Cells. Molecules 2022, 27, 6849. https://doi.org/10.3390/molecules27206849
Zheng Y-D, Ma L-M, Lu J-J, Chai T, Naghavi MR, Ma J-Y, Sang C-Y, Yang J-L. Nardoguaianone L Isolated from Nardostachys jatamansi Improved the Effect of Gemcitabine Chemotherapy via Regulating AGE Signaling Pathway in SW1990 Cells. Molecules. 2022; 27(20):6849. https://doi.org/10.3390/molecules27206849
Chicago/Turabian StyleZheng, Yi-Dan, Li-Mei Ma, Jin-Jian Lu, Tian Chai, Mohammad Reza Naghavi, Jun-Yi Ma, Chun-Yan Sang, and Jun-Li Yang. 2022. "Nardoguaianone L Isolated from Nardostachys jatamansi Improved the Effect of Gemcitabine Chemotherapy via Regulating AGE Signaling Pathway in SW1990 Cells" Molecules 27, no. 20: 6849. https://doi.org/10.3390/molecules27206849
APA StyleZheng, Y.-D., Ma, L.-M., Lu, J.-J., Chai, T., Naghavi, M. R., Ma, J.-Y., Sang, C.-Y., & Yang, J.-L. (2022). Nardoguaianone L Isolated from Nardostachys jatamansi Improved the Effect of Gemcitabine Chemotherapy via Regulating AGE Signaling Pathway in SW1990 Cells. Molecules, 27(20), 6849. https://doi.org/10.3390/molecules27206849






