β-Cyclocitral: Emerging Bioactive Compound in Plants
Abstract
1. Introduction
2. Biosynthesis of βCC
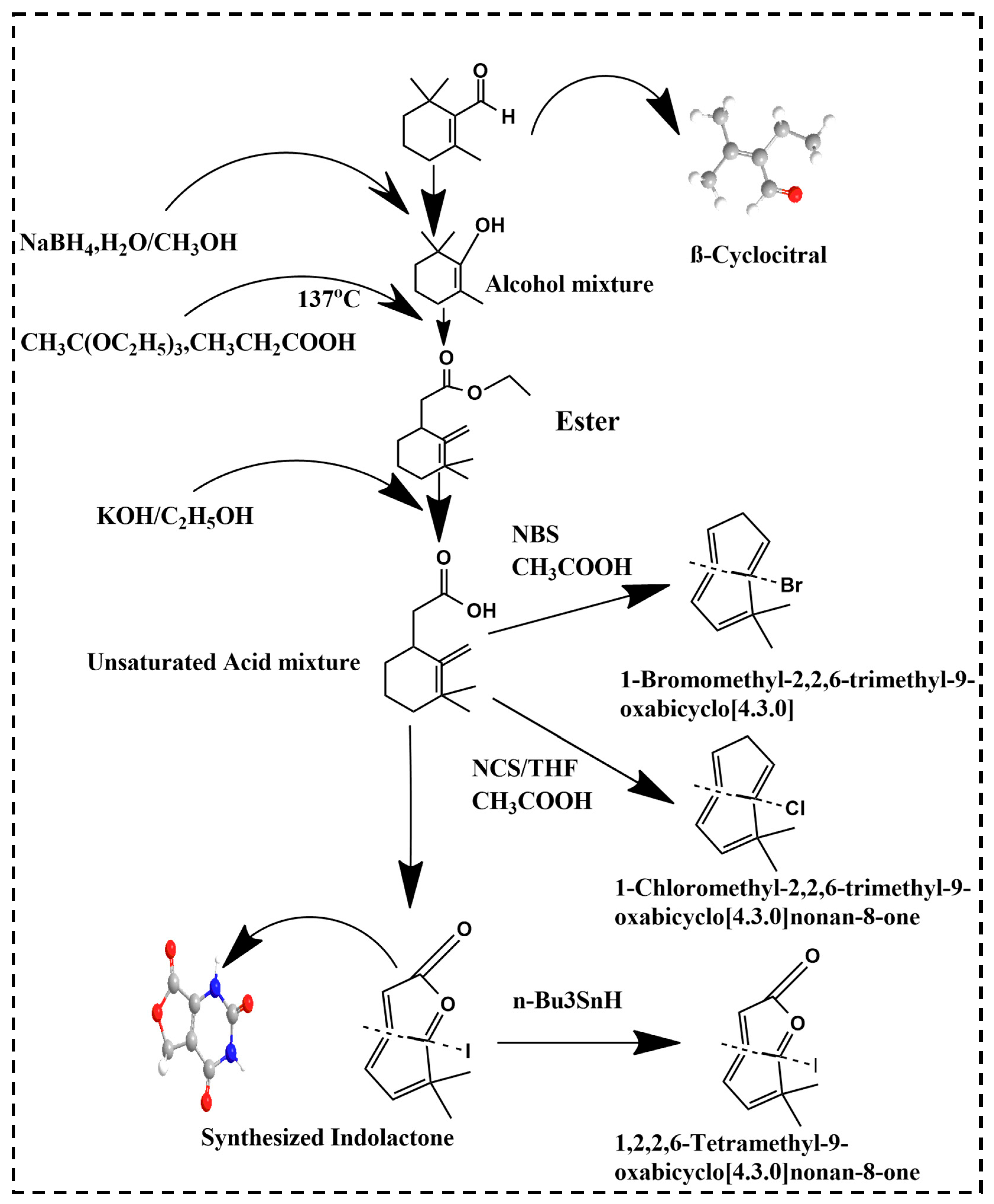
3. Derivatives of βCC
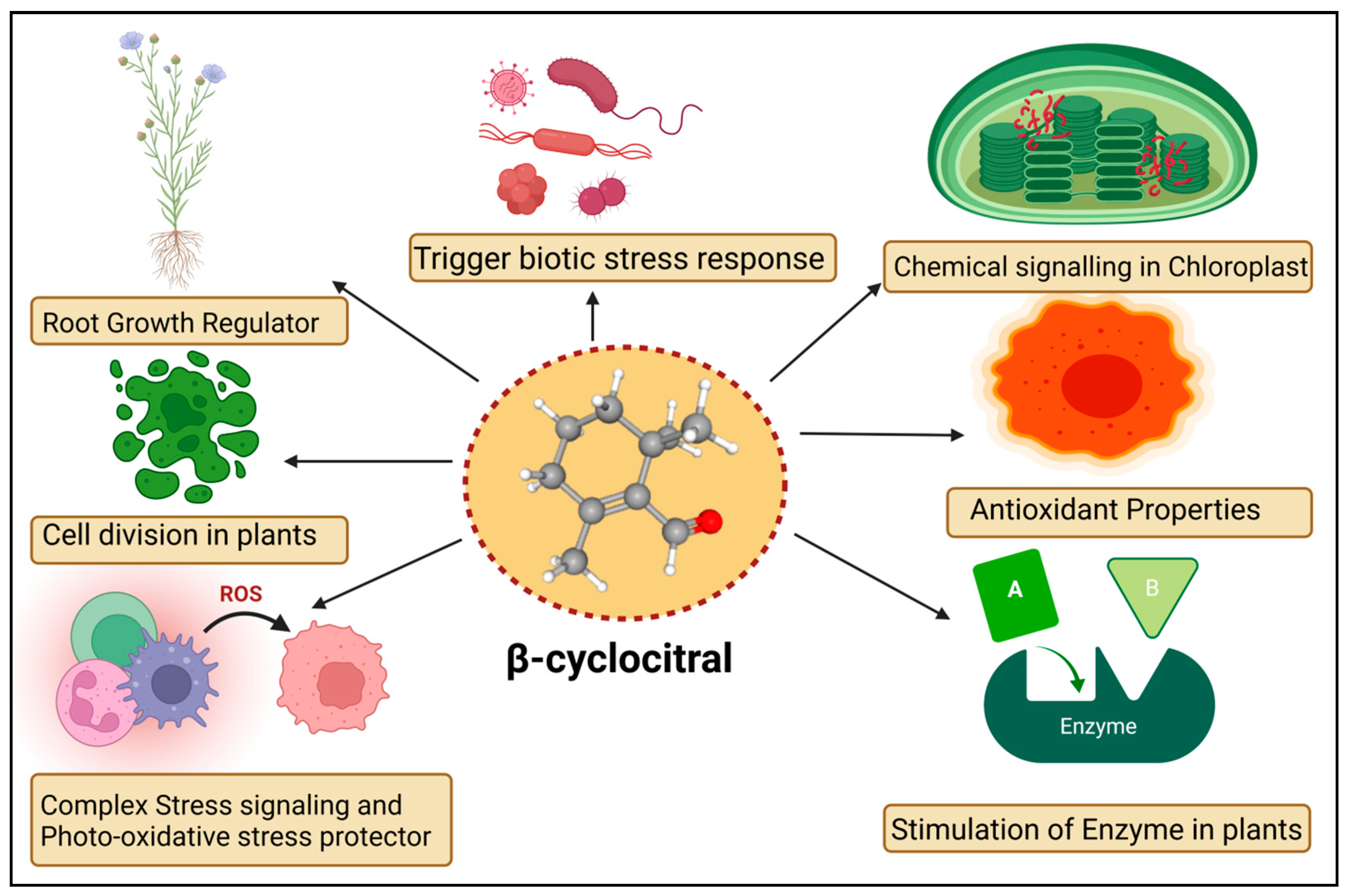
4. Signalling of βCC in Plants
5. Functions of βCC in Plants
| Plant Species | Functions | References |
|---|---|---|
 Nitzschia palea | Cell rupture at 0.1–0.5 mg mL−1 dose, cyanobacterial cell degradation, change in water color | [59] |
 Cyanidium caldarium | Unpalatable water odor | [71] |
 Chlorella pyrenoidosa | Inhibition of cell growth and development | [60] |
 Chlamydomonas reinhardtii | Induce programmed cell death, cause poison to other algae | [61] |
 Microcystis aeruginosa | Increase βCC emission, expose high ion concentration | [72] |
 Solanum lycopersicum | Retro nasal olfactory (smell) add to flavor to the fruit, volatile compound induces taste | [73] |
 Oryza sativa | Scented rice varieties have aroma, more leaves present in vegetative stage | [74] |
 Petroselinum crispum | Helps to produce essential oil and contribute in anti-fungal activity | [75] |
 Camellia sinensis | Improve odorant properties and structural functions | [76] |
 Grapevines | Inhibit infestation of spider mite and reduce symptoms | [77] |
6. βCC Involved in the Regulation of Stress Response
| Plants Name | Stress | Effect in Plants | References |
|---|---|---|---|
| Arabidopsis thaliana | Light stress | βCC acts as a secondary messenger, alters transcription of singlet oxygen via MBS protein | [89] |
| Solanum lycopersicum | Drought stress | Increases the osmolyte accumulation, root and branching, superoxide exclusion and improves stress tolerance | [7] |
| Arabidopsis thaliana | Photo-oxidative stress | ROS production, changes gene expression | [10] |
| Viola tricolor | Drought stress | Elicits drought stress, no wilting occurs in water lack condition | [31] |
| Arabidopsis thaliana | Oxidative stress | Influences gene expression, suppression in transgenic plants, Cu/Zn SOD is induced | [106] |
| Oryza sativa | Salt stress | Tolerates the adverse salinity stress condition and increased vigor of plants | [24] |
| Arabidopsis thaliana | Water stress | Overlaps genetic responses, showing the impact of singlet oxygen | [107] |
| Microcystis cyanobacteria | Water stress | Oxidation and acidification of βCC; Blue color forms, pH is reduced | [47] |
| Arabidopsis thaliana | Light stress | SCL14 enhances genetic responses, upregulation of lipid peroxidation | [68] |
| Oryza sativa | Salt stress | Promotes cell division in root meristems and stimulates lateral root branching | [24] |
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernhoft, A. A brief review on bioactive compounds in plants. In Bioactive Compounds in Plants—Benefits and Risks for Man and Animals; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; Volume 50, pp. 11–17. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S. Plants: A rich source of herbal medicine. J. Nat. Prod. 2008, 1, 27–35. [Google Scholar]
- Couladis, M.; Tzakou, O.; Verykokidou, E.; Harvala, C. Screening of some Greek aromatic plants for antioxidant activity. Phyther. Res. 2003, 17, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and inductrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Deshpande, S.; Manoharan, R.; Mitra, S. Exogenous β-cyclocitral treatment primes tomato plants against drought by inducing tolerance traits, independent of abscisic acid. Plant Biol. 2021, 23, 170–180. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Medina, V.; Carmona, L.; Bruno, M.; Al-Babili, S.; Zacarías, L. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J. Exp. Bot. 2013, 64, 4461–4478. [Google Scholar] [CrossRef]
- Hayward, S.; Cilliers, T.; Swart, P. Lipoxygenases: From isolation to application. Compr. Rev. Food Sci. Food Saf. 2017, 16, 199–211. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef]
- Moretto, J.A.S.; de Freitas, P.N.N.; de Almeida, E.C.; Altarugio, L.M.; da Silva, S.V.; Fiore, M.F.; Pinto, E. Effects of different cultivation conditions on the production of β-cyclocitral and β-ionone in Microcystis aeruginosa. BMC Microbiol. 2022, 22, 78. [Google Scholar] [CrossRef]
- Mitra, S.; Estrada-Tejedor, R.; Volke, D.C.; Phillips, M.A.; Gershenzon, J.; Wright, L.P. Negative regulation of plastidial isoprenoid pathway by herbivore-induced β-cyclocitral in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2008747118. [Google Scholar] [CrossRef] [PubMed]
- Sharoni, Y.; Danilenko, M.; Dubi, N.; Ben-Dor, A.; Levy, J. Carotenoids and transcription. Arch. Biochem. Biophys. 2004, 430, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, E.; Hoeller, U.; Greatrix, B.; Lankin, C.; Seifert, N.; Acharya, S.; Riss, G.; Buchwald-Hunziker, P.; Hunziker, W.; Goralczyk, R. β-Carotene and apocarotenals promote retinoid signaling in BEAS-2B human bronchioepithelial cells. Arch. Biochem. Biophys. 2006, 455, 48–60. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Dong, H.; Sun, C.; Sun, W.; Chen, B.; Song, Y.; Yang, B. β-Ionone suppresses mammary carcinogenesis, proliferative activity and induces apoptosis in the mammary gland of the Sprague-Dawley rat. Int. J. Cancer 2008, 122, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Mahattanatawee, K.; Rouseff, R.; Valim, M.F.; Naim, M. Identification and aroma impact of norisoprenoids in orange juice. J. Agric. Food Chem. 2005, 53, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Lalko, J.; Lapczynski, A.; McGinty, D.; Bhatia, S.; Letizia, C.S.; Api, A.M. Fragrance material review on ionone. Food Chem. Toxicol. 2007, 45, S251–S257. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shi, C.; Ji, M.; Xu, X.; Zhang, Z.; Ma, J.; Wang, G. Taste and odor compounds associated with aquatic plants in Taihu Lake: Distribution and producing potential. Environ. Sci. Pollut. Res. 2019, 26, 34510–34520. [Google Scholar] [CrossRef]
- Moraga, Á.R.; Rambla, J.L.; Ahrazem, O.; Granell, A.; Gómez-Gómez, L. Metabolite and target transcript analyses during Crocus sativus stigma development. Phytochemistry 2009, 70, 1009–1016. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid pigmentation affects the volatile composition of tomato and watermelon fruits, as revealed by comparative genetic analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef]
- Arii, S.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Bober, B.; Harada, K.-I. Cyanobacterial blue color formation during lysis under natural conditions. Appl. Environ. Microbiol. 2015, 81, 2667–2675. [Google Scholar] [CrossRef]
- Juttner, F.; Watson, S.B.; von Elert, E.; Koster, O.J. β-cyclocitral, a grazer defense signal unique to the cyanobacterium Microcystis. Chem. Ecol. 2010, 36, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Ohta, A.; Iwata, C.; Horikawa, A.; Tsuji, K.; Ito, E.; Ikai, Y.; Harada, K.I. Lysis of cyanobacteria with volatile organic compounds. Chemosphere 2008, 71, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.-P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral is a conserved root growth regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, K.; Ye, J.; Price, E.J.; Deng, X.; Fraser, P.D. The effect of β-cyclocitral treatment on the carotenoid content of transgenic Marsh grapefruit (Citrus paradisi Macf.) suspension-cultured cells. Phytochemistry 2020, 180, 112509. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Pedreño, M.A.; Fraser, P.D.; Sabater-Jara, A.B.; Almagro, L. Effect of diflufenican on total carotenoid and phytoene production in carrot suspension-cultured cells. Planta 2019, 249, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. β-Cyclocitral and Derivatives: Emerging Molecular Signals Serving Multiple Biological Functions. Plant Physiol. Biochem. 2020, 155, 35–41. [Google Scholar] [CrossRef]
- Shumbe, L.; d’Alessandro, S.; Shao, N.; Chevalier, A.; Ksas, B.; Bock, R.; Havaux, M. Methylene Blue Sensitivity 1 (MBS1) is required for acclimation of Arabidopsis to singlet oxygen and acts downstream of β-cyclocitral. Plant Cell Environ. 2017, 40, 216–226. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Portillo-Estrada, M.; Fernández-Marín, B.; Kännaste, A.; Niinemets, Ü. Emissions of carotenoid cleavage products upon heat shock and mechanical wounding from a foliose lichen. Environ. Exp. Bot. 2017, 133, 87–97. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Havaux, M. Sensing β-carotene oxidation in photosystem II to master plant stress tolerance. New Phytol. 2019, 223, 1776–1783. [Google Scholar] [CrossRef]
- Noctor, G.; Arisi, A.-C.M.; Jouanin, L.; Kunert, K.J.; Rennenberg, H.; Foyer, C.H. Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 1998, 49, 623–647. [Google Scholar] [CrossRef]
- Jamaluddin, N.D.; Rohani, E.R.; Mohd Noor, N.; Goh, H.-H. Transcriptome-wide effect of DE-ETIOLATED1 (DET1) suppression in embryogenic callus of Carica papaya. J. Plant Res. 2019, 132, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Al-Babili, S.; Von Lintig, J. Carotenoid oxygenases: Cleave it or leave it. Trends Plant Sci. 2003, 8, 145–149. [Google Scholar] [CrossRef]
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci. 2016, 21, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165. [Google Scholar] [CrossRef]
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 2013, 64, 799–805. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Qin, S. Genomewide analysis of carotenoid cleavage dioxygenases in unicellular and filamentous cyanobacteria. Comp. Funct. Genom. 2012, 2012, 164690. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef]
- Rubio-Moraga, A.; Rambla, J.L.; Fernández-de-Carmen, A.; Trapero-Mozos, A.; Ahrazem, O.; Orzáez, D.; Granell, A.; Gómez-Gómez, L. New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Mol. Biol. 2014, 86, 555–569. [Google Scholar] [CrossRef]
- Meng, N.; Yan, G.-L.; Zhang, D.; Li, X.-Y.; Duan, C.-Q.; Pan, Q.-H. Characterization of two Vitis vinifera carotenoid cleavage dioxygenases by heterologous expression in Saccharomyces cerevisiae. Mol. Biol. Rep. 2019, 46, 6311–6323. [Google Scholar] [CrossRef]
- Zorn, H.; Langhoff, S.; Scheibner, M.; Nimtz, M.; Berger, R.G. A peroxidase from Lepista irina cleaves β, β-carotene to flavor compounds. Biol. Chem. 2003, 384, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Ma, Z.; Niu, Y.; Xie, P.; Chen, J.; Tao, M.; Deng, X. Off-flavor compounds from decaying cyanobacterial blooms of Lake Taihu. J. Environ. Sci. 2013, 25, 495–501. [Google Scholar] [CrossRef]
- Crombie, B.S.; Smith, C.; Varnavas, C.Z.; Wallace, T.W. A conjugate addition–radical cyclisation approach to sesquiterpene-phenol natural products. J. Chem. Soc. Perkin Trans. 2001, 1, 206–215. [Google Scholar] [CrossRef]
- Mazur, M.; Gładkowski, W.; Podkowik, M.; Bania, J.; Nawrot, J.; Białońska, A.; Wawrzeńczyk, C. Lactones 43. New biologically active lactones: β-cyclocitral derivatives. Pest Manag. Sci. 2014, 70, 286–294. [Google Scholar] [CrossRef]
- Tomita, K.; Hasegawa, M.; Arii, S.; Tsuji, K.; Bober, B.; Harada, K. Characteristic oxidation behavior of β-cyclocitral from the cyanobacterium Microcystis. Environ. Sci. Pollut. Res. 2016, 23, 11998–12006. [Google Scholar] [CrossRef]
- Van Berkel, W.J.H.; Kamerbeek, N.M.; Fraaije, M. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 2006, 124, 670–689. [Google Scholar] [CrossRef]
- Kim, T.-W.; Hwang, J.-Y.; Kim, Y.-S.; Joo, S.-H.; Chang, S.C.; Lee, J.S.; Takatsuto, S.; Kim, S.-K. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.-K.; Zoh, K.-D. Degradation kinetics and pathways of β-cyclocitral and β-ionone during UV photolysis and UV/chlorination reactions. J. Environ. Manag. 2019, 239, 8–16. [Google Scholar] [CrossRef]
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids involved in plant development and stress response. Front. Plant Sci. 2019, 10, 1168. [Google Scholar] [CrossRef]
- Baba, S.A.; Jain, D.; Abbas, N.; Ashraf, N. Overexpression of Crocus carotenoid cleavage dioxygenase, CsCCD4b, in Arabidopsis imparts tolerance to dehydration, salt and oxidative stresses by modulating ROS machinery. J. Plant Physiol. 2015, 189, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.B.; Estavillo, G.M.; Field, K.J.; Pornsiriwong, W.; Carroll, A.J.; Howell, K.A.; Woo, N.S.; Lake, J.A.; Smith, S.M.; Harvey Millar, A. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 2009, 58, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Rossel, J.B.; Walter, P.B.; Hendrickson, L.; Chow, W.S.; Poole, A.; Mullineaux, P.M.; Pogson, B.J. A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant Cell Environ. 2006, 29, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Von Arnim, A.G. FIERY1 regulates light-mediated repression of cell elongation and flowering time via its 3′(2′), 5′-bisphosphate nucleotidase activity. Plant J. 2009, 58, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sharkey, T.D. Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant Cell Environ. 2013, 36, 429–437. [Google Scholar] [CrossRef]
- Zuo, Z. Why algae release volatile organic compounds—The emission and roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef]
- Chang, D.W.; Hsieh, M.-L.; Chen, Y.-M.; Lin, T.-F.; Chang, J.-S. Kinetics of cell lysis for Microcystis aeruginosa and Nitzschia palea in the exposure to β-cyclocitral. J. Hazard. Mater. 2011, 185, 1214–1220. [Google Scholar] [CrossRef]
- Ikawa, M.; Sasner, J.J.; Haney, J.F. Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth. Hydrobiologia 2001, 443, 19–22. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, M.; Zuo, Z. Toxic mechanism of eucalyptol and β-cyclocitral on Chlamydomonas reinhardtii by inducing programmed cell death. J. Hazard. Mater. 2020, 389, 121910. [Google Scholar] [CrossRef]
- Saritas, Y.; Sonwa, M.M.; Iznaguen, H.; König, W.A.; Muhle, H.; Mues, R. Volatile constituents in mosses (Musci). Phytochemistry 2001, 57, 443–457. [Google Scholar] [CrossRef]
- Schwachtje, J.; Minchin, P.E.H.; Jahnke, S.; van Dongen, J.T.; Schittko, U.; Baldwin, I.T. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc. Natl. Acad. Sci. USA 2006, 103, 12935–12940. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Duan, G.Y.; Bock, R. A mediator of singlet oxygen responses in Chlamydomonas reinhardtii and Arabidopsis identified by a luciferase-based genetic screen in algal cells. Plant Cell 2013, 25, 4209–4226. [Google Scholar] [CrossRef] [PubMed]
- Riechers, D.E.; Kreuz, K.; Zhang, Q. Detoxification without intoxication: Herbicide safeners activate plant defense gene expression. Plant Physiol. 2010, 153, 3–13. [Google Scholar] [CrossRef]
- Sandermann, H., Jr. Plant metabolism of xenobiotics. Trends Biochem. Sci. 1992, 17, 82–84. [Google Scholar] [CrossRef]
- Huang, L.J.; Li, N.; Thurow, C.; Wirtz, M.; Hell, R.; Gatz, C. Ectopically expressed glutaredoxin ROXY19 negatively regulates the detoxification pathway in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 200. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, S.; Ksas, B.; Havaux, M. Decoding β-cyclocitral-mediated retrograde signaling reveals the role of a detoxification response in plant tolerance to photooxidative stress. Plant Cell 2018, 30, 2495–2511. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive carbonyl species: A missing link in ROS signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Mizokami, Y.; Légeret, B.; Havaux, M. The apocarotenoid β-cyclocitric acid elicits drought tolerance in plants. iScience 2019, 19, 461–473. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, L.; Yang, W.; Bai, Y.; Hou, P.; Zhao, J.; Zhou, L.; Zuo, Z. Volatile organic compounds released from Microcystis flos-aquae under nitrogen sources and their toxic effects on Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2017, 135, 191–200. [Google Scholar] [CrossRef]
- Hasegawa, M.; Nishizawa, A.; Tsuji, K.; Kimura, S.; Harada, K. Volatile organic compounds derived from 2-keto-acid decarboxylase in Microcystis aeruginosa. Microbes Environ. 2012, 27, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodríguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2012, 22, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Hinge, V.; Patil, H.; Nadaf, A. Comparative characterization of aroma volatiles and related gene expression analysis at vegetative and mature stages in basmati and non-basmati rice (Oryza sativa L.) cultivars. Appl. Biochem. Biotechnol. 2016, 178, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Linde, G.A.; Gazim, Z.C.; Cardoso, B.K.; Jorge, L.F.; Tešević, V.; Glamočlija, J.; Soković, M.; Colauto, N.B. Antifungal and antibacterial activities of Petroselinum crispum essential oil. Genet. Mol. Res. 2016, 15, gmr.15038538. [Google Scholar] [CrossRef] [PubMed]
- Ojha, P.K.; Roy, K. PLS regression-based chemometric modeling of odorant properties of diverse chemical constituents of black tea and coffee. RSC Adv. 2018, 8, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Lazazzara, V.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Downy mildew symptoms on grapevines can be reduced by volatile organic compounds of resistant genotypes. Sci. Rep. 2018, 8, 1618. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.; Solomon, S.; Mall, A.K.; Prajapati, C.P.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Morphological assessment of water stressed sugarcane: A comparison of waterlogged and drought affected crop. Saudi J. Biol. Sci. 2020, 27, 1228–1236. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Laloi, C.; Havaux, M. Key players of singlet oxygen-induced cell death in plants. Front. Plant Sci. 2015, 6, 39. [Google Scholar] [CrossRef]
- Dogra, V.; Rochaix, J.; Kim, C. Singlet oxygen-triggered chloroplast-to-nucleus retrograde signalling pathways: An emerging perspective. Plant. Cell Environ. 2018, 41, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Krieger-Liszkay, A. Regulation of photosynthetic electron transport and photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.M.; Suorsa, M.; Rokka, A.; Allahverdiyeva, Y.; Paakkarinen, V.; Saleem, A.; Battchikova, N.; Rintamäki, E. Dynamics of photosystem II: A proteomic approach to thylakoid protein complexes. J. Exp. Bot. 2005, 56, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Garab, G.; Adams III, W.; Govindjee, U. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Ouchane, S.; Picaud, M.; Vernotte, C.; Astier, C. Photooxidative stress stimulates illegitimate recombination and mutability in carotenoid-less mutants of Rubrivivax gelatinosus. EMBO J. 1997, 16, 4777–4787. [Google Scholar] [CrossRef]
- Shumbe, L.; Bott, R.; Havaux, M. Dihydroactinidiolide, a high light-induced β-carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis. Mol. Plant 2014, 7, 1248–1251. [Google Scholar] [CrossRef]
- Liu, T.; Ye, X.; Li, M.; Li, J.; Qi, H.; Hu, X. H2O2 and NO are involved in trehalose-regulated oxidative stress tolerance in cold-stressed tomato plants. Environ. Exp. Bot. 2020, 171, 103961. [Google Scholar] [CrossRef]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Lycopersicon esculentum under low temperature stress: An approach toward enhanced antioxidants and yield. Environ. Sci. Pollut. Res. 2015, 22, 14178–14188. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Isayenkov, S.V. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant. Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.M.M.; Kozuleva, M.A.; Rudenko, N.N.; Naydov, I.A.; Klenina, I.B.; Ivanov, B.N. Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Juvany, M.; Müller, M.; Munné-Bosch, S. Photo-oxidative stress in emerging and senescing leaves: A mirror image? J. Exp. Bot. 2013, 64, 3087–3098. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Queval, G.; Foyer, C.H. Redox regulation of photosynthetic gene expression. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3475–3485. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Queval, G.; Foyer, C.H. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 2013, 161, 5–19. [Google Scholar] [CrossRef]
- Mozzo, M.; Dall’Osto, L.; Hienerwadel, R.; Bassi, R.; Croce, R. Photoprotection in the antenna complexes of photosystem II: Role of individual xanthophylls in chlorophyll triplet quenching. J. Biol. Chem. 2008, 283, 6184–6192. [Google Scholar] [CrossRef]
- Fischer, B.B.; Hideg, E.; Krieger-Liszkay, A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid. Redox Signal. 2013, 18, 2145–2162. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Purkar, V.; Mitra, S. β-Cyclocitral, a Master Regulator of Multiple Stress-Responsive Genes in Solanum lycopersicum L. Plants. Plants 2021, 10, 2465. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Baur, T.; Kranner, I. β-Cyclocitral Does Not Contribute to Singlet Oxygen-Signalling in Algae, but May Down-Regulate Chlorophyll Synthesis. Plants 2022, 11, 2155. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Kapoor, A.; Zhu, J.-K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef]
- Koh, E.; Carmieli, R.; Mor, A.; Fluhr, R. Singlet oxygen-induced membrane disruption and serpin-protease balance in vacuolar-driven cell death. Plant Physiol. 2016, 171, 1616–1625. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizan, M.; Tonny, S.H.; Afzal, S.; Farooqui, Z.; Alam, P.; Ahmed, S.M.; Yu, F.; Hayat, S. β-Cyclocitral: Emerging Bioactive Compound in Plants. Molecules 2022, 27, 6845. https://doi.org/10.3390/molecules27206845
Faizan M, Tonny SH, Afzal S, Farooqui Z, Alam P, Ahmed SM, Yu F, Hayat S. β-Cyclocitral: Emerging Bioactive Compound in Plants. Molecules. 2022; 27(20):6845. https://doi.org/10.3390/molecules27206845
Chicago/Turabian StyleFaizan, Mohammad, Sadia Haque Tonny, Shadma Afzal, Zeba Farooqui, Pravej Alam, S. Maqbool Ahmed, Fangyuan Yu, and Shamsul Hayat. 2022. "β-Cyclocitral: Emerging Bioactive Compound in Plants" Molecules 27, no. 20: 6845. https://doi.org/10.3390/molecules27206845
APA StyleFaizan, M., Tonny, S. H., Afzal, S., Farooqui, Z., Alam, P., Ahmed, S. M., Yu, F., & Hayat, S. (2022). β-Cyclocitral: Emerging Bioactive Compound in Plants. Molecules, 27(20), 6845. https://doi.org/10.3390/molecules27206845









