Effect of Chitosan and Amphiphilic Polymers on the Photosensitizing and Spectral Properties of Rose Bengal
Abstract
1. Introduction
2. Results and Discussion
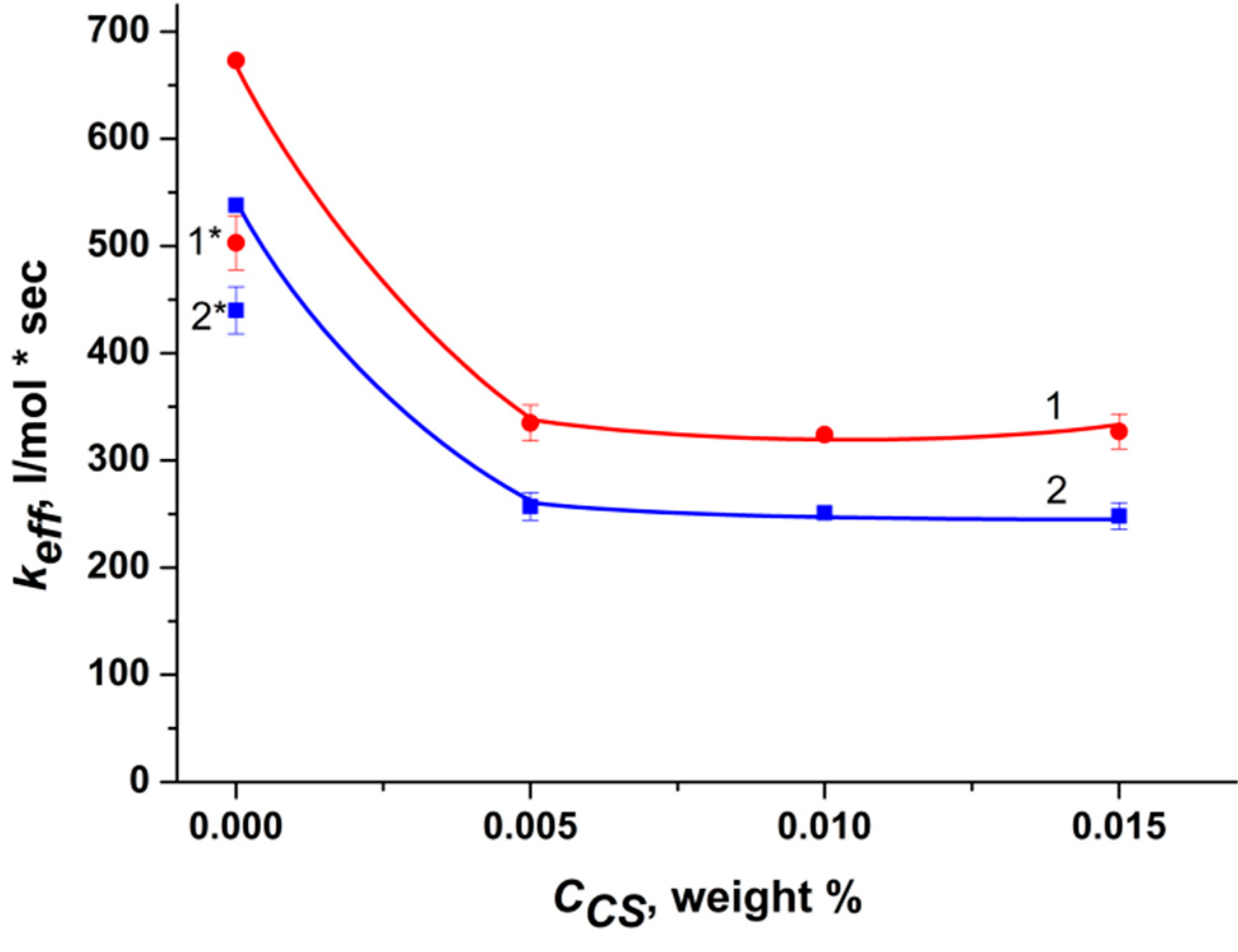
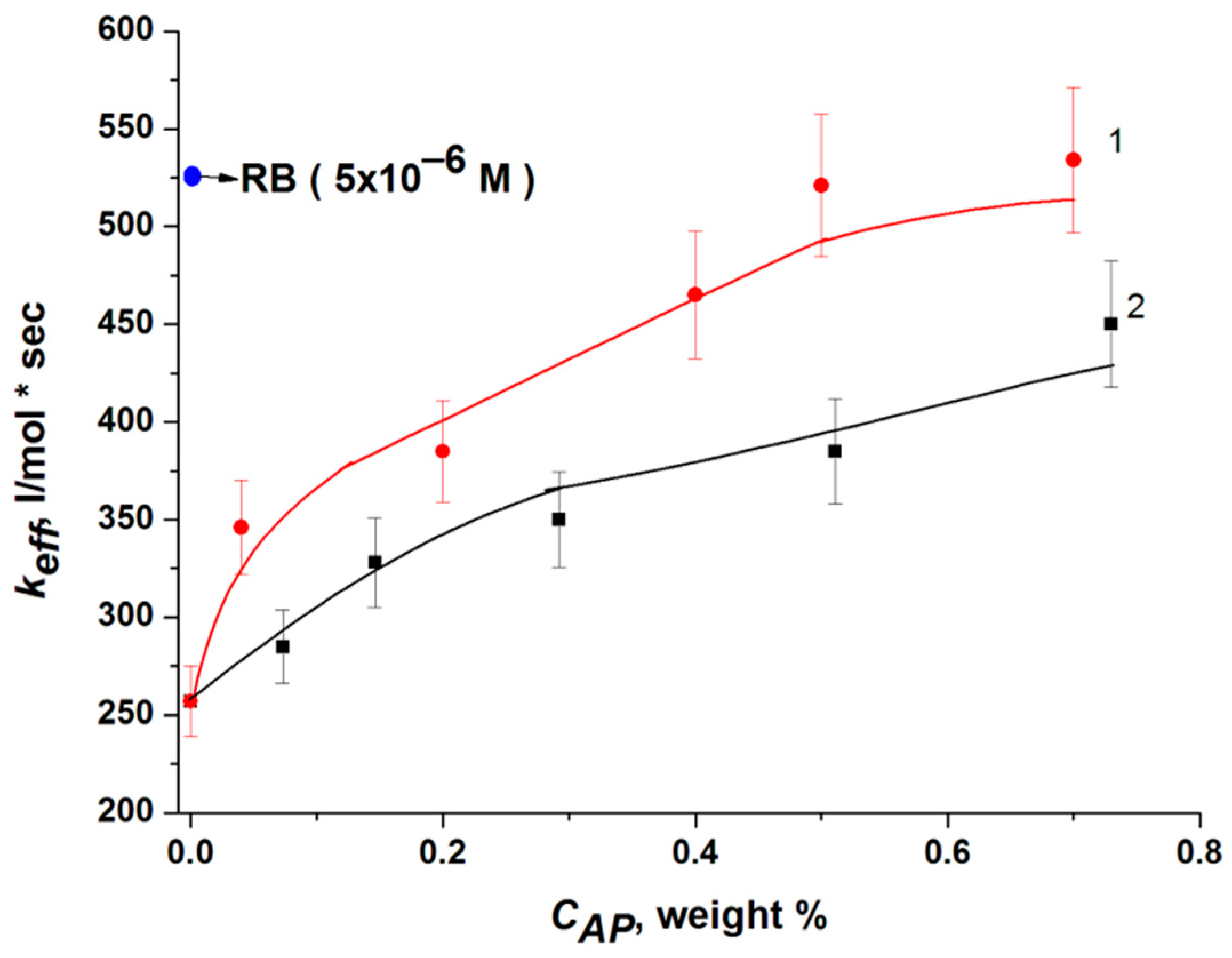
2.1. Effect of Polymers on the Photocatalytic Activity of RB
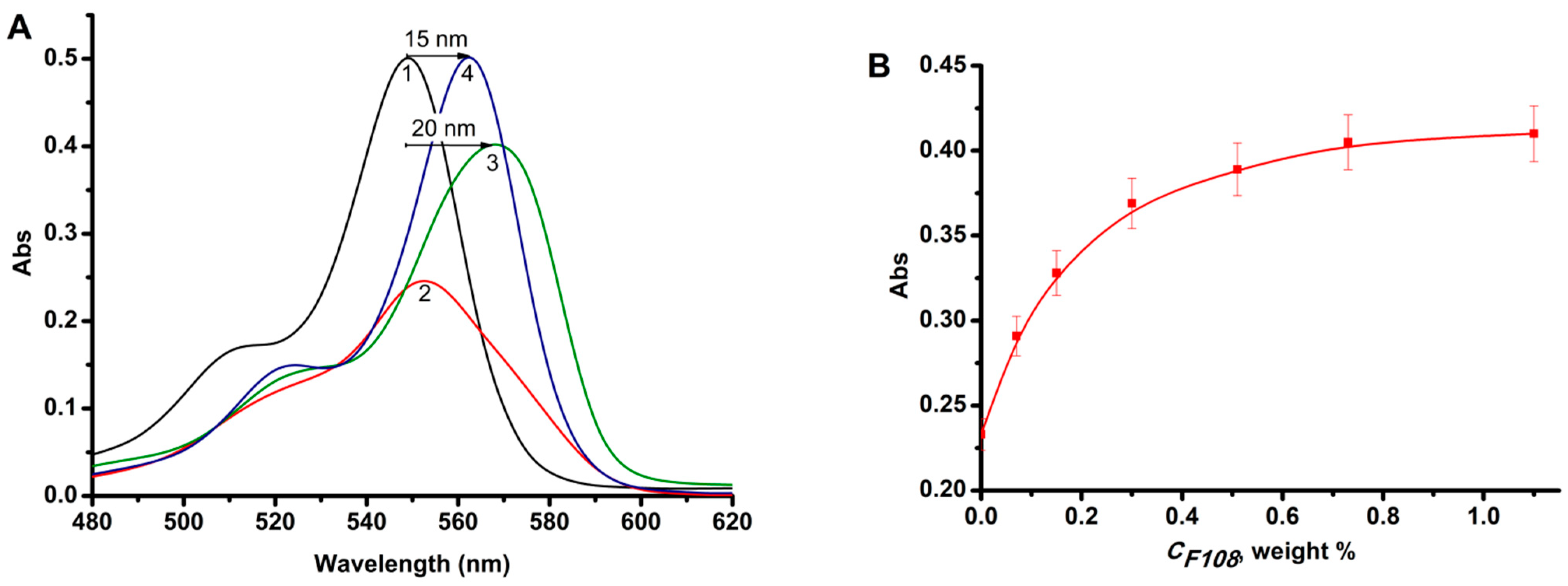
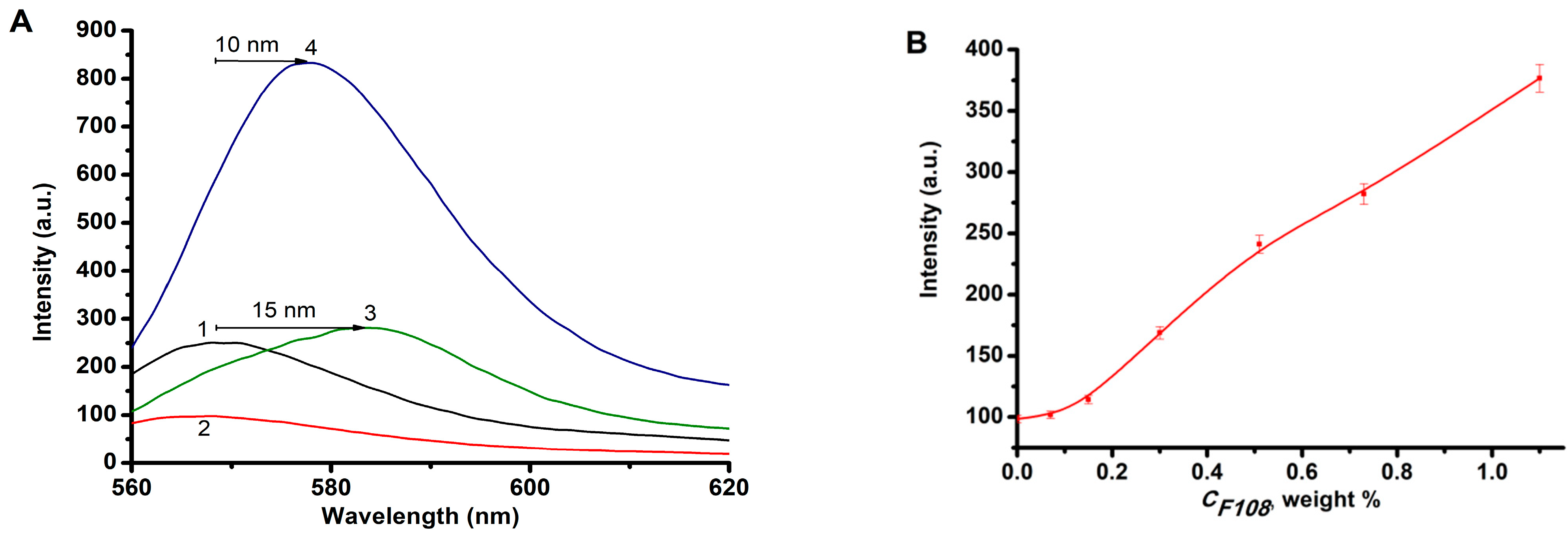
2.2. Effect of the Nature of Polymers on the RB Absorption and Fluorescence Spectra
3. Materials and Methods
4. Conclusions
- It has been shown that effective photosensitizing systems for the generation of singlet oxygen 1O2 based on rose bengal and chitosan (dissolved in 2% acetic acid) are formed when a number of amphiphilic polymers, primarily PVP and pluronic F108, are used as the third component, and buffer medium PBS is used as a solvent. Such RB-AP-CS systems may be promising in the treatment of hard-to-heal purulent wounds and trophic ulcers by aPDT.
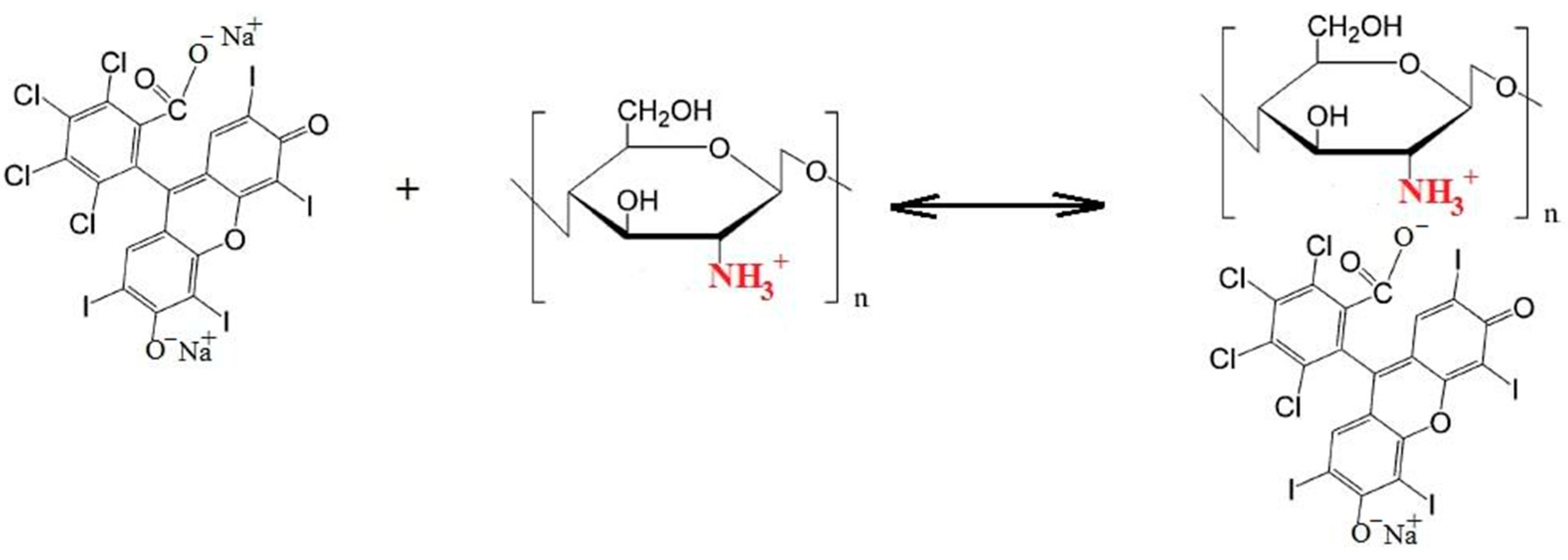
- It was shown that the activity of rose bengal in the generation of singlet oxygen 1O2 in the presence of chitosan decreases due to ionic interaction with protonated CS groups; however, the presence of amphiphilic polymers protects RB from binding to CS, and the photocatalytic activity of the dye is restored. At the same time, if focusing on the rate constant of generation of singlet oxygen 1O2, the efficiency of the ternary system with PVP is higher than the efficiency of the ternary system with pluronic F108.
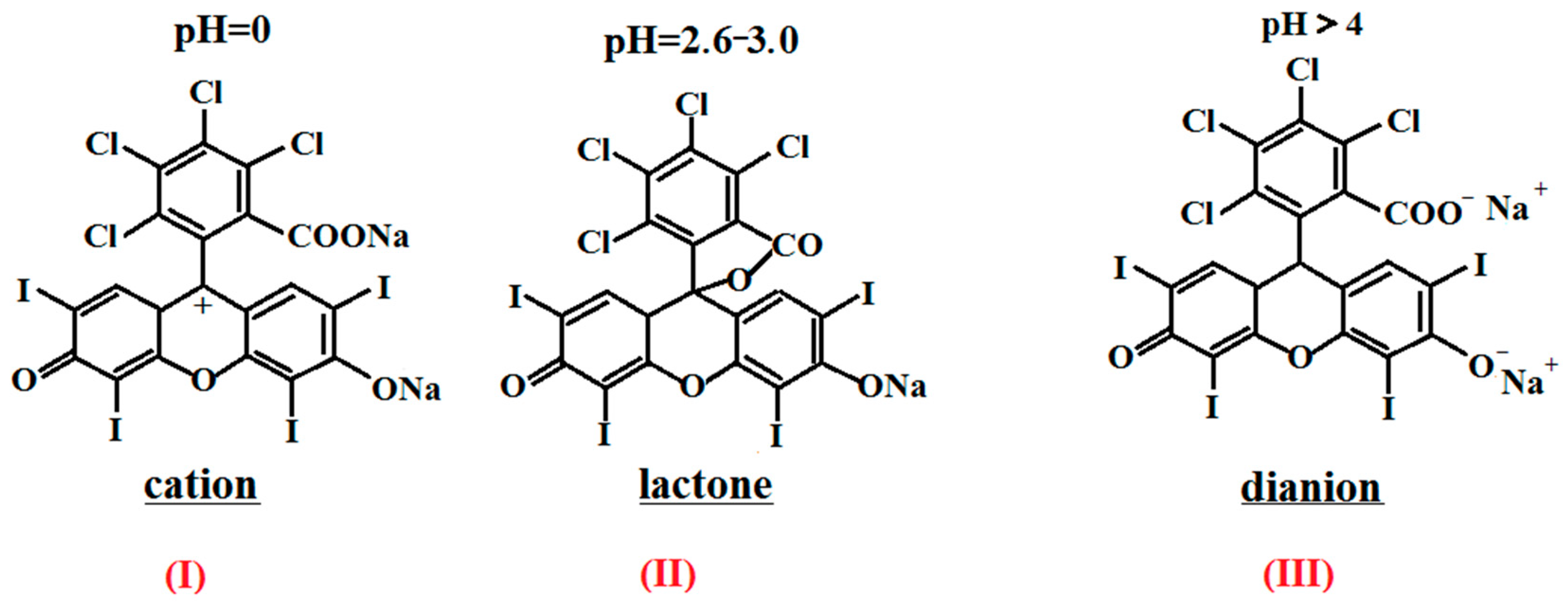
- The introduction of chitosan into an aqueous and PBS solution containing RB led to a decrease in the optical density of both absorption bands in the EAS and the RB luminescence intensity, which is associated with the RB-CS ionic interaction and the formation of the lactone photoinactive form of RB.
- The formation of RB-AP complexes was confirmed by the methods of electron and fluorescence spectroscopy. In particular, when pluronic F108 was introduced into a solution containing RB and CS, a bathochromic shift of the absorption band by 20 nm and the fluorescence band by 15 nm (λ = 550 nm) in the EAS of RB was observed.
- The effect of polymers on the degree of fluorescence anisotropy (r) of RB was studied. It was shown that in the presence of chitosan, an increase in r is observed, which is obviously associated with the formation of ionic bonds between RB and chitosan.
- The addition of pluronic to the RB-CS system in aqueous and PBS media leads to a decrease of the degree of RB fluorescence anisotropy (r), which may be due to the dispersal of RB molecules in pluronic F108 micelles. In the presence of PVP, which does not form micellar structures in water, the effect of reducing the value of r was not observed.
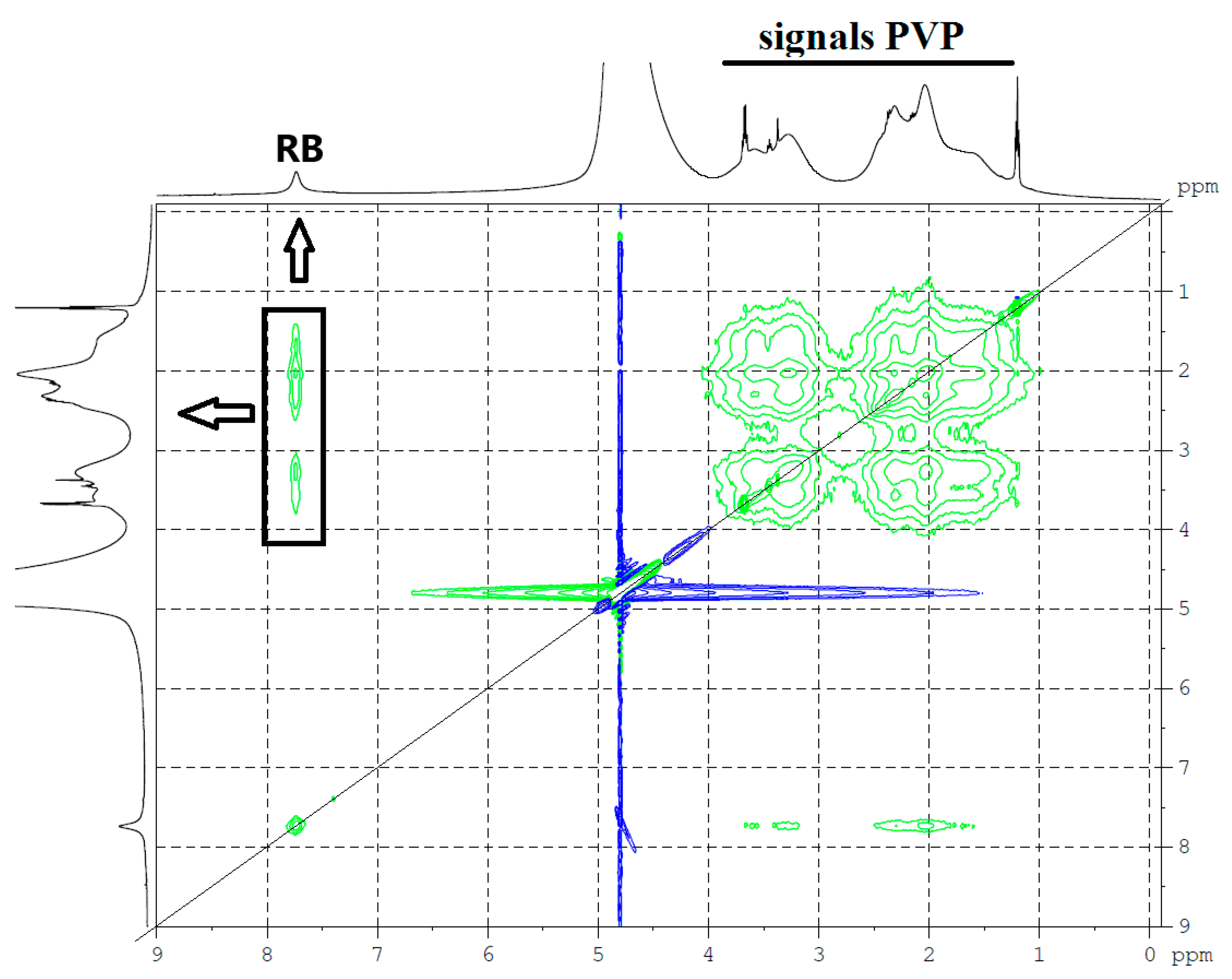
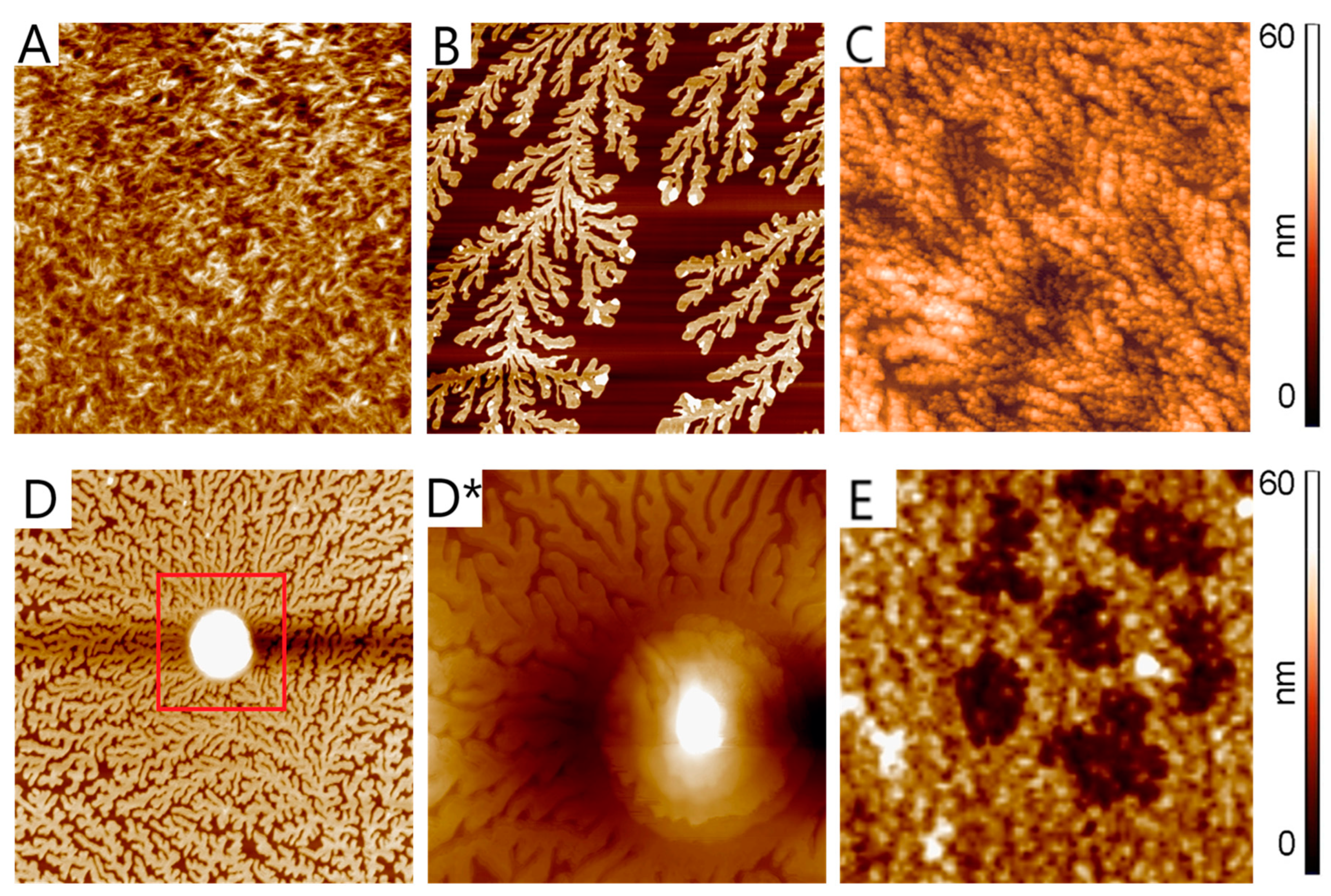
- The presence of intermolecular interactions of RB with PVP was shown by the proton NMR method. In addition, the formation of RB-F108 macromolecular complexes, which form associates during solution concentration (in particular, during evaporation), was shown by AFM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dougherty, T.J. An Update on Photodynamic Therapy Applications. J. Clin. Laser Med. Surg. 2002, 20, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. JCMT 2019, 5, 25. [Google Scholar] [CrossRef]
- Oyama, J.; Fernandes Herculano Ramos-Milaré, Á.C.; Lopes Lera-Nonose, D.S.S.; Nesi-Reis, V.; Galhardo Demarchi, I.; Alessi Aristides, S.M.; Juarez Vieira Teixeira, J.; Gomes Verzignassi Silveira, T.; Campana Lonardoni, M.V. Photodynamic Therapy in Wound Healing in Vivo: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Azari-Marhabi, S.; Mojahedi, S.M.; Namdari, M.; Rankohi, Z.E.; Jafari, S. Comparing Clinical Effects of Photodynamic Therapy as a Novel Method with Topical Corticosteroid for Treatment of Oral Lichen Planus. Photodiagnosis Photodyn. Ther. 2017, 20, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One—Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Günsel, A.; Taslimi, P.; Atmaca, G.Y.; Bilgiçli, A.T.; Pişkin, H.; Ceylan, Y.; Erdoğmuş, A.; Yarasir, M.N.; Gülçin, İ. Novel Potential Metabolic Enzymes Inhibitor, Photosensitizer and Antibacterial Agents Based on Water-Soluble Phthalocyanine Bearing Imidazole Derivative. J. Mol. Struct. 2021, 1237, 130402. [Google Scholar] [CrossRef]
- Ziental, D.; Mlynarczyk, D.T.; Czarczynska-Goslinska, B.; Lewandowski, K.; Sobotta, L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials 2021, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Noweski, A.; Roosen, A.; Lebdai, S.; Barret, E.; Emberton, M.; Benzaghou, F.; Apfelbeck, M.; Gaillac, B.; Gratzke, C.; Stief, C.; et al. Medium-Term Follow-up of Vascular-Targeted Photodynamic Therapy of Localized Prostate Cancer Using TOOKAD Soluble WST-11 (Phase II Trials). Eur. Urol. Focus 2019, 5, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, V.G.; Somers, M.L. Photofrin-Mediated Photodynamic Therapy for Treatment of Early Stage (Tis-T2N0M0) SqCCa of Oral Cavity and Oropharynx: Photofrin-mediated photodynamic therapy. Lasers Surg. Med. 2010, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Méndez, D.A.C.; Gutierrez, E.; Dionísio, E.J.; Oliveira, T.M.; Buzalaf, M.A.R.; Rios, D.; Machado, M.A.A.M.; Cruvinel, T. Effect of Methylene Blue-Mediated Antimicrobial Photodynamic Therapy on Dentin Caries Microcosms. Lasers Med. Sci 2018, 33, 479–487. [Google Scholar] [CrossRef]
- Manoil, D.; Filieri, A.; Schrenzel, J.; Bouillaguet, S. Rose Bengal Uptake by E. Faecalis and F. Nucleatum and Light-Mediated Antibacterial Activity Measured by Flow Cytometry. J. Photochem. Photobiol. B Biol. 2016, 162, 258–265. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kang, S.-M.; Jeong, S.-H.; Chung, K.-H.; Kim, B.-I. Antibacterial Photodynamic Therapy with Curcumin and Curcuma Xanthorrhiza Extract against Streptococcus Mutans. Photodiagnosis Photodyn. Ther. 2017, 20, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. The Role of the Methylene Blue and Toluidine Blue Monomers and Dimers in the Photoinactivation of Bacteria. J. Photochem. Photobiol. B Biol. 2003, 71, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Barroso, R.A.; Navarro, R.; Tim, C.R.; de Paula Ramos, L.; de Oliveira, L.D.; Araki, Â.T.; Fernandes, K.G.C.; Macedo, D.; Assis, L. Antimicrobial Photodynamic Therapy against Propionibacterium Acnes Biofilms Using Hypericin (Hypericum Perforatum) Photosensitizer: In Vitro Study. Lasers Med. Sci 2021, 36, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Samper, S.; Soria-Lozano, P.; Rezusta, A.; Gilaberte, Y. Antimicrobial Photodynamic Activity of Rose Bengal, Alone or in Combination with Gentamicin, against Planktonic and Biofilm Staphylococcus Aureus. Photodiagnosis Photodyn. Ther. 2018, 21, 211–216. [Google Scholar] [CrossRef]
- Xu, D.; Neckers, D.C. Aggregation of Rose Bengal Molecules in Solution. J. Photochem. Photobiol. A Chem. 1987, 40, 361–370. [Google Scholar] [CrossRef]
- Simões, J.C.S.; Sarpaki, S.; Papadimitroulas, P.; Therrien, B.; Loudos, G. Conjugated Photosensitizers for Imaging and PDT in Cancer Research. J. Med. Chem. 2020, 63, 14119–14150. [Google Scholar] [CrossRef]
- Gündüz, E.Ö.; Gedik, M.E.; Günaydın, G.; Okutan, E. Amphiphilic Fullerene-BODIPY Photosensitizers for Targeted Photodynamic Therapy. ChemMedChem 2022, 17, e202100693. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Ouk, T.-S.; Calvete, M.J.F.; Pereira, M.M.; Villandier, N.; Leroy-Lhez, S. Conjugating Biomaterials with Photosensitizers: Advances and Perspectives for Photodynamic Antimicrobial Chemotherapy. Photochem. Photobiol. Sci. 2020, 19, 445–461. [Google Scholar] [CrossRef]
- Ma, R.; Yan, C.; Fong, P.W.-K.; Yu, J.; Liu, H.; Yin, J.; Huang, J.; Lu, X.; Yan, H.; Li, G. In Situ and Ex Situ Investigations on Ternary Strategy and Co-Solvent Effects towards High-Efficiency Organic Solar Cells. Energy Environ. Sci. 2022, 15, 2479–2488. [Google Scholar] [CrossRef]
- Hegge, A.B.; Andersen, T.; Melvik, J.E.; Kristensen, S.; Tønnesen, H.H. Evaluation of Novel Alginate Foams as Drug Delivery Systems in Antimicrobial Photodynamic Therapy (APDT) of Infected Wounds—An In Vitro Study: Studies on Curcumin and Curcuminoides XL. J. Pharm. Sci. 2010, 99, 3499–3513. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Zhiyentayev, T.M.; Boltaev, U.T.; Solov’eva, A.B.; Aksenova, N.A.; Glagolev, N.N.; Chernjak, A.V.; Melik-Nubarov, N.S. Complexes of Chlorin E6 with Pluronics and Polyvinylpyrrolidone: Structure and Photodynamic Activity in Cell Culture. Photochem. Photobiol. 2014, 90, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Gorokh, Y.A.; Aksenova, N.A.; Solov’eva, A.B.; Ol’shevskaya, V.A.; Zaitsev, A.V.; Lagutina, M.A.; Luzgina, V.N.; Mironov, A.F.; Kalinin, V.N. The Influence of Amphiphilic Polymers on the Photocatalytic Activity of Water-Soluble Porphyrin Photosensitizers. Russ. J. Phys. Chem. 2011, 85, 871–875. [Google Scholar] [CrossRef]
- Aksenova, N.A.; Zhientaev, T.M.; Brilkina, A.A.; Dubasova, L.V.; Ivanov, A.V.; Timashev, P.S.; Melik-Nubarov, N.S.; Solovieva, A.B. Polymers as Enhancers of Photodynamic Activity of Chlorin Photosensitizers for Photodynamic Therapy. Photonics Lasers Med. 2013, 2. [Google Scholar] [CrossRef]
- Solovieva, A.B.; Rudenko, T.G.; Shekhter, A.B.; Glagolev, N.N.; Spokoinyi, A.L.; Fayzullin, A.L.; Aksenova, N.A.; Shpichka, A.I.; Kardumyan, V.V.; Timashev, P.S. Broad-Spectrum Antibacterial and pro-Regenerative Effects of Photoactivated Photodithazine-Pluronic F127-Chitosan Polymer System: In Vivo Study. J. Photochem. Photobiol. B Biol. 2020, 210, 111954. [Google Scholar] [CrossRef]
- Amescua, G.; Arboleda, A.; Nikpoor, N.; Durkee, H.; Relhan, N.; Aguilar, M.C.; Flynn, H.W.; Miller, D.; Parel, J.-M. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea 2017, 36, 1141–1144. [Google Scholar] [CrossRef]
- Rossoni, R.D.; Junqueira, J.C.; Santos, E.L.S.; Costa, A.C.B.; Jorge, A.O.C. Comparison of the Efficacy of Rose Bengal and Erythrosin in Photodynamic Therapy against Enterobacteriaceae. Lasers Med. Sci. 2010, 25, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Demartis, S.; Obinu, A.; Gavini, E.; Giunchedi, P.; Rassu, G. Nanotechnology-Based Rose Bengal: A Broad-Spectrum Biomedical Tool. Dye. Pigment. 2021, 188, 109236. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Polycationic Chitosan-Conjugated Photosensitizer for Antibacterial Photodynamic Therapy†: Photochemistry and Photobiology. Photochem. Photobiol. 2012, 88, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Doughty, M.J. Rose Bengal Staining as an Assessment of Ocular Surface Damage and Recovery in Dry Eye Disease—A Review. Contact Lens Anterior Eye 2013, 36, 272–280. [Google Scholar] [CrossRef]
- Sebrão, C.C.N.; Bezerra, A.G.; de França, P.H.C.; Ferreira, L.E.; Westphalen, V.P.D. Comparison of the Efficiency of Rose Bengal and Methylene Blue as Photosensitizers in Photodynamic Therapy Techniques for Enterococcus Faecalis Inactivation. Photomed. Laser Surg. 2017, 35, 18–23. [Google Scholar] [CrossRef]
- Labban, N.; Taweel, S.M.A.; ALRabiah, M.A.; Alfouzan, A.F.; Alshiddi, I.F.; Assery, M.K. Efficacy of Rose Bengal and Curcumin Mediated Photodynamic Therapy for the Treatment of Denture Stomatitis in Patients with Habitual Cigarette Smoking: A Randomized Controlled Clinical Trial. Photodiagnosis Photodyn. Ther. 2021, 35, 102380. [Google Scholar] [CrossRef]
- Moczek, L.; Nowakowska, M. Novel Water-Soluble Photosensitizers from Chitosan. Biomacromolecules 2007, 8, 433–438. [Google Scholar] [CrossRef]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-Based Blends for Biomedical Applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Amat-Guerri, F.; López-González, M.M.C.; Sastre, R.; Martinez-Utrilla, R. Spectrophotometric Determination of Ionization and Isomerization Constants of Rose Bengal, Eosin Y and Some Derivatives. Dye. Pigment. 1990, 13, 219–232. [Google Scholar] [CrossRef]
- Martin, M.M.; Lindqvist, L. The PH Dependence of Fluorescein Fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Islam, S.D.-M.; Ito, O. Solvent Effects on Rates of Photochemical Reactions of Rose Bengal Triplet State Studied by Nanosecond Laser Photolysis. J. Photochem. Photobiol. A Chem. 1999, 123, 53–59. [Google Scholar] [CrossRef]
- Neckers, D.C. Rose Bengal. J. Photochem. Photobiol. A Chem. 1989, 47, 1–29. [Google Scholar] [CrossRef]
- Kuryanova, A.S.; Aksenova, N.A.; Savko, M.A.; Glagolev, N.N.; Dubovik, A.S.; Plashchina, I.G.; Timashev, P.S.; Solov’eva, A.B. Effect of Amphiphilic Polymers on the Activity of Rose Bengal during the Photooxidation of Tryptophan in an Aqueous Medium. Russ. J. Phys. Chem. 2022, 96, 1106–1111. [Google Scholar] [CrossRef]
- Yang, B.; Guo, C.; Chen, S.; Ma, J.; Wang, J.; Liang, X.; Zheng, L.; Liu, H. Effect of Acid on the Aggregation of Poly(Ethylene Oxide)−Poly(Propylene Oxide)−Poly(Ethylene Oxide) Block Copolymers. J. Phys. Chem. B 2006, 110, 23068–23074. [Google Scholar] [CrossRef]
- Kardumyan, V.V.; Aksenova, N.A.; Timofeeva, V.A.; Krivandin, A.V.; Shatalova, O.V.; Dubovik, A.S.; Plashchina, I.G.; Timashev, P.S.; Solovieva, A.B. Effect of Chitosan on the Activity of Water-Soluble and Hydrophobic Porphyrin Photosensitizers Solubilized by Amphiphilic Polymers. Polymers 2021, 13, 1007. [Google Scholar] [CrossRef]
- Maruthamuthu, M.; Sobhana, M. Hydrophobic Interactions in the Binding of Polyvinylpyrrolidone. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 3159–3167. [Google Scholar] [CrossRef]
- Vlasova, I.M.; Kuleshova, A.A.; Vlasov, A.A.; Saletskii, A.M. Polarized Fluorescence in Investigation of Rotational Diffusion of the Fluorescein Family Markers in Bovine Serum Albumin Solutions. Mosc. Univ. Phys. 2014, 69, 401–405. [Google Scholar] [CrossRef]
- Gvozdev, D.A.; Maksimov, E.G.; Strakhovskaya, M.G.; Ivanov, M.V.; Paschenko, V.Z.; Rubin, A.B. The Effect of Ionic Strength on Spectral Properties of Quantum Dots and Aluminum Phthalocyanine Complexes. Nanotechnol Russ. 2017, 12, 73–85. [Google Scholar] [CrossRef]
- Kalvinkovskaya, J.A.; Tsaplev, Y.B.; Trofimov, A.V.; Romanenko, A.A.; Bushuk, S.B.; Pavich, T.A.; Lapina, V.A. Anisotropy and Spectroscopic Properties of the Complexes of Meso-Tetra(4-Carboxyphenyl)Porphyrin Molecules with Diamond Nanoparticles. Opt. Spectrosc. 2020, 128, 1475–1480. [Google Scholar] [CrossRef]
- Aksenova, N.A.; Timofeeva, V.A.; Rogovina, S.Z.; Timashev, P.S.; Glagolev, N.N.; Solov’eva, A.B. Photocatalytic Properties and Structure of Chitosan-Based Porphyrin-Containing Systems. Polym. Sci. Ser. B 2010, 52, 67–72. [Google Scholar] [CrossRef]
- Kotova, S.L.; Timofeeva, V.A.; Belkova, G.V.; Aksenova, N.A.; Solovieva, A.B. Porphyrin Effect on the Surface Morphology of Amphiphilic Polymers as Observed by Atomic Force Microscopy. Micron 2012, 43, 445–449. [Google Scholar] [CrossRef] [PubMed]

| Absorption | ||||
|---|---|---|---|---|
| RB in PBS | RB in Water | |||
| RB | Dmax (λ = 550 nm) | Dshoulder (λ = 515 nm) | Dmax (λ = 550 nm) | Dshoulder (λ = 515 nm) |
| 0.50 | 0.16 | 0.50 | 0.16 | |
| CS (0.005%) | 0.25 | 0.11 | 0.24 (**) | 0.15 (**) |
| AcOH (0.1%) | 0.32 | 0.12 | 0.03 (*) | 0.01 (*) |
| AcOH (0.25%) | 0.09 | 0.04 | - | - |
| AcOH (0.35%) | 0.05 | 0.03 | - | - |
| Fluorescence | ||||
| RB in PBS | RB in Water | |||
| RB | λmax | Intensity | λmax | Intensity |
| 568 | 252 | 568 | 241 | |
| CS (0.005%) | 568 | 98 | 568 | 60.5 |
| AcOH (0.1%) | 569 | 180 | 9.5 | |
| AcOH (0.25%) | 566 | 79.5 | - | - |
| AcOH (0.35%) | 566 | 39 | - | - |
| Sample | r of the Samples in Aqueous Solution (585 nm) | r of the Samples in PBS Solution (585 nm) |
|---|---|---|
| RB | 0.26 ± 0.01 | 0.27 ± 0.01 |
| RB-AcOH | 0.26 ± 0.01 | 0.26 ± 0.01 |
| RB-CS | 0.32 ± 0.01 | 0.28 ± 0.01 |
| RB-F108 | 0.27 ± 0.01 | 0.27 ± 0.01 |
| RB-F108-CS | 0.09 ± 0.01 | 0.23 ± 0.01 |
| RB-PVP | 0.31 ± 0.01 | 0.31 ± 0.01 |
| RB-PVP-CS | 0.23 ± 0.01 | 0.31 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuryanova, A.S.; Savko, M.A.; Kaplin, V.S.; Aksenova, N.A.; Timofeeva, V.A.; Chernyak, A.V.; Glagolev, N.N.; Timashev, P.S.; Solovieva, A.B. Effect of Chitosan and Amphiphilic Polymers on the Photosensitizing and Spectral Properties of Rose Bengal. Molecules 2022, 27, 6796. https://doi.org/10.3390/molecules27206796
Kuryanova AS, Savko MA, Kaplin VS, Aksenova NA, Timofeeva VA, Chernyak AV, Glagolev NN, Timashev PS, Solovieva AB. Effect of Chitosan and Amphiphilic Polymers on the Photosensitizing and Spectral Properties of Rose Bengal. Molecules. 2022; 27(20):6796. https://doi.org/10.3390/molecules27206796
Chicago/Turabian StyleKuryanova, Anastasia S., Marina A. Savko, Vladislav S. Kaplin, Nadezhda A. Aksenova, Victoria A. Timofeeva, Aleksandr V. Chernyak, Nicolay N. Glagolev, Petr S. Timashev, and Anna B. Solovieva. 2022. "Effect of Chitosan and Amphiphilic Polymers on the Photosensitizing and Spectral Properties of Rose Bengal" Molecules 27, no. 20: 6796. https://doi.org/10.3390/molecules27206796
APA StyleKuryanova, A. S., Savko, M. A., Kaplin, V. S., Aksenova, N. A., Timofeeva, V. A., Chernyak, A. V., Glagolev, N. N., Timashev, P. S., & Solovieva, A. B. (2022). Effect of Chitosan and Amphiphilic Polymers on the Photosensitizing and Spectral Properties of Rose Bengal. Molecules, 27(20), 6796. https://doi.org/10.3390/molecules27206796






