The Effect of pH and Sodium Caseinate on the Aqueous Solubility, Stability, and Crystallinity of Rutin towards Concentrated Colloidally Stable Particles for the Incorporation into Functional Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. The Effect of pH on the Solubility, Crystallinity, and Morphology of Rutin
2.3. The Effect of NaCas on the Solubility of Rutin under Various pH Conditions
2.4. Formation of the Colloidally Stable Systems (Rutin–NaCas Particles)
2.5. Particle Size and Zeta Potential Analyses
2.6. Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.7. Release Kinetics of Rutin during the Simulated Gastric Digestion
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Effect of pH on Aqueous Solubility, Stability, and Crystallinity of Rutin
3.2. The Effect of NaCas on Solubility and Stability of Rutin
3.3. Characteristics of the Concentrated Rutin–NaCas Particles
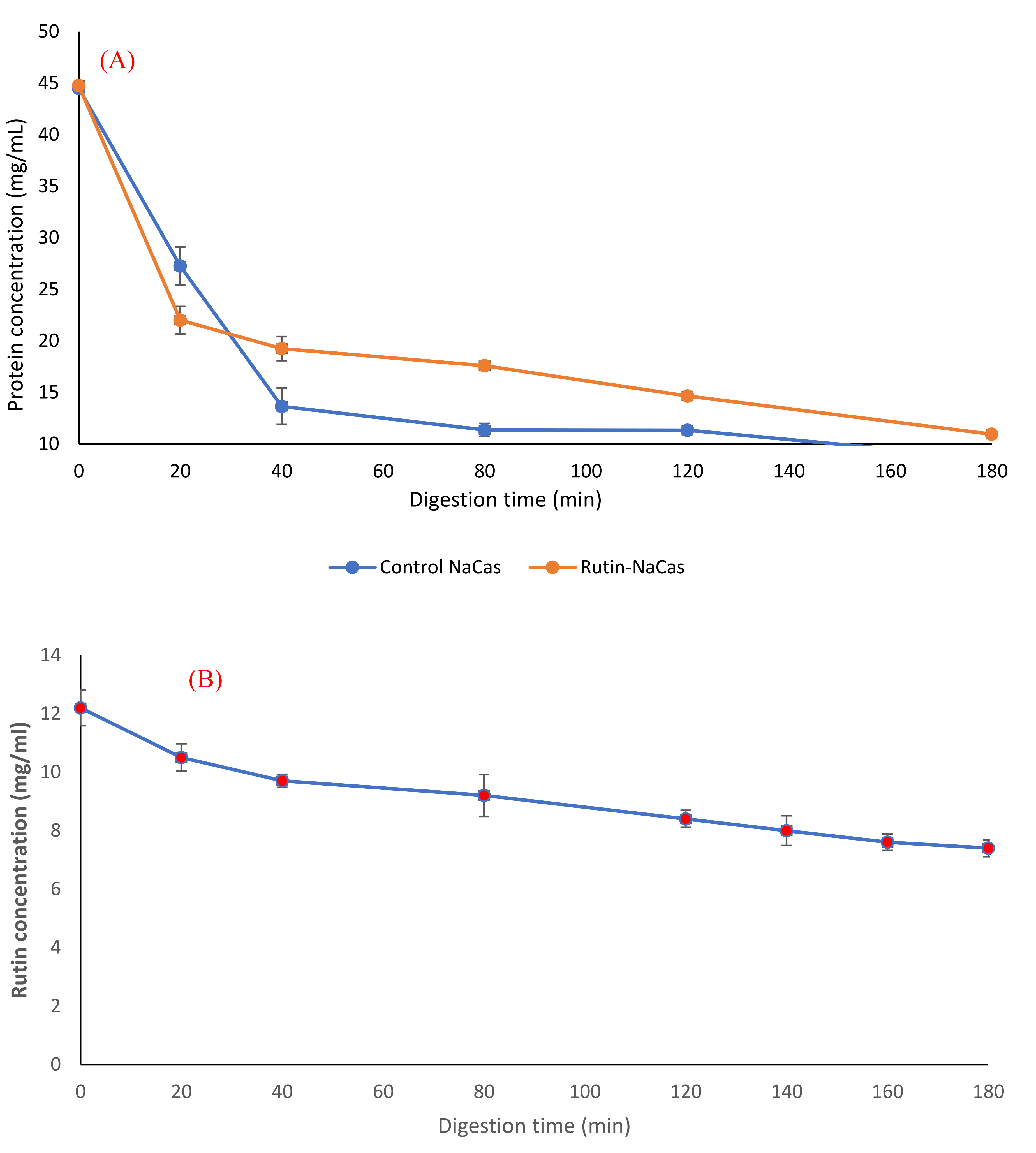
3.4. In Vitro Digestion of the Rutin–NaCas Complexes and Rutin Release Behaviour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Sattanathan, K.; Dhanapal, C.; Umarani, R.; Manavalan, R. Beneficial health effects of rutin supplementation in patients with diabetes mellitus. J. Appl. Pharm. Sci. 2011, 1, 227. [Google Scholar]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chem.-Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Quantification of rutin in rat’s brain by UHPLC/ESI-Q-TOF-MS/MS after intranasal administration of rutin loaded chitosan nanoparticles. EXCLI J. 2016, 15, 518. [Google Scholar] [PubMed]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Ashafaq, M.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016, 91, 640–655. [Google Scholar] [CrossRef]
- Prasad, R.; Prasad, S.B. A review on the chemistry and biological properties of Rutin, a promising nutraceutical agent. Asian J. Pharm. Pharm. 2019, 5, 1–20. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Comer, J.; Tam, K. Lipophilicity profiles: Theory and measurement. In Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical and Computational Strategies; Wiley: Hoboken, NJ, USA, 2001; pp. 275–304. [Google Scholar]
- Baldisserotto, A.; Vertuani, S.; Bino, A.; De Lucia, D.; Lampronti, I.; Milani, R.; Gambari, R.; Manfredini, S. Design, synthesis and biological activity of a novel Rutin analogue with improved lipid soluble properties. Bioorg. Med. Chem. 2015, 23, 264–271. [Google Scholar] [CrossRef]
- Vogrincic, M.; Timoracka, M.; Melichacova, S.; Vollmannova, A.; Kreft, I. Degradation of rutin and polyphenols during the preparation of tartary buckwheat bread. J. Agric. Food Chem. 2010, 58, 4883–4887. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Koval’skii, I.; Krasnyuk, I.; Nikulina, O.; Belyatskaya, A.; Kharitonov, Y.Y.; Feldman, N.; Lutsenko, S. Mechanisms of rutin. Pharm. Chem. J. 2014, 48, 73–76. [Google Scholar] [CrossRef]
- Kumar, R.; Vijayalakshmi, S.; Nadanasabapathi, S. Health benefits of quercetin. Def. Life Sci. J. 2017, 2, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems. Drug Discov. Today 2020, 25, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Acvedo-Fani, A.; Singh, H.; Loveday, S.; Nui, Z.; Thompson, A. Flavonoid Delivery System. New Zealand Patent WO2020095238A1, 7 November 2019. [Google Scholar]
- Rashidinejad, A.; Loveday, S.; Jameson, G.; Singh, H. Rutin-casein co-precipitates as potential delivery vehicles for flavonoid rutin. Food Hydrocoll. 2019, 96, 451–462. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Kerdudo, A.; Dingas, A.; Fernandez, X.; Faure, C. Encapsulation of rutin and naringenin in multilamellar vesicles for optimum antioxidant activity. Food Chem. 2014, 159, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Polo, J.; Silva-Weiss, A.; Giménez, B.; Cantero-López, P.; Vega, R.; Osorio, F.A. Effect of lyophilization on the physicochemical and rheological properties of food grade liposomes that encapsulate rutin. Food Res. Int. 2020, 130, 108967. [Google Scholar] [CrossRef]
- Mel, M.; Gunathilake, K.; Fernando, C. Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. Int. J. Biol. Macromol. 2020, 159, 316–323. [Google Scholar] [CrossRef]
- Cândido, T.M.; De Oliveira, C.A.; Ariede, M.B.; Velasco MV, R.; Rosado, C.; Baby, A.R. Safety and antioxidant efficacy profiles of rutin-loaded ethosomes for topical application. AAPS PharmSciTech 2018, 19, 1773–1780. [Google Scholar] [CrossRef]
- Hooresfand, Z.; Ghanbarzadeh, S.; Hamishehkar, H. Preparation and characterization of rutin-loaded nanophytosomes. Pharm. Sci. 2015, 21, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Mehranfar, F.; Bordbar, A.-K.; Parastar, H. A combined spectroscopic, molecular docking and molecular dynamic simulation study on the interaction of quercetin with β-casein nanoparticles. J. Photochem. Photobiol. B Biol. 2013, 127, 100–107. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D.; Dalgleish, D.G. Casein Micelles for Nanoencapsulation of Hydrophobic Compounds. U.S. Patent 658,303, 20 August 2015. [Google Scholar]
- Huppertz, T.; De Kruif, C.G. Disruption and reassociation of casein micelles under high pressure: Influence of milk serum composition and casein micelle concentration. J. Agric. Food Chem. 2006, 54, 5903–5909. [Google Scholar] [CrossRef]
- Teng, Z.; Li, Y.; Luo, Y.; Zhang, B.; Wang, Q. Cationic β-lactoglobulin nanoparticles as a bioavailability enhancer: Protein characterization and particle formation. Biomacromolecules 2013, 14, 2848–2856. [Google Scholar] [CrossRef]
- Pereyra, R.; Schmidt, K.A.; Wicker, L. Interaction and stabilization of acidified casein dispersions with low and high methoxyl pectins. J. Agric. Food Chem. 1997, 45, 3448–3451. [Google Scholar] [CrossRef]
- Pan, K.; Chen, H.; Davidson, P.M.; Zhong, Q. Thymol nanoencapsulated by sodium caseinate: Physical and antilisterial properties. J. Agric. Food Chem. 2014, 62, 1649–1657. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Walstra, P.; Walstra, P.; Wouters, J.T.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Elzoghby, A.O.; El-Fotoh, W.S.A.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Morr, C.V. Milk proteins: Physicochemical and functional properties. Crit. Rev. Food Sci. Nutr. 1984, 21, 197–262. [Google Scholar] [CrossRef]
- Dammak, I.; do Amaral Sobral, P.J. Formulation and Stability Characterization of Rutin-Loaded Oil-in-Water Emulsions. Food Bioprocess Technol. 2017, 10, 926–939. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. The formation and breakdown of structured clots from whole milk during gastric digestion. Food Funct. 2016, 7, 4259–4266. [Google Scholar] [CrossRef] [PubMed]
- Watters, C. A one-step biuret assay for protein in the presence of detergent. Anal. Biochem. 1978, 88, 695–698. [Google Scholar] [CrossRef]
- Das, M.K.; Kalita, B. Design and evaluation of phyto-phospholipid complexes (phytosomes) of rutin for transdermal application. J. Appl. Pharm. Sci. 2014, 4, 51–57. [Google Scholar] [CrossRef]
- Mielczarek, C. Acid–base properties of selected flavonoid glycosides. Eur. J. Pharm. Sci. 2005, 25, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Pedriali, C.A.; Fernandes, A.U.; Bernusso, L.d.C.; Polakiewicz, B. The synthesis of a water-soluble derivative of rutin as an antiradical agent. Química Nova 2008, 31, 2147–2151. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Pan, K.; Zhong, Q. Casein/pectin nanocomplexes as potential oral delivery vehicles. Int. J. Pharm. 2015, 486, 59–68. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]
- Peng, B.; Li, R.; Yan, W. Solubility of rutin in ethanol+ water at (273.15 to 323.15) K. J. Chem. Eng. Data 2009, 54, 1378–1381. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of rutin with β-cyclodextrin as potential delivery system. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dechene, E.B. The Relative Stability of Rutin and Quercetin in Alkaline Solution. J. Am. Pharm. Assoc. 1951, 40, 495–497. [Google Scholar] [CrossRef]
- Baracat, M.M.; Nakagawa, A.M.; Casagrande, R.; Georgetti, S.R.; Verri, W.A.; de Freitas, O. Preparation and characterization of microcapsules based on biodegradable polymers: Pectin/casein complex for controlled drug release systems. AAPS Pharmscitech 2012, 13, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Konecsni, K.; Low, N.; Nickerson, M. Chitosan–tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem. 2012, 134, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, G.; Zhang, M.; Zhou, Z.; Li, Q.; Strappe, P.; Blanchard, C. Epigallocatechin gallate (EGCG) decorating soybean seed ferritin as a rutin nanocarrier with prolonged release property in the gastrointestinal tract. Plant Foods Hum. Nutr. 2016, 71, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Carvalho, A.F.; Bouhallab, S. Milk proteins as encapsulation devices and delivery vehicles: Applications and trends. Trends Food Sci. Technol. 2014, 37, 5–20. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.-A. Interaction of milk α- and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, F.; Yang, F.; Zhao, Y.; Zhang, C.; Yamamoto, K. Interaction of dietary polyphenols with bovine milk proteins: Molecular structure–affinity relationship and influencing bioactivity aspects. Mol. Nutr. Food Res. 2011, 55, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Zhou, C.; Yagoub, A.E.A.; Ma, H. Effects of collagen and casein with phenolic compounds interactions on protein in vitro digestion and antioxidation. LWT 2020, 124, 109192. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S.; Kroll, J. Influence of a sugar moiety (rhamnosylglucoside) at 3-O position on the reactivity of quercetin with whey proteins. Int. J. Biol. Macromol. 2003, 32, 109–120. [Google Scholar] [CrossRef]
- Keppler, J.K.; Schwarz, K.; van der Goot, A.J. Covalent modification of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): A commonplace reaction with novel utilization potential. Trends Food Sci. Technol. 2020, 101, 38–49. [Google Scholar] [CrossRef]
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004, 18, 81–89. [Google Scholar] [CrossRef]
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- MaHam, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-based nanomedicine platforms for drug delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, J.; Mulvihill, D.M. Preparation, characterisation and selected functional properties of sodium caseinate–maltodextrin conjugates. Food Chem. 2009, 115, 1257–1267. [Google Scholar] [CrossRef]
- Ye, A.; Flanagan, J.; Singh, H. Formation of stable nanoparticles via electrostatic complexation between sodium caseinate and gum arabic. Biopolym. Orig. Res. Biomol. 2006, 82, 121–133. [Google Scholar] [CrossRef] [PubMed]
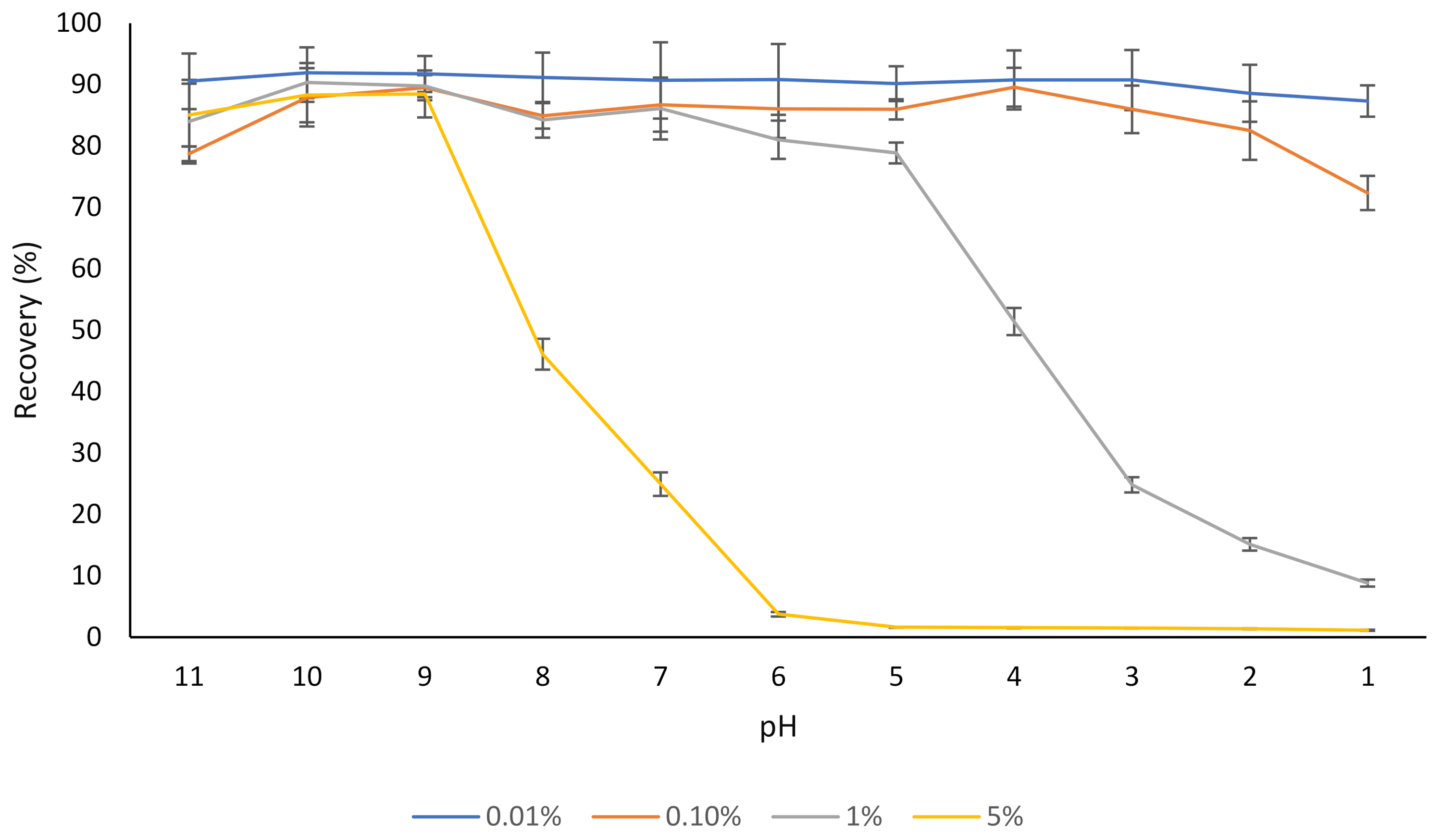
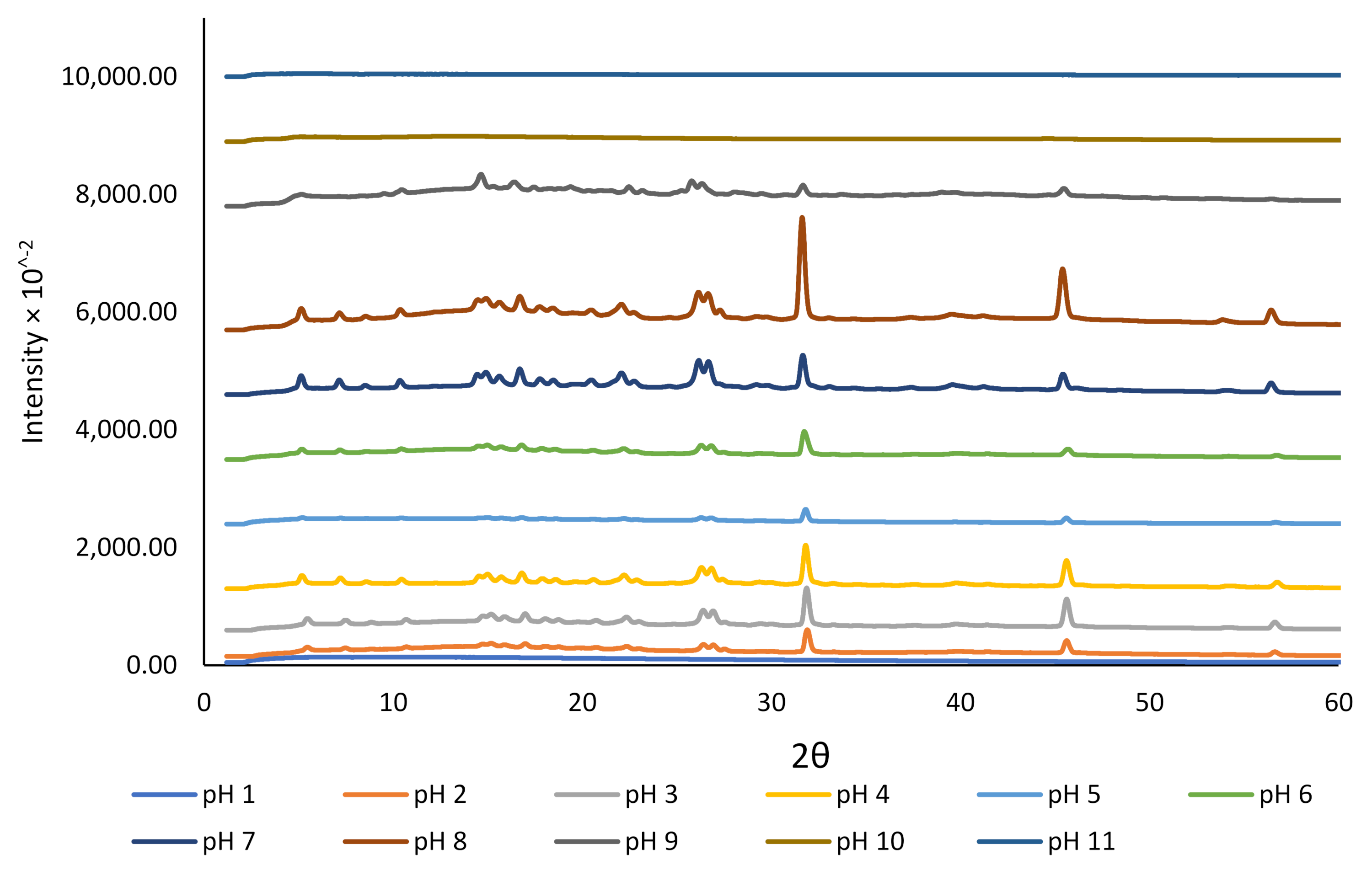
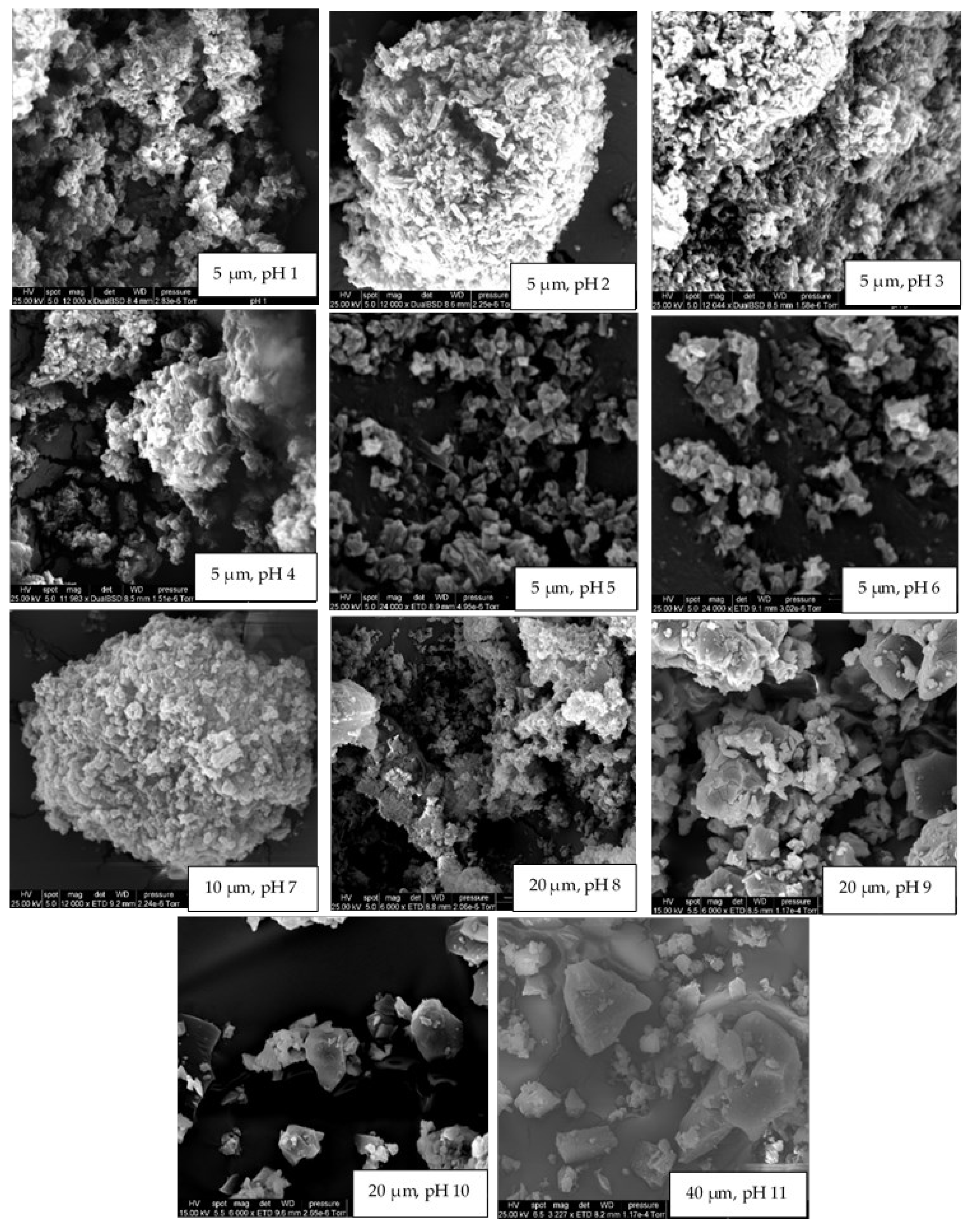

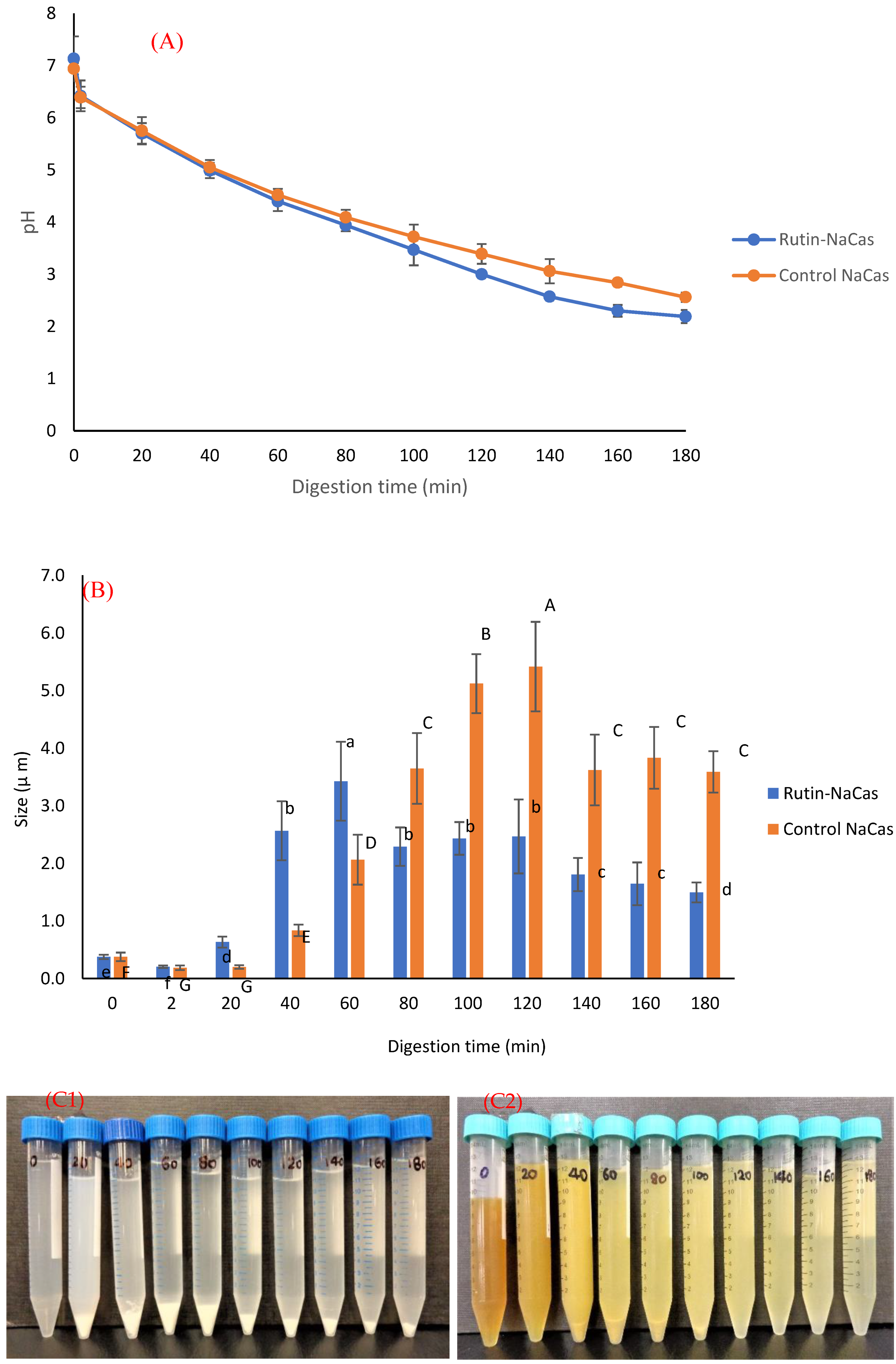
| Formulation | NaCas Concentration (% w/v) | Rutin Concentration (% w/v) | NaCas: Rutin Ratio (w/w) | Size (nm) | Zeta Potential (mV) | Colloidally Stable? |
|---|---|---|---|---|---|---|
| A | 5 | 1 | 5 | 1880 ± 50 b | −21.3 ± 2.2 a | No |
| B | 5 | 2 | 2.5 | 2120 ± 40 a | −22.8 ± 1.7 a | No |
| C | 5 | 4 | 1.25 | 1850 ± 30 b | −23.0 ± 1.5 a | No |
| D | 1 | 0.2 | 5 | 1380 ± 40 f | −22.8 ± 1.4 a | No |
| E | 1 | 0.4 | 2.5 | 145 ± 70 e | −23.6 ± 2.6 ab | No |
| F | 1 | 0.8 | 1.25 | 1490 ± 40 d | −24.6 ± 1.8 c | No |
| G | 1 | 0.1 | 10 | 832 ± 22 h | −22.6 ± 1.2 a | No |
| H | 5 | 0 | NA | 214 ± 5 qp | −30.2 ± 1.5d | Yes |
| I | 1 | 0 | NA | 203 ± 8 r | −31.2 ± 2.3 de | Yes |
| J | 2 | 0 | NA | 259 ± 14 o | −29.7 ± 1.0 d | Yes |
| K | 2 | 0.1 | 20 | 1520 ± 60 d | −24.2 ± 1.03 c | No |
| L | 2 | 0.2 | 10 | 1670 ± 60 c | −25.31 ± 1.1 c | No |
| M | 2 | 0.4 | 5 | 1400 ± 80 f | −22.4 ± 1.4 a | No |
| N | 2 | 0.8 | 2.5 | 1320 ± 70 g | −21.1 ± 1.0 a | No |
| O | 2 | 1.6 | 1.25 | 1740 ± 40 i | −23.5 ± 1.3 ab | No |
| P | 0.2 | 0 | NA | 172 ± 17 s | −24.5 ± 0.9 c | Yes |
| Q | 0.2 | 0.01 | 20 | 236 ± 21 p | −23.1 ± 0.9 b | Yes |
| R | 0.2 | 0.02 | 10 | 321 ± 27 n | −22.7 ± 1.2 a | Yes |
| S | 0.2 | 0.04 | 5 | 450 ± 30 k | −21.3 ± 1.6a | Yes |
| T | 0.2 | 0.08 | 2.5 | 460 ± 40 k | −23.5 ± 1.4 ab | Yes |
| U | 0.2 | 0.16 | 1.25 | 620 ± 40 j | −22.7 ± 1.4 a | No |
| W | 8 | 0.2 | 40 | 362 ± 17 m | −25.0 ± 1.0 c | Yes |
| X | 8 | 0.1 | 80 | 422 ± 18 l | −24.8 ± 0.8 c | Yes |
| Y | 4 | 0.1 | 40 | 445 ± 29 kl | −30.4 ± 2.0 d | Yes |
| Z | 4 | 0.05 | 80 | 271 ± 22 o | −31.4 ± 2.2 de | Yes |
| WC | 8 | 0 | NA | 226 ± 10 p | −32.7 ± 1.2 e | Yes |
| YC | 4 | 0 | NA | 220 ± 6 p | −30.3 ± 0.9 d | Yes |
| Formulation | NaCas Concentration (%) | Rutin Concentration (%) | NaCas: Rutin Ratio (w/w) | Size (nm) | Zeta Potential (mV) | Encapsulation Efficiency (EE, %) | Loading Capacity (LC, %) |
|---|---|---|---|---|---|---|---|
| UF1C | 66.81 | 0 | NA | 218 ± 7 b | −26 ± 3 b | NA | NA |
| UF1 | 66.81 | 1.67 | 40 | 208 ± 5 c | −38.7 ± 1.5 d | 83 ± 4 a | 2.03 ± 0.06 d |
| UF2C | 39.64 | 0 | NA | 167 ± 13 e | −13.5 ± 0.9 a | NA | NA |
| UF2 | 39.64 | 1.98 | 20 | 230 ± 4 a | −36.8 ± 1.5 c | 80 ± 4 bc | 3.78 ± 0.12 c |
| EUF3C | 5.68 | 0 | NA | 157 ± 4 f | −16.2 ± 0.8 b | NA | NA |
| FUF3 | 5.68 | 1.14 | 4.98 | 185 ± 6 d | −37.0 ± 1.1 c | 81 ± 3 b | 13.4 ± 0.5 b |
| UF4C | 5.04 | 0 | NA | 162 ± 9 e | −16.7 ± 0.9 b | NA | NA |
| UF4 | 5.04 | 2.02 | 2.5 | 204 ± 6 c | −38.1 ± 1.7 d | 75.8 ± 2.2 c | 21.7 ± 1.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashidinejad, A.; Jameson, G.B.; Singh, H. The Effect of pH and Sodium Caseinate on the Aqueous Solubility, Stability, and Crystallinity of Rutin towards Concentrated Colloidally Stable Particles for the Incorporation into Functional Foods. Molecules 2022, 27, 534. https://doi.org/10.3390/molecules27020534
Rashidinejad A, Jameson GB, Singh H. The Effect of pH and Sodium Caseinate on the Aqueous Solubility, Stability, and Crystallinity of Rutin towards Concentrated Colloidally Stable Particles for the Incorporation into Functional Foods. Molecules. 2022; 27(2):534. https://doi.org/10.3390/molecules27020534
Chicago/Turabian StyleRashidinejad, Ali, Geoffrey B. Jameson, and Harjinder Singh. 2022. "The Effect of pH and Sodium Caseinate on the Aqueous Solubility, Stability, and Crystallinity of Rutin towards Concentrated Colloidally Stable Particles for the Incorporation into Functional Foods" Molecules 27, no. 2: 534. https://doi.org/10.3390/molecules27020534
APA StyleRashidinejad, A., Jameson, G. B., & Singh, H. (2022). The Effect of pH and Sodium Caseinate on the Aqueous Solubility, Stability, and Crystallinity of Rutin towards Concentrated Colloidally Stable Particles for the Incorporation into Functional Foods. Molecules, 27(2), 534. https://doi.org/10.3390/molecules27020534







