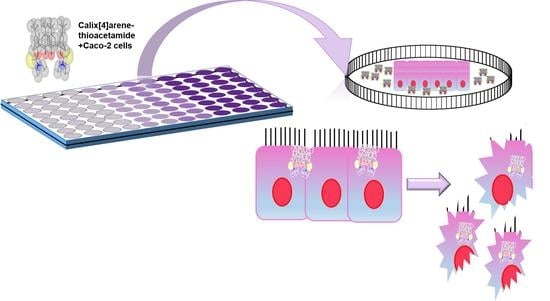
Targeting Colorectal Cancer Cells with a Functionalised Calix[4]arene Receptor: Biophysical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Thioacetamide Calix[4]arene Derivative, 5,11,17,23-Tetra-Tert-Butyl[25,27-bis(Diethylthiocarbamoyl)oxy]calix[4]arene, CAII
2.3. Cell Culture
2.4. CAII Treatment
2.5. CAII Preparation
2.6. Cell Viability
2.7. Apoptosis Analysis by Annexin V-FITC Staining
2.8. Structural Insights on the CAII and Caco-2 Interactions Using 1H NMR Measurements
2.9. 1H NMR Spectroscopy of Caco-2 Cell Extracts
2.10. Confocal Raman Microscopy Studies
2.11. Statistical Analysis
3. Results and Discussion
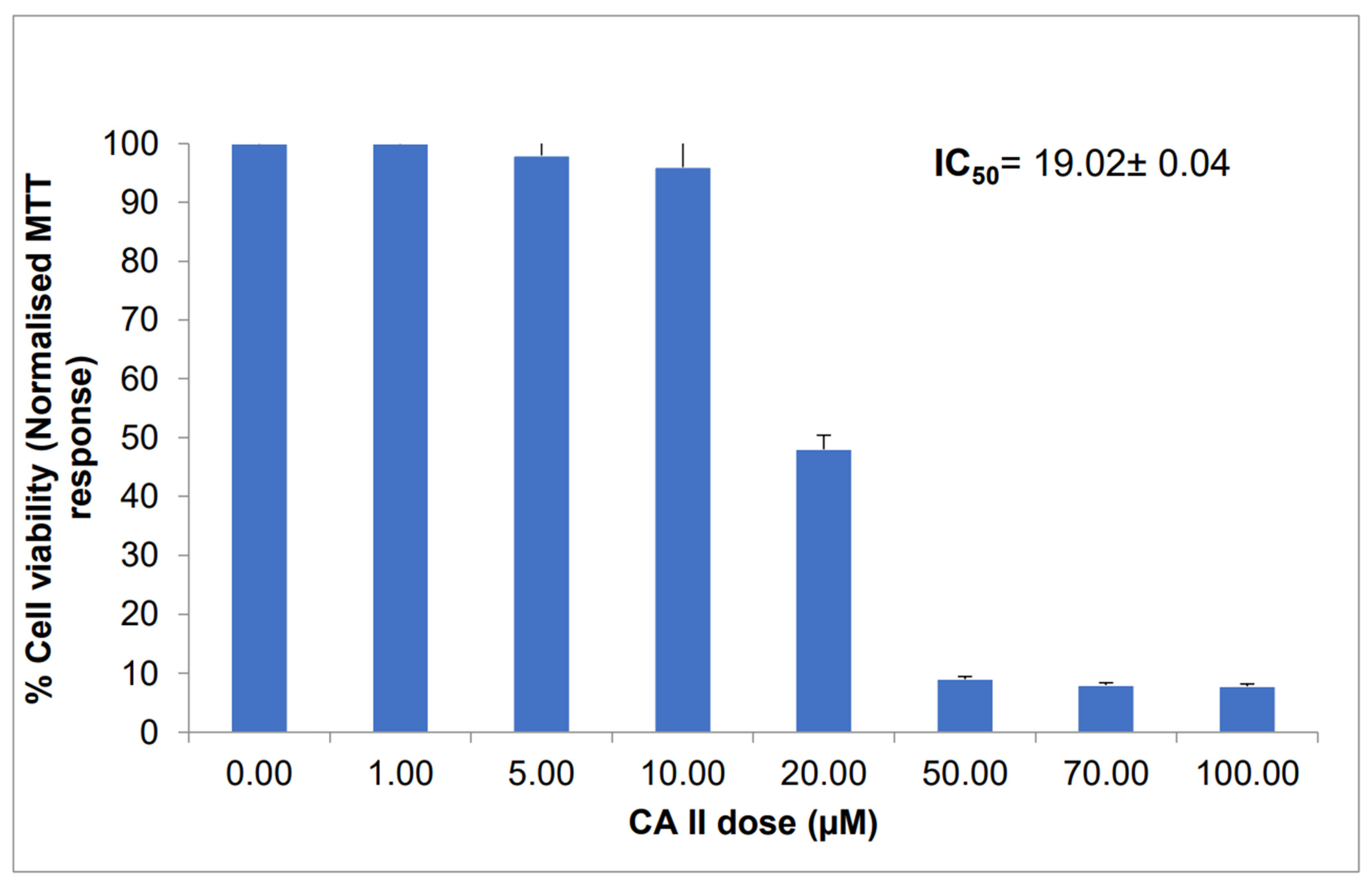
3.1. CAII Cytotoxicity against Caco-2 Cell Viability
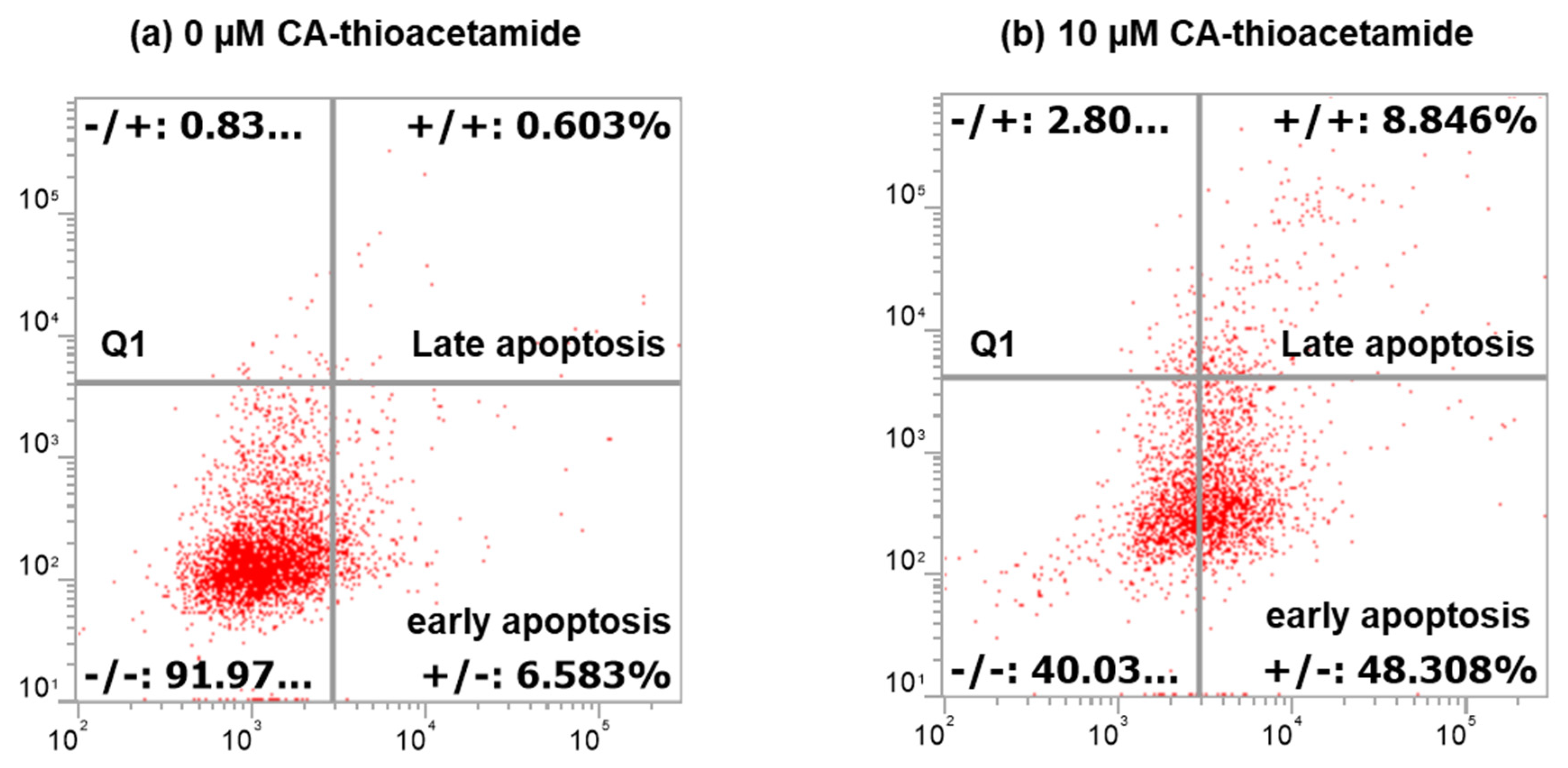
3.2. CAII Inducing Death on Caco-2 Cells
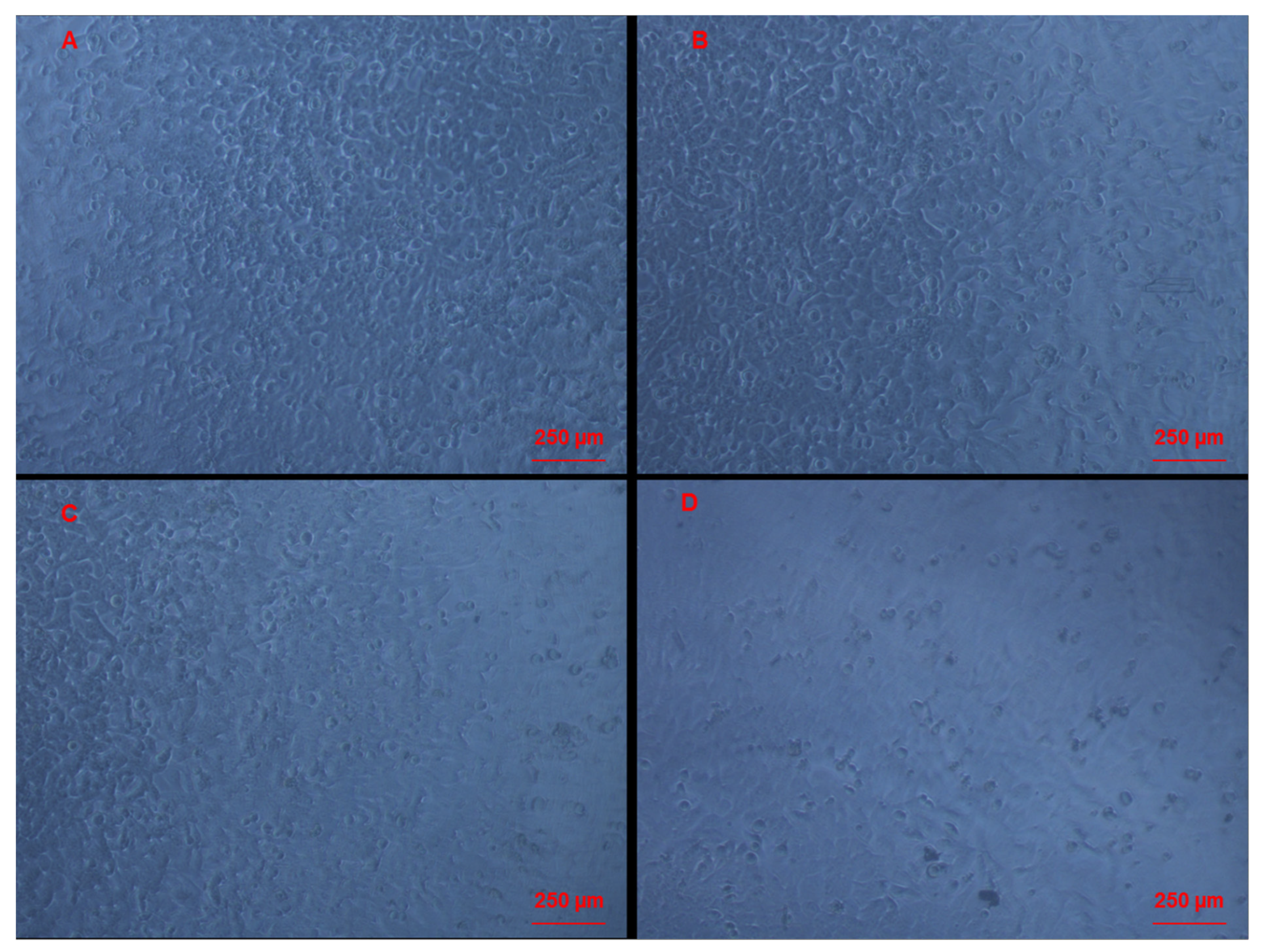
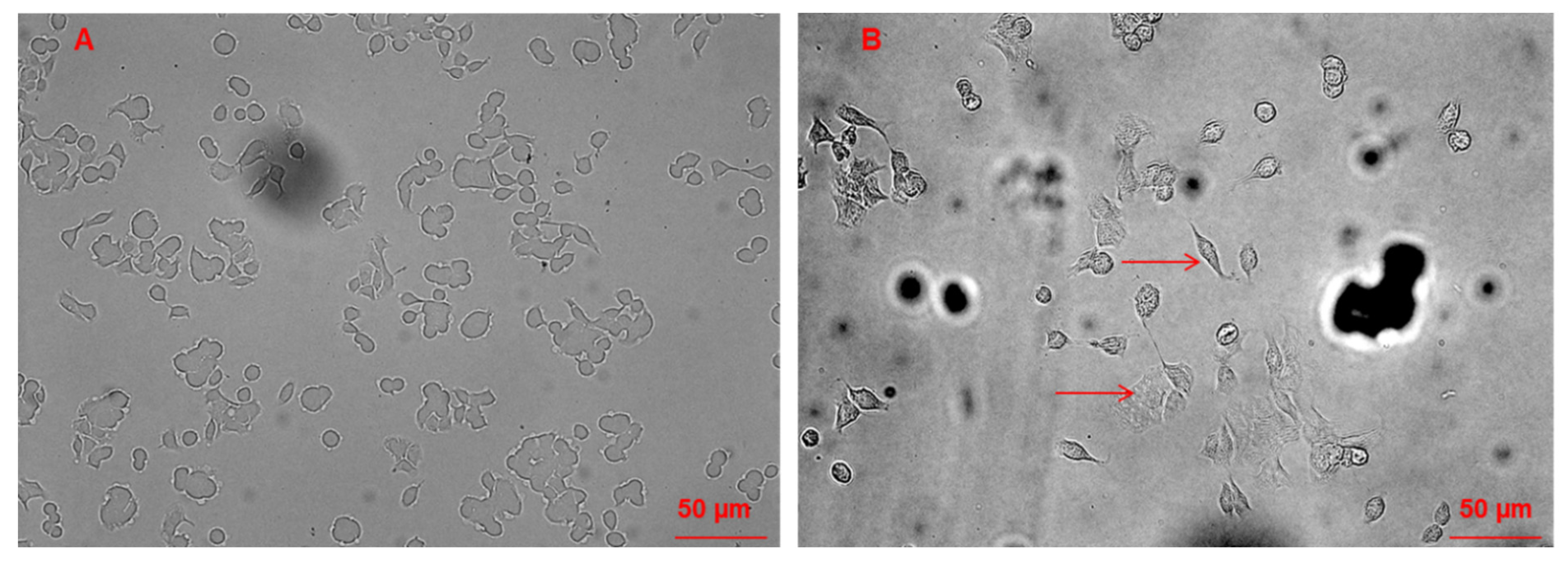
3.3. Morphological Changes of Caco-2 Cells Induced by CAII Treatment
3.4. Metabolite Analysis of Untreated and CAII-Treated Caco-2 Cells Using 1H NMR Spectroscopy
3.5. Confocal Raman Spectroscopy Measurements
4. Conclusions
- (i)
- CAII inhibits the Caco-2 cell proliferation in a concentration-dependent manner. The compound mode of action is mediated by an apoptosis mechanism that is considered to be an important aspect in defeating cancer.
- (ii)
- The MTT assay results demonstrate the anticancer effect of CAII against Caco-2 cells with an IC50 value of 19.02 ± 0.04 µM, revealing the fast action mechanism of this receptor relative to other reported chemotherapeutic drugs.
- (iii)
- Flow cytometry analysis shows that, at a starting dose of 10 µM, an apoptotic cascade in Caco-2 cells is observed. This cell death mechanism was corroborated by 1H NMR and Raman spectroscopic analyses. Thus, the reduction of the level of water soluble metabolites treated with CAII as well as the phospholipid component of the cell membrane clearly indicate a change in the metabolic profile of the cancer cell following the treatment with the calix[4]-based receptor.
- (iv)
- Raman studies provide evidence that the calix[4]arene–thioacetamide receptor interacts with the protein metabolites of Caco-2 cells. This is indeed an important aspect to highlight in this paper since it proves the effect of the applied anti-cancer agent on the protein transporters which play an essential role in the efflux mechanism of drugs, thus leaving the cells resistant to them.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Den Bakker, C.M.; Schaafsma, F.G.; Huirne, J.A.F.; Consten, E.C.J.; Stockmann, H.B.A.C.; Rodenburg, C.J.; de Klerk, G.J.; Bonjer, H.J.; Anema, J.R. Cancer survivors’ needs during various treatment phases after multimodal treatment for colon cancer—Is there a role for eHealth? BMC Cancer 2018, 18, 1207. [Google Scholar] [CrossRef]
- Ouakrim, D.; Pizot, C.; Boniol, M.; Malvezzi, M.; Boniol, M.; Jenkins, M.; Bleiberg, H.; Autier, P. Trends in colorectal cancer mortality in Europe: Retrospective analysis of the WHO mortality database. BMJ 2015, 351, h4970. [Google Scholar] [CrossRef] [Green Version]
- National Bowel Cancer Audits. 2016 Annual Report; National Health Service: London, UK, 2016. [Google Scholar]
- Melina, A.; Mònica, S.S.; Mathieu, L.; Isabelle, S.; Ahmedin, J.; Freddie, B. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar]
- Murphy, C.C.; Harlan, L.C.; Lund, J.L.; Lynch, C.F.; Geiger, A.M. Patterns of Colorectal Cancer Care in the United States: 1990–2010. J. Natl. Cancer Inst. 2015, 107, djv198. [Google Scholar] [CrossRef] [Green Version]
- Agur, Z.; Arnon, R.; Schechter, B. Reduction of cytotoxicity to normal tissues by new regimens of phase-specific drugs. Math Biosci. 1988, 92, 1–15. [Google Scholar] [CrossRef]
- Robella, M.; Vaira, M.; Argenziano, M.; Spagnolo, R.; Cavalli, R.; Borsano, A.; Zhang, Q.; Zhou, H.; Wu, J.; Tian, Y.; et al. Exploring the use of pegylated liposomal doxorubicin (Caelyx(R)) as pressurized intraperitoneal aerosol chemotherapy. Front. Pharmacol. 2019, 9, 2158–2166. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, M.; Luo, L.; Gill, M.R.; De Pace, C.; Battaglia, G.; Zhang, Q.; Zhou, H.; Wu, J.; Tian, Y.; et al. NF-kappaB hijacking theranostic Pt(ll) complex in cancer therapy. Theranostics 2019, 9, 2158–2166. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Z.; Yin, H.; Yang, G. Interaction between p53 and Ras signaling controls cisplatin resistance via HDAC4- and HIF-1alpha-mediated regulation of apoptosis and autophagy. Theranostics 2019, 9, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Ashwin, B.C.M.A.; Arun Baby, A.H.; Prakash, M.; Hochlaf, M.; Mareeswaran, P.M. A combined experimental and theoretical study on p-sulfonatocalix[4]arene encapsulated 7-methoxycoumarin. J. Org. Phys. Chem. 2018, 31, e3788. [Google Scholar] [CrossRef]
- Nimse, S.B.; Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef]
- Baggetto, L.G.; Coleman, W.A.; Lazar, A.N.; Magnard, S.; Michaud, M.H. Calixarene Derivatives as Anticancer Agent. U.S. Patent Application No. 20100056482A1, 4 March 2010. [Google Scholar]
- Pur, F.N.; Dilmaghani, K.A. Calixplatin: Novel potential anticancer agent based on the platinum complex with functionalized calixarene. J. Coord. Chem. 2014, 67, 440–448. [Google Scholar]
- Da Silva, E.; Lazar, A.N.; Coleman, A.W. Biopharmaceutical applications of calixarenes. J. Drug Del. Sci. Tech. 2004, 14, 3–20. [Google Scholar] [CrossRef]
- Uyar Arpaci, P.; Ozcan, F.; Omar, N.; Ozcan, F.; Ertul, S. Evaluation and biocompatibility of supramolecular calixarene on L-929 by real time cell analysis. In Proceedings of the IEEE 7th Internet, Conference Nanomaterials: Applications and Properties, Zatoka, Ukraine, 10–15 September 2017. [Google Scholar]
- Dawn, A.; Yao, X.; Jiang, J.; Kumari, H. Assessment of the In Vitro toxicity of calixarenes and a metal seamid calixarene: A chemical pathway for clinical applications. Supramol. Chem. 2019, 31, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Craigg, P.J. Antimicrobial activity of calixarenes and related macrocycles. Molecules 2020, 25, 5145. [Google Scholar] [CrossRef]
- Coleman, A.W.; Jebors, S.; Cecillon, S.; Perret, P.; Garin, D.; Marti-Battle, D.; Moulin, M. Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J. Chem. 2008, 32, 780–782. [Google Scholar] [CrossRef]
- Hulίková, K.; Grobárová, V.; Křivohlavá, R.; Fišerová, A. Antitumor activity of N-acetyl-D-glucosamine-substituted glycoconjugates and combined therapy with keyhole limpet hemocyanin in B16F10 mouse melanoma model. Folia Microbiol. 2010, 55, 528–532. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Connelly, J.; Lindon, J.C.; Holmes, E. Metabonomics: A platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002, 1, 153–161. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D. Understanding ‘Global’ Systems Biology: Metabonomics and the Continuum of Metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Xu, H.; Qiu, S.; Wang, X. Cell Metabolomics. OMICS 2013, 17, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Danil de Namor, A.F.; Pawlowski, T. A new calix[4]arene derivative and its ionic recognition for silver(i) and mercury(ii): The solvent effect. New J. Chem. 2011, 35, 375–384. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- McConkey, D.J. Therapy-induced apoptosis in primary tumors. Adv. Exp. Med. Biol. 2007, 608, 31–51. [Google Scholar] [PubMed]
- Schmitt, J.M. Optical coherence tomography (OCT): A review. IEEE J. Sel. Top. Quantum. Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef] [Green Version]
- DiMilla, P.A.; Stone, J.A.; Quinn, J.A.; Albelda, S.M.; Lauffenburger, D.A. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an indermediate attachment strength. J. Cell. Biol. 1993, 122, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan-Jun, Y.; Jun, B.; Li-Li, W.; Yong-Long, H.; Bin, L.; Qi, Y.; Yan, L.; Cheng, G.; Gen-Jin, Y. NMR-based metabolomics reveals distinct pathways mediated by curcumin in cachexia mice bearing CT26 tumor. RSC Adv. 2015, 5, 11766–11775. [Google Scholar] [CrossRef]
- Arminan, A.; Palomino-Schatzlein, M.; Deladriere, C.; Arroyo-Crespo, J.J.; Vicente-Ruiz, S.; Vicent, M.J. Pineda-Lucena, A. Metabolomics facilitates the discrimination of the specific anti-cancer effects of free- and polymer-conjugated doxorubicin in breast cancer models. Biomaterials 2018, 162, 144–153. [Google Scholar] [CrossRef]
- Lauri, I.; Savorani, F.; Iaccarino, N.; Zizza, P.; Pavone, L.M.; Novellino, E.; Engelsen, S.B.; Randazzo, A. Development of an Optimized Protocol for NMR Metabolomics Studies of Human Colon Cancer Cell Lines and First Insight from Testing of the Protocol Using DNA G-Quadruplex Ligands as Novel Anti-Cancer Drugs. Metabolites 2016, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Halama, A. Metabolomics in cell cultureea strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch. Biochem. Biophys. 2014, 564, e100–e109. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—the human metabolome database in 2013. Nucleic. Acids Res. Spec. Publ. 2013, 41, D801–D807. [Google Scholar] [CrossRef]


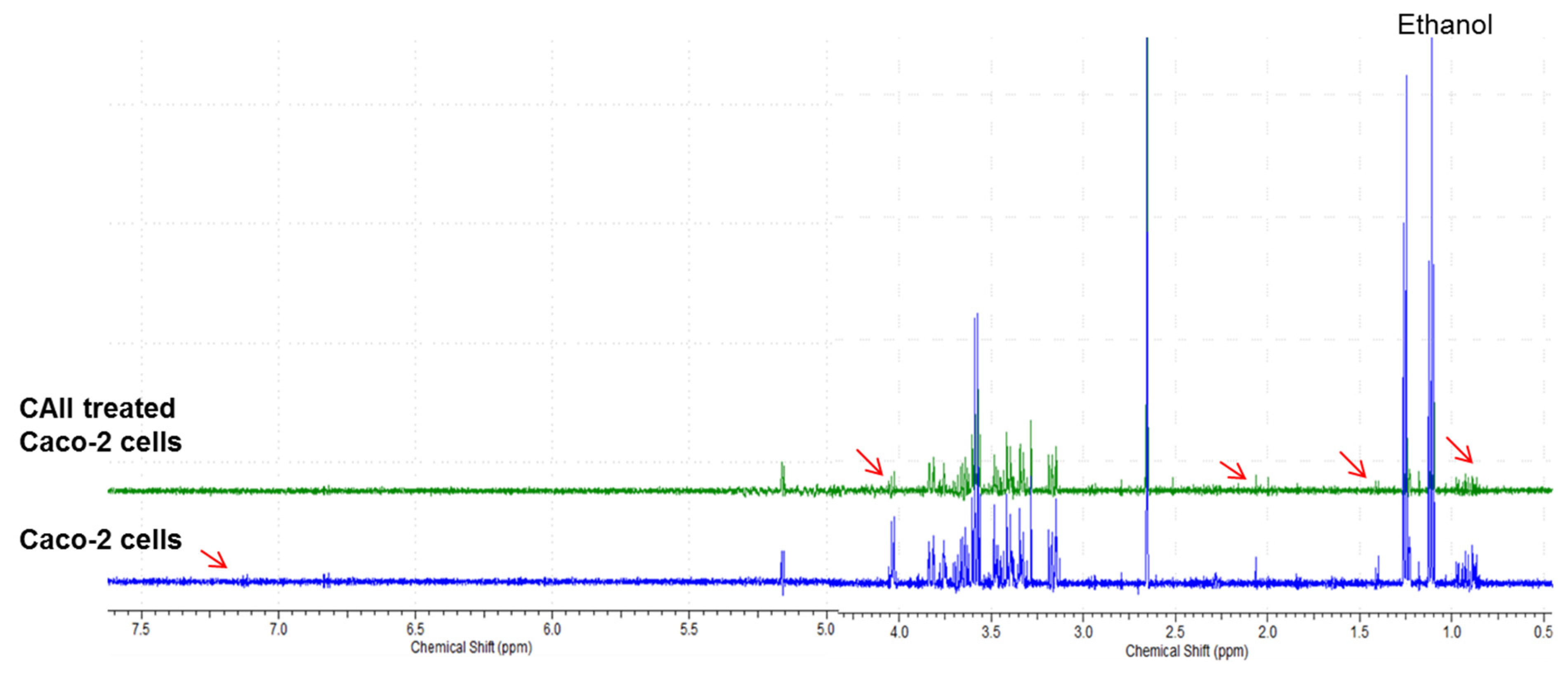
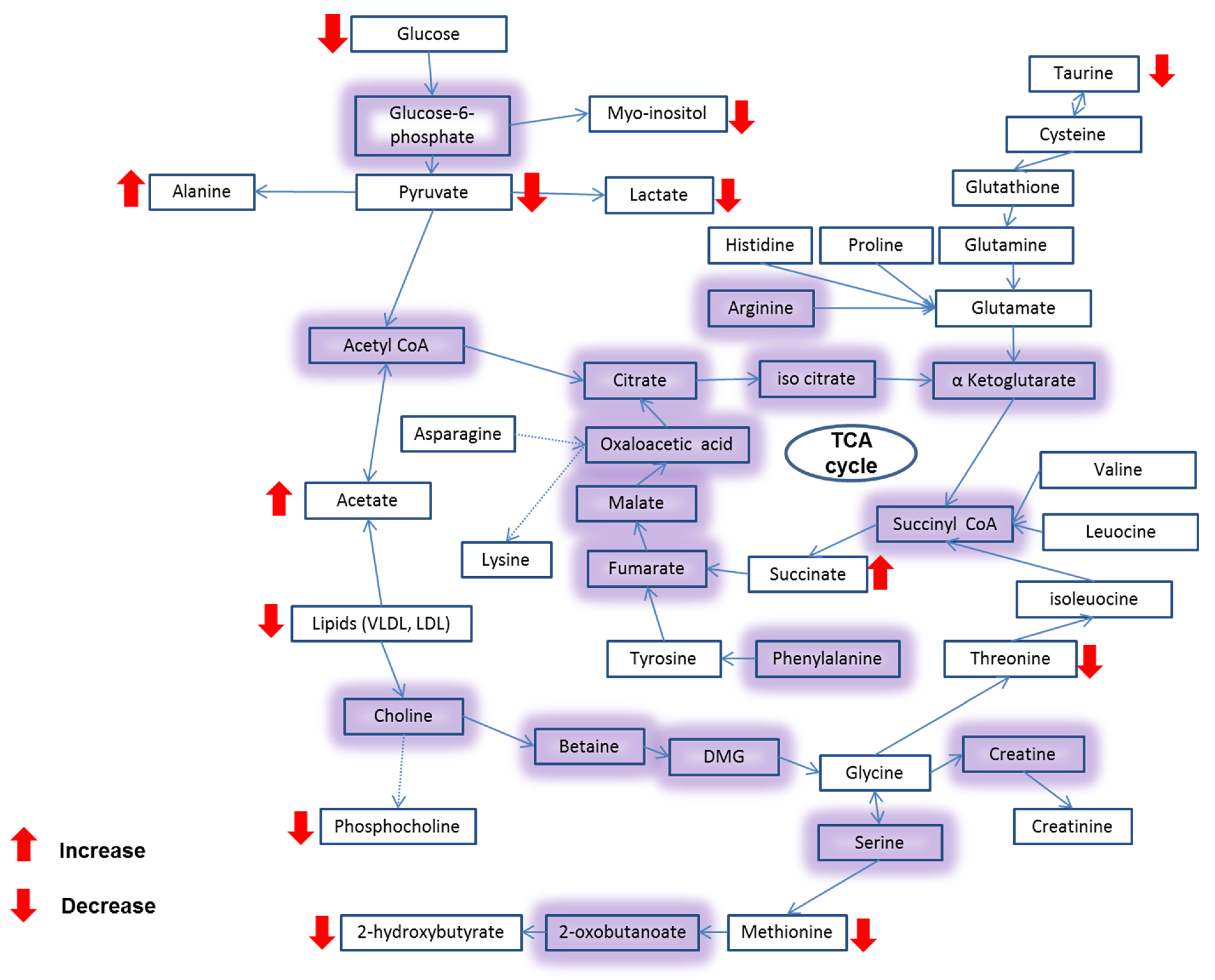
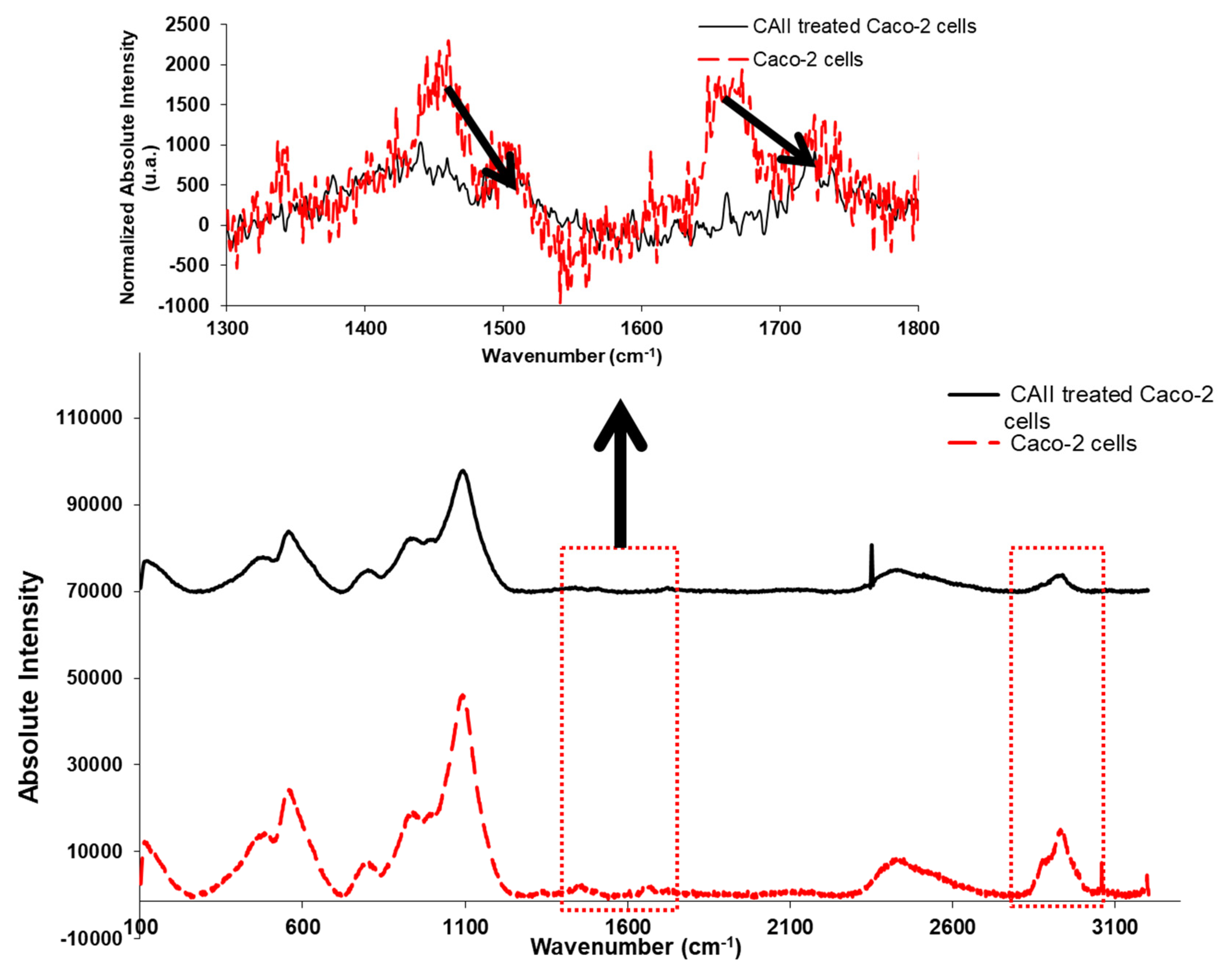
| Metabolite Number | Metabolites |
Caco-2 Chemical Shifts (ppm) |
>Caco-2 Treated with CAII Chemical Shifts (ppm) |
|---|---|---|---|
| 1 | Lactate | 1.33, 4.12 | - |
| 2 | Acetate | 1.91 | 1.91 |
| 3 | Glutamine | 2.12, 2.16, 2.45 | 2.12,2.16 |
| 4 | Glutamate | 2.05, 2.36 | 2.05, 2.36 |
| 5 | VLDL | 0.88, 1.29 | 0.88, 1.29 |
| 6 | LDL | 0.84 | - |
| 7 | Glycoprotein | - | 2.10 |
| 8 | Lysine | 1.44, 1.75, 3.02, 3.76 | 1.44, 1.75, 3.76 |
| 9 | Asparagine | 2.94, 3.95 | 2.94, 3.95 |
| 10 | Creatinine | 3.04, 3.93 | - |
| 11 | Methionine | 2.14 | - |
| 12 | α-Glucose | 3.45, 3.54, 3.71, 3.73, 3.85 | 3.45, 3.54, 3.71, 3.73, 3.85 |
| 13 | β-Glucose | 3.23, 3.43, 3.49, 3.90 | 3.43, 3.49, 3.90 |
| 14 | Glycine | 3.56 | 3.56 |
| 15 | Myo-Inositol | 3.55, 3.63, 4.07 | 3.55, 3.63, 4.07 |
| 16 | Taurine | 3.27 | - |
| 17 | Glycerophosphocholine | 3.24 | - |
| 18 | Glutathione | 2.57, 2.97 | 2.57, 2.97 |
| 19 | Threonine | 1.34, 4.27 | 1.34 |
| 20 | Succinate | 2.39 | - |
| 21 | Alanine | 1.49 | 1.49 |
| 22 | Tyrosine | 6.91 | - |
| 23 | 2-hydroxybutyrate | 1.19, 2.28 | 2.28 |
| 24 | Citrate | 2.70 | 2.70 |
| 25 | Pyruvate | 2.37 | 2.37 |
| 26 | Histidine | 7.11 | - |
| 27 | S-Sulfocysteine | 3.48, 3.66 | 3.48, 3.66 |
| 28 | O-phosphocholine | 4.17 | 4.17 |
| 29 | Carnitine | 3.17 | 3.17 |
| 30 | Valine | 1.05 | 1.05 |
| 31 | Isoleucine, leucine | 0.94, 0.97 | 0.94, 0.97 |
| 32 | Ethanolamine | 3.16, 3.82 | 3.16, 3.82 |
| 33 | AMP | 4.01, 4.15, 4.36, 6.15 | 4.15, 6.15 |
| Assignments | Wavenumber (cm−1) |
Intensity Caco-2 Cells |
Intensity CAII-Treated Caco-2 Cells |
|---|---|---|---|
| C=C (aromatic) | 3055 | 885 | 85 |
| CH3 (stretching) | 2971 | 6720 | 1204 |
| CH2 (stretching) | 2882 | 8309 | 1480 |
| C=O (stretching) | 1728 | 913 | 591 |
| Amide I | 1650 | 1162 | −28 |
| CH2 deformation of nucleic acids | 1450 | 1576 | 583 |
| CH2 twist and bend | 1338 | 625 | 124 |
| PO2− symmetric stretching | 1108 | 41,436 | 25,425 |
| C–O (ribose) | 982 | 17,719 | 11,463 |
| Skeletal mode of polysaccharides | 939 | 18,311 | 12,195 |
| Cytosine and uracil (stretching) | 789 | 6985 | 4398 |
| >S-S< (stretching) | 550 | 23,519 | 13,055 |
| >S-S< (stretching) | 511 | 12,230 | 7169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danil de Namor, A.F.; Al Hakawati, N.; Farhat, S.Y. Targeting Colorectal Cancer Cells with a Functionalised Calix[4]arene Receptor: Biophysical Studies. Molecules 2022, 27, 510. https://doi.org/10.3390/molecules27020510
Danil de Namor AF, Al Hakawati N, Farhat SY. Targeting Colorectal Cancer Cells with a Functionalised Calix[4]arene Receptor: Biophysical Studies. Molecules. 2022; 27(2):510. https://doi.org/10.3390/molecules27020510
Chicago/Turabian StyleDanil de Namor, Angela F, Nawal Al Hakawati, and Sami Y Farhat. 2022. "Targeting Colorectal Cancer Cells with a Functionalised Calix[4]arene Receptor: Biophysical Studies" Molecules 27, no. 2: 510. https://doi.org/10.3390/molecules27020510
APA StyleDanil de Namor, A. F., Al Hakawati, N., & Farhat, S. Y. (2022). Targeting Colorectal Cancer Cells with a Functionalised Calix[4]arene Receptor: Biophysical Studies. Molecules, 27(2), 510. https://doi.org/10.3390/molecules27020510