Chemical Changes in the Broccoli Volatilome Depending on the Tissue Treatment
Abstract
:1. Introduction
2. Results
2.1. Aspects Related to SPME Extraction
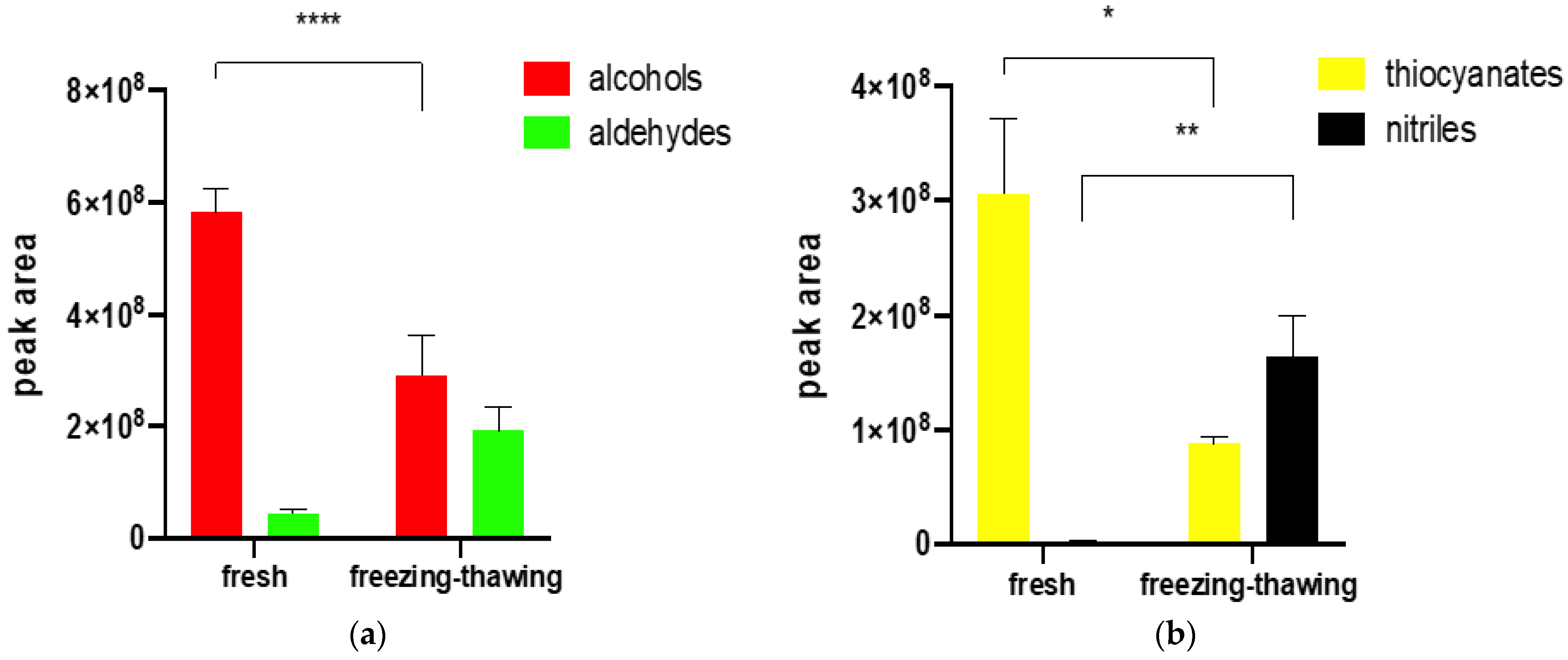
2.2. The Influence of Freezing and Thawing on the Profile of Volatile Compounds
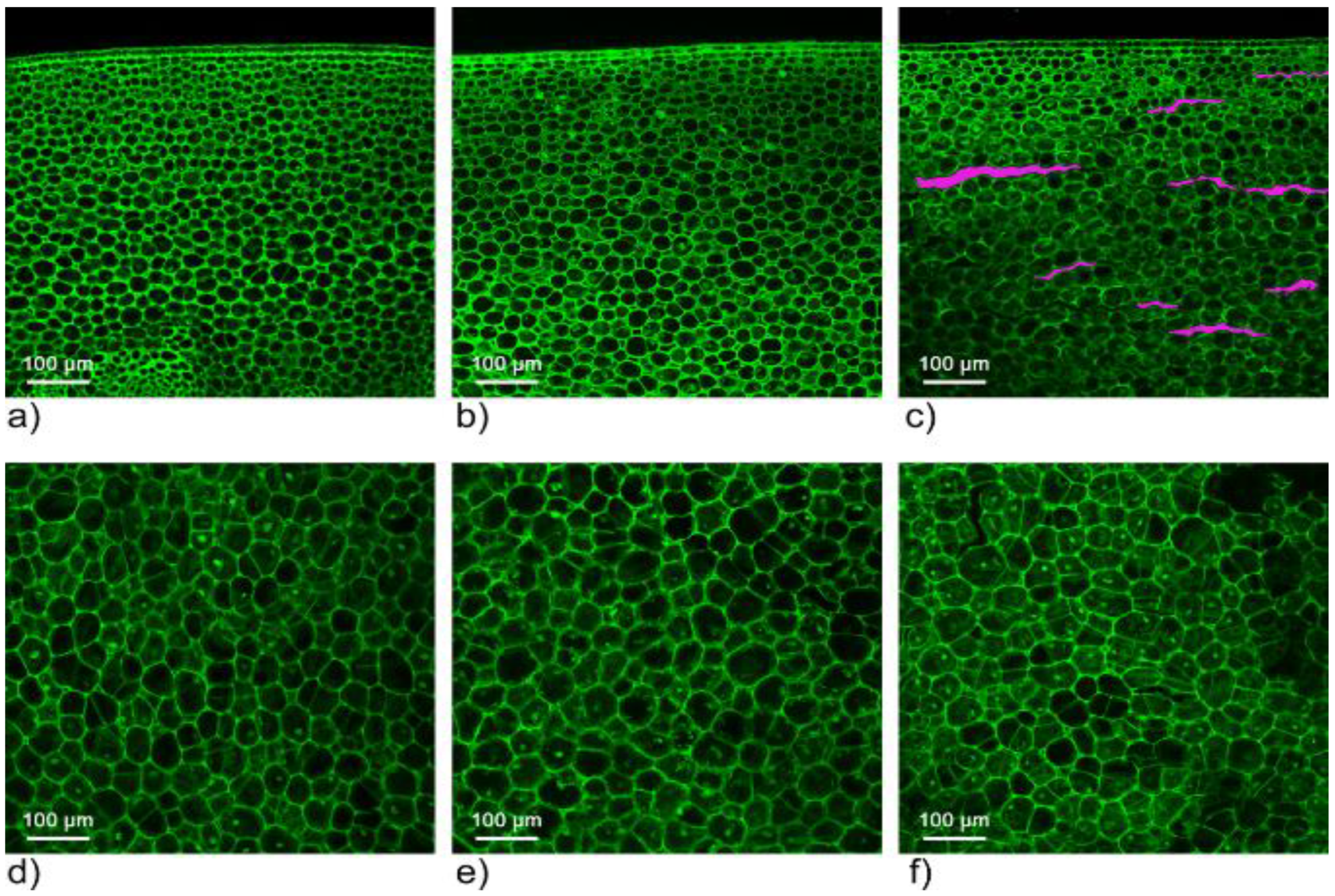
2.3. The Confocal Microscopy Scanning Results
2.4. The pH Measurement
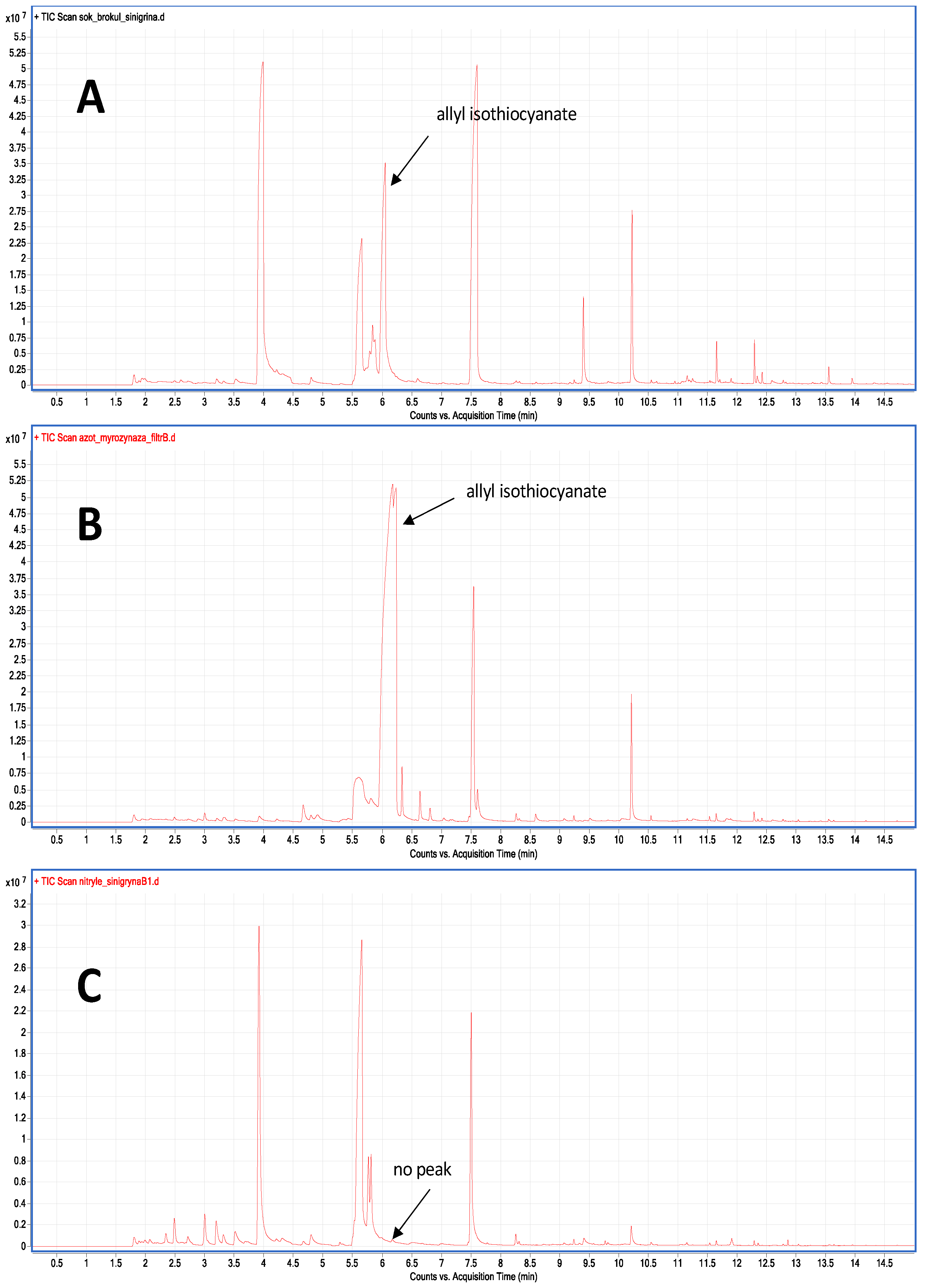
2.5. Enzyme Activity Assays
2.6. The Influence of Cutting on the Profile of Volatile Compounds
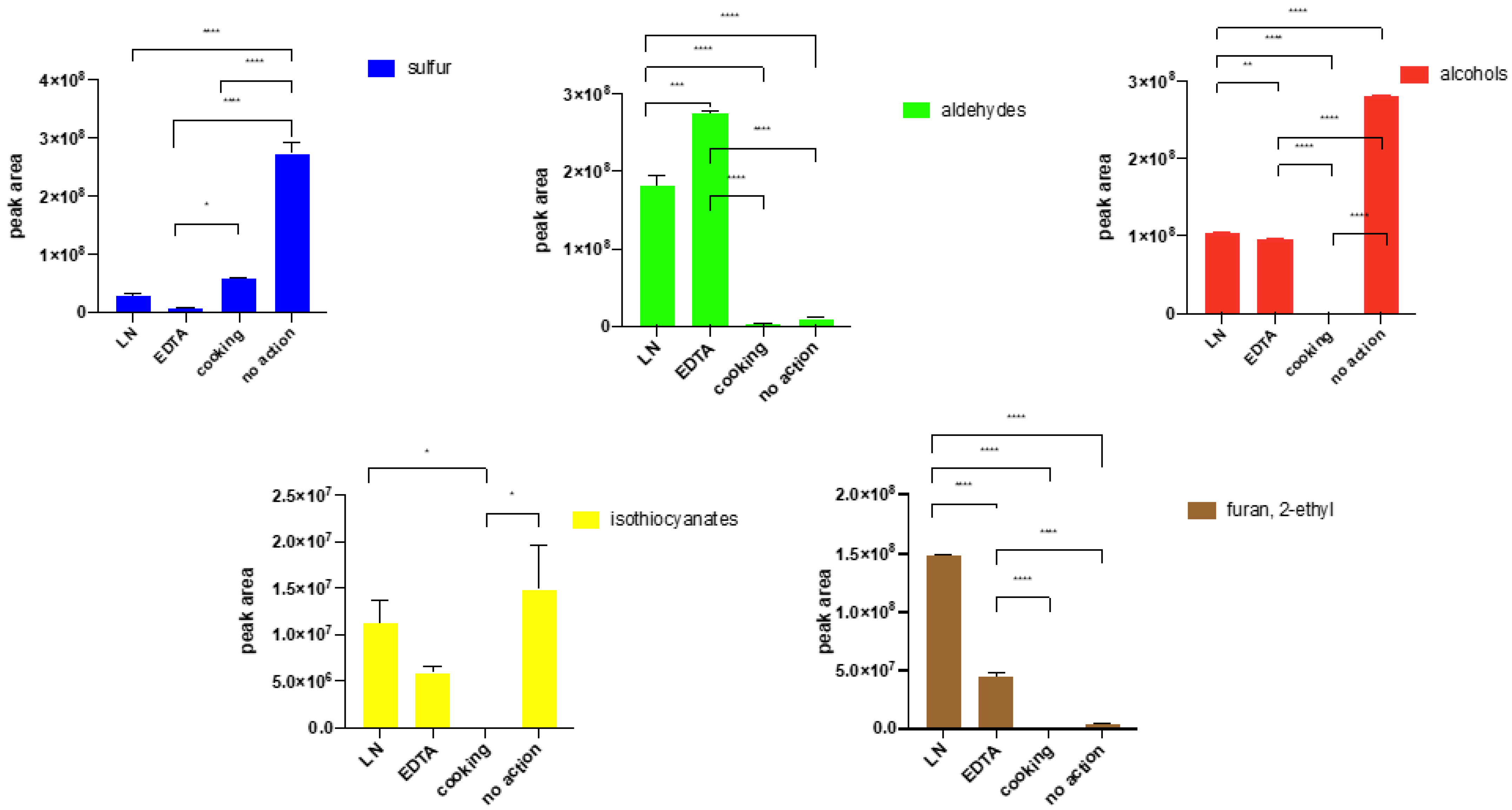
2.7. The Influence of Enzyme Reaction Quenching on the Profile of Volatile Compounds
3. Discussion
4. Materials and Methods
4.1. Materials, Reagents and Standards
4.2. The SPME Extraction Procedure
4.2.1. The Effect of Tissue Disintegration Technique on Volatile Profile
4.2.2. The Enzyme Inactivation and Freezing-Thawing Influence on Volatile Composition
4.3. Instrumentation for Analyzing Volatiles
4.4. Confocal Microscopy
4.5. The Myrosinase and Nitrile-Specifier Protein Activity
4.6. The pH Measurement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, 180007. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.V.; Foss, F.W.; Rady, J.M.; Macdonald, T.; Templeton, D. The isothiocyanate class of bioactive nutrients covalently inhibit the MEKK1 protein kinase. BMC Cancer 2007, 7, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieczorek, M.N.; Walczak, M.; Skrzypczak-Zielińska, M.; Jeleń, H. Bitter taste of Brassica vegetables: The role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit. Rev. Food Sci. Nutr. 2018, 58, 3130–3140. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Jeleń, H. Volatile Compounds of Selected Raw and Cooked Brassica Vegetables. Molecules 2019, 24, 391. [Google Scholar] [CrossRef] [Green Version]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Kiefl, J.; Schieberle, P.; Reichenbach, S.E.; Bicchi, C. Comprehensive two-dimensional gas chromatography and food sensory properties: Potential and challenges. Anal. Bioanal. Chem. 2015, 407, 169–191. [Google Scholar] [CrossRef]
- Cordero, C.; Cagliero, C.; Liberto, E.; Nicolotti, L.; Rubiolo, P.; Sgorbini, B.; Bicchi, C. High concentration capacity sample preparation techniques to improve the informative potential of two-dimensional comprehensive gas chromatography–mass spectrometry: Application to sensomics. J. Chromatogr. A 2013, 1318, 1–11. [Google Scholar] [CrossRef]
- Koek, M.M.; van der Kloet, F.M.; Kleemann, R.; Kooistra, T.; Verheij, E.R.; Hankemeier, T. Semi-automated non-target processing in GC × GC-MS metabolomics analysis: Applicability for biomedical studies. Metabolomics 2011, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and flavor perceptions of glu-cosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.; Laustsen, A.M.; Olsen, C.E.; Poll, L.; Sørensen, H. Chemical and Sensory Quality of Broccoli (Brassica oleracea L. Var Italica). J. Food Qual. 1997, 20, 441–459. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Guadagni, D.G.; Ling, L.C.; Seifert, R.M.; Lipton, W. Additional volatile components of cabbage, broccoli, and cauliflower. J. Agric. Food Chem. 1976, 24, 829. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef]
- Maruyama, F.T. Identification of Dimethyl Trisulfide as a Major Aroma Component of Cooked Brassicaceous Vegetables. J. Food Sci. 1970, 35, 540–543. [Google Scholar] [CrossRef]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Markert, J.; Gershenzon, J.; Wittstock, U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. FEBS J. 2006, 273, 2432–2436. [Google Scholar] [CrossRef]
- Tulio, A.Z.; Yamanaka, H.; Ueda, Y.; Imahori, Y. Formation of methanethiol and dimethyl disulfide in crushed tissues of broccoli florets and their inhibition by freeze-thawing. J. Agric. Food Chem. 2002, 50, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Springett, M.B.; Adams, J.B. Properties of Brussels Sprouts Thioglueosidase. Food Chem. 1989, 33, 173–186. [Google Scholar] [CrossRef]
- Yen, G.-C.; Wei, Q.-K. Myrosinase activity and total glucosinolate content of cruciferous vegetables, and some properties of cabbage myrosinase in Taiwan. J. Sci. Food Agric. 1993, 61, 471–475. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Rodrigo, L.; Hendrickx, M. The Activity of Myrosinase from Broccoli (Brassica oleracea L. cv. Italica): Influence of Intrinsic and Extrinsic Factors. J. Food Prot. 2000, 63, 400–403. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lestremau, F.; Andersson, F.A.T.; Desauziers, V. Investigation of Artefact Formation During Analysis of Volatile Sulphur Compounds Using Solid Phase Microextraction (SPME). Chromatographia 2004, 59, 607–613. [Google Scholar] [CrossRef]
- Warren, J.M.; Parkinson, D.-R.; Pawliszyn, J. Assessment of thiol compounds from garlic by automated headspace derivatized in-needle-NTD-GC-MS and derivatized in-fiber-SPME-GC-MS. J. Agric. Food Chem. 2013, 61, 492–500. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, M.N.; Pieczywek, P.M.; Cybulska, J.; Zdunek, A.; Jeleń, H.H. Chemical Changes in the Broccoli Volatilome Depending on the Tissue Treatment. Molecules 2022, 27, 500. https://doi.org/10.3390/molecules27020500
Wieczorek MN, Pieczywek PM, Cybulska J, Zdunek A, Jeleń HH. Chemical Changes in the Broccoli Volatilome Depending on the Tissue Treatment. Molecules. 2022; 27(2):500. https://doi.org/10.3390/molecules27020500
Chicago/Turabian StyleWieczorek, Martyna N., Piotr Mariusz Pieczywek, Justyna Cybulska, Artur Zdunek, and Henryk H. Jeleń. 2022. "Chemical Changes in the Broccoli Volatilome Depending on the Tissue Treatment" Molecules 27, no. 2: 500. https://doi.org/10.3390/molecules27020500
APA StyleWieczorek, M. N., Pieczywek, P. M., Cybulska, J., Zdunek, A., & Jeleń, H. H. (2022). Chemical Changes in the Broccoli Volatilome Depending on the Tissue Treatment. Molecules, 27(2), 500. https://doi.org/10.3390/molecules27020500








