Interactions of Linearly Polarized and Unpolarized Light on Kiwifruit Using Aquaphotomics
Abstract
:1. Introduction
2. Materials and Methods
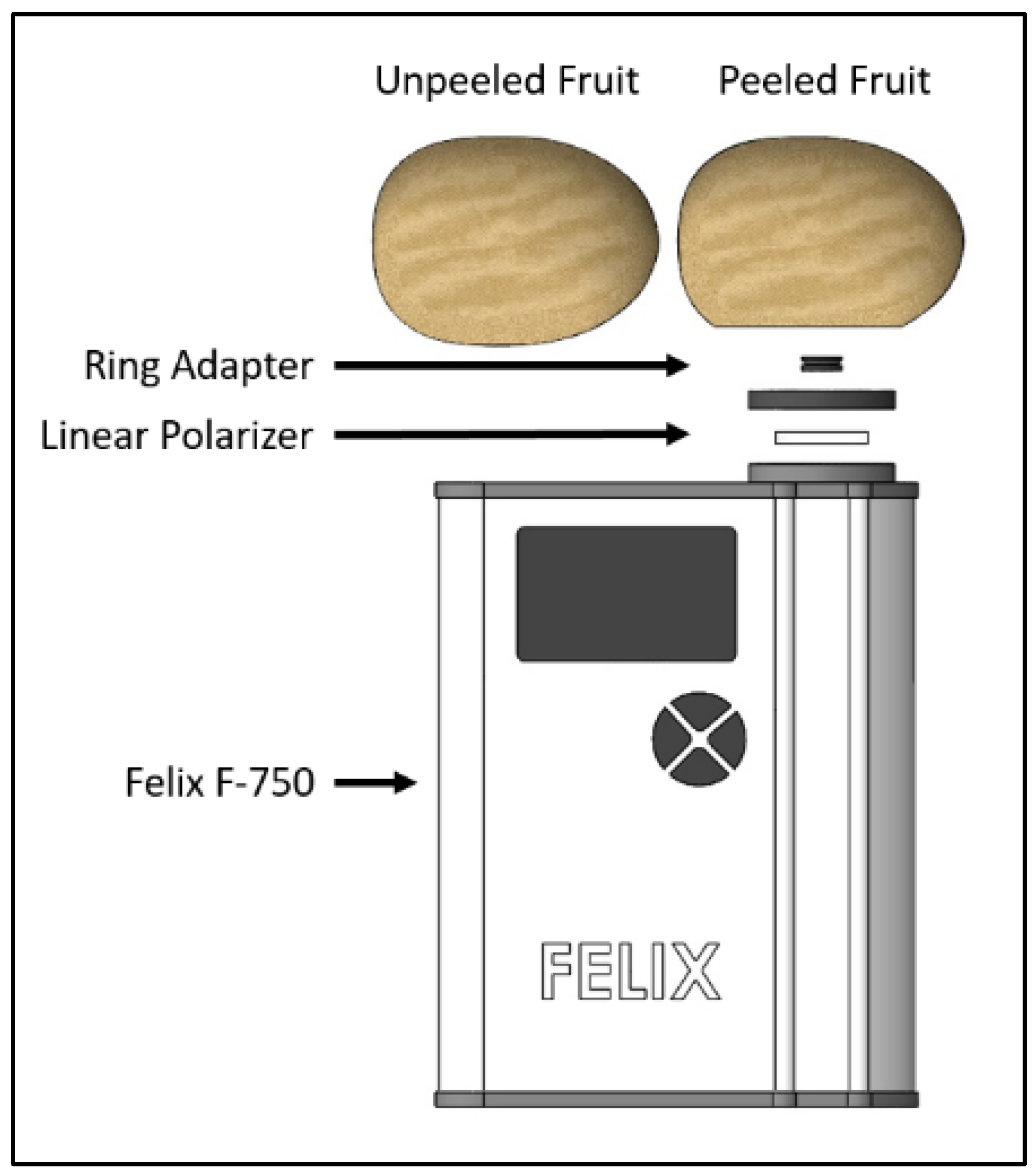
2.1. Sample Preparation
2.2. Spectra Collection
2.3. Destructive Measurements
2.4. Multivariate and Aquaphotomics Analysis
- Mean centering;
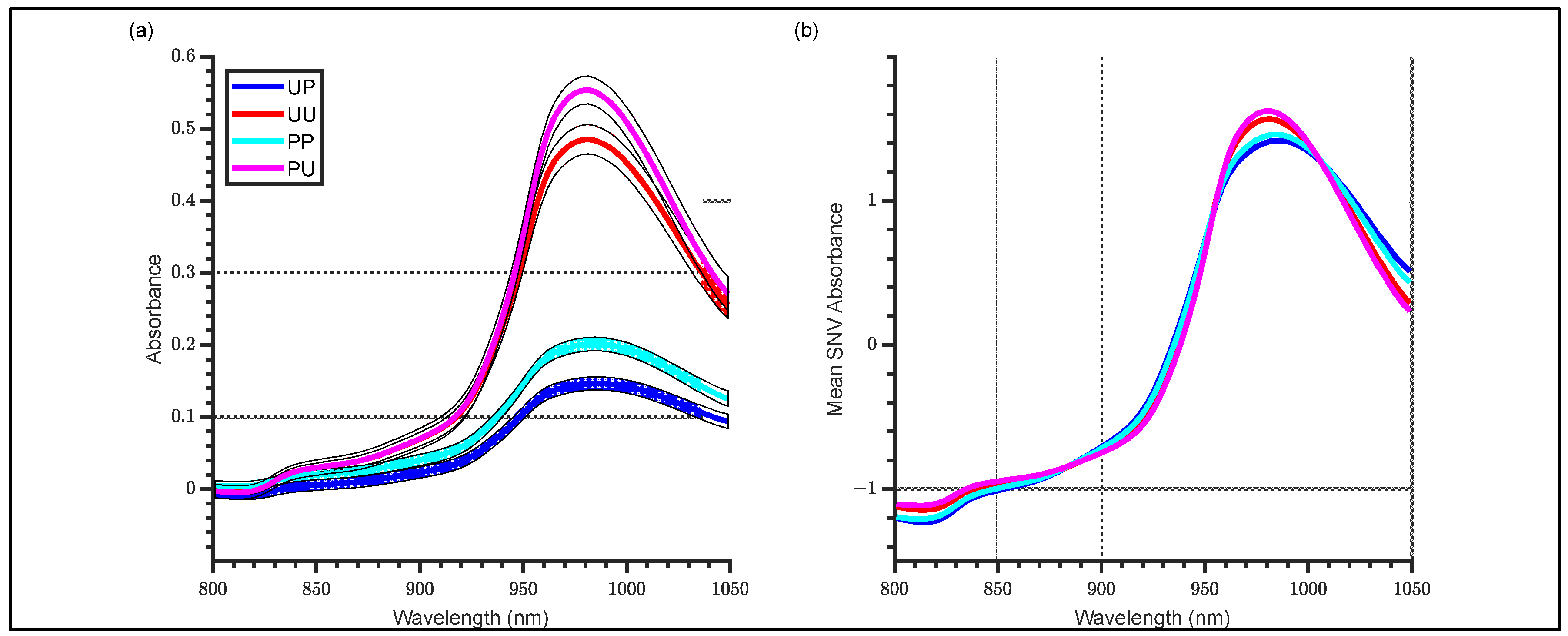
- Standard Normal Variate (SNV);
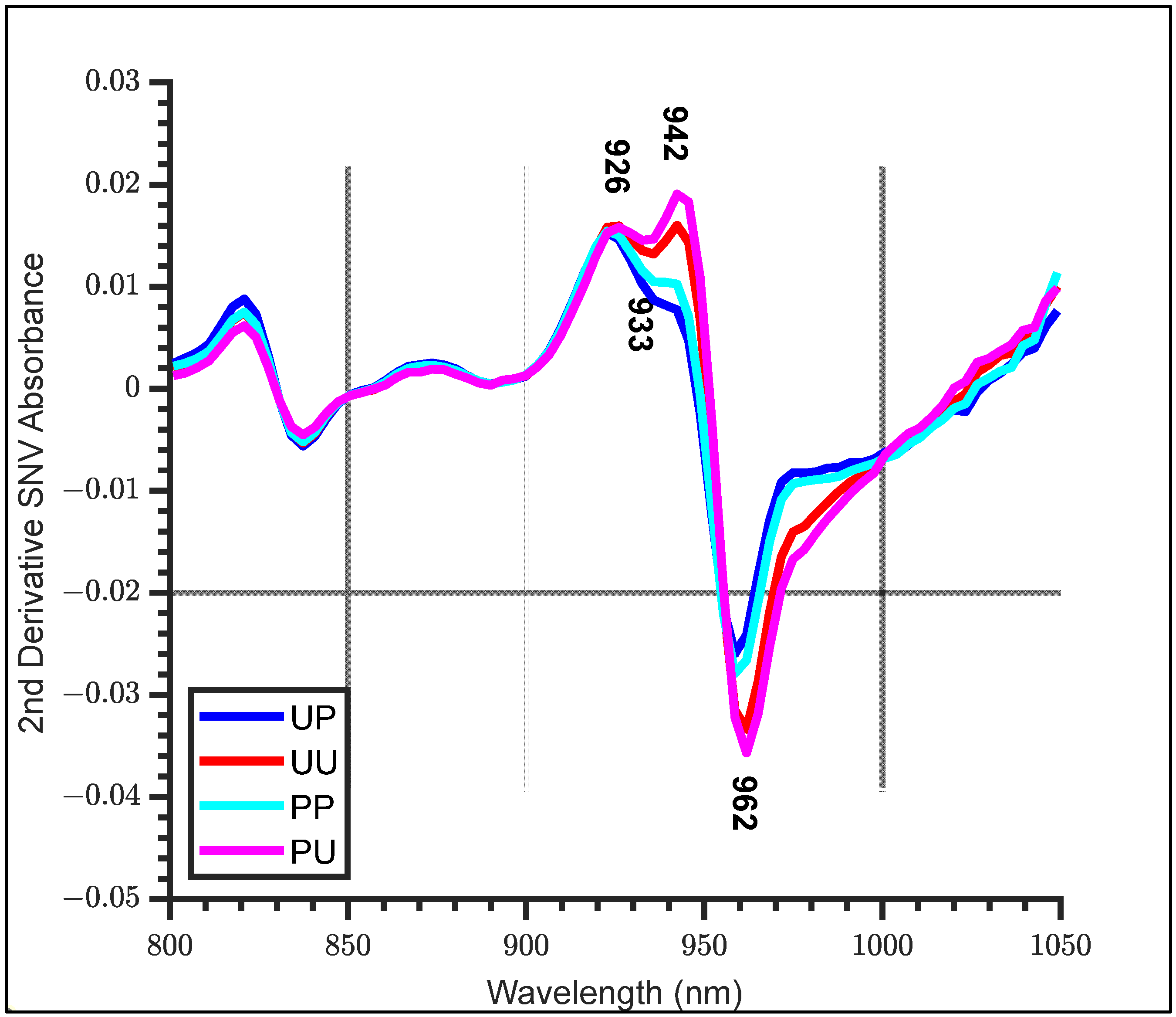
- SNV followed by second derivative processing (SNV + 2D). The second derivative was calculated using the Savitzky–Golay method with a filter window width of 7 nm and second order polynomial smoothing. A filter width of seven was chosen as higher widths may ‘wash’ out any interesting features, and to improve the resolution.
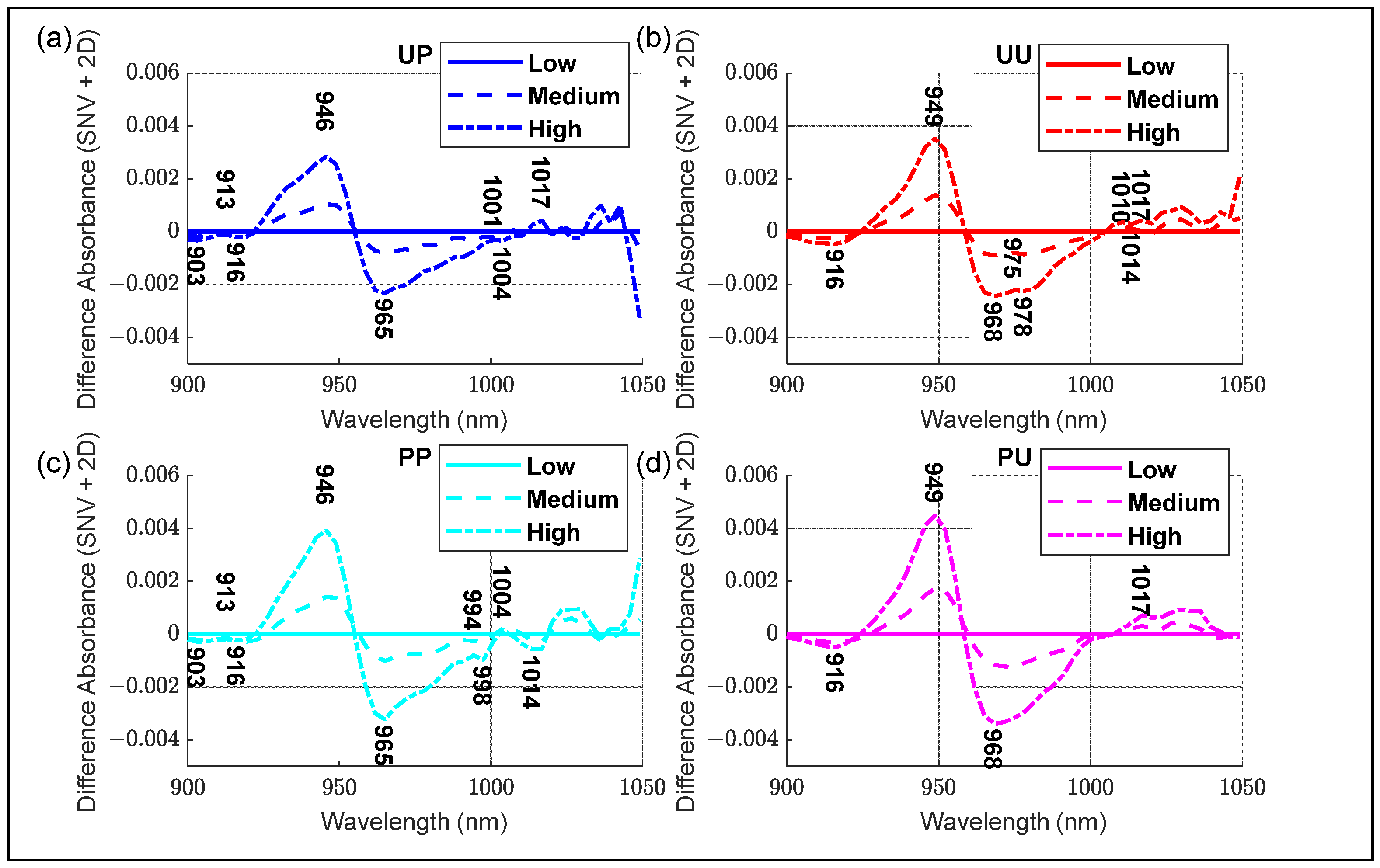
- SNV + 2D absorbance spectra of all configurations, noting any features.
- Average SSC difference spectra (SNV + 2D absorbance) between the Low, Medium and High SSC groups.
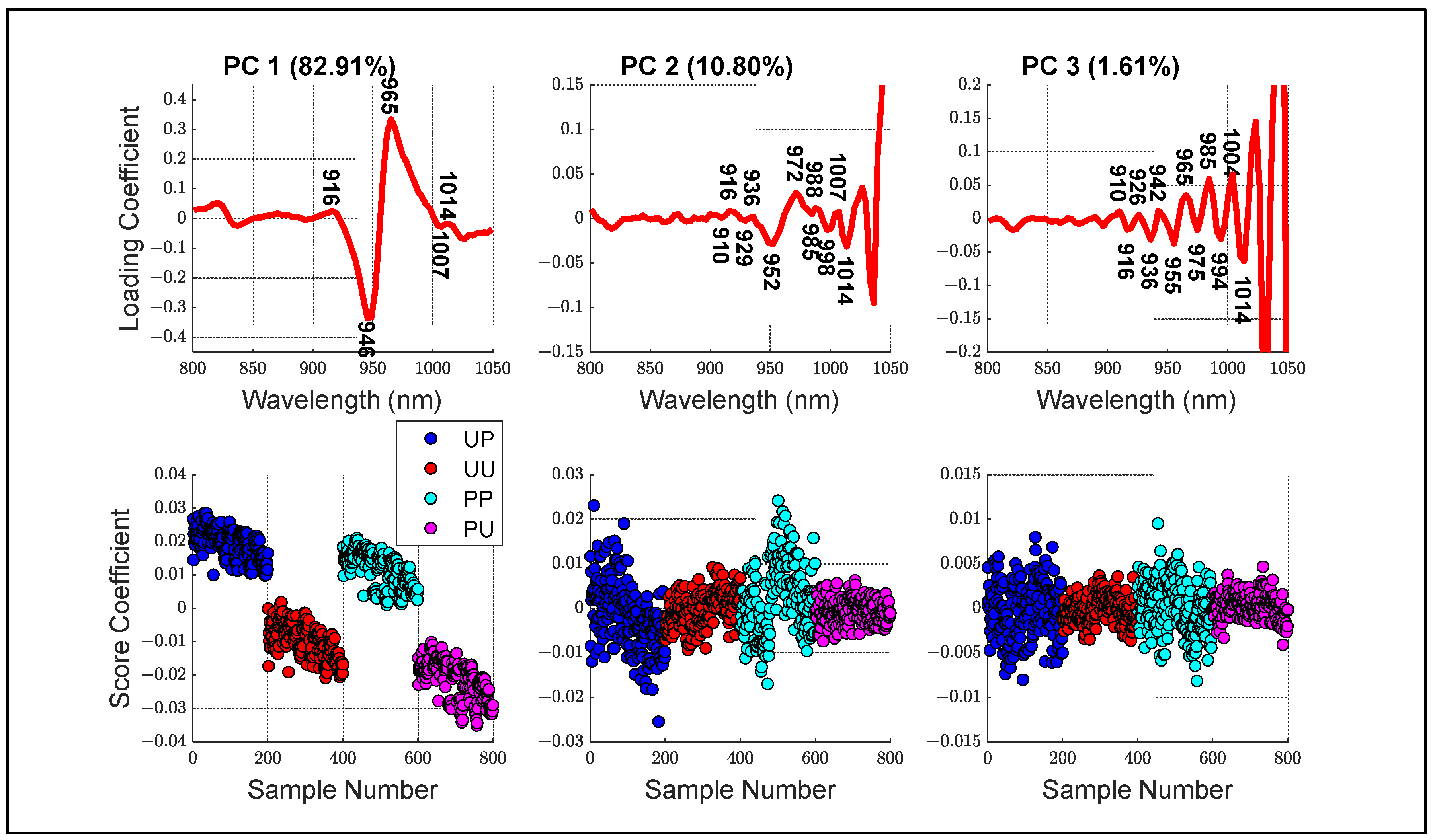
- Principal components analysis (PCA) of different configurations, including loading and score plots.
3. Results and Discussion
3.1. Raw and SNV Spectra
3.2. WAMACS Wavelength Selection
3.3. Aquaphotomics Analysis
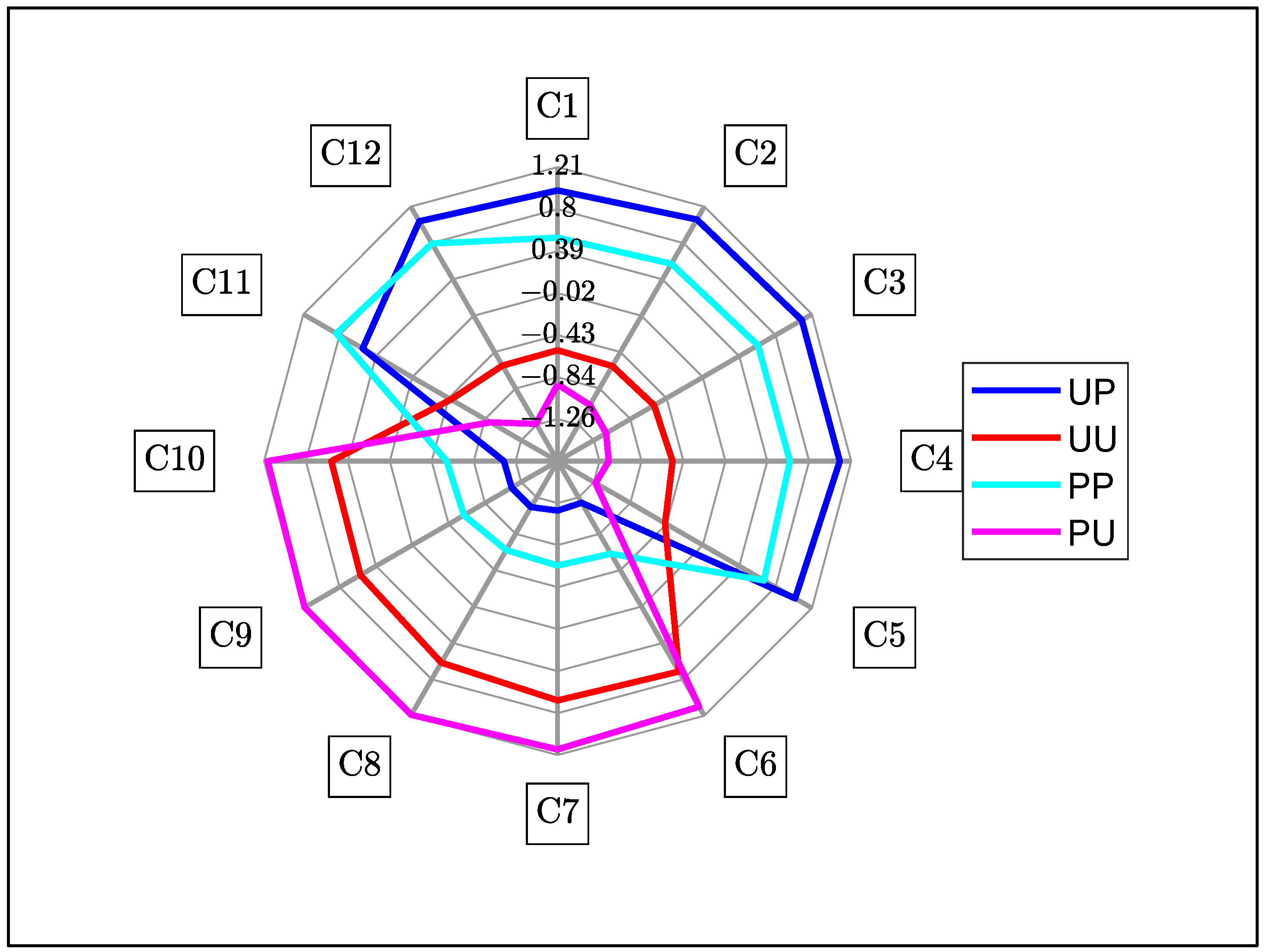
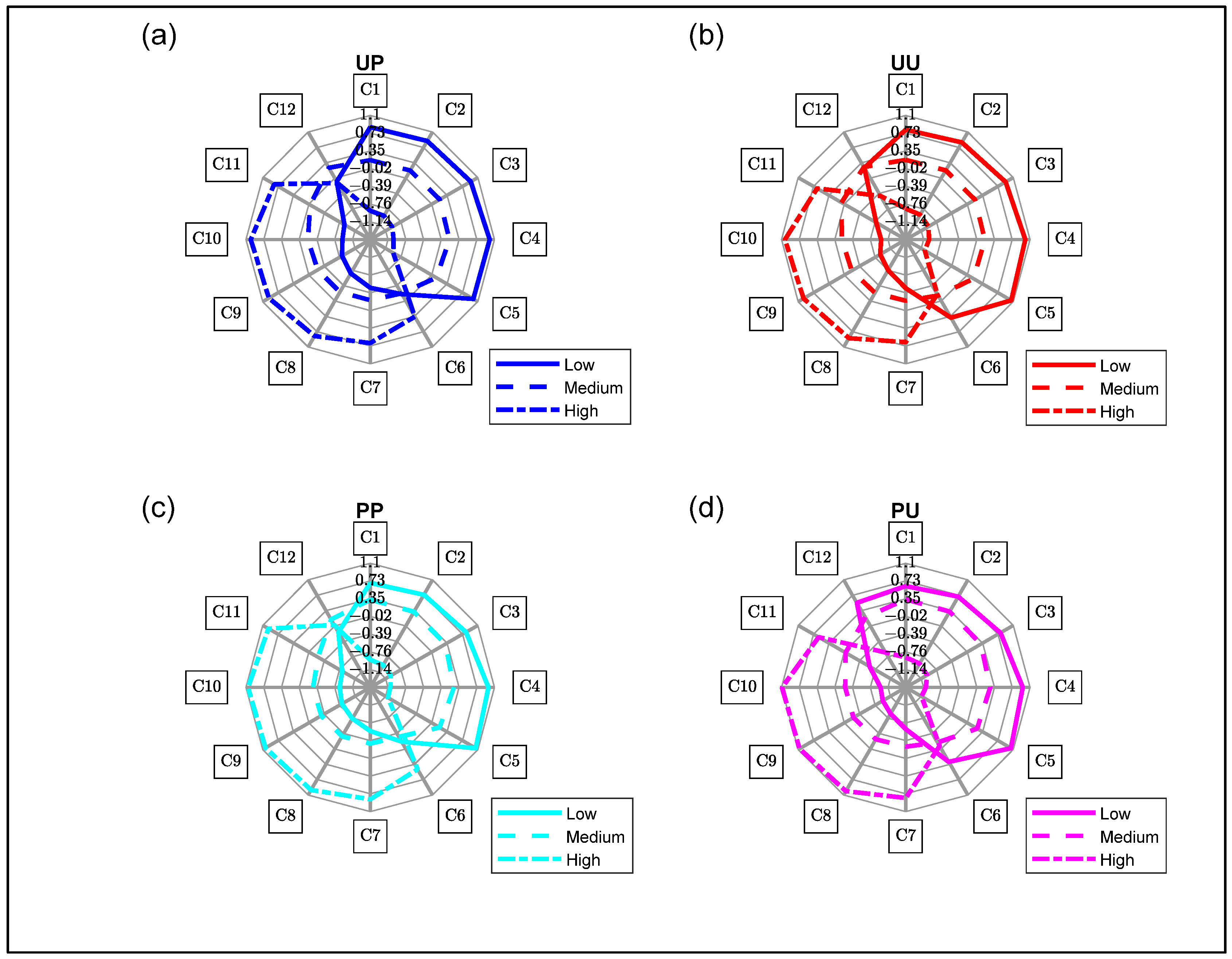
3.4. Aquagrams
3.4.1. Aquagram for Polarized and Unpolarized Light
3.4.2. Aquagram for Soluble Solids Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abbott, J.A. Quality measurement of fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 207–225. [Google Scholar] [CrossRef]
- Schaare, P.; Fraser, D. Comparison of reflectance, interactance and transmission modes of visible-near infrared spectroscopy for measuring internal properties of kiwifruit (Actinidia chinensis). Postharvest Biol. Technol. 2000, 20, 175–184. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Xie, L.; Wang, A.; Xu, H.; Fu, X.; Ying, Y. Applications of near-infrared systems for quality evaluation of fruits: A review. Trans. ASABE 2016, 59, 399–419. [Google Scholar]
- Nguyen-Do-Trong, N.; Dusabumuremyi, J.C.; Saeys, W. Cross-polarized VNIR hyperspectral reflectance imaging for non-destructive quality evaluation of dried banana slices, drying process monitoring and control. J. Food Eng. 2018, 238, 85–94. [Google Scholar] [CrossRef]
- Nassif, R.; Pellen, F.; Magné, C.; Le Jeune, B.; Le Brun, G.; Abboud, M. Scattering through fruits during ripening: Laser speckle technique correlated to biochemical and fluorescence measurements. Opt. Express 2012, 20, 23887–23897. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, M.; Gupta, N.; Assaad, M. Monitoring of fruit freshness using phase information in polarization reflectance spectroscopy. Appl. Opt. 2019, 58, 6396–6405. [Google Scholar] [CrossRef]
- Tuchin, V.V. Polarized light interaction with tissues. J. Biomed. Opt. 2016, 21, 071114. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.J.; Turzhitsky, V.; Ruderman, S.; Backman, V. Monte Carlo model of the penetration depth for polarization gating spectroscopy: Influence of illumination-collection geometry and sample optical properties. Appl. Opt. 2012, 51, 4627–4637. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.; Deumie, C.; Vanzetta, I. Elliptically polarized light for depth resolved optical imaging. Biomed. Opt. Express 2012, 3, 2907–2915. [Google Scholar] [CrossRef] [Green Version]
- Vignolini, S.; Rudall, P.J.; Rowland, A.V.; Reed, A.; Moyroud, E.; Faden, R.B.; Baumberg, J.J.; Glover, B.J.; Steiner, U. Pointillist structural color in Pollia fruit. Proc. Natl. Acad. Sci. USA 2012, 109, 15712–15715. [Google Scholar] [CrossRef] [Green Version]
- Muncan, J.; Tsenkova, R. Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Künnemeyer, R.; McGlone, A. Investigating aquaphotomics for temperature-independent prediction of soluble solids content of pure apple juice. J. Near Infrared Spectrosc. 2020, 28, 103–112. [Google Scholar] [CrossRef]
- Osborne, B.G.; Fearn, T.; Hindle, P.H. Practical NIR Spectroscopy with Applications in Food and Beverage Analysis; Longman Scientific and Technical: Essex, England, 1993. [Google Scholar]
- McGlone, V.A.; Jordan, R.B.; Seelye, R.; Martinsen, P.J. Comparing density and NIR methods for measurement of kiwifruit dry matter and soluble solids content. Postharvest Biol. Technol. 2002, 26, 191–198. [Google Scholar] [CrossRef]
- Lu, R.; Guyer, D.E.; Beaudry, R.M. Determination of firmness and sugar content of apples using near-infrared diffuse reflectance. J. Texture Stud. 2000, 31, 615–630. [Google Scholar] [CrossRef]
- Compton, R.; Mahurin, S.; Zare, R.N. Demonstration of optical rotatory dispersion of sucrose. J. Chem. Educ. 1999, 76, 1234. [Google Scholar] [CrossRef]
- Gobrecht, A.; Bendoula, R.; Roger, J.-M.; Bellon-Maurel, V. Combining linear polarization spectroscopy and the Representative Layer Theory to measure the Beer–Lambert law absorbance of highly scattering materials. Anal. Chim. Acta 2015, 853, 486–494. [Google Scholar] [CrossRef]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of aquaphotomics and its chemometrics approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef]
- Kaur, H. Investigating Aquaphotomics for Fruit Quality Assessment. Ph.D. Thesis, The University of Waikato, Hamilton, New Zealand, 2020. [Google Scholar]
- Moses. Spider_Plot. Moses. GitHub. 2021. Available online: https://github.com/NewGuy012/spider_plot/releases/tag/16.6 (accessed on 24 November 2021).
- Liu, Y.; Kim, Y.L.; Li, X.; Backman, V. Investigation of depth selectivity of polarization gating for tissue characterization. Opt. Express 2005, 13, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Redgwell, R.J.; MacRae, E.; Hallett, I.; Fischer, M.; Perry, J.; Harker, R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta 1997, 203, 162–173. [Google Scholar] [CrossRef]
- Fang, Z.-h.; Fu, X.-p.; He, X.-m. Investigation of absorption and scattering characteristics of kiwifruit tissue using a single integrating sphere system. J. Zhejiang Univ.-Sci. B 2016, 17, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tongdang, T. Some properties of starch extracted from three Thai aromatic fruit seeds. Starch-Stärke 2008, 60, 199–207. [Google Scholar] [CrossRef]

| SSC Group | SSC (°Brix) | Average (°Brix) |
|---|---|---|
| Low SSC (N = 61) | 7–11.9 | 10.4 |
| Medium SSC (N = 69) | 12.1–14.9 | 13.5 |
| High SSC (N = 70) | 15–20.5 | 16.9 |
| All SSC (N = 200) | 7–20.5 | 13.7 |
| WAMACS | Assignment | Water Bands (nm) | Activated Wavelengths (nm) |
|---|---|---|---|
| C1 | v3—asymmetric stretching vibration | 900–908 | 903 |
| C2 | OH stretch—(water solvation shell) | 916–920 | 916 |
| C3 | symmetric stretching and asymmetric stretching vibration | 923–927 | 926 |
| C4 | OH stretch (water solvation shell) | 930–935 | 933 |
| C5 | S0 (free water) | 942–955 | 946 |
| C6 | 957–963 | 962 | |
| C7 | S1—Water molecules with one hydrogen bond | 965–973 | 965 |
| C8 | bending andasymmetric stretching vibration | 975–979 | 975 |
| C9 | S2—Water molecules with 2 hydrogen bonds | 982–989 | 988 |
| C10 | S3—Water molecules with 3 hydrogen bonds | 992–998 | 994 |
| C11 | S4—Water molecules with 4 hydrogen bonds | 998–1007 | 1007 |
| C12 | Strongly bonded water (v1, v2) | 1014–1021 | 1014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkumar, D.; Künnemeyer, R.; Kaur, H.; Longdell, J.; McGlone, A. Interactions of Linearly Polarized and Unpolarized Light on Kiwifruit Using Aquaphotomics. Molecules 2022, 27, 494. https://doi.org/10.3390/molecules27020494
Rajkumar D, Künnemeyer R, Kaur H, Longdell J, McGlone A. Interactions of Linearly Polarized and Unpolarized Light on Kiwifruit Using Aquaphotomics. Molecules. 2022; 27(2):494. https://doi.org/10.3390/molecules27020494
Chicago/Turabian StyleRajkumar, Damenraj, Rainer Künnemeyer, Harpreet Kaur, Jevon Longdell, and Andrew McGlone. 2022. "Interactions of Linearly Polarized and Unpolarized Light on Kiwifruit Using Aquaphotomics" Molecules 27, no. 2: 494. https://doi.org/10.3390/molecules27020494
APA StyleRajkumar, D., Künnemeyer, R., Kaur, H., Longdell, J., & McGlone, A. (2022). Interactions of Linearly Polarized and Unpolarized Light on Kiwifruit Using Aquaphotomics. Molecules, 27(2), 494. https://doi.org/10.3390/molecules27020494






