Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms
Abstract
1. Introduction
2. Human Data: Epidemiological Studies on the Effects of Mediterranean Diet and Olive Oil on Breast Cancer Risk
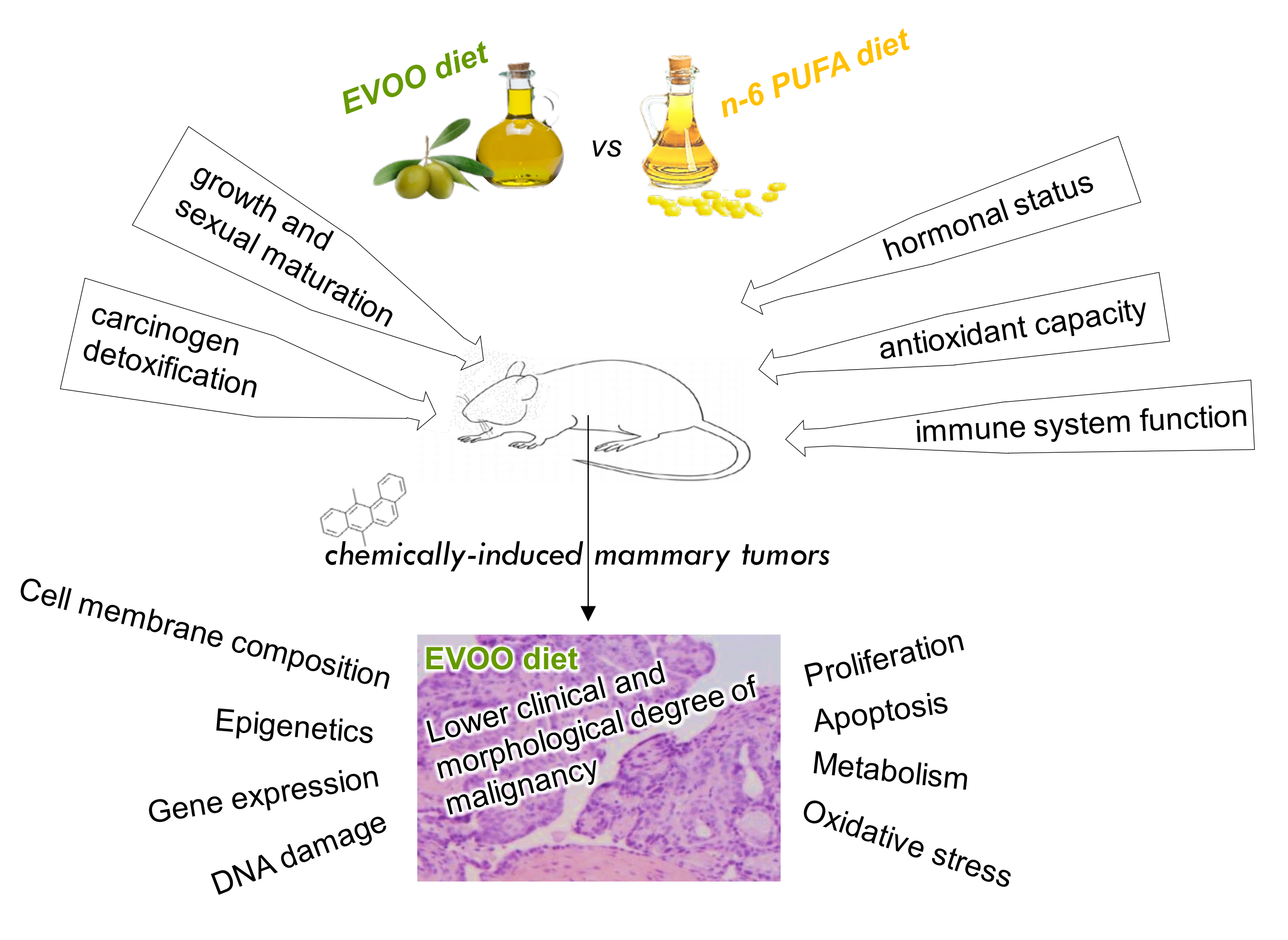
3. Effects of Olive Oil on Experimental Mammary Carcinogenesis
3.1. Molecular Mechanisms of the Effects of Olive Oil on Experimental Mammary Carcinogenesis
3.1.1. Effects on Animal Susceptibility and Tumor Initiation
3.1.2. Effects on Tumor Lipid Profile
3.1.3. Effects on Tumor Gene Expression
3.1.4. Effects on Tumor Epigenetic Mechanisms
3.1.5. Effects on Tumor Proliferation and Apoptosis Pathways
3.1.6. Effects on Tumor Metabolism
3.1.7. Effects on Tumor Oxidative Stress
3.1.8. Effects on Angiogenesis and Metastasis
| Animal Model | Dietary Intervention | Carcinogenesis | Molecular/Cellular Mechanisms | Ref. |
|---|---|---|---|---|
| NMU (50 mg/kg body weight at day 50) | Safflower oil (23%, 5%), corn oil (23%, 5%), olive oil (23%, 5%), coconut oil (23%); post- induction. | Promoting effect of high-safflower-oil and high-corn-oil diets (increased incidence and decreased latent period). | Lipid profile | [62,63,92] |
| NMU (40 mg/kg body weight at day 50) | Diets at 20% different varieties of olive oil 54/20, 70/15, 80/5 (% oleic acid/% linoneic acid); post-induction. | Lower degree of morphological malignancy by olive oil (80/5). | [64] | |
| NMU (3 × 50 mg/kg body weight at 50, 80 and 110 days) | Diets of 4% EVOO, 4% sunflower oil, 4% oleic acid-enriched sunflower oil; post-weaning. | Protective effect of olive oil (longer latency period, lowest mortality). | [68] | |
| Diet of 4% oleic acid-enriched sunflower oil induced the highest tumor volume but the lowest morphological malignancy. | Renin–angiotensin system | [100] | ||
| Tumor implantation (2 mm3 of metastatic mammary tumor) | Corn oil (23%, 5%), olive oil (20%, 5%), beef tallow (20%); 4 weeks pre-implantation. | Increased metastases in 23% corn oil vs. all others. | [66] | |
| DMBA (65 mg/kg body weight at day 50) | Diets of 20% high-linoleic safflower oil, 20% high-oleic safflower oil, 20% olive oil, 20% linoleic-supplemented olive oil. | Preventive effect of olive oil (longer tumor-free time, fewer tumors per rat and lower tumor incidence). | Lipid profile | [65,90] |
| DMBA (2 × 2 mg/rat at 5 and 6 weeks of age) | Corn oil (7%, 15%), EVOO (7%, 15%); prenatal and lactation. | Smaller tumors with 7% olive oil diet. Promoting effect of high-fat diets. | Hormones (estradiol), apoptosis (Bcl2, Bak, Casp3) | [69] |
| DMBA (2 × 10 mg/rat at 5 and 6 weeks of age) | Corn oil (7%, 15%), olive oil (7%, 15%); prenatal and lactation. | Preventive effect of olive oil. Promoting effect of high-fat diets. | Immune function, apoptotic index | [87] |
| DMBA (2 × of 10 mg/rat) | Low-fat, 15% olive oil. | Spleen cellular components, tumor leukocyte infiltrates, apoptosis | [86] | |
| DMBA (5 mg/rat at day 53) | Diets of 3% low-fat, 20% corn oil, 20% EVOO; post-induction. | EVOO vs. corn oil preventive effect. Low histologic grade, few necrotic and invasive areas. | [74,77] | |
| EVOO vs. corn oil preventive effect. Higher latency time, lower incidence, multiplicity, volume; lower degree of histopathological malignancy. | [70] | |||
| Gene expression—proliferation genes (EGFR, neu) | [71] | |||
| Gene expression—differentiation genes (igf2, H19, VDUP1) | [94] | |||
| Gene expression—differentiation genes (transferrin, β-actin); ZBP1 protein | [81] | |||
| Proliferation and apoptosis pathways (PCNA, ErbB4, Ras, ERK1/2, AKT, Casp3), DNA damage | [75] | |||
| DMBA (5 mg/rat at day 53) | Diets of 3% low-fat, 20% corn oil, 20% EVOO; post-weaning/post-induction. | EVOO vs. corn oil preventive effect. Lower tumor incidence and yield. | Growth and sexual maturation (hypothalamic Kiss1) | [78] |
| Body mass (plasma OEA, hypothalamic oxytocin) | [80] | |||
| Transcriptomics in mammary gland (immune system, apoptosis, metabolism genes) Liver metabolism (UCP2) | [73] | |||
| EVOO vs. corn oil preventive effect. Lower tumor incidence, yield, volume; lower degree of histopatho- logical malignancy (degree, stromal reaction, necrosis, mitoses). | Transcriptomics in tumor (proliferation, immune system, apoptosis, metabolism genes) | [76,93] | ||
| Expression of Scd, Pfkl, Sema3A, Jak2, Smad1, Casp3, Arg1, Tgfβ1; serum ILα, leptin; CD8 infiltration | [76] | |||
| Epigenetics: DNA methylation (DNMT, Rassf1A, Timp3), histone modifications | [96,97] | |||
| Metabolism (Glut1, PFKL, GAPDH, CS, IDH, UCP2) | [99] | |||
| Carcinogen detoxification (liver and mammary gland Cyp1A1, Cyp1A2, Cyp1B1, Nqo1, AhR, Nfr2, Gstp1) | [84,85] | |||
| Oxidative stress (GSSG/GSH, lipid oxidation, DNA damage) | [83] | |||
| DMBA (10 mg/rat at day 53) | Diets of 3% low-fat, 20% corn oil, 20% EVOO; post-weaning. | EVOO vs. corn oil preventive effect. Promoting effect of high-fat diets. | Carcinogen detoxification (Cyp1A1, Cyp1A2, Cyp1B1, Nqo1, AhR, Nfr2, Gstp1), DMBA metabolites and DNA adducts | [72] |
| DMBA (20 mg/kg body weight at day 21) | Diets of 23,4% olive oil, 23,4% butterfat, 23,4% safflower oil; prenatal. | High safflower oil increased carcinogenesis. | Gene transcription Cadm4, Bbn1a1 | [95] |
| MMTV-neu(ndl)-YD5 mouse | Diets of 10% safflower oil (SA), 3% menhaden oil + 7% SA, 3% flaxseed oil + 7% SA, 10% olive oil, 10% lard. | Menhaden oil better prevented carcinogenesis; safflower oil was the strongest promoter. | Lipid profile | [67] |
| N-ethyl-N-nitrosourea (180 mg/kg) | Diets of 4% fish oil, 4% olive oil, 4% maize oil; post-induction. | Protective effect of fish oil. | Lipid profile | [91] |
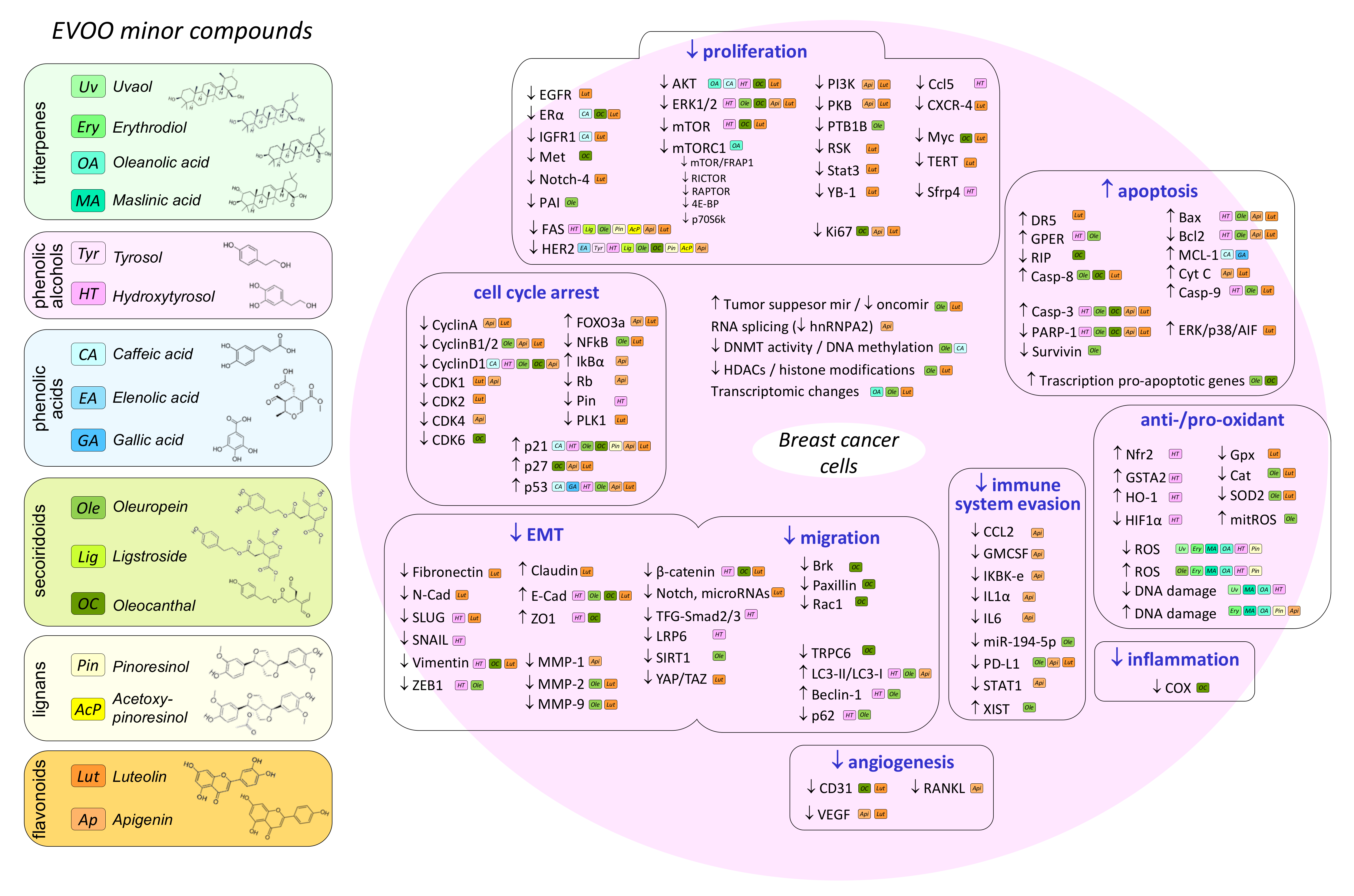
4. Effect of Olive Oil Components on Mammary Carcinogenesis in In Vivo and In Vitro Models
4.1. Oleic Acid
4.2. Hydroxytyrosol
4.3. Oleuropein
4.4. Oleocanthal
4.5. Luteolin and Apigenin
4.6. Other Minor Compounds
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Today-International Agency for Research on Cancer: World Health Organisation Global Cancer Observatory (GLOBOCAN). Global Cancer Observatory. Cancer Today-International Agency for Research on Cancer: World Health Organisation. 2018. Available online: https://gco.iarc.fr/today/home (accessed on 20 October 2021).
- Albuquerque, R.C.; Baltar, V.T.; Marchioni, D.M. Breast cancer and dietary patterns: A systematic review. Nutr. Rev. 2014, 72, 1–17. [Google Scholar] [CrossRef]
- Escrich, E.; Solanas, M.; Moral, R. Olive oil, and other dietary lipids, in cancer: Experimental approaches. In Olive Oil and Health; Quiles, J.L., Ramirez-Tortosa, M.C., Yaqoob, P., Eds.; CABI Publishing: Wallingford, UK; Cambridge, MA, USA, 2006; pp. 317–374. [Google Scholar]
- Escrich, E.; Solanas, M.; Moral, R.; Escrich, R. Modulatory Effects and Molecular Mechanisms of Olive Oil and Other Dietary Lipids in Breast Cancer. Curr. Pharm. Des. 2011, 17, 813–830. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Ramos, F.; Serra-Majem, L.; La Vecchia, C.; Ramon, J.M.; Tresserras, R.; Salleras, L. Mortality trends and past and current dietary factors of breast cancer in Spain. Eur. J. Epidemiol. 1996, 12, 141–148. [Google Scholar] [CrossRef]
- Barjol, J.-L. Introduction. In Handbook of Olive Oil; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 1–17. [Google Scholar]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Ho-Min Kang, M.A.; Mele, M.Z.I.; Giuffre, A.M. Pre-and post-harvest factors and their impact on oil composition and quality of olive fruit. Emirates J. Food Agric. 2018, 30, 592–603. [Google Scholar] [CrossRef]
- Ramírez-Tortosa, M.C.; Granados, S.; Quiles, J.L. Chemical composition, types and characteristics of olive oil. In Olive oil and health; Quiles, J.L., Ramirez-Tortosa, M.C., Yaqoob, P., Eds.; CABI Publishing: Wallingford, UK; Cambridge, MA, USA, 2006; pp. 45–62. [Google Scholar]
- Olmo-García, L.; Polari, J.J.; Li, X.; Bajoub, A.; Fernández-Gutiérrez, A.; Wang, S.C.; Carrasco-Pancorbo, A. Study of the minor fraction of virgin olive oil by a multi-class GC–MS approach: Comprehensive quantitative characterization and varietal discrimination potential. Food Res. Int. 2019, 125, 108649. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; de Gennaro, B.C.; Franchini, C.; Corbo, F.; Apetrei, C. Extra Virgin Olive Oils: Bioactive Compounds and Health Benefits. In Frontiers in Bioactive Compounds; Apetrei, C., Ed.; Bentham Science Publishers: Sharjah, UAE, 2016; pp. 3–31. [Google Scholar]
- Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Stefanoudaki, E.; Kotsifaki, F.; Koutsaftakis, A. Classification of virgin olive oils of the two major cretan cultivars based on their fatty acid composition. J. Am. Oil Chem. Soc. 1999, 76, 623–626. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Morales-Pérez, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship of quality parameters, antioxidant capacity and total phenolic content of EVOO with ripening state and olive variety. Food Chem. 2020, 325, 126926. [Google Scholar] [CrossRef]
- International Olive Council. Trade Standard Applying to Olive Oils and Olive Pomace Oils. 2019. Available online: http://www.internationaloliveoil.org (accessed on 22 December 2021).
- Lou-Bonafonte, J.M.; Arnal, C.; Navarro, M.A.; Osada, J. Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Mol. Nutr. Food Res. 2012, 56, 1043–1057. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Uceda, M.; Aguilera, M.P.; Gaforio, J.J.; Beltrán, G. Triterpenic Content and Chemometric Analysis of Virgin Olive Oils from Forty Olive Cultivars. J. Agric. Food Chem. 2009, 57, 3604–3610. [Google Scholar] [CrossRef]
- Giuffrè, A.M. n-Alkanes and n-Alkenes in Virgin Olive Oil from Calabria (South Italy): The Effects of Cultivar and Harvest Date. Foods 2021, 10, 290. [Google Scholar] [CrossRef]
- Arrizabalaga-Larrañaga, A.; Rodríguez, P.; Medina, M.; Santos, F.J.; Moyano, E. Pigment profiles of Spanish extra virgin olive oils by ultra-high-performance liquid chromatography coupled to high-resolution mass spectrometry. Food Addit. Contam. Part. A 2020, 37, 1075–1086. [Google Scholar] [CrossRef]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct study of minor extra-virgin olive oil components without any sample modification. 1H NMR multisupression experiment: A powerful tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to olive oil and maintenance of normal blood LDL-cholesterol concentrations (ID 1316, 1332), maintenance of normal (fasting) blood concentrations of triglycerides (ID 1316, 1332), maintenance of normal blood HDL cholesterol concentrations (ID 1316, 1332) and maintenance of normal blood glucose concentrations (ID 4244) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2044. [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Z.; Yang, X.; Liu, L.; Ahn, K.S. An updated review on the potential antineoplastic actions of oleuropein. Phyther. Res. 2021. (ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Pang, K.-L.; Chin, K.-Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Şoica, C.; Voicu, M.; Ghiulai, R.; Dehelean, C.; Racoviceanu, R.; Trandafirescu, C.; Roșca, O.-J.; Nistor, G.; Mioc, M.; Mioc, A. Natural Compounds in Sex Hormone-Dependent Cancers: The Role of Triterpenes as Therapeutic Agents. Front. Endocrinol. 2021, 11, 612396. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Katsouyanni, K.; Stuver, S.; Tzala, L.; Gnardellis, C.; Rimm, E.; Trichopoulos, D. Consumption of Olive Oil and Specific Food Groups in Relation to Breast Cancer Risk in Greece. JNCI J. Natl. Cancer Inst. 1995, 87, 110–116. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Negri, E.; Franceschi, S.; Decarli, A.; Giacosa, A.; Lipworth, L. Olive oil, other dietary fats, and the risk of breast cancer (Italy). Cancer Causes Control. 1995, 6, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.; Reno, W.; Ibrahim, G.; Behbehani, A.; Dashti, H.; Asfar, S. The first pilot study on characteristics and practice patterns of Kuwaiti breast cancer patients. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 61–75. [Google Scholar] [CrossRef]
- Martin-Moreno, J.M.; Willett, W.C.; Gorgojo, L.; Banegas, J.R.; Rodriguez-Artalejo, F.; Fernandez-Rodriguez, J.C.; Maisonneuve, P.; Boyle, P. Dietary fat, olive oil intake and breast cancer risk. Int. J. Cancer 1994, 58, 774–780. [Google Scholar] [CrossRef]
- García-Segovia, P.; Sánchez-Villegas, A.; Doreste, J.; Santana, F.; Serra-Majem, L. Olive oil consumption and risk of breast cancer in the Canary Islands: A population-based case–control study. Public Health Nutr. 2006, 9, 163–167. [Google Scholar] [CrossRef]
- Simonsen, N.R.; Navajas, J.F.C.; Martin-Moreno, J.M.; Strain, J.J.; Huttunen, J.K.; Martin, B.C.; Thamm, M.; Kardinaal, A.F.M.; Van’t Veer, P.; Kok, F.J.; et al. Tissue stores of individual monounsaturated fatty acids and breast cancer: The EURAMIC study. Am. J. Clin. Nutr. 1998, 68, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Gardeazabal, I.; Romanos-Nanclares, A.; Martínez-González, M.Á.; Sánchez-Bayona, R.; Vitelli-Storelli, F.; Gaforio, J.J.; Aramendiá-Beitia, J.M.; Toledo, E. Total polyphenol intake and breast cancer risk in the Seguimiento Universidad de Navarra (SUN) cohort. Br. J. Nutr. 2019, 122, 542–551. [Google Scholar] [CrossRef]
- Pelucchi, C.; Bosetti, C.; Negri, E.; Lipworth, L.; La Vecchia, C. Olive oil and cancer risk: An update of epidemiological findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Bamia, C.; Lagiou, P.; Trichopoulos, D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am. J. Clin. Nutr. 2010, 92, 620–625. [Google Scholar] [CrossRef]
- Fung, T.T.; Hu, F.B.; McCullough, M.L.; Newby, P.K.; Willett, W.C.; Holmes, M.D. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J. Nutr. 2006, 136, 466–472. [Google Scholar] [CrossRef]
- Van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef]
- Lavalette, C.; Adjibade, M.; Srour, B.; Sellem, L.; Fiolet, T.; Hercberg, S.; Latino-Martel, P.; Fassier, P.; Deschasaux, M.; Kesse-Guyot, E.; et al. Cancer-specific and general nutritional scores and cancer risk: Results from the prospective NutriNet-Santé cohort. Cancer Res. 2018, 78, 4427–4435. [Google Scholar] [CrossRef]
- Männistö, S.; Harald, K.; Härkänen, T.; Maukonen, M.; Eriksson, J.G.; Heikkinen, S.; Jousilahti, P.; Kaartinen, N.E.; Kanerva, N.; Knekt, P.; et al. Association between overall diet quality and postmenopausal breast cancer risk in five Finnish cohort studies. Sci. Rep. 2021, 11, 1–9. [Google Scholar]
- Petimar, J.; Park, Y.M.M.; Smith-Warner, S.A.; Fung, T.T.; Sandler, D.P. Dietary index scores and invasive breast cancer risk among women with a family history of breast cancer. Am. J. Clin. Nutr. 2019, 109, 1393–1401. [Google Scholar] [CrossRef]
- Haridass, V.; Ziogas, A.; Neuhausen, S.L.; Anton-Culver, H.; Odegaard, A.O. Diet Quality Scores Inversely Associated with Postmenopausal Breast Cancer Risk Are Not Associated with Premenopausal Breast Cancer Risk in the California Teachers Study. J. Nutr. 2018, 148, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, S.H.; Mitchell, C.; Fung, T.T. Associations between diet quality scores and risk of postmenopausal estrogen receptor-negative breast cancer: A systematic review. J. Nutr. 2018, 148, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Agudo, A.; Fonseca-Nunes, A.; Navarro, C.; Lagiou, P.; Demetriou, C.; Amiano, P.; Dorronsoro, M.; Chirlaque, M.D.; et al. Olive oil intake and breast cancer risk in the Mediterranean countries of the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2012, 131, 2465–2469. [Google Scholar] [CrossRef]
- Sealy, N.; Hankinson, S.E.; Houghton, S.C. Olive oil and risk of breast cancer: A systematic review and dose-response meta-analysis of observational studies. Br. J. Nutr. 2021, 125, 1148–1156. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Boucher, P.; Mamelle, N. Mediterranean dietary pattern in a randomized trial: Prolonged survival and possible reduced cancer rate. Arch. Intern. Med. 1998, 158, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Salas-Salvado, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fito, M.; Hu, F.B.; Aros, F.; et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the predimed trial a randomized clinical trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef]
- Álvarez-Pérez, J.; Sánchez-Villegas, A.; Díaz-Benítez, E.M.; Ruano-Rodríguez, C.; Corella, D.; Martínez-González, M.Á.; Estruch, R.; Salas-Salvadó, J.; Serra-Majem, L. Influence of a Mediterranean Dietary Pattern on Body Fat Distribution: Results of the PREDIMED–Canarias Intervention Randomized Trial. J. Am. Coll. Nutr. 2016, 35, 568–580. [Google Scholar] [CrossRef]
- Razquin, C.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Mitjavila, M.T.; Estruch, R.; Marti, A. A 3 years follow-up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. Eur. J. Clin. Nutr. 2009, 63, 1387–1393. [Google Scholar] [CrossRef]
- Buckland, G.; Travier, N.; Cottet, V.; González, C.A.; Luján-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, W.; Chen, C.S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef]
- Guthrie, N.; Carroll, K.K. Specific versus non-specific effects of dietary fat on carcinogenesis. Prog. Lipid Res. 1999, 38, 261–271. [Google Scholar] [CrossRef]
- Tannenbaum, A. The Genesis and Growth of Tumors: III. Effects of a High-Fat Diet. Cancer Res. 1942, 2, 468–475. [Google Scholar]
- Cohen, L.A.; Thompson, D.O.; Maeura, Y.; Choi, K.; Blank, M.E.; Rose, D.P. Dietary fat and mammary cancer. I. promoting effects of different dietary fats on N-nitrosomethylurea-induced rat mammary tumorigenesis. J. Natl. Cancer Inst. 1986, 77, 33–42. [Google Scholar]
- Cohen, L.A. Fat and endocrine-responsive cancer in animals. Prev. Med. 1987, 16, 468–474. [Google Scholar] [CrossRef]
- Cohen, L.A.; Epstein, M.; Pittman, B.; Rivenson, A. The influence of different varieties of olive oil on N-methylnitrosourea(NMU)-induced mammary tumorigenesis. Anticancer Res. 2000, 20, 2307–2312. [Google Scholar]
- Lasekan, J.B.; Clayton, M.K.; Gendron-Fitzpatrick, A.; Ney, D.M. Dietary olive and safflower oils in promotion of DMBA-induced mammary tumorigenesis in rats. Nutr. Cancer 1990, 13, 153–163. [Google Scholar] [CrossRef]
- Katz, E.B.; Boylan, E.S. Effect of the quality of dietary fat on tumor growth and metastasis from a rat mammary adenocarcinoma. Nutr. Cancer 1989, 12, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.A.; Abdelmagid, S.A.; Perez, K.; Muller, W.J.; Ma, D.W.L. Mammary tumour development is dose-dependently inhibited by n-3 polyunsaturated fatty acids in the MMTV-neu(ndl)-YD5 transgenic mouse model. Lipids Health Dis. 2014, 13, 96. [Google Scholar] [CrossRef]
- Ramírez-Expósito, M.J.; Carrera, M.P.; Cortés, P.; Martínez-Martos, J.M. Dietary Fat Including Olive Oil and Breast Cancer in the N-methyl Nitrosourea (NMU) Animal Model. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V., Watson, R., Eds.; Elsevier: Oxford, UK, 2010; pp. 969–979. [Google Scholar]
- Stark, A.H.; Kossoy, G.; Zusman, I.; Yarden, G.; Madar, Z. Olive oil consumption during pregnancy and lactation in rats influences mammary cancer development in female offspring. Nutr. Cancer 2003, 46, 59–65. [Google Scholar] [CrossRef]
- Solanas, M.; Hurtado, A.; Costa, I.; Moral, R.; Menéndez, J.A.; Colomer, R.; Escrich, E. Effects of a high olive oil diet on the clinical behavior and histopathological features of rat DMBA-induced mammary tumors compared with a high corn oil diet. Int. J. Oncol. 2002, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Solanas, M.; García, G.; Colomer, R.; Escrich, E. Modulation of EGFR and neu expression by n-6 and n-9 high-fat diets in experimental mammary adenocarcinomas. Oncol. Rep. 2003, 10, 1417–1424. [Google Scholar] [CrossRef]
- Manzanares, M.Á.; de Miguel, C.; Ruiz de Villa, M.C.; Santella, R.M.; Escrich, E.; Solanas, M. Dietary lipids differentially modulate the initiation of experimental breast carcinogenesis through their influence on hepatic xenobiotic metabolism and DNA damage in the mammary gland. J. Nutr. Biochem. 2017, 43, 68–77. [Google Scholar] [CrossRef]
- Moral, R.; Escrich, R.; Solanas, M.; Vela, E.; Ruiz de Villa, M.C.; Escrich, E. Diets high in corn oil or extra-virgin olive oil differentially modify the gene expression profile of the mammary gland and influence experimental breast cancer susceptibility. Eur. J. Nutr. 2016, 55, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Moral, R.; Solanas, M.; Escrich, E. High-fat corn oil diet promotes the development of high histologic grade rat DMBA-induced mammary adenocarcinomas, while high olive oil diet does not. Breast Cancer Res. Treat. 2004, 86, 225–235. [Google Scholar] [CrossRef]
- Solanas, M.; Grau, L.; Moral, R.; Vela, E.; Escrich, R.; Escrich, E. Dietary olive oil and corn oil differentially affect experimental breast cancer through distinct modulation of the p21Ras signaling and the proliferation-apoptosis balance. Carcinogenesis 2010, 31, 871–879. [Google Scholar] [CrossRef]
- Escrich, R.; Costa, I.; Moreno, M.; Cubedo, M.; Vela, E.; Escrich, E.; Moral, R. A high-corn-oil diet strongly stimulates mammary carcinogenesis, while a high-extra-virgin-olive-oil diet has a weak effect, through changes in metabolism, immune system function and proliferation/apoptosis pathways. J. Nutr. Biochem. 2019, 64, 218–227. [Google Scholar] [CrossRef]
- Costa, I.; Moral, R.; Solanas, M.; Andreu, F.J.; Ruiz De Villa, M.C.; Escrich, E. High corn oil and extra virgin olive oil diets and experimental mammary carcinogenesis: Clinicopathological and immunohistochemical p21Ha-Ras expression study. Virchows Arch. 2011, 458, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Escrich, R.; Solanas, M.; Vela, E.; Costa, I.; Ruiz de Villa, M.C.; Escrich, E. Diets high in corn oil or extra-virgin olive oil provided from weaning advance sexual maturation and differentially modify susceptibility to mammary carcinogenesis in female rats. Nutr. Cancer 2011, 63, 410–420. [Google Scholar] [CrossRef]
- Goldberg, M.; D’Aloisio, A.A.; O’Brien, K.M.; Zhao, S.; Sandler, D.P. Pubertal timing and breast cancer risk in the Sister Study cohort. Breast Cancer Res. 2020, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, I.; Moral, R.; Escrich, R.; Solanas, M.; Vela, E.; Escrich, E. Effect of High Fat Diets on Body Mass, Oleylethanolamide Plasma Levels and Oxytocin Expression in Growing Rats. J. Food Sci. 2015, 80, H1425–H1431. [Google Scholar] [CrossRef]
- Moral, R.; Solanas, M.; Garcia, G.; Grau, L.; Vela, E.; Escrich, R.; Escrich, E. High corn oil and high extra virgin olive oil diets have different effects on the expression of differentiation-related genes in experimental mammary tumors. Oncol. Rep. 2008, 20, 429–435. [Google Scholar]
- Eder, E.; Wacker, M.; Lutz, U.; Nair, J.; Fang, X.; Bartsch, H.; Beland, F.A.; Schlatter, J.; Lutz, W.K. Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive- or coconut oil supplemented diets. Chem. Biol. Interact. 2006, 159, 81–89. [Google Scholar] [CrossRef]
- Escrich, R.; Vela, E.; Solanas, M.; Moral, R. Effects of diets high in corn oil or in extra virgin olive oil on oxidative stress in an experimental model of breast cancer. Mol. Biol. Rep. 2020, 47, 4923–4932. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, M.Á.; Solanas, M.; Moral, R.; Escrich, R.; Vela, E.; Costa, I.; Escrich, E. Dietary extra-virgin olive oil and corn oil differentially modulate the mRNA expression of xenobiotic-metabolizing enzymes in the liver and in the mammary gland in a rat chemically induced breast cancer model. Eur. J. Cancer Prev. 2015, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, M.A.; Solanas, M.; Moral, R.; Escrich, R.; Vela, E.; Escrich, E. Ontogeny of the Major Xenobiotic-Metabolizing Enzymes Expression and the Dietary Lipids Modulatory Effect in the Rat Dimethylbenz(a)anthracene-Induced Breast Cancer Model. J. Biochem. Mol. Toxicol. 2014, 28, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Kossoy, G.; Yarden, G.; Ben-Hur, H.; Kossoy, N.; Stark, A.; Madar, Z.; Zusman, I. Comparative effects of dimethylbenz(a)anthacene and a 15% olive-oil diet on cellular components and expression of apoptosis-related proteins in the spleen and mammary gland tumors of rats. Oncol. Rep. 2001, 8, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Kossoy, G.; Stark, A.; Tendler, Y.; Ben-Hur, H.; Beniashvili, D.; Madar, Z.; Zusman, I. Transplacental effects of high fat diets on functional activity of the spleen and lymph nodes, cell kinetics and apoptosis in mammary gland tumors in female rat offspring. Int. J. Mol. Med. 2002, 10, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev. 2005, 80, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Escrich, E.; Solanas, M.; Soler, M.; Ruiz de Villa, M.C.; Sanchez, J.A.; Segura, R. Dietary polyunsaturated n-6 lipids effects on the growth and fatty acid composition of rat mammary tumors. J. Nutr. Biochem. 2001, 12, 536–549. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Ney, D.M. Mammary tumor lipids and plasma lipoproteins in DMBA-intubated rats fed olive or safflower oils. Nutr. Cancer 1990, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Maunder, K. The influence of dietary fatty acid composition on N-ethyl-N-nitrosourea-induced mammary tumour incidence in the rat and on the composition of inositol- and ethanolamine-phospholipids of normal and tumour mammary tissue. Br. J. Nutr. 1994, 71, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.A.; Thompson, D.O.; Choi, K.; Karmali, R.A.; Rose, D.P. Dietary fat and mammary cancer. II. Modulation of serum and tumor lipid composition and tumor prostaglandins by different dietary fats: Association with tumor incidence patterns. J. Natl. Cancer Inst. 1986, 77, 43–51. [Google Scholar]
- Escrich, R.; Cubedo, M.; Escrich, E.; Moral, R. Gene ontology analysis of transcriptome data from DMBA-induced mammary tumors of rats fed a high-corn oil and a high-extra virgin olive oil diet. Data Br. 2019, 22, 104–108. [Google Scholar] [CrossRef]
- Solanas, M.; Moral, R.; Garcia, G.; Grau, L.; Vela, E.; Escrich, R.; Costa, I.; Escrich, E. Differential expression of H19 and vitamin D3 upregulated protein 1 as a mechanism of the modulatory effects of high virgin olive oil and high corn oil diets on experimental mammary tumours. Eur. J. Cancer Prev. 2009, 18, 153–161. [Google Scholar] [CrossRef]
- Govindarajah, V.; Leung, Y.-K.; Ying, J.; Gear, R.; Bornschein, R.L.; Medvedovic, M.; Ho, S.-M. In utero exposure of rats to high-fat diets perturbs gene expression profiles and cancer susceptibility of prepubertal mammary glands. J. Nutr. Biochem. 2016, 29, 73–82. [Google Scholar] [CrossRef]
- Rodríguez-Miguel, C.; Moral, R.; Escrich, R.; Vela, E.; Solanas, M.; Escrich, E. The role of dietary extra virgin olive oil and corn oil on the alteration of epigenetic patterns in the rat DMBA-induced breast cancer model. PLoS ONE 2015, 10, e0138980. [Google Scholar] [CrossRef]
- Moral, R.; Escrich, E. Extra virgin olive oil and corn oil and epigenetic patterns in breast cancer. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V., Preedy, V., Eds.; Springer: Cham, Switzerland, 2019; pp. 1877–1896. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Guasch, M.; Navarro, L.; Rivero, V.; Costa, I.; Escrich, E.; Moral, R. A high extra-virgin olive oil diet induces changes in metabolic pathways of experimental mammary tumors. J. Nutr. Biochem. 2022, 99, 108833. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Sanjuan, M.D.; Martínez-Martos, J.M.; Carrera-González, M.P.; Mayas, M.D.; García, M.J.; Arrazola, M.; Ramírez-Expósito, M.J. Normolipidic dietary fat modifies circulating Renin-Angiotensin system-regulating aminopeptidase activities in rat with breast cancer. Integr. Cancer Ther. 2015, 14, 149–155. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.; Langelier, Y.; Prentki, M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000, 60, 6353–6358. [Google Scholar]
- Hardy, S.; St-Onge, G.G.; Joly, É.; Langelier, Y.; Prentki, M. Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J. Biol. Chem. 2005, 280, 13285–13291. [Google Scholar] [CrossRef]
- Soto-Guzman, A.; Robledo, T.; Lopez-Perez, M.; Salazar, E.P. Oleic acid induces ERK1/2 activation and AP-1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol. Cell. Endocrinol. 2008, 294, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tito, N.; Soto-Guzman, A.; Castro-Sanchez, L.; Martinez-Orozco, R.; Salazar, E.P. Oleic acid promotes migration on MDA-MB-231 breast cancer cells through an arachidonic acid-dependent pathway. Int. J. Biochem. Cell Biol. 2010, 42, 306–317. [Google Scholar] [CrossRef]
- Soto-Guzman, A.; Villegas-Comonfort, S.; Cortes-Reynosa, P.; Perez Salazar, E. Role of arachidonic acid metabolism in Stat5 activation induced by oleic acid in MDA-MB-231 breast cancer cells. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Katoh, K.; Obara, Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem. Biophys. Res. Commun. 2004, 314, 805–809. [Google Scholar] [CrossRef]
- Marcial-Medina, C.; Ordoñez-Moreno, A.; Gonzalez-Reyes, C.; Cortes-Reynosa, P.; Salazar, E.P. Oleic acid induces migration through a FFAR1/4, EGFR and AKT-dependent pathway in breast cancer cells. Endocr. Connect. 2019, 8, 252–265. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erb B-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (HerceptinTM) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef]
- Menendez, J.A.; Mehmi, I.; Atlas, E.; Colomer, R.; Lupu, R. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB. Int. J. Oncol. 2004, 24, 591–608. [Google Scholar] [CrossRef]
- Li, S.; Zhou, T.; Li, C.; Dai, Z.; Che, D.; Yao, Y.; Li, L.; Ma, J.; Yang, X.; Gao, G. High metastaticgastric and breast cancer cells consume oleic acid in an AMPK dependent manner. PLoS ONE 2014, 9, e97330. [Google Scholar] [CrossRef] [PubMed]
- Przybytkowski, E.; Joly, É.; Nolan, C.J.; Hardy, S.; Francoeur, A.M.; Langelier, Y.; Prentki, M. Upregulation of cellular triacylglycerol—Free fatty acid cycling by oleate is associated with long-term serum-free survival of human breast cancer cells. Biochem. Cell Biol. 2007, 85, 301–310. [Google Scholar] [CrossRef]
- Al-Bahlani, S.; Al-Lawati, H.; Al-Adawi, M.; Al-Abri, N.; Al-Dhahli, B.; Al-Adawi, K. Fatty acid synthase regulates the chemosensitivity of breast cancer cells to cisplatin-induced apoptosis. Apoptosis 2017, 22, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.; Camacho-Corencia, P.; Sanchez-Rovira, P.; Vera-Ramirez, L.; Ramirez-Tortosa, M. Hydroxytyrosol inhibits growth and cell proliferation and promotes high expression of sfrp4 in rat mammary tumours. Mol. Nutr. Food Res. 2011, 55, S117–S126. [Google Scholar] [CrossRef] [PubMed]
- El-azem, N.; Pulido-Moran, M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Cara, F.E.; Sanchez-Rovira, P.; Granados-Principal, S.; Ramirez-Tortosa, M. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur. J. Nutr. 2019, 58, 1203–1211. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, R.; Chimento, A.; de Luca, A.; Casaburi, I.; Rizza, P.; Onofrio, A.; Iacopetta, D.; Puoci, F.; Andò, S.; Maggiolini, M.; et al. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Mol. Nutr. Food Res. 2010, 54, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Analyzing effects of extra-virgin olive polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008, 22, 433–439. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Fernandez-Gutierrez, A.; Segura-Carretero, A. tabAnti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial Extra-Virgin Olive Oil (EVOO). BMC Cancer 2008, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Sarsour, E.H.; Goswami, M.; Kalen, A.L.; Lafin, J.T.; Goswami, P.C. Hydroxytyrosol inhibits chemokine C-C motif ligand 5 mediated aged quiescent fibroblast-induced stimulation of breast cancer cell proliferation. Age 2014, 36, 9645. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Santolla, M.F.; Maggiolini, M.; Pezzi, V.; et al. Oleuropein and hydroxytyrosol activate GPER/GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 478–489. [Google Scholar] [CrossRef]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA Damage Is Prevented by Extracts of Olive Oil, Hydroxytyrosol, and Other Olive Phenolic Compounds in Human Blood Mononuclear Cells and HL60 Cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar] [CrossRef]
- Fabiani, R.; Sepporta, M.V.; Rosignoli, P.; De Bartolomeo, A.; Crescimanno, M.; Morozzi, G. Anti-proliferative and pro-apoptotic activities of hydroxytyrosol on different tumour cells: The role of extracellular production of hydrogen peroxide. Eur. J. Nutr. 2012, 51, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Odiatou, E.M.; Skaltsounis, A.L.; Constantinou, A.I. Identification of the factors responsible for the in vitro pro-oxidant and cytotoxic activities of the olive polyphenols oleuropein and hydroxytyrosol. Cancer Lett. 2013, 330, 113–121. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Sepporta, M.V.; Fabiani, R. In vitro chemo-preventive activities of hydroxytyrosol: The main phenolic compound present in extra-virgin olive oil. Food Funct. 2016, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; De Dios, C.; Siles, E. Hypoxia modulates the antioxidant effect of hydroxytyrosol in MCF-7 breast cancer cells. PLoS ONE 2018, 13, e0203892. [Google Scholar] [CrossRef] [PubMed]
- Calahorra, J.; Martínez-Lara, E.; Granadino-Roldán, J.M.; Martí, J.M.; Cañuelo, A.; Blanco, S.; Oliver, F.J.; Siles, E. Crosstalk between hydroxytyrosol, a major olive oil phenol, and HIF-1 in MCF-7 breast cancer cells. Sci. Rep. 2020, 10, 6361. [Google Scholar] [CrossRef]
- Cruz-Lozano, M.; González-González, A.; Marchal, J.A.; Muñoz-Muela, E.; Molina, M.P.; Cara, F.E.; Brown, A.M.; García-Rivas, G.; Hernández-Brenes, C.; Lorente, J.A.; et al. Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/β-catenin and TGFβ signaling pathways. Eur. J. Nutr. 2019, 58, 3207–3219. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Zhang, Z.; Shen, W.-J.; Jiang, S.; Long, Y.-F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anticancer. Agents Med. Chem. 2019, 19, 1983–1990. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Xie, J.; Zhang, Z.; Zhu, J.; Jiang, S.; Shen, W.-J.; Wu, B.; Ding, T.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion via Induction of Autophagy in ER-Positive Breast Cancer Cell Lines (MCF7 and T47D). Nutr. Cancer 2021, 73, 350–360. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Oppong, M.B.; Guo, Y.; Wang, L.-Z.; Fang, S.-M.; Deng, Y.-R.; Gao, X.-M. The Oleaceae family: A source of secoiridoids with multiple biological activities. Fitoterapia 2019, 136, 104155. [Google Scholar] [CrossRef] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Gene Expression Alterations Associated with Oleuropein-Induced Antiproliferative Effects and S-Phase Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Nutrients 2020, 12, 3755. [Google Scholar] [CrossRef]
- Asgharzade, S.; Sheikhshabani, S.H.; Ghasempour, E.; Heidari, R.; Rahmati, S.; Mohammadi, M.; Jazaeri, A.; Amini-Farsani, Z. The effect of oleuropein on apoptotic pathway regulators in breast cancer cells. Eur. J. Pharmacol. 2020, 886, 173509. [Google Scholar] [CrossRef]
- Abtin, M.; Alivand, M.R.; Khaniani, M.S.; Bastami, M.; Zaeifizadeh, M.; Derakhshan, S.M. Simultaneous downregulation of miR-21 and miR-155 through oleuropein for breast cancer prevention and therapy. J. Cell. Biochem. 2018, 119, 7151–7165. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Faja, B.; Cuyàs, E.; Lozano-Sánchez, J.; Cufí, S.; Verdura, S.; Fernández-Arroyo, S.; Borrás-Linares, I.; Martin-Castillo, B.; Martin, Á.G.; Lupu, R.; et al. Extra-virgin olive oil contains a metabolo-epigenetic inhibitor of cancer stem cells. Carcinogenesis 2018, 39, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Alivand, M.R.; Bayat, S.; Khaniani, M.S.; Derakhshan, S.M. The hopeful anticancer role of oleuropein in breast cancer through histone deacetylase modulation. J. Cell. Biochem. 2019, 120, 17042–17049. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Mansoori Derakhshan, S.; Mansoori Derakhshan, N.; Shekari Khaniani, M.; Alivand, M.R. Downregulation of HDAC2 and HDAC3 via oleuropein as a potent prevention and therapeutic agent in MCF-7 breast cancer cells. J. Cell. Biochem. 2019, 120, 9172–9180. [Google Scholar] [CrossRef]
- Przychodzen, P.; Kuban-Jankowska, A.; Wyszkowska, R.; Barone, G.; Bosco, G.L.; Celso, F.L.; Kamm, A.; Daca, A.; Kostrzewa, T.; Gorska-Ponikowska, M. PTP1B phosphatase as a novel target of oleuropein activity in MCF-7 breast cancer model. Toxicol. In Vitro 2019, 61, 104624. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.K.; Elamin, M.H.; Omer, S.A.; Daghestani, M.H.; Al-Olayan, E.S.; Elobeid, M.A.; Virk, P. Oleuropein induces apoptosis via the p53 pathway in breast cancer cells. Asian Pac. J. Cancer Prev. 2014, 14, 6739–6742. [Google Scholar] [CrossRef]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor–negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Tzekaki, E.E.; Geromichalos, G.; Lavrentiadou, S.N.; Tsantarliotou, M.P.; Pantazaki, A.A.; Papaspyropoulos, A. Oleuropein is a natural inhibitor of PAI-1-mediated proliferation in human ER-/PR- breast cancer cells. Breast Cancer Res. Treat. 2021, 186, 305–316. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Carrasco-Pancorbo, A.; Garcia-Villalba, R.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Olive oil’s bitter principle reverses acquired autoresistance to trastuzumab (Herceptin) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80. [Google Scholar] [CrossRef]
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic Anti-Breast-Cancer Effects of Combined Treatment with Oleuropein and Doxorubicin In Vivo. Altern. Ther. Health Med. 2019, 25, 17–24. [Google Scholar]
- Benot-Dominguez, R.; Tupone, M.G.; Castelli, V.; D’Angelo, M.; Benedetti, E.; Quintiliani, M.; Cinque, B.; Forte, I.M.; Cifone, M.G.; Ippoliti, R.; et al. Olive leaf extract impairs mitochondria by pro-oxidant activity in MDA-MB-231 and OVCAR-3 cancer cells. Biomed. Pharmacother. 2021, 134, 111139. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F.; et al. Characterization of phenolic extracts from Brava extra virgin olive oils and their cytotoxic effects on MCF-7 breast cancer cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Choupani, J.; Alivand, M.R.; Derakhshan, S.M.; Zaeifizadeh, M.; Khaniani, M.S. Oleuropein inhibits migration ability through suppression of epithelial-mesenchymal transition and synergistically enhances doxorubicin-mediated apoptosis in MCF-7 cells. J. Cell. Physiol. 2019, 234, 9093–9104. [Google Scholar] [CrossRef]
- Hamed, M.M.; Handoussa, H.; Hussein, N.H.; Eissa, R.A.; Abdel-Aal, L.K.; El Tayebi, H.M. Oleuropin controls miR-194/XIST/PD-L1 loop in triple negative breast cancer: New role of nutri-epigenetics in immune-oncology. Life Sci. 2021, 277, 119353. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Bianchini, F.; Andreucci, E.; Urciuoli, S.; Romani, A.; Tortora, K.; Caderni, G.; Nediani, C.; Calorini, L. Cancer Glycolytic Dependence as a New Target of Olive Leaf Extract. Cancers 2020, 12, 317. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (−)-Oleocanthal as a c-Met Inhibitor for the Control of Metastatic Breast and Prostate Cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Qusa, M.H.; Abdelwahed, K.S.; Siddique, A.B.; El Sayed, K.A. Comparative gene signature of (−)-oleocanthal formulation treatments in heterogeneous triple negative breast tumor models: Oncological therapeutic target insights. Nutrients 2021, 13, 1706. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; Sayed, K.A.E. Olive Phenolics as c-Met Inhibitors: (−)-Oleocanthal Attenuates Cell Proliferation, Invasiveness, and Tumor Growth in Breast Cancer Models. PLoS ONE 2014, 9, e97622. [Google Scholar]
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Meyer, S.A.; Hill, R.A.; El Sayed, K.A. (−)-Oleocanthal Prevents Breast Cancer Locoregional Recurrence After Primary Tumor Surgical Excision and Neoadjuvant Targeted Therapy in Orthotopic Nude Mouse Models. Cancers 2019, 11, 637. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (−)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Olive Oil-derived Oleocanthal as Potent Inhibitor of Mammalian Target of Rapamycin: Biological Evaluation and Molecular Modeling Studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Diez-Bello, R.; Jardin, I.; Lopez, J.J.; El Haouari, M.; Ortega-Vidal, J.; Altarejos, J.; Salido, G.M.; Salido, S.; Rosado, J.A. (−)-Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 474–485. [Google Scholar] [CrossRef]
- Mafuvadze, B.; Liang, Y.; Besch-Williford, C.; Zhang, X.; Hyder, S.M. Apigenin Induces Apoptosis and Blocks Growth of Medroxyprogesterone Acetate-Dependent BT-474 Xenograft Tumors. Horm. Cancer 2012, 3, 160–171. [Google Scholar] [CrossRef]
- Chen, D.; Landis-Piwowar, K.R.; Chen, M.S.; Dou, Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007, 9, R80. [Google Scholar] [CrossRef]
- Yin, F.; Giuliano, A.E.; Law, R.E.; Van Herle, A.J. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001, 21, 413–420. [Google Scholar]
- Lee, E.J.; Oh, S.Y.; Sung, M.K. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem. Toxicol. 2012, 50, 4136–4143. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.-Q.; Xie, K.-P.; Xie, M.-J. Inhibitory effect of luteolin on the proliferation of human breast cancer cell lines induced by epidermal growth factor. Sheng Li Xue Bao 2016, 68, 27–34. [Google Scholar]
- Wang, L.-M.; Xie, K.-P.; Huo, H.-N.; Shang, F.; Zou, W.; Xie, M.-J. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac. J. Cancer Prev. 2012, 13, 1431–1437. [Google Scholar] [CrossRef]
- Markaverich, B.M.; Shoulars, K.; Rodriguez, M.A. Luteolin Regulation of Estrogen Signaling and Cell Cycle Pathway Genes in MCF-7 Human Breast Cancer Cells. Int. J. Biomed. Sci. 2011, 7, 101–111. [Google Scholar]
- Baier, A.; Szyszka, R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, S.-H.; Shin, J.; Harikishore, A.; Lim, J.-K.; Jung, Y.; Lyu, H.-N.; Baek, N.-I.; Choi, K.Y.; Yoon, H.S.; et al. Luteolin Suppresses Cancer Cell Proliferation by Targeting Vaccinia-Related Kinase 1. PLoS ONE 2014, 9, e109655. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, C.-Y.; Lee, K.-R.; Lin, H.-J.; Chen, T.-H.; Wan, L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef]
- Park, S.-H.; Ham, S.; Kwon, T.H.; Kim, M.S.; Lee, D.H.; Kang, J.-W.; Oh, S.-R.; Yoon, D.-Y. Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 219–231. [Google Scholar] [CrossRef]
- Chew, B.P.; Brown, C.M.; Park, J.S.; Mixter, P.F. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res. 2003, 23, 3333–3339. [Google Scholar] [PubMed]
- Cao, X.; Liu, B.; Cao, W.; Zhang, W.; Zhang, F.; Zhao, H.; Meng, R.; Zhang, L.; Niu, R.; Hao, X.; et al. Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells. Chin. J. Cancer Res. 2013, 25, 212–222. [Google Scholar]
- Kim, M.J.; Woo, J.S.; Kwon, C.H.; Kim, J.H.; Kim, Y.K.; Kim, K.H. Luteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell lines. Cell Biol. Int. 2012, 36, 339–344. [Google Scholar] [CrossRef]
- Cook, M.; Liang, Y.; Besch-Williford, C.; Hyder, S. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer Targets Ther. 2016, 9, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902. [Google Scholar] [CrossRef]
- Reipas, K.M.; Law, J.H.; Couto, N.; Islam, S.; Li, Y.; Li, H.; Cherkasov, A.; Jung, K.; Cheema, A.S.; Jones, S.J.M.; et al. Luteolin is a novel p90 ribosomal S6 kinase (RSK) inhibitor that suppresses Notch4 signaling by blocking the activation of Y-box binding protein-1 (YB-1). Oncotarget 2013, 4, 329–345. [Google Scholar] [CrossRef]
- Sun, D.-W.; Zhang, H.-D.; Mao, L.; Mao, C.-F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.-X.; Jing, C.-W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell. Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef]
- Wu, H.-T.; Lin, J.; Liu, Y.-E.; Chen, H.-F.; Hsu, K.-W.; Lin, S.-H.; Peng, K.-Y.; Lin, K.-J.; Hsieh, C.-C.; Chen, D.-R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhu, G.-Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.-J.; Peng, S.-X.; Chen, L.-W.; Li, Y.-W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, J.-H.; Lee, D.-H.; Kang, J.-W.; Song, H.-H.; Oh, S.-R.; Yoon, D.-Y. Luteolin 8-C-β-fucopyranoside inhibits invasion and suppresses TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-κB signaling in MCF-7 breast cancer cells. Biochimie 2013, 95, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Fristiohady, A.; Nguyen, C.H.; Milovanovic, D.; Huttary, N.; Krieger, S.; Hong, J.; Geleff, S.; Birner, P.; Jäger, W.; et al. Apigenin and Luteolin Attenuate the Breaching of MDA-MB231 Breast Cancer Spheroids Through the Lymph Endothelial Barrier in Vitro. Front. Pharmacol. 2018, 9, 220. [Google Scholar] [CrossRef]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Goyette, S.; Mafuvadze, B.; Hyder, S.M. Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. Springerplus 2015, 4, 444. [Google Scholar] [CrossRef]
- Cook, M.T.; Mafuvadze, B.; Besch-Williford, C.; Ellersieck, M.R.; Goyette, S.; Hyder, S.M. Luteolin suppresses development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Oncol. Rep. 2016, 35, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Coombs, M.R.P.; Harrison, M.E.; Hoskin, D.W. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 2016, 380, 424–433. [Google Scholar] [CrossRef]
- Bauer, D.; Redmon, N.; Mazzio, E.; Soliman, K.F. Apigenin inhibits TNFα/IL-1α-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells. PLoS ONE 2017, 12, e0175558. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Morohashi, K.; Yilmaz, A.; Kuramochi, K.; Parihar, A.; Brahimaj, B.; Grotewold, E.; Doseff, A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA 2013, 110, E2153–E2162. [Google Scholar] [CrossRef]
- Du, G.-J.; Song, Z.-H.; Lin, H.-H.; Han, X.; Zhang, S.; Yang, Y. Luteolin as a glycolysis inhibitor offers superior efficacy and lesser toxicity of doxorubicin in breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 372, 497–502. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Ignacimuthu, S. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem. Biol. Interact. 2006, 164, 1–14. [Google Scholar] [CrossRef]
- Vrhovac Madunić, I.; Madunić, J.; Antunović, M.; Paradžik, M.; Garaj-Vrhovac, V.; Breljak, D.; Marijanović, I.; Gajski, G. Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 2018, 391, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-W.; Suh, Y.J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol. Rep. 2013, 29, 819–925. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Ahn, Y.E.; Chung, W.S.; Choi, H.J.; Suh, Y.J. Synergistic effect between celecoxib and luteolin is dependent on estrogen receptor in human breast cancer cells. Tumour Biol. 2015, 36, 6349–6359. [Google Scholar] [CrossRef]
- Tu, S.-H.; Ho, C.-T.; Liu, M.-F.; Huang, C.-S.; Chang, H.-W.; Chang, C.-H.; Wu, C.-H.; Ho, Y.-S. Luteolin sensitises drug-resistant human breast cancer cells to tamoxifen via the inhibition of cyclin E2 expression. Food Chem. 2013, 141, 1553–1561. [Google Scholar] [CrossRef]
- Liu, R.; Ji, P.; Liu, B.; Qiao, H.; Wang, X.; Zhou, L.; Deng, T.; Ba, Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol. Lett. 2017, 13, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Dai, Q.; Si, H.; Zhang, L.; Eltom, S.E.; Si, H. Combined Luteolin and Indole-3-Carbinol Synergistically Constrains ERα-Positive Breast Cancer by Dual Inhibiting Estrogen Receptor Alpha and Cyclin-Dependent Kinase 4/6 Pathway in Cultured Cells and Xenograft Mice. Cancers 2021, 13, 2116. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Wang, C.-J.; Chen, N.-F.; Ho, W.-H.; Lu, F.-J.; Tseng, T.-H. Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem. Biol. Interact. 2014, 213, 60–68. [Google Scholar] [CrossRef]
- Sato, Y.; Sasaki, N.; Saito, M.; Endo, N.; Kugawa, F.; Ueno, A. Luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 human breast cancer cells. Biol. Pharm. Bull. 2015, 38, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Extra-virgin olive oil polyphenols inhibit HER2 (erbB-2)-induced malignant transformation in human breast epithelial cells: Relationship between the chemical structures of extra-virgin olive oil secoiridoids and lignans and their inhibitory activities on. Int. J. Oncol. 2009, 34, 43–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chin, Y.W.; Jones, W.P.; Rachman, I.; Riswan, S.; Kardono, L.B.S.; Chai, H.B.; Farnsworth, N.R.; Cordell, G.A.; Swanson, S.M.; Cassady, J.M.; et al. Cytotoxic lignans from the stems of Helicteres hirsuta collected in Indonesia. Phyther. Res. 2006, 20, 62–65. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Mazza, T.; Morozzi, G.; Fabiani, R. Pinoresinol inhibits proliferation and induces differentiation on human HL60 leukemia cells. Nutr. Cancer 2013, 65, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado-Rodríguez, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomed. 2019, 9, 574–586. [Google Scholar]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.P.; Jernström, H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, Antiproliferative, and Pro-apoptotic Capacities of Pentacyclic Triterpenes Found in the Skin of Olives on MCF-7 Human Breast Cancer Cells and Their Effects on DNA Damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. The differential localization of a methyl group confers a different anti-breast cancer activity to two triterpenes present in olives. Food Funct. 2015, 6, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. Oleanolic Acid, a Compound Present in Grapes and Olives, Protects against Genotoxicity in Human Mammary Epithelial Cells. Molecules 2015, 20, 13670–13688. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Barone, I.; Gelsomino, L.; Giordano, C.; Bonofiglio, D.; Statti, G.; Menichini, F.; Catalano, S.; Andò, S. Oldenlandia diffusa extracts exert antiproliferative and apoptotic effects on human breast cancer cells through ERα/Sp1-mediated p53 activation. J. Cell. Physiol. 2012, 227, 3363–3372. [Google Scholar] [CrossRef]
- Chu, R.; Zhao, X.; Griffin, C.; Staub, R.E.; Shoemaker, M.; Climent, J.; Leitman, D.; Cohen, I.; Shtivelman, E.; Fong, S. Selective concomitant inhibition of mTORC1 and mTORC2 activity in estrogen receptor negative breast cancer cells by BN107 and oleanolic acid. Int. J. Cancer 2009, 127, 1209–1219. [Google Scholar] [CrossRef]
- Liang, Z.; Pan, R.; Meng, X.; Su, J.; Guo, Y.; Wei, G.; Zhang, Z.; He, K. Transcriptome study of oleanolic acid in the inhibition of breast tumor growth based on high-throughput sequencing. Aging 2021, 13, 22883–22897. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef]
- Newmark, H.L. Squalene, Olive Oil, and Cancer Risk: Review and Hypothesis. Ann. N. Y. Acad. Sci. 1999, 889, 193–203. [Google Scholar] [CrossRef]
- Warleta, F.; Campos, M.; Allouche, Y.; Sánchez-Quesada, C.; Ruiz-Mora, J.; Beltrán, G.; Gaforio, J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010, 48, 1092–1100. [Google Scholar] [CrossRef]
- Feltrin, S.; Ravera, F.; Traversone, N.; Ferrando, L.; Bedognetti, D.; Ballestrero, A.; Zoppoli, G. Sterol synthesis pathway inhibition as a target for cancer treatment. Cancer Lett. 2020, 493, 19–30. [Google Scholar] [CrossRef]
- Mura, S.; Fattal, E.; Nicolas, J. From poly(alkyl cyanoacrylate) to squalene as core material for the design of nanomedicines. J. Drug Target. 2019, 27, 470–501. [Google Scholar] [CrossRef] [PubMed]
| Composition of Olive Oil |
|---|
| Saponifiable Fraction (>98%) |
| Triacylglycerols and derivatives |
| 16:0 Palmitic acid |
| 16:1n-7 Palmitoleic acid |
| 18:0 Stearic acid |
| 18:1n-9 Oleic acid |
| 18:2n-6 Linoleic acid |
| 18:3n-3 Linolenic acid |
| Unsaponifiable Fraction (<2%) |
| Non-glyceride esters and waxes |
| Aliphatic alcohols |
| Volatile compounds: aldehydes, ketones, alcohols, acids, esters, etc. |
| Triterpenes: erythrodiol, uvaol, oleanolic acid and maslinic acid |
| Sterols: β-sitosterol, campesterol, stigmasterol and avenasterol |
| Hydrocarbons |
| Squalene |
| n-alkanes and n-alkenes |
| Carotenoids: β-carotene and lycopene |
| Pigments: chlorophylls and pheophytins |
| Lipophilic phenolics: tocopherols and tocotrienols |
| Hydrophilic phenolics |
| Phenolic acids: gallic, vanillic, cinnamic, caffeic, coumanic and elenolic acids |
| Phenolic alcohols: hydroxytyrosol, tyrosol and their glucosides |
| Secoiridoids: oleuropein and ligstroside derivates (oleocanthal and oleacein) |
| Lignans: pinoresinol and acetoxypinoresinol |
| Flavonoids: luteolin and apigenin |
| Component | Model | Carcinogenesis | Molecular/Cellular Mechanisms | Ref. |
|---|---|---|---|---|
| Triterpenes | ||||
| Uvaol | MCF-7 | Anti-proliferative | ↓ ROS, ↓ H2O2-induced DNA damage | [209] |
| MCF-10A, MDA-MB-231 | Decrease in proliferation and survival | ↓ ROS, ↓ basal DNA damage (at low doses), ↑ H2O2-induced DNA damage | [210] | |
| Erythrodiol | MCF-7 | Anti-proliferative, pro-apoptotic | ↑ ROS | [209] |
| MCF-10A, MDA-MB-231 | Decrease in proliferation and survival | ↓ ROS, ↑ DNA damage; cycle arrest and apoptosis in MCF-10A | [210] | |
| Maslinic acid | MCF-7 | ↓ ROS, ↓ H2O2-induced DNA damage | [209] | |
| MCF-10A, MCF-7, MDA-MB-23 | Decrease in proliferation and survival | ↓ basal ROS in MCF-10A; ↑ basal ROS in MCF-7 ↑ H2O2-induced DNA damage | [211] | |
| Oleanolic acid | MCF-7 | Anti-proliferative, pro-apoptotic | ↓ ROS, ↓ H2O2-induced DNA damage | [209] |
| MCF-10A, MCF-7, MDA-MB-23 | Decrease in proliferation and survival | ↑ ROS and H2O2-induced DNA damage in MDA-MB-231 ↓ ROS and H2O2-induced DNA damage in MCF-10A | [211] | |
| MCF-7, T47D, SKBR3 | Growth inhibition, pro-apoptotic | ERα/Sp1-mediated activation of the p53 gene | [212] | |
| MCF-7, MDA-MB-231 | ↓ mTOR-Complex 1 and -Complex2 activity (↓mTOR/FRAP1, RICTOR, RAPTOR, AKT, 4E-BP, p70S6k) | [213] | ||
| MCF-7 | Anti-proliferative, pro-apoptotic | Transcriptomic changes; modulation of p53-, TNF- and mTOR-signaling pathways genes ↓THBS1, EDN1, CACNG4, CCN2, AXIN2, BMP4 ↑ATF4, SERPINE1, SESN2, PPARGC1A, EGR1 and JAG1 | [214] | |
| Phenolic acids | ||||
| Caffeic acid | MCF-7 | Decreased viability | ↓ p53, ↑ Mcl-1, ↓ p21 (short treatment), ↑ p21 (long treatment) | [206] |
| MCF-7, MDA-MB-231 | Anti-proliferative, cycle arrest, pro-apoptotic | ↓ IGFIR, ↓ AKT activation; ↓ ER, ↓ Cyclin D1 in MCF-7 cells | [207] | |
| MCF-7, MDA-MB-231 | ↓ RAR-β methylation | [208] | ||
| Elenolic acid | SKBR3, MCF-7/HER2 | Anti-proliferative | ↓ HER2 | [121] |
| Gallic acid | MCF-7 | Decreased viability | ↑ p53, ↑ Mcl-1, ↓ p21 (short treatment), ↑ p21 (long treatment) | [206] |
| Phenolic alcohols | ||||
| Tyrosol | SKBR3, MCF-7/HER2 | Anti-proliferative | ↓ HER2 | [121] |
| Hydroxyty-rosol | In vivo (DMBA) | Growth inhibition, anti- proliferative | Transcriptomic changes in tumors; modulation of apoptosis, cell cycle, proliferation, differentiation, survival and transformation pathways genes; ↑ sfrp4 | [115] |
| Plasma: ↑ antioxidant capacity, ↓ DNA and protein damage | [116] | |||
| MCF-7 | Decreased cell viability, anti-proliferative, blocked G(1)-to-S transition, pro-apoptotic | ↓ Pin1, ↓ Cyclin D1 | [117,118] | |
| MCF-7 | Anti-proliferative | ↓ ERK1/2 | [119] | |
| SKBR3, MCF-7/HER2 | Anti-proliferative, pro-apoptotic | ↓ FAS, ↓ HER2 | [120,121] | |
| cocultures MCF-7- fibroblast | Inhibition of fibroblast-stimulated MCF-7 proliferation | ↓ CCL5 expression in aging fibroblasts | [122] | |
| SKBR3 | Pro-apoptotic | ↑ GPER, ↑ ERK1/2, ↑ Bax, ↓ Bcl-2, ↑ Casp-9, ↑ Casp-3, ↓ PARP-1, ↑ p21, ↑ p53, ↓ Cyclin D1 | [123] | |
| MDA and MCF-7 | Anti-proliferative, pro-apoptotic | Extracellular production of hydrogen peroxide | [125] | |
| MCF-10A, MDA-MB-231, MCF-7 | Prevents oxidative DNA damage ↓ intracellular ROS level in MCF-10A | [128] | ||
| MCF-10A, MDA-MB-231 | Pro-oxidant under specific growth conditions | [126] | ||
| MCF-7 | Antioxidant; ↑ Nrf2, ↑ GSTA2, ↑ HO-1 | [129] | ||
| MCF-7 under hypoxic conditions | ↓ PI3K/AKT/mTOR pathway, ↓ HIF-1α, ↓ PARP-1 At high doses ↑ VEGF, ↑ AM, ↑ Glut1 | [130] | ||
| MDA-MB-231, BT549, Hs578T | Inhibition of EMT, migration and metastatic potential | ↓ SMAD2/3-dependent TGFβ signaling, ↓ p-LRP6, ↓ LRP6, ↓ β-catenin, ↓ cyclin D1 ↓ SLUG, ↓ ZEB1, ↓ SNAIL, ↓ Vimentin; ↑ ZO-1 | [131] | |
| MCF-7 and T47D | Inhibition of migration and invasion | Induction of autophagy | [133] | |
| MDA-MB-231 | Inhibition of migration and invasion | Induction of autophagy; ↑ LC3-II/LC3-I, ↑ Beclin-1, ↓ p63 | [132] | |
| Secoiridoids | ||||
| Ligstroside | SKBR3, MCF-7/HER2 | Anti-proliferative, pro-apoptotic | ↓ FAS, ↓ HER2 | [120,121] |
| Oleuropein | In vivo (cancer- stem-cell-enriched orthotopic model) | Treatment with decarboxymethyl oleuropein reduced carcinogenesis | ↓ DNMT, ↓ mTOR | [138] |
| MCF-7 | Decreased cell viability, inhibited cell proliferation, blocked G(1)-to-S transition, pro-apoptotic | [117] | ||
| MDA-MB-468, MDA-MB-231 | Growth inhibition, S-phase cell-cycle arrest-mediated apoptosis | Transcriptomic changes in apoptosis-involved genes (Casp1, Casp14, FADD, TNFRSF21, GADD45A, CYCS and BNIP2) | [135] | |
| MCF-7, MDA-MB-231 | Pro-apoptotic | Increased the expression of pro-apoptotic genes and tumor-suppressor miRNAs; decreased the expression of anti-apoptotic genes and oncomiR | [136] | |
| MCF-7 | Anti-proliferative, pro-apoptotic, inhibition of migration | ↓ mir-21, ↓ mir-155 | [137] | |
| MCF-7 | Reduced viability and invasiveness, pro-apoptotic | ↓ HDAC2, ↓ HDAC3, ↓ HDAC4 | [139,140] | |
| MCF-7 | Anti-proliferative | ↓ ERK1/2 | [119] | |
| MCF-7 | Reduced viability, cell- cycle arrest | ↓ PTP1B | [141] | |
| MCF-7 | Pro-apoptotic | ↑ p53, ↑ Bax, ↓ Bcl-2 | [142] | |
| MDA-MB-231 | Anti-proliferative, pro-apoptotic, cell-cycle arrest | ↑ Bax, ↑ Casp3, ↓ Bcl2, ↓ Survivin; ↓ NF-kB, ↓ CycD1, ↑ p21 | [143] | |
| MDA-MB-231 | Pro-apoptotic | ↑ ROS, ↓ NF-kB | [144] | |
| MDA-MB-231 | Cell growth inhibition | ↓ PAI-1, ↑ Casp8 | [145] | |
| SKBR3, MCF-7/HER2 | ↓ FAS | [120] | ||
| SKBR3, MCF-7/HER2, MCF-10A/HER2 | Anti-proliferative, pro-apoptotic | ↓ HER2 | [121,146] | |
| SKBR3 | Pro-apoptotic | ↑ GPER, ↑ Bax, ↓ Bcl-2; ↑ Casp-9, ↑ Casp-3, ↓ PARP-1; ↑ p21, ↑ p53, ↓ Cyclin D1 | [123] | |
| MCF-10A, MDA-MB-231 | Pro-oxidant under specific growth conditions | [126] | ||
| MDA-MB-231 | Anti-proliferative, pro-apoptotic | Pro-oxidant activity, ↓ SOD2 ↓ catalase, ↑ intracellular and mitochondrial ROS ↓ CycB2, ↓ CycD1, ↑ Casp9, ↓ PARP-1 | [148] | |
| MCF-7 and T47D | Inhibition of migration and invasion | Induction of autophagy | [133] | |
| MDA-MB-231 | Inhibition of migration and invasion | Induction of autophagy; ↑ LC3-II/LC3-I, ↑ Beclin-1, ↓ p62 | [132] | |
| MCF-7 | Inhibition of migration | ↓ Sirt1, ↑ ECad, ↓ ZEB1, ↓ MMP-2, ↓ MMP-9, ↑ p53 | [150] | |
| MDA-MB-231 | Decreased viability and migration | ↓ miR-194-5p, ↓ PD-L1, ↑ XIST | [151] | |
| MDA-MB-231 | ↓ glycolysis rate | [152] | ||
| Oleocanthal | In vivo (MMTV-PyVT; patient-derived xenograft) | Suppressed initiation and incidence | Transcriptomic changes, ↓ Myc | [155] |
| In vivo (MDA- MB-231 xenograft) | Inhibition of tumor proliferation and growth | ↓ c-Met, ↓ Ki-67, ↓ CD31 | [156] | |
| In vivo (BT-474 and MDA-MB-231 xenografts) | Prevention of locoregional recurrence, tumor growth inhibition | ↓ c-Met, ↓ HER2; ↑ ECad, ↓ Vimentin; ↓ CA 15-3 in serum | [157] | |
| In vivo (BT-474 xenograft) | Tumor growth inhibition | ↓ ERα | [158] | |
| MCF-7, BT-474, MDA-MB-231 | Inhibition of proliferation and survival | ↓ Met, ↓ AKT, ↓ ERK; ↓ CycD1, ↓ Cdk6, ↑ p21, ↑ p27; ↓ Brk/Paxillin/Rac1; ↑ ECad, ↑ ZO-1, ↓ Vimentin, ↓ β-catenin; ↑ Casp8, ↑ Casp3, ↓ RIP, ↓ PARP-1 | [156] | |
| MCF-7, MDA-MB-231 | Anti-proliferative, inhibition of migration and invasion | ↓ c-Met activation. ↓ microvessel density marker (CD31) | [154] | |
| MCF-7, T47D, MDA-MB-231 | Anti-proliferative | ↓ mTOR and inducing apoptosis in MDA-MB-231 cells | [159] | |
| MCF-7, BT-474, T47D | Inhibition of estrogen- induced proliferation | ↓ ERα in BT-474 | [158] | |
| MCF-7, MDA-MB-231 | Anti-proliferative, inhibition of migration | Modulation of Ca2+ entry through TRPC6 | [160] | |
| Lignans | ||||
| Pinoresinol | SKBR3, MCF-7/HER2 | ↓ FAS | [120] | |
| SKBR3, MCF-7/HER2, MCF-10A/HER2 | Anti-proliferative, pro-apoptotic | ↓ HER2 | [121,202] | |
| MCF-7 and TD47D | Cytotoxicity | [203] | ||
| MDA-MB-231 | Anti-proliferation | ↑ p21 | [204] | |
| MDA-MB-231, MCF-7, MCF-10A | Cytotoxic, anti-proliferative and pro-oxidant | ↓ ROS, ↓ DNA damage in MCF-10A cells; ↑ ROS in cancer cells after H2O2 treatment | [205] | |
| Acetoxypinoresinol | SKBR3, MCF-7/HER2 | ↓ FAS | [120] | |
| SKBR3, MCF-7/HER2, MCF-10A/HER2 | Anti-proliferative, pro-apoptotic | ↓ HER2 | [121,202] | |
| Flavonoids | ||||
| Apigenin | In vivo (BT-474 xenograft model) | Tumor growth inhibition, anti-proliferative, pro- apoptotic | ↓ Ki-67, ↓ HER2, ↓ VEGF, ↑ RANKL | [161] |
| In vivo (MDA- MB-231 xenograft) | Tumor growth inhibition, pro-apoptotic | ↑ ubiquitinated proteins, ↑ Bax, ↑ IκBα | [162] | |
| Hs578T, MDA- MB-231, MCF-7 | Anti-proliferative, cell- cycle arrest, pro-apoptotic | ↓ PI3K, ↓ PKB, ↑ FOXO3a, ↑ p21, ↑ p27; ↑ p53; ↑ PARP-1; ↑ Cyt C | [170] | |
| SKBR3, MCF-7/HER2 | ↓ FAS | [120] | ||
| MDA-MB-231 | Anti-proliferative, pro-apoptotic | ↑ Casp-3, ↑ proteosome activity, ↑ ubiquitinated proteins, ↑ Bax, ↑ IκBα, ↑ PARP-1 | [162] | |
| MCF-7, MDA-MB-468 | Growth inhibition, cycle arrest | ↓ cyclin B1, ↓ cyclin D1, ↓ cyclin A, ↓ CDK1, ↓ CDK4, ↓ Rb (in MCF-7), ↓ ERK (in MDA-MB-468) | [163] | |
| T47D, MDA-MB-231 | Anti-proliferative, pro-apoptotic | ↑ Casp3, ↓ PARP-1, ↑ Bax ↓ Bcl-2; ↑ LC3-II | [156] | |
| MDA-MB-231 spheroids—lymph endothelial cells | ↓ MMP-1, ↓ CYP1A1 in MDA-MB-231 cells ↓ FAK in lymph endothelial cells | [185] | ||
| MDA-MB-468, SKBR3, mouse 4T1 cells | ↓ PD-L1, ↓ STAT1 activation, ↑ T-cell proliferation in co-culture | [188] | ||
| MDA-MB-231 | Decreased viability | ↓ CCL2, ↓ GMCSF, ↓ IL-1α, ↓ IL-6, ↓ IKBK-e | [189] | |
| human breast tumor phage display cDNA librar; MDA-MB-231 | 160 direct targets, hnRNPA2 top candidate ↓ hnRNPA2 activation, modulation of splicing in MDA-MB-231 cells | [190] | ||
| MCF-7, MDA MB-231 | Decreased viability, pro-apoptotic | ↑ lipid peroxidation, ↑ DNA damage | [193] | |
| Luteolin | In vivo (MDA-MB-231 xenograft) | Reduced tumor burden Reduced tumor growth | ↓ Ki-67 | [164,180] |
| In vivo (mouse mammary tumor cells) | Tumor growth inhibition, pro-apoptotic, angiogene- sis inhibition | ↑ p53, ↑ Bax, ↓ Bcl-2 | [174] | |
| In vivo (DMBA- induced) | Tumor growth inhibition | Antioxidant, ↑ SOD, ↑ CAT, ↑ GPx | [192] | |
| In vivo (DMBA- induced) | Tumor growth inhibition, anti-angiogenic | ↓ VEGF, ↓ CD31 | [187] | |
| In vivo (T47D xenograft) | Tumor growth inhibition, anti-angiogenic | ↓ VEGF, ↓ CD31 | [186] | |
| In vivo (MDA-MB-435, MDA-MB-231(4175)LM2 xenograft) | Inhibition of lung metastases | [177] | ||
| In vivo (MDA-MB-231 xenograft) | Inhibition of lung metastases | ↓ Slug, ↓ Vimentin | [178] | |
| In vivo (4T1 implantation) | Tumor growth inhibition | ↓ YAP, ↓ TAZ | [183] | |
| In vivo (MCF-7, 4T1 implantation) | ↑ SOD, CAT in serum ↓ SOD, ↓ CAT in tumor | [191] | ||
| MDA-MB-231 | Cell growth inhibition, cell-cycle arrest, pro-apoptotic | ↑ p21, ↓ PLK1, ↓CycB1, ↓ CycA, ↓ CDK1, ↓ CDK2; ↑ Bax; ↓ EGFR, ↓ AKT, ↓ ERK1/2, ↓ p38 | [164] | |
| MCF-7 | Anti-proliferative, cell- cycle arrest, pro-apoptotic | ↓ EGFR, ↓ AKT, ↓ ERK1/2, ↓ Stat3 | [165] | |
| ↓ IGFR1, ↓ AKT, ↓ ERα | [166] | |||
| MCF-7 | Anti-proliferative | Regulation of gene expression (estrogen receptor pathway and cell cycle genes) | [167] | |
| Hs578T, MDA- MB-231, MCF-7 | Anti-proliferative, cell- cycle arrest, pro-apoptotic | ↓ PI3K, ↓ PKB, ↑ FOXO3a, ↑ p21, ↑ p27; ↑ p53; ↑ PARP-1; ↑ Cyt C | [170] | |
| MDA-MB-231 | Anti-proliferative, pro-apoptotic | ↓ FAS | [171] | |
| SKBR3, MCF-7/HER2 | ↓ FAS | [120] | ||
| MDA-MB-231 | Anti-proliferative, cell- cycle arrest, pro-apoptotic | ↓ NF-kB, ↓ Myc, ↓TERT | [172] | |
| MCF-7 | Anti-proliferative, cell- cycle arrest, pro-apoptotic | ↑ DR5, ↑ Casp8, ↑ Bax, ↓ Bcl-2, ↑ Casp9, ↑ Casp3 | [173] | |
| MCF-7, MDA- MB-231, SKBR3 | Decreased viability, pro-apoptotic | ↑ ERK/p38 activation, AIF translocation | [176] | |
| MDA-MB-435, MDA-MB-231(4175)LM2 | Anti-proliferative, pro-apoptotic, reduced migration | ↓ VEGF secretion | [177] | |
| MDA-MB-231, BT5-4 | Inhibition of migration and invasion | ↓ β-catenin, ↓ N-cadherin, ↓Vimentin, ↑ E-cadherin, | [178] | |
| ↑ Claudin | ||||
| SUM-149 | Reduced enrichment in stem cells and growth, pro-apoptotic | ↓ RSK, ↓ YB-1, ↓ Notch4 | [179] | |
| MCF-7, MDA-MB-231 | Anti-proliferative, cycle arrest, decreased migration | ↓ Notch1, ↓ Hes1, ↓ Hey1, ↓ Hey2, ↓ VEGF, ↓ MMP-2, ↓ MMP-9 ↑ miR-34a, ↑ miR-181a, ↑ miR-139-5p, ↑ miR-224, ↑ miR-246, ↓ miR-155 | [180] | |
| BT-20 | Anti-proliferative, reduced migration and invasion | ↓ AKT, ↓ mTOR, ↓ MMP-9, ↓ H3K27ac, ↓ H3K56ac | [181] | |
| MDA-MB-231 | Reduced viability, cycle arrest, pro-apoptotic | ↓ CXCR4, ↓ MMP-2, ↓ MMP-9 | [182] | |
| MDA-MB-231, 4T1 | Reduced viability, inhibition of colony formation | ↓ YAP, ↓ TAZ, ↓ N-Cad, ↓ Vimentin, ↓ FN1, ↑ E-Cad | [183] | |
| MDA-MB-231 spheroids—lymph endothelial cells | ↓ MMP-1, ↓ CYP1A1 in MDA-MB-231 cells ↓ FAK, ↓ Ca2+ release in lymph endothelial cells | [185] | ||
| T47D, BT-474 | Reduced viability, pro-apoptotic | ↓ VEGF | [186] | |
| Reduced mammosphere formation in T47D cells | ||||
| MDA-MB-468 | ↓ PD-L1 | [188] | ||
| MCF-7, 4T1 | ↓ glycolytic flux (under hypoxia) | [191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. https://doi.org/10.3390/molecules27020477
Moral R, Escrich E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules. 2022; 27(2):477. https://doi.org/10.3390/molecules27020477
Chicago/Turabian StyleMoral, Raquel, and Eduard Escrich. 2022. "Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms" Molecules 27, no. 2: 477. https://doi.org/10.3390/molecules27020477
APA StyleMoral, R., & Escrich, E. (2022). Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules, 27(2), 477. https://doi.org/10.3390/molecules27020477






