27 Years of Catalytic Carbonylative Coupling Reactions in Hungary (1994–2021)
Abstract
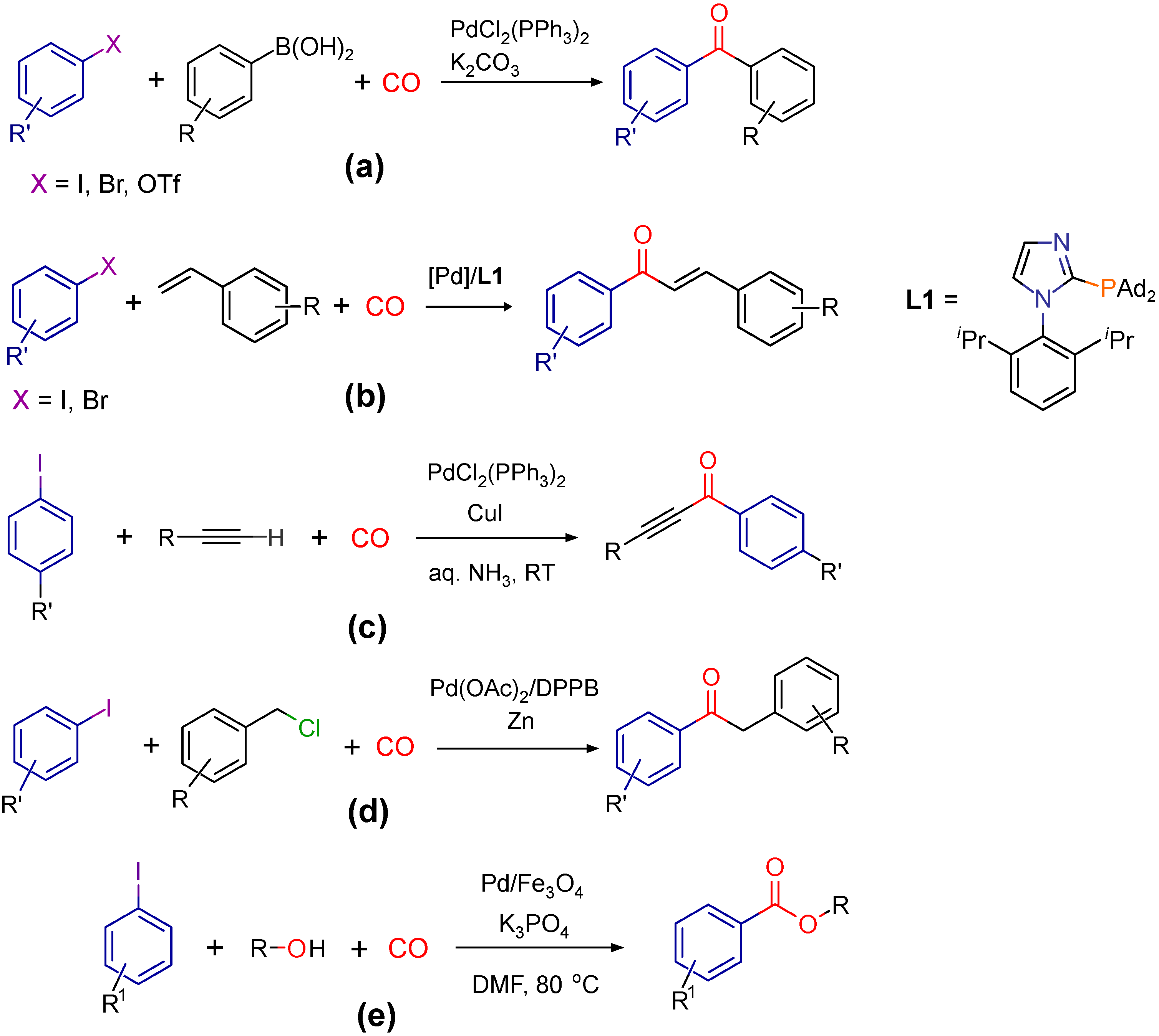
1. Introduction
2. Formation of the Active Catalyst
3. Reactions in Conventional Solvents
3.1. Preparation of Carbonyl Compounds with Steroid Scaffolds
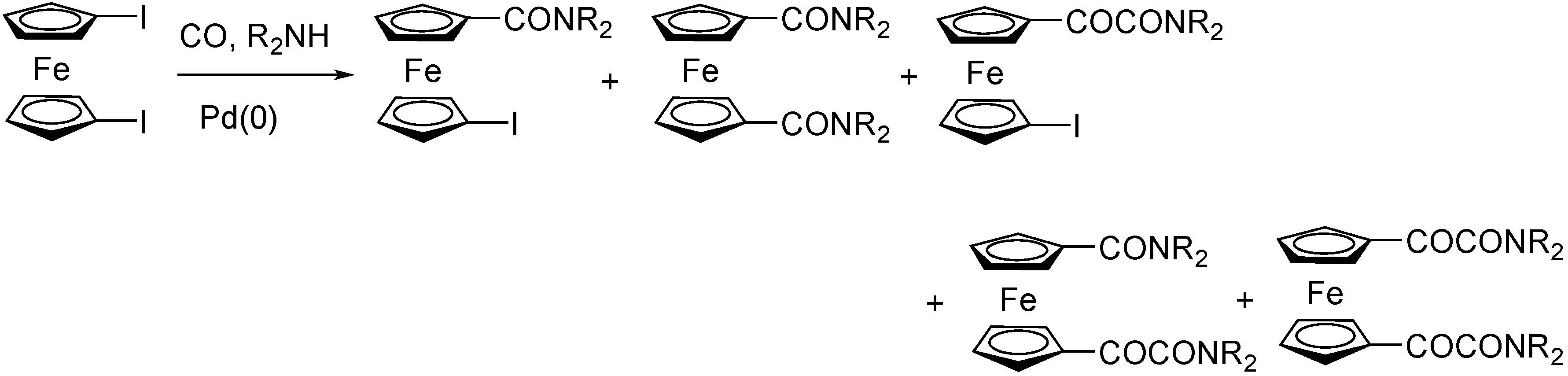
3.2. Ferrocene-Based Substrates
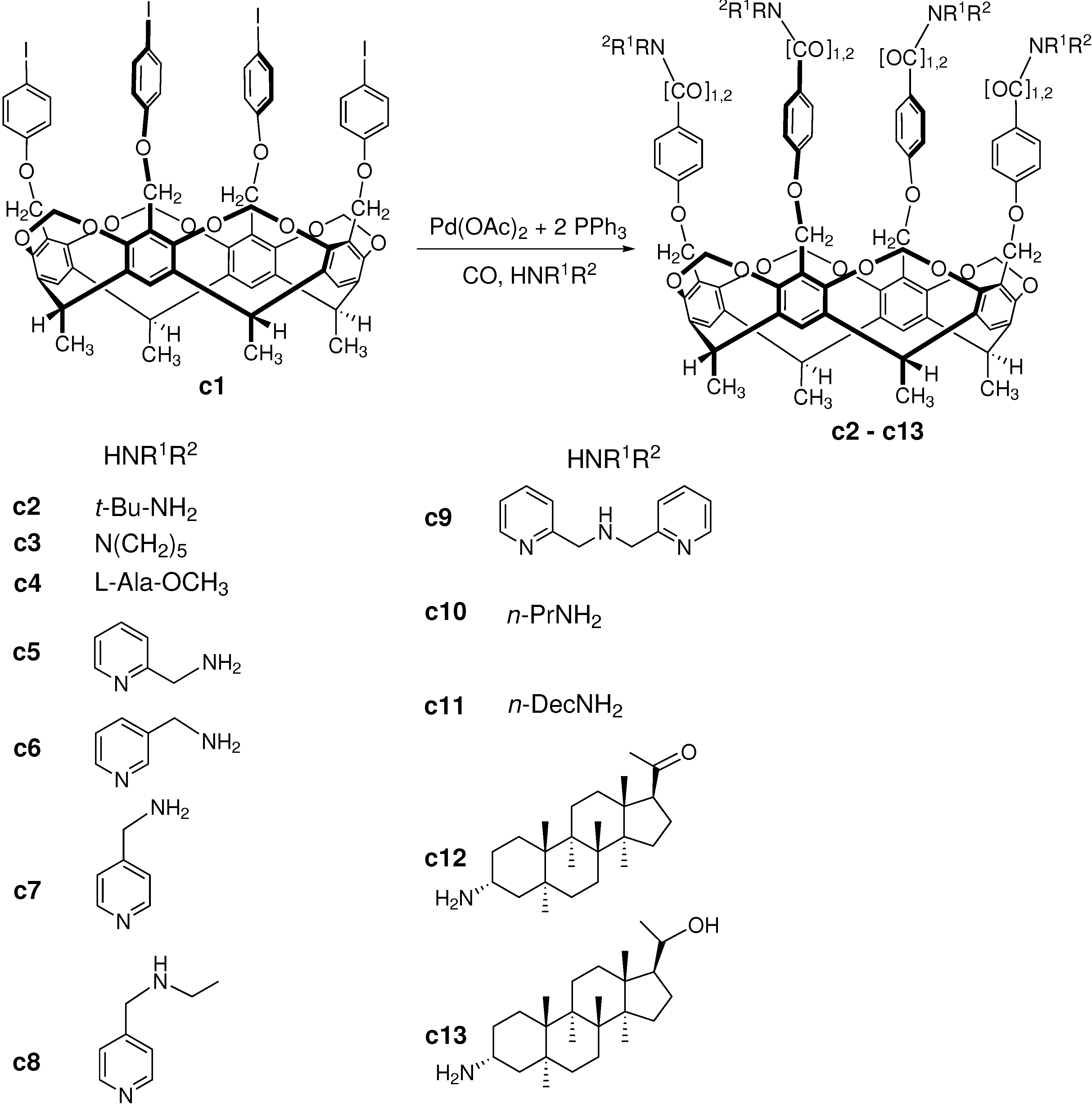
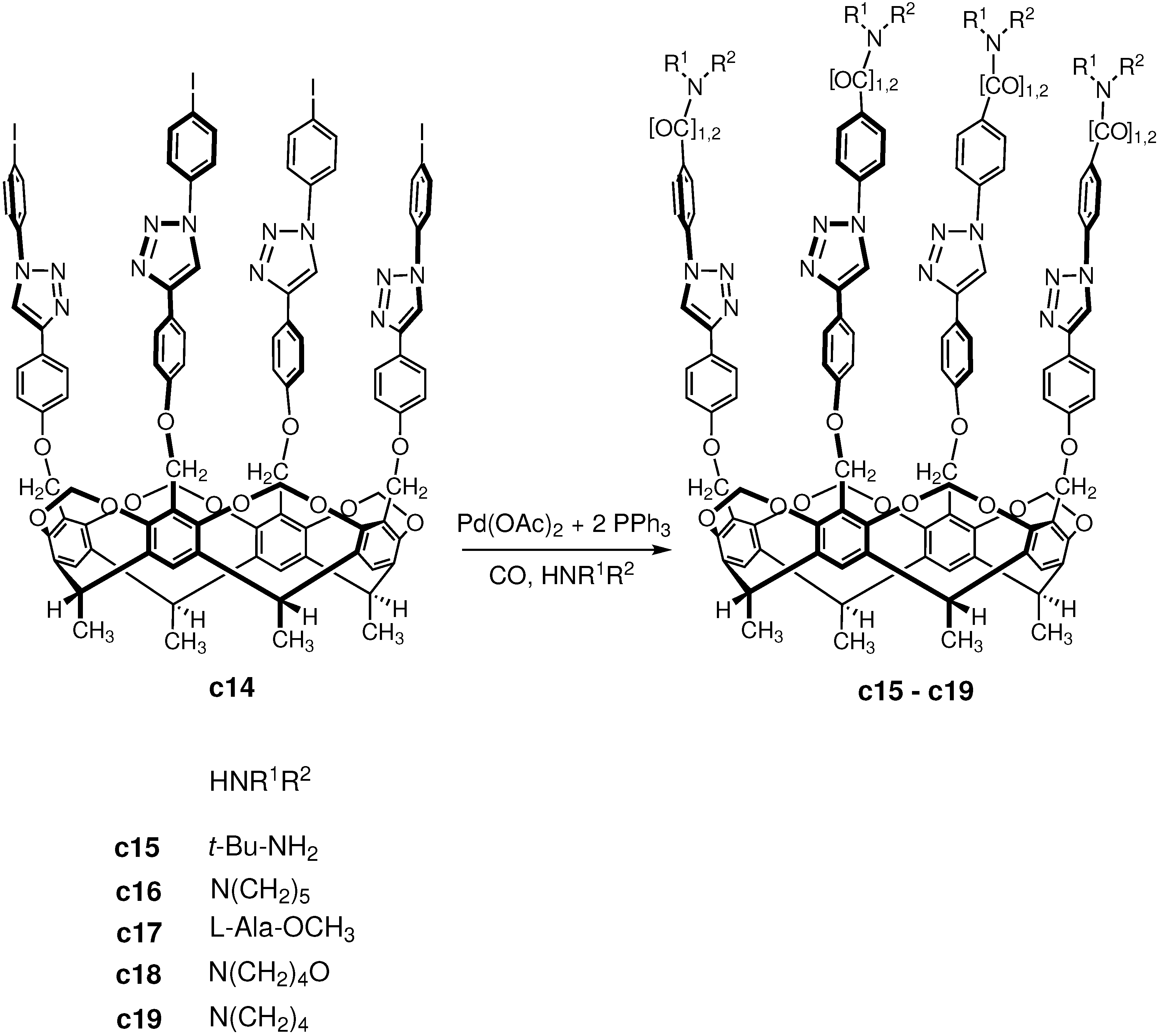
3.3. Carbonylation on Macromolecular Cavitand Scaffolds
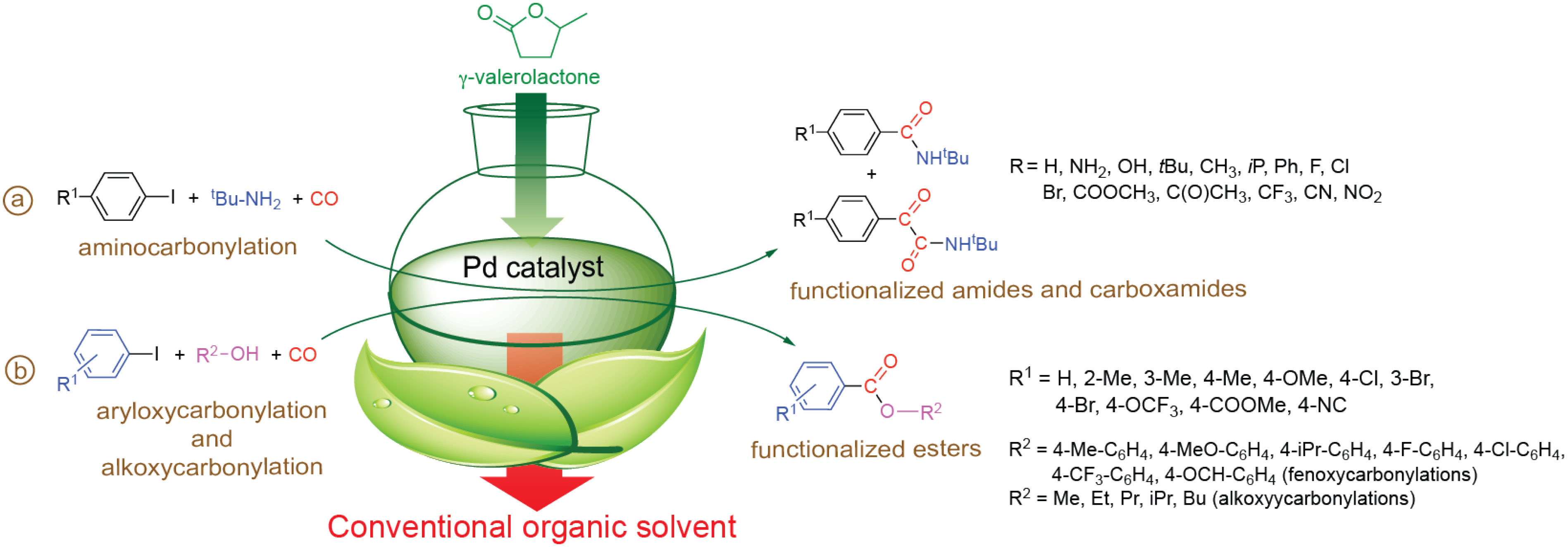
4. Reactions in Biomass-Based Solvents
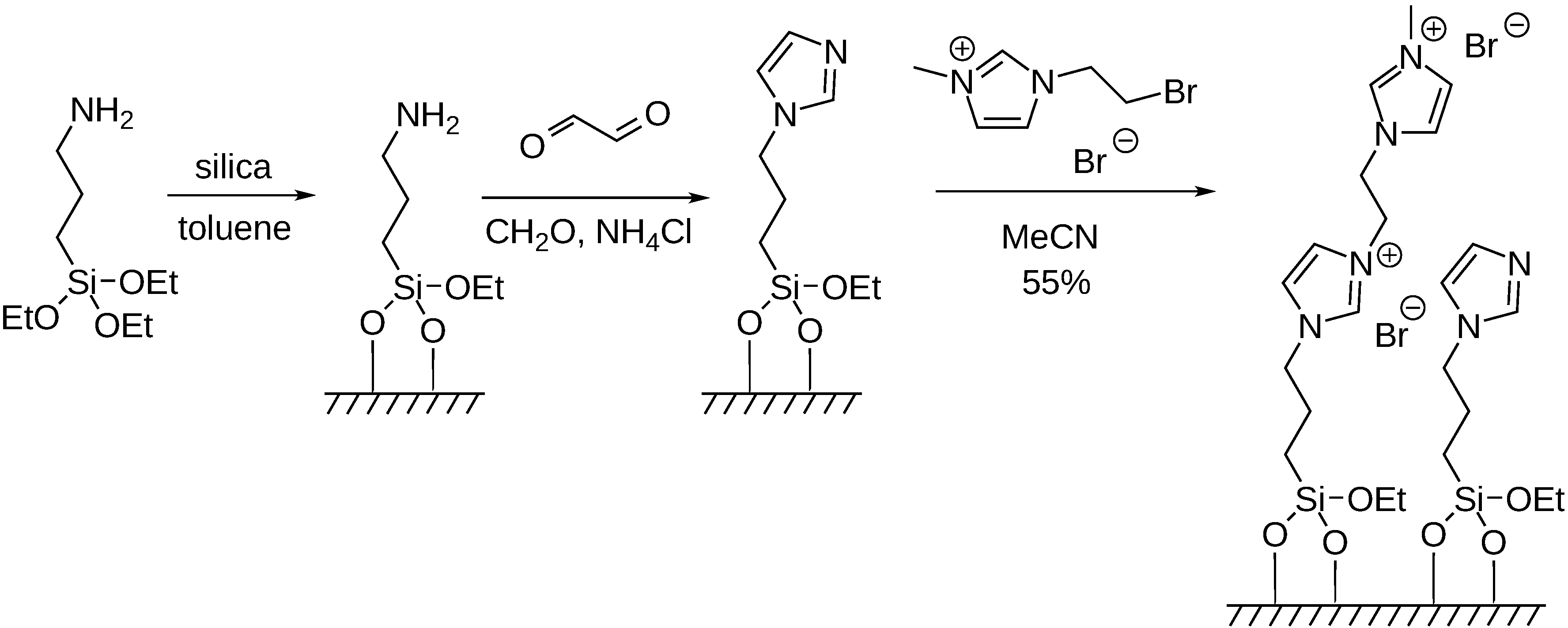
5. Carbonylation Reactions in Ionic Liquid and on Supported Ionic Liquid Phase (SILP)
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Gabriele, B. (Ed.) Carbon Monoxide in Organic Synthesis–Carbonylation Chemistry; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Skoda-Foldes, R.; Kollár, L. Synthetic applications of palladium catalysed carbonylation of organic halides. Curr. Org. Chem. 2002, 6, 1097–1119. [Google Scholar] [CrossRef]
- Kollár, L. (Ed.) Modern Carbonylation Methods; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Ishiyama, T.; Kizaki, H.; Hayashi, T.; Suzuki, A.; Miyaura, N. Palladium-catalyzed carbonylative cross-coupling reaction of arylboronic acids with aryl electrophiles: Synthesis of biaryl ketones. J. Org. Chem. 1998, 63, 4726–4731. [Google Scholar] [CrossRef]
- Wu, X.F.; Neumann, H.; Spannenberg, A.; Schulz, T.; Jiao, H.; Beller, M. Development of a general palladium-catalyzed carbonylative Heck reaction of aryl halides. J. Am. Chem. Soc 2010, 132, 14596–14602. [Google Scholar] [CrossRef]
- Mohamed Ahmed, M.S.; Mori, A. Carbonylative sonogashira coupling of terminal alkynes with aqueous ammonia. Org. Lett. 2003, 5, 3057–3060. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Schranck, J.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylative Negishi-type Coupling of Aryl Iodides with Benzyl Chlorides. Chem. Asian J. 2012, 7, 40–44. [Google Scholar] [CrossRef] [PubMed]
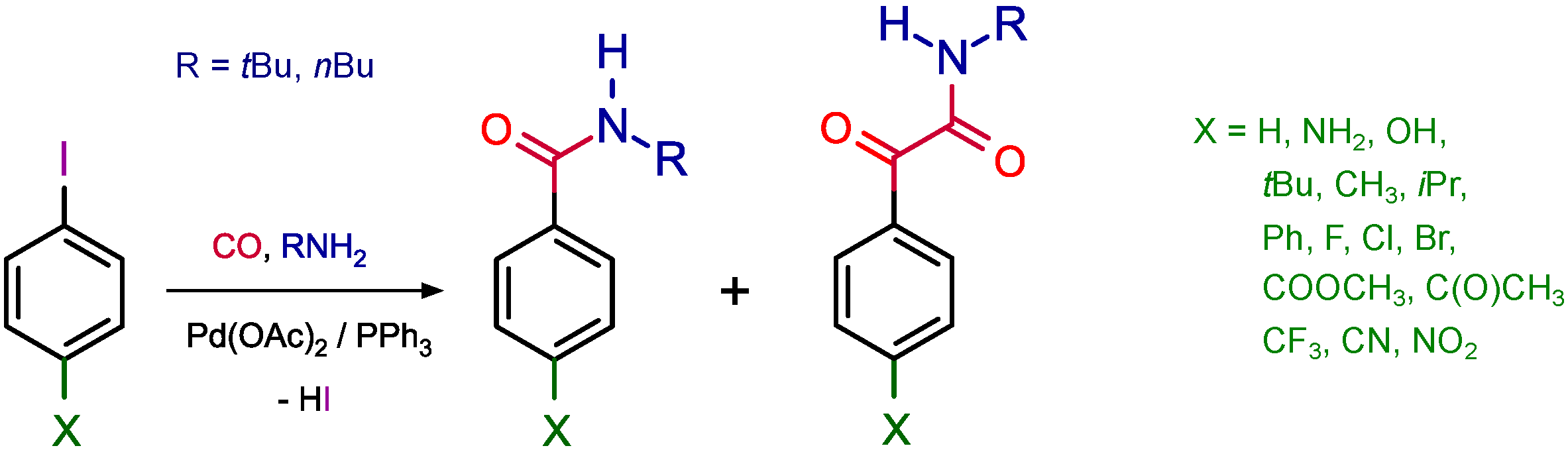
- Ozawa, F.; Sugimoto, T.; Yuasa, Y.; Santra, M.; Yamamoto, T.; Yamamoto, A. Palladium-promoted double-carbonylation reactions. Reactions of organopalladium compounds with carbon monoxide and amines to give α-keto amides. Organometallics 1984, 3, 683–692. [Google Scholar] [CrossRef]
- Ozawa, F.; Sugimoto, T.; Yamamoto, T.; Yamamoto, A. Preparation of trans-Pd(COCOR)Cl(PMePh2)2 complexes (R = Ph and Me) and their reactivities relative to double carbonylation promoted by palladium. Organometallics 1984, 3, 692–697. [Google Scholar] [CrossRef]
- Ozawa, F.; Soyama, H.; Yanagihara, H.; Aoyama, I.; Takino, H.; Izawa, K.; Yamamoto, T.; Yamamoto, A. Palladium-catalyzed double carbonylation of aryl halides to give α-keto amides. Mechanistic studies. J. Am. Chem. Soc. 1985, 107, 3235–3245. [Google Scholar] [CrossRef]
- Marosvölgyi-Haskó, D.; Kégl, T.; Kollár, L. Substituent effects in aminocarbonylation of para-substituted iodobenzenes. Tetrahedron 2016, 72, 7509–7516. [Google Scholar] [CrossRef]
- Prasad, A.S.; Satyanarayana, B. Fe3O4 supported Pd(0) nanoparticles catalyzed alkoxycarbonylation of aryl halides. J. Mol. Catal. A Chem. 2013, 370, 205–209. [Google Scholar] [CrossRef]
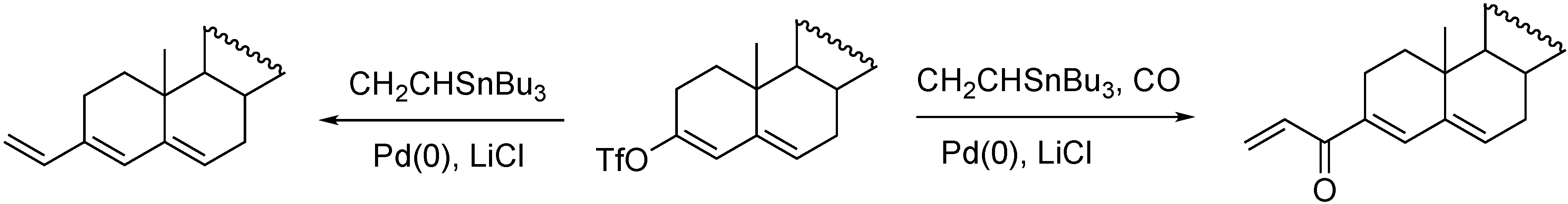
- Skoda-Földes, R.; Kollár, L.; Marinelli, F.; Arcadi, A. Direct and carbonylative vinylation of steroidal triflates in the presence of homogeneous palladium catalysts. Steroids 1994, 59, 691–695. [Google Scholar] [CrossRef]
- Kollár, L.; Floris, B.; Pino, P. Hydroformylation of Vinylferrocene with Rhodium and Platinum Catalysts. Chimia 1986, 40, 201–202. [Google Scholar]
- Kollár, L.; Consiglio, G.; Pino, P. Asymmetric Hydroformylation of Unsaturated Esters with PtCl(SnCl3)[(R,R)-DIOP] Catalyst. J. Organomet. Chem. 1987, 330, 305–314. [Google Scholar] [CrossRef]
- Kollár, L.; Bakos, J.; Tóth, I.; Heil, B. Temperature dependence of the asymmetric induction in the PtCl(SnCl3)[(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane]-catalyzed enantioselective hydroformylation reaction. J. Organomet. Chem. 1988, 350, 277–284. [Google Scholar] [CrossRef]
- Kollár, L.; Bakos, J.; Tóth, I.; Heil, B. Asymmetric hydroformylation with Pt-phosphine-SnCl2 and Pt-bisphosphine-CuCl2 (or CuCl) catalytic systems. J. Organomet. Chem. 1989, 370, 257–261. [Google Scholar] [CrossRef]
- Kollár, L.; Bakos, J.; Heil, B.; Sándor, P.; Szalontai, G. Hydroformylation of chiral terpenes with PtCl(SnCl3)-(bis-phosphine) as catalyst. J. Organomet. Chem. 1990, 385, 147–152. [Google Scholar] [CrossRef]
- Kollár, L.; Sándor, P.; Szalontai, G.; Heil, B. The role of additives in platinum-catalyzed hydroformylation. J. Organomet. Chem. 1990, 393, 153–158. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Kollár, L.; Heil, B.; Gálik, G.; Tuba, Z.; Arcade, A. A possible way for the introduction of α-and β-formyl-ethyl-substituents into the steroid-skeleton via coupling and carbonylation reactions. Tetrahedron Asymmetry 1991, 2, 633–634. [Google Scholar] [CrossRef]
- Kollár, L.; Sándor, P.; Szalontai, G. Temperature dependence of the enantioselective hydroformylation with PtCl2[(S)-BINAP] + SnCl2 catalyst and the dynamic NMR study of the catalytic precursor. J. Mol. Catal. 1991, 67, 191–198. [Google Scholar] [CrossRef]
- Kollár, L.; Floris, B. Highly selective hydroformylation and dimerization reactions of 2-ferrocenylpropene. J. Organomet. Chem. 1992, 441, 117–123. [Google Scholar] [CrossRef]
- Kollár, L.; Wada, T.; Lautens, M. Asymmetric hydroformylation of deltacyclene. Tetrahedron Asymmetry 1992, 3, 1011–1014. [Google Scholar] [CrossRef]
- Kollár, L.; Sándor, P. Highly stereoselective hydroformylation of a (2R)-2-tert-butyl-Δ4-1, 3-oxazoline derivative. J. Organomet. Chem. 1993, 445, 257–259. [Google Scholar] [CrossRef]
- Kollár, L.; Kégl, T.; Bakos, J. Platinum-catalysed enantioselective hydroformylation of styrene. Platinum-diphosphine-tin (II) fluoride catalytic system: A novel asymmetric hydroformylation catalyst. J. Organomet. Chem. 1993, 453, 155–158. [Google Scholar] [CrossRef]
- Kollár, L.; Skoda-Földes, R.; Mahó, S.; Tuba, Z. Functionalization of the estrone skeleton via homogeneous coupling and hydroformylation reactions. J. Organomet. Chem. 1993, 453, 159–162. [Google Scholar] [CrossRef]
- Tóth, I.; Kégl, T.; Elsevier, C.J.; Kollár, L. CO Insertion in Four-Coordinate cis-Methyl(carbonyl)platinum-Diphosphine Compounds. An Ionic Mechanism for Platinum-Diphosphine-Catalyzed Hydroformylation. Inorg. Chem. 1994, 33, 5708–5712. [Google Scholar] [CrossRef]
- Gladiali, S.; Fabbri, D.; Kollár, L. Asymmetric hydroformylation of styrene catalysed by platinum-tin complexes with chiral bis-binaphthophosphole ligands. J. Organomet. Chem. 1995, 491, 91–96. [Google Scholar] [CrossRef]
- Kollár, L.; Bódi, G. Asymmetric hydroformylation of mono-and sesquiterpenes. Chirality 1995, 7, 121–127. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Jeges, G.; Kollár, L.; Horváth, J.; Tuba, Z. Synthesis of Pentacyclic Steroids via Tandem Stille Coupling and Diels- Alder Reactions. J. Org. Chem. 1997, 62, 1326–1332. [Google Scholar] [CrossRef]
- Csákai, Z.; Skoda-Földes, R.; Kollár, L. NMR investigation of Pd(II)-Pd(0) reduction in the presence of mono- and ditertiary phosphines. Inorg. Chim. Acta 1999, 286, 93–97. [Google Scholar] [CrossRef]
- Pálinkás, N.; Kollár, L.; Kégl, T. Viable pathways for the oxidative addition of iodobenzene to palladium (0)-triphenylphosphine-carbonyl complexes: A theoretical study. Dalton Trans. 2017, 46, 15789–15802. [Google Scholar] [CrossRef]
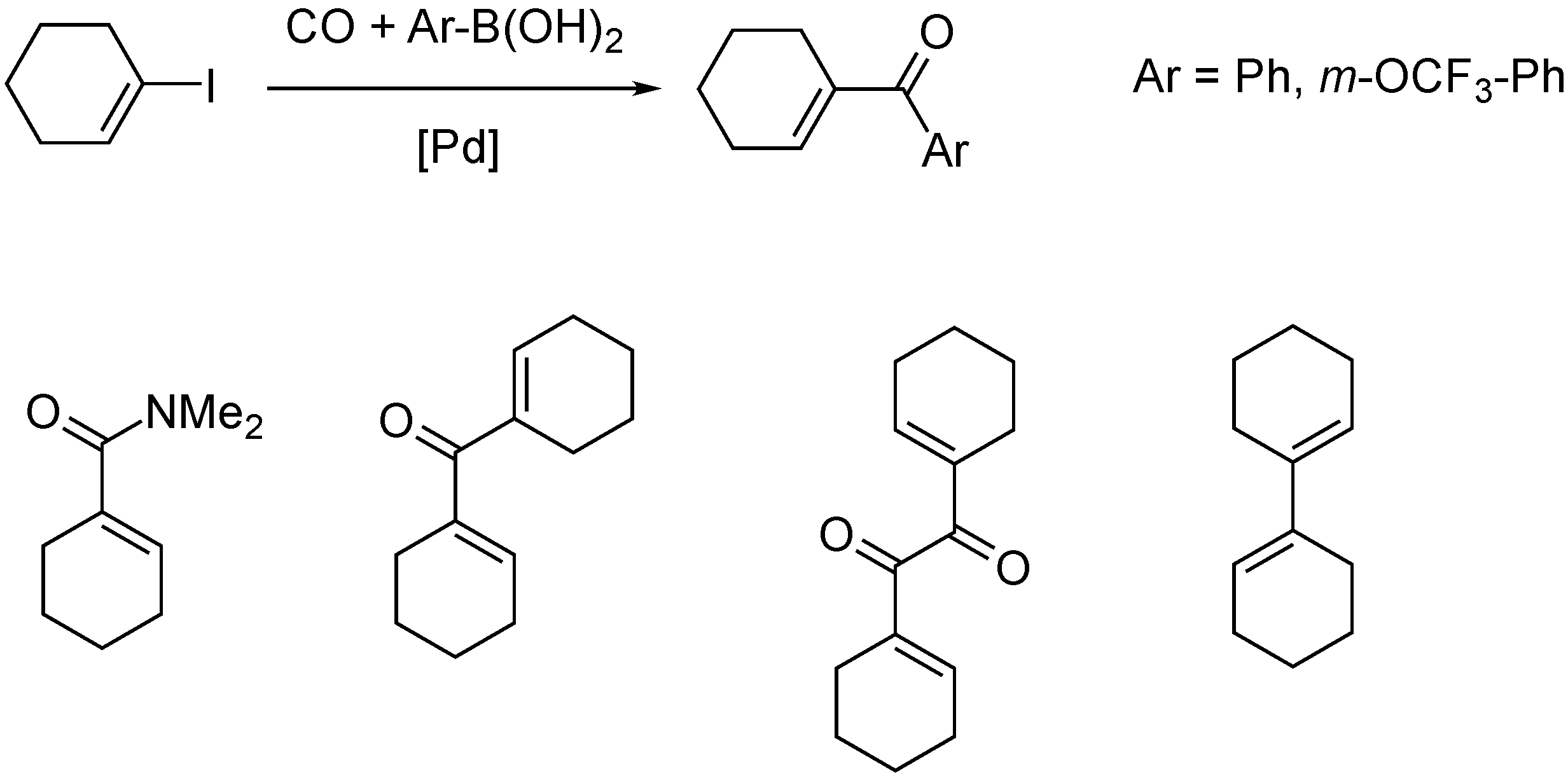
- Petz, A.; Pintér, Z.; Kollár, L. Mass spectrometric studies on the coupling model reaction towards alkenyl–aryl ketones. J. Biochem. Biophys. Methods 2004, 61, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Petz, A.; Péczely, G.; Pintér, Z.; Kollár, L. Carbonylative and direct Suzuki–Miyaura cross-coupling reactions with 1-iodo-cyclohexene. J. Mol. Catal. A Chem. 2006, 255, 97–102. [Google Scholar] [CrossRef]
- Takács, E.; Varga, C.; Skoda-Földes, R.; Kollar, L. Facile synthesis of primary amides and ketoamides via a palladium-catalysed carbonylation–deprotection reaction sequence. Tetrahedron Lett. 2007, 48, 2453–2456. [Google Scholar] [CrossRef]
- Takács, A.; Abreu, A.R.; Peixoto, A.F.; Pereira, M.M.; Kollár, L. Synthesis of Ortho-alkoxy-aryl Carboxamides via Palladium-Catalyzed Aminocarbonylation. Synth. Commun. 2009, 39, 1534–1548. [Google Scholar] [CrossRef]
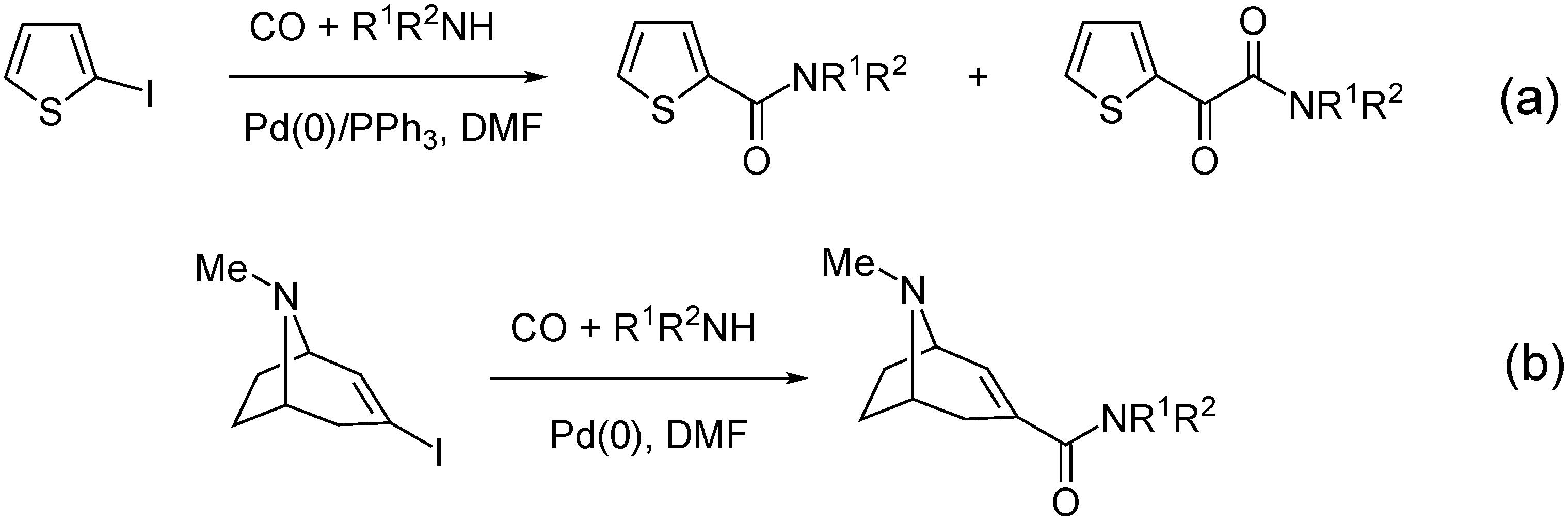
- Takacs, A.; Petz, A.; Jakab, B.; Kollar, L. Aminocarbonylation of 2-iodothiophene: High-yielding synthesis of thiophen-2-yl-glyoxylamides. Lett. Org. Chem. 2007, 4, 590–594. [Google Scholar] [CrossRef]
- Horvath, L.; Berente, Z.; Kollar, L. High-yielding aminocarbonylation of 3-iodo-2-tropene by using amino acid esters as N-nucleophiles. Lett. Org. Chem. 2007, 4, 236–238. [Google Scholar] [CrossRef]
- Takács, E.; Skoda-Földes, R.; Ács, P.; Müller, E.; Kokotos, G.; Kollár, L. Prolinates as secondary amines in aminocarbonylation: Synthesis of N-acylated prolinates. Lett. Org. Chem. 2006, 3, 62–67. [Google Scholar] [CrossRef]
- Kollár, L.; Erdélyi, Á.; Rasheed, H.; Takács, A. Selective Synthesis of N-Acylnortropane Derivatives in Palladium-Catalysed Aminocarbonylation. Molecules 2021, 26, 1813. [Google Scholar] [CrossRef] [PubMed]
- Szőke, G.; Takács, A.; Kollár, L. Synthesis of Pyridazine Dicarboxamides via Highly Selective Palladium-catalyzed Aminocarbonylation. J. Heterocycl. Chem. 2015, 53, 2020–2024. [Google Scholar] [CrossRef]
- Gergely, M.; Takács, A.; Kollár, L. 4-Amino-TEMPO asN-Nucleophile in Palladium-Catalyzed Aminocarbonylation. J. Heterocycl. Chem. 2016, 54, 634–640. [Google Scholar] [CrossRef]
- Takács, A.; Jakab, B.; Petz, A.; Kollár, L. Homogeneous catalytic aminocarbonylation of nitrogen-containing iodo-heteroaromatics. Synthesis of N-substituted nicotinamide related compounds. Tetrahedron 2007, 63, 10372–10378. [Google Scholar] [CrossRef]
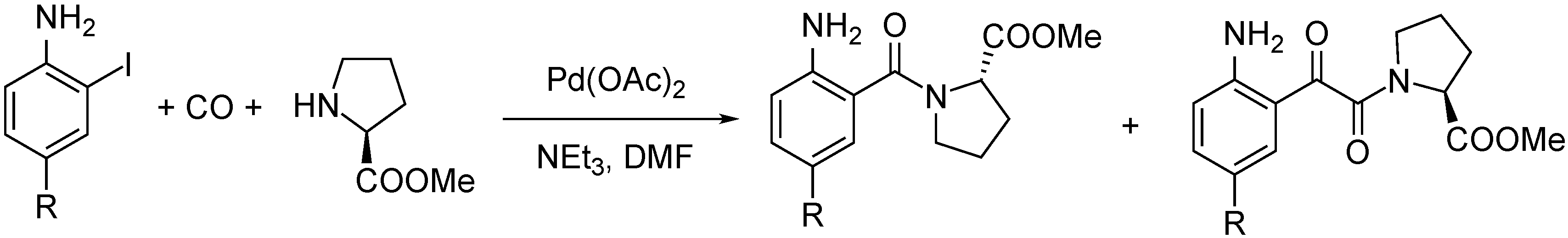
- Ács, P.; Müller, E.; Rangits, G.; Lóránd, T.; Kollár, L. Palladium-catalysed carbonylation of 4-substituted 2-iodoaniline derivatives: carbonylative cyclisation and aminocarbonylation. Tetrahedron 2006, 62, 12051–12056. [Google Scholar] [CrossRef]
- Takács, A.; Farkas, R.; Kollár, L. High-yielding synthesis of 2-arylacrylamides via homogeneous catalytic aminocarbonylation of α-iodostyrene and α, α′-diiodo-1,4-divinylbenzene. Tetrahedron 2008, 64, 61–66. [Google Scholar] [CrossRef]
- Takács, A.; Ács, P.; Kollár, L. Facile synthesis of 1,8-naphthalimides in palladium-catalysed aminocarbonylation of 1,8-diiodo-naphthalene. Tetrahedron 2008, 64, 983–987. [Google Scholar] [CrossRef]
- Takács, A.; Ács, P.; Farkas, R.; Kokotos, G.; Kollár, L. Homogeneous catalytic aminocarbonylation of 1-iodo-1-dodecene. The facile synthesis of odd-number carboxamides via palladium-catalysed aminocarbonylation. Tetrahedron 2008, 64, 9874–9878. [Google Scholar] [CrossRef]
- Takács, A.; Farkas, R.; Petz, A.; Kollár, L. Synthesis of 2-naphthylacrylamides and 2-naphthylacrylates via homogeneous catalytic carbonylation of 1-iodo-1-naphthylethene derivatives. Tetrahedron 2009, 65, 4795–4800. [Google Scholar] [CrossRef]
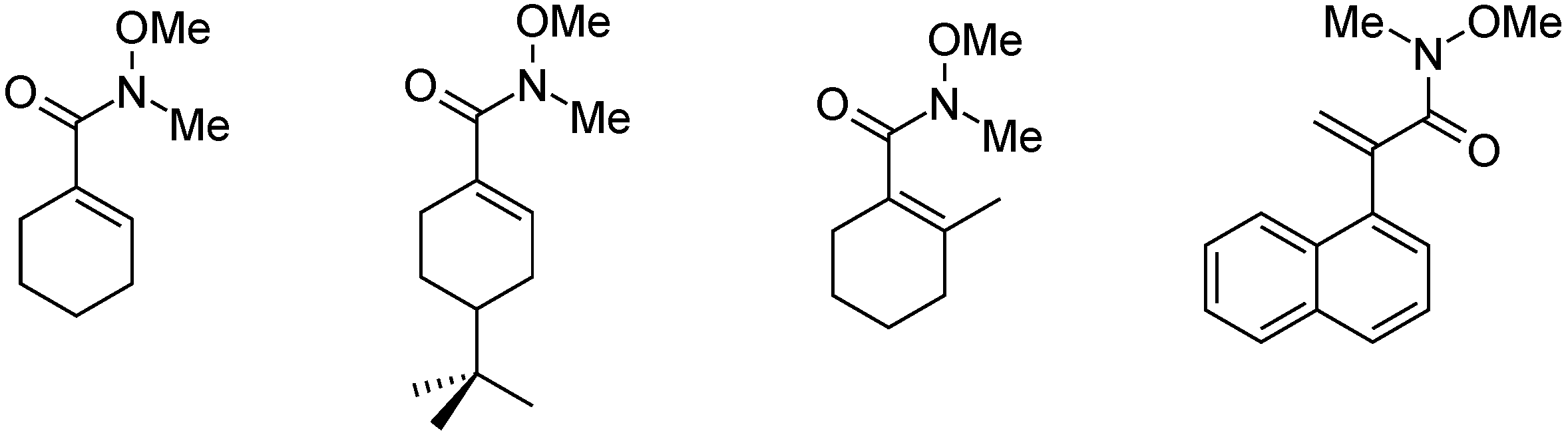
- Takács, A.; Petz, A.; Kollár, L. High-yielding synthesis of Weinreb amides via homogeneous catalytic carbonylation of iodoalkenes and iodoarenes. Tetrahedron 2010, 66, 4479–4483. [Google Scholar] [CrossRef]
- Marosvölgyi-Haskó, D.; Takács, A.; Riedl, Z.; Kollár, L. High-yielding synthesis of 1-isoindolinone derivatives via palladium-catalysed cycloaminocarbonylation. Tetrahedron 2011, 67, 1036–1040. [Google Scholar] [CrossRef]
- Takács, A.; Szilágyi, A.; Ács, P.; László, M.; Peixoto, A.F.; Pereira, M.M.; Kollár, L. Palladium-catalysed reactions of 8-hydroxy- and 8-benzyloxy-5,7-diiodoquinoline under aminocarbonylation conditions. Tetrahedron 2011, 67, 2402–2406. [Google Scholar] [CrossRef]
- Marosvölgyi-Haskó, D.; Petz, A.; Takács, A.; Kollár, L. Synthesis of tetrahydrophthalazine and phthalamide (phthalimide) derivatives via palladium-catalysed carbonylation of iodoarenes. Tetrahedron 2011, 67, 9122–9128. [Google Scholar] [CrossRef]
- Takács, A.; Czompa, A.; Krajsovszky, G.; Mátyus, P.; Kollár, L. Functionalization of the pyridazin-3(2H)-one ring via palladium-catalysed aminocarbonylation. Tetrahedron 2012, 68, 7855–7860. [Google Scholar] [CrossRef][Green Version]
- Farkas, R.; Molnár, E.A.; Ács, P.; Takács, A.; Kollár, L. High-yielding synthesis of 1-carboxamido-3,4-dihydronaphthalenes via palladium-catalyzed aminocarbonylation. Tetrahedron 2013, 69, 500–504. [Google Scholar] [CrossRef]
- Gergely, M.; Farkas, R.; Takács, A.; Petz, A.; Kollár, L. Synthesis of N-picolylcarboxamides via palladium-catalysed aminocarbonylation of iodobenzene and iodoalkenes. Tetrahedron 2014, 70, 218–224. [Google Scholar] [CrossRef]
- Szőke, G.; Takács, A.; Berente, Z.; Petz, A.; Kollár, L. Synthesis of amino-substituted pyridylglyoxylamides via palladium-catalysed aminocarbonylation. Tetrahedron 2016, 72, 3063–3067. [Google Scholar] [CrossRef]
- Mikle, G.; Bede, F.; Kollár, L. Synthesis of N-picolylcarboxamides in aminocarbonylation. Tetrahedron 2021, 88, 132128. [Google Scholar] [CrossRef]
- Takács, A.; Varga, G.M.; Kardos, J.; Kollár, L. Palladium-catalysed aminocarbonylation of diiodopyridines. Tetrahedron 2017, 73, 2131–2138. [Google Scholar] [CrossRef]
- Kollár, L.; Varga, M.G.; Dörnyei, Á.; Takács, A. Functionalisation of the uracil ring via palladium-catalysed aminocarbonylation. Tetrahedron 2019, 75, 4632–4639. [Google Scholar] [CrossRef]
- Kollár, L.; Takács, A. Novel synthesis of 3-carboxamidolactam derivatives via palladium-catalysed aminocarbonylation. Tetrahedron 2018, 74, 6116–6128. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Szarka, Z.; Kollár, L.; Dinya, Z.; Horváth, J.; Tuba, Z. Synthesis of N-substituted steroidal hydrazides in homogeneous catalytic hydrazinocarbonylation reaction. J. Org. Chem. 1999, 64, 2134–2136. [Google Scholar] [CrossRef]
- Kollár, L.; Szarka, Z.; Horváth, J.; Tuba, Z. Facile, high-yielding synthesis of steroidal hydrazides via homogeneous hydrazinocarbonylation reaction. Tetrahedron Lett. 1997, 38, 4467–4468. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Horváth, J.; Tuba, Z.; Kollár, L. Homogeneous coupling and carbonylation reactions of steroids possessing iodoalkene moieties. Catalytic and mechanistic aspects. J. Organomet. Chem. 1999, 586, 94–100. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Székvölgyi, Z.; Kollár, L.; Berente, Z.; Horváth, J.; Tuba, Z. Facile synthesis of steroidal phenyl ketones via homogeneous catalytic carbonylation. Tetrahedron 2000, 56, 3415–3418. [Google Scholar] [CrossRef]
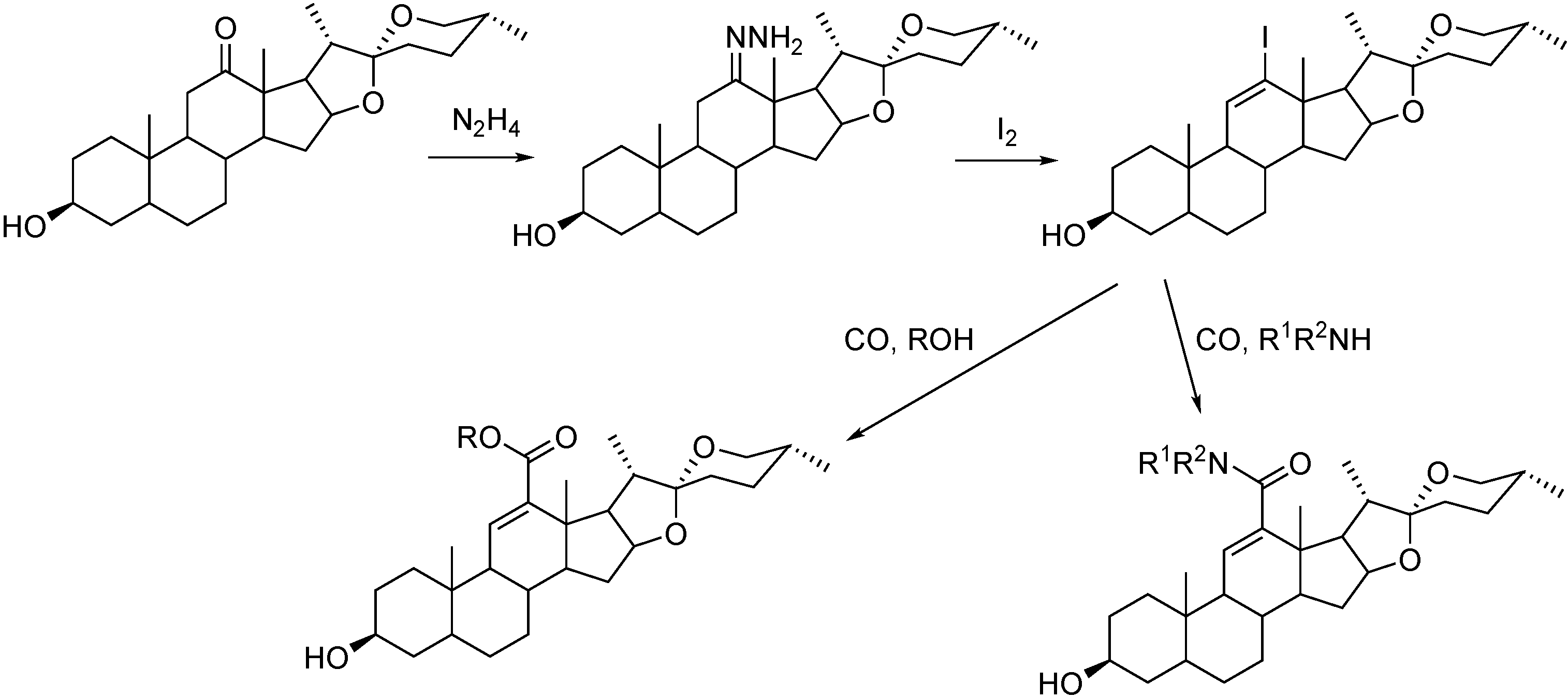
- Szarka, Z.; Skoda-Földes, R.; Kollar, L.; Horváth, J.; Tuba, Z. Novel method for the high-yielding synthesis of steroidal hydroxamic acid derivatives. Synth. Commun. 2000, 30, 1945–1953. [Google Scholar] [CrossRef]
- Szarka, Z.; Skoda-Földes, R.; Horváth, J.; Tuba, Z.; Kollár, L. Synthesis of steroidal diacyl hydrazines and their 1,3,4-oxadiazole derivatives. Steroids 2002, 67, 581–586. [Google Scholar] [CrossRef]
- Szarka, Z.; Skoda-Földes, R.; Kollár, L.; Berente, Z.; Horváth, J.; Tuba, Z. Highly efficient synthesis of steroidal hydroxamic acid derivatives via homogeneous catalytic carbonylation reaction. Tetrahedron 2000, 56, 5253–5257. [Google Scholar] [CrossRef]
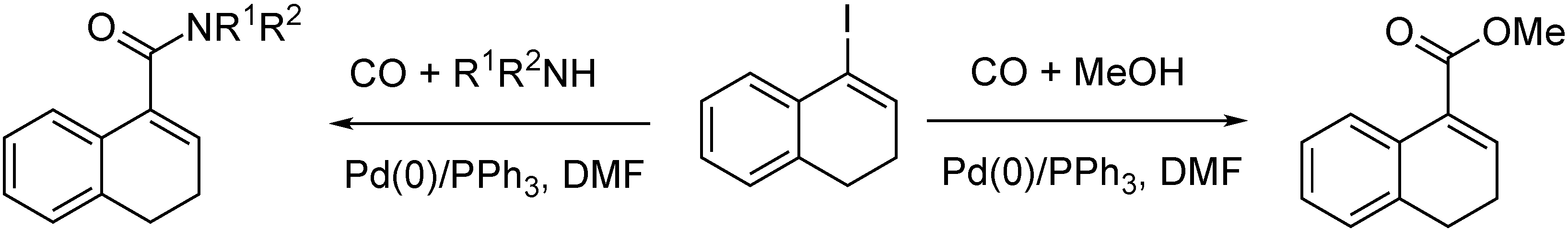
- Ács, P.; Takács, A.; Szilágyi, A.; Wölfling, J.; Schneider, G.; Kollár, L. The synthesis of 17-alkoxycarbonyl-and 17-carboxamido-13α-estra-1,3,5(10),16-tetraene derivatives via palladium-catalyzed carbonylation reactions. Steroids 2008, 73, 669–675. [Google Scholar] [CrossRef]
- Ács, P.; Takács, A.; Szilágyi, A.; Wölfling, J.; Schneider, G.; Kollár, L. The synthesis of 13α-androsta-5,16-diene derivatives with carboxylic acid, ester and carboxamido functionalities at position-17 via palladium-catalyzed carbonylation. Steroids 2009, 74, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Takács, A.; Ács, P.; Berente, Z.; Wölfling, J.; Schneider, G.; Kollár, L. Novel 13β- and 13α-D-homo steroids: 17a-carboxamido-D-homoestra-1,3,5(10),17-tetraene derivatives via palladium-catalyzed aminocarbonylations. Steroids 2010, 75, 1075–1081. [Google Scholar] [CrossRef]
- Kiss, M.; Pálinkás, N.; Takács, A.; Mahó, S.; Kollár, L. A systematic approach to the synthesis of androstane-based 3,17-dicarboxamides (homo- and mixed dicarboxamides) via palladium-catalyzed aminocarbonylation. Steroids 2013, 78, 693–699. [Google Scholar] [CrossRef] [PubMed]
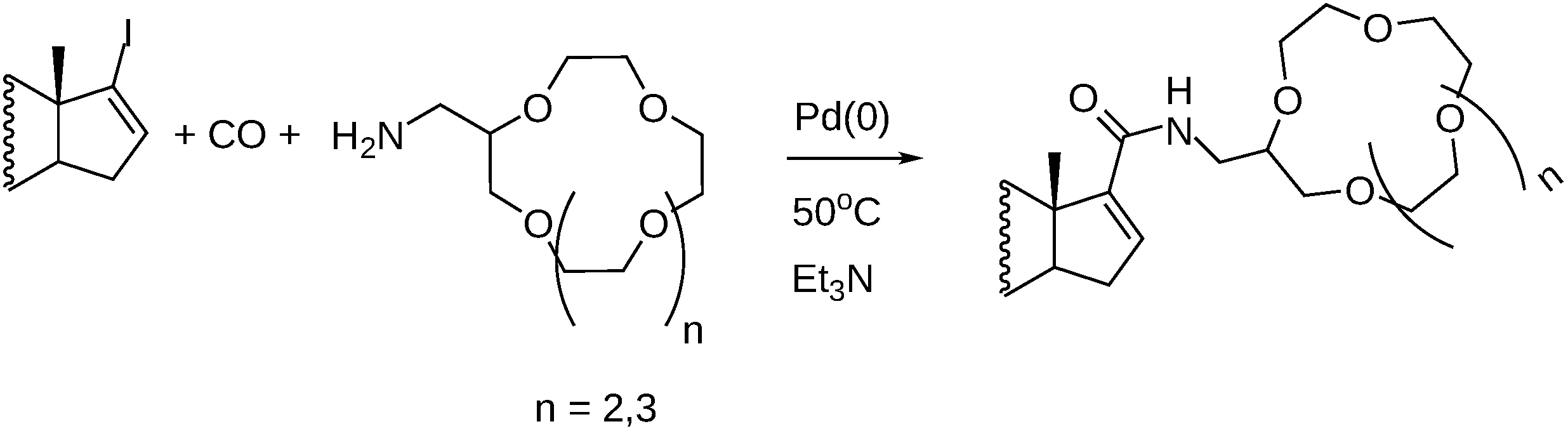
- Petz, A.; Gálik, G.; Horváth, J.; Tuba, Z.; Berente, Z.; Pintér, Z.; Kollár, L. Facile, high-yielding synthesis of steroidal crown ethers via palladium-catalyzed carbonylation reaction. Synth. Commun. 2001, 31, 335–341. [Google Scholar] [CrossRef]
- Ács, P.; Müller, E.; Czira, G.; Mahó, S.; Perreira, M.; Kollár, L. Facile synthesis of 12-carboxamido-11-spirostenes via palladium-catalyzed carbonylation reactions. Steroids 2006, 71, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Ács, P.; Jakab, B.; Takács, A.; Kollár, L. Facile synthesis of 11-carboxamido-androst-4, 9(11)-dienes via palladium-catalyzed aminocarbonylation. Steroids 2007, 72, 627–632. [Google Scholar] [CrossRef]
- Mikle, G.; Zugó, A.; Szatnik, E.; Maxim, A.; Mahó, S.; Kollár, L. Synthesis of novel pregnane-based 20-carboxamides via palladium-catalysed aminocarbonylation. Chem. Pap. 2021, 75, 1861–1867. [Google Scholar] [CrossRef]
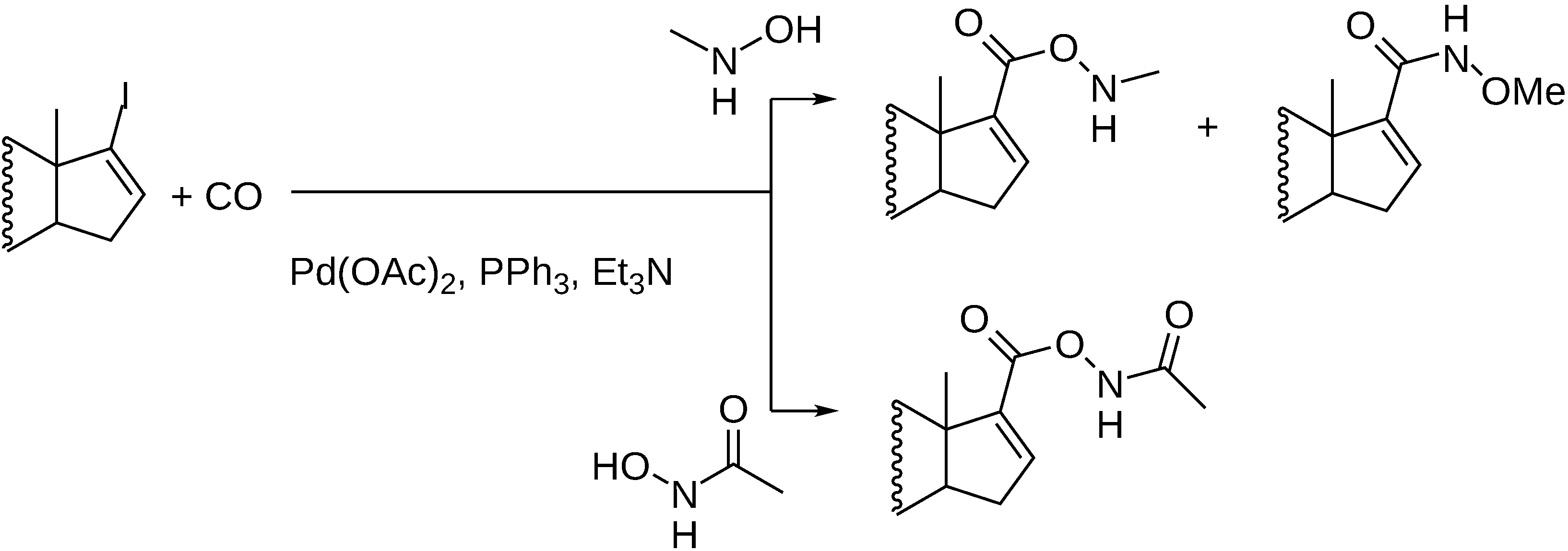
- Petz, A.; Horváth, J.; Tuba, Z.; Pintér, Z.; Kollár, L. Facile synthesis of 17-formyl steroids via palladium-catalyzed homogeneous carbonylation reaction. Steroids 2002, 67, 777–781. [Google Scholar] [CrossRef]
- Kuik, Á.; Skoda-Földes, R.; Balogh, J.; Kollár, L. Synthesis of ferrocenoyl amino acid derivatives via homogeneous catalytic aminocarbonylation. J. Organomet. Chem. 2005, 690, 3237–3242. [Google Scholar] [CrossRef]
- Szarka, Z.; Kuik, Á.; Skoda-Földes, R.; Kollár, L. Aminocarbonylation of 1,1′-diiodoferrocene, two-step synthesis of heterodisubstituted ferrocene derivatives via homogeneous catalytic carbonylation/coupling reactions. J. Organomet. Chem. 2004, 689, 2770–2775. [Google Scholar] [CrossRef]
- Kuik, Á.; Skoda-Földes, R.; Bényei, A.C.; Rangits, G.; Kollár, L. Formation of intramolecular hydrogen bonds in heterodisubstituted ferrocene diamides with a secondary and a tertiary amido group: X-ray structure of 1′-(N′-butyl-carbamoyl)-morpholino ferrocenecarboxamide. J. Organomet. Chem. 2006, 691, 3037–3042. [Google Scholar] [CrossRef]
- Szarka, Z.; Skoda-Földes, R.; Kuik, Á.; Berente, Z.; Kollár, L. Synthesis of ferrocene amides and α-ketoamides via palladium-catalyzed homogeneous carbonylation reaction. Synthesis 2003, 2003, 0545–0550. [Google Scholar]
- Szarka, Z.; Skoda-Földes, R.; Kollár, L. Facile synthesis of novel ferrocene α-ketoamides via homogeneous catalytic carbonylation. Tetrahedron Lett. 2001, 42, 739–741. [Google Scholar] [CrossRef]
- Kuik, Á.; Skoda-Földes, R.; Jánosi, L.; Kollar, L. Facile Synthesis of Unsymmetrical 1, n′-Disubstituted Ferrocenoyl Amino Acid Derivatives by Palladium-Catalyzed Aminocarbonylation. Synthesis 2007, 2007, 1456–1458. [Google Scholar]
- Csók, Z.; Kégl, T.; Li, Y.; Skoda-Földes, R.; Kiss, L.; Kunsági-Máté, S.; Todd, M.H.; Kollár, L. Synthesis of elongated cavitands via click reactions and their use as chemosensors. Tetrahedron 2013, 69, 8186–8190. [Google Scholar] [CrossRef]
- Filotás, D.; Nagy, L.; Kégl, T.R.; Csók, Z.; Kollár, L.; Nagy, G. Synthesis and Electrochemical Properties of the Tetraferrocenyl-Cavitand in Dimethyl Formamide Solvent Using Platinum and Carbon Working Electrodes. Electroanalysis 2015, 27, 799–807. [Google Scholar] [CrossRef]
- Janosi, T.Z.; Makkai, G.; Kegl, T.; Matyus, P.; Kollar, L.; Erostyak, J. Light-enhanced fluorescence of multi-level cavitands possessing pyridazine upper rim. J. Fluoresc. 2016, 26, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Kégl, T.; Csekő, G.; Mikle, G.; Takátsy, A.; Kollár, L.; Kégl, T. The Role of Weak Interactions in Supramolecular Compounds: A Synthetic and Theoretical Study of Novel Elongated Cavitands. ChemistrySelect 2017, 2, 8337–8345. [Google Scholar] [CrossRef]
- Csók, Z.; Takátsy, A.; Kollár, L. Highly selective palladium-catalyzed aminocarbonylation and cross-coupling reactions on a cavitand scaffold. Tetrahedron 2012, 68, 2657–2661. [Google Scholar] [CrossRef]
- Nagymihály, Z.; Kollár, L. High-yielding synthesis of deepened cavitands bearing picolyl moieties on the upper rim. Tetrahedron 2015, 71, 2555–2560. [Google Scholar] [CrossRef]
- Nagymihály, Z.; Wölfling, J.; Schneider, G. Synthesis of 2-Methylresorcinol-Based Deepened Cavitands with Chiral Inlet Bearing Steroidal Moieties on the Upper Rim. ChemistrySelect 2020, 5, 6933–6938. [Google Scholar] [CrossRef]
- Kégl, T.R.; Kégl, T. Palladium-catalyzed carbonylative synthesis and theoretical study of elongated tubular cavitands. J. Organomet. Chem. 2020, 923, 121387. [Google Scholar] [CrossRef]
- Nagymihály, Z.; Csók, Z.; Kollár, L. Influence of base additives on the selectivity of palladium-catalysed aminocarbonylation: Highly selective functionalization of a cavitand scaffold. Mol. Catal. 2018, 444, 70–75. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvent Effects: An Introduction. Org. Process Res. Dev. 2006, 11, 105–113. [Google Scholar] [CrossRef]
- Horváth, I.T. Solvents from nature. Green Chem. 2008, 10, 1024. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. Γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Novák, M.; Dibó, G.; Mika, L.T. An improved catalytic system for the reduction of levulinic acid to γ-valerolactone. Catal. Sci. Technol. 2014, 4, 2908–2912. [Google Scholar] [CrossRef]
- Pongrácz, P.; Bartal, B.; Kollár, L.; Mika, L.T. Rhodium-catalyzed hydroformylation in γ-valerolactone as a biomass-derived solvent. J. Organomet. Chem. 2017, 847, 140–145. [Google Scholar] [CrossRef]
- Pongrácz, P.; Kollár, L.; Mika, L.T. A step towards hydroformylation under sustainable conditions: platinum-catalysed enantioselective hydroformylation of styrene in gamma-valerolactone. Green Chem. 2016, 18, 842–847. [Google Scholar] [CrossRef]
- Marosvölgyi-Haskó, D.; Lengyel, B.; Tukacs, J.M.; Kollár, L.; Mika, L.T. Application of γ-Valerolactone as an Alternative Biomass-Based Medium for Aminocarbonylation Reactions. ChemPlusChem 2016, 81, 1224–1229. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Marton, B.; Albert, E.; Tóth, I.; Mika, L.T. Palladium-catalyzed aryloxy- and alkoxycarbonylation of aromatic iodides in γ-valerolactone as bio-based solvent. J. Organomet. Chem. 2020, 923, 121407. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Takács, E.; Horváth, J.; Tuba, Z.; Kollár, L. Palladium-catalysed aminocarbonylation of steroidal 17-iodo-androst-16-ene derivatives in N,N′-dialkyl-imidazolium-type ionic liquids. Green Chem. 2003, 5, 643–645. [Google Scholar] [CrossRef]
- Müller, E.; Péczely, G.; Skoda-Földes, R.; Takács, E.; Kokotos, G.; Bellis, E.; Kollár, L. Homogeneous catalytic aminocarbonylation of iodoalkenes and iodobenzene with amino acid esters under conventional conditions and in ionic liquids. Tetrahedron 2005, 61, 797–802. [Google Scholar] [CrossRef]
- Takács, E.; Berente, Z.; Háda, V.; Mahó, S.; Kollár, L.; Skoda-Földes, R. Synthesis of new steroidal derivatives by the reaction of steroid–amino acid conjugates with N,N′-dicyclohexyl-carbodiimide. Unusual formation of steroidal imide derivatives. Tetrahedron 2009, 65, 4659–4663. [Google Scholar] [CrossRef]
- Papp, M.; Szabó, P.; Srankó, D.; Sáfrán, G.; Kollár, L.; Skoda-Földes, R. Mono- and double carbonylation of aryl iodides with amine nucleophiles in the presence of recyclable palladium catalysts immobilised on a supported dicationic ionic liquid phase. RSC Adv. 2017, 7, 44587–44597. [Google Scholar] [CrossRef]
- Urbán, B.; Nagy, E.; Nagy, P.; Papp, M.; Skoda-Földes, R. Double carbonylation of iodoarenes in the presence of a pyridinium SILP-Pd catalyst. J. Organomet. Chem. 2020, 918, 121287. [Google Scholar] [CrossRef]
- Urbán, B.; Skoda-Földes, R. Development of palladium catalysts immobilized on supported phosphonium ionic liquid phases. Phosphorus Sulfur Silicon Relat. Elem. 2018, 194, 302–306. [Google Scholar] [CrossRef]
- Adamcsik, B.; Nagy, E.; Urbán, B.; Szabó, P.; Pekker, P.; Skoda-Földes, R. Palladium nanoparticles on a pyridinium supported ionic liquid phase: A recyclable and low-leaching palladium catalyst for aminocarbonylation reactions. RSC Adv. 2020, 10, 23988–23998. [Google Scholar] [CrossRef]
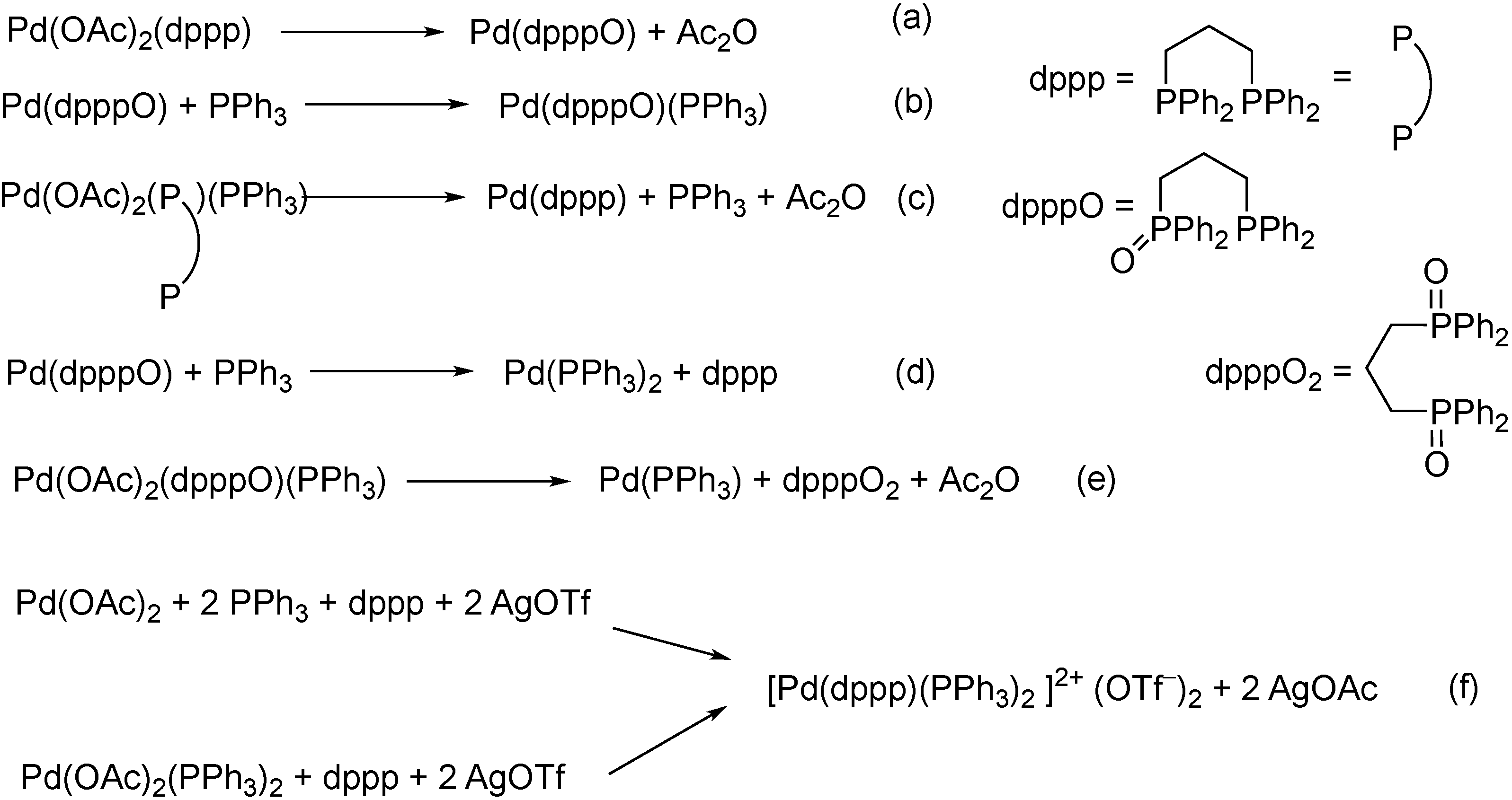
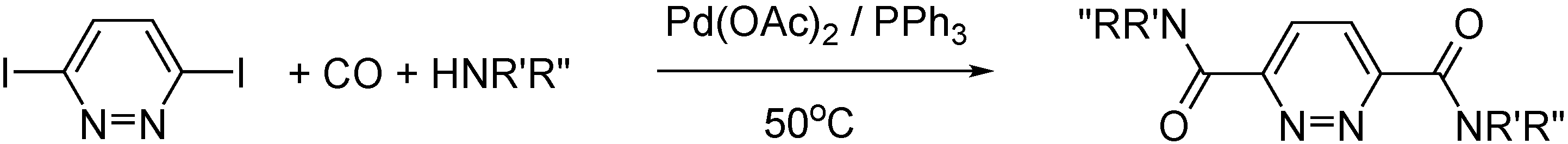
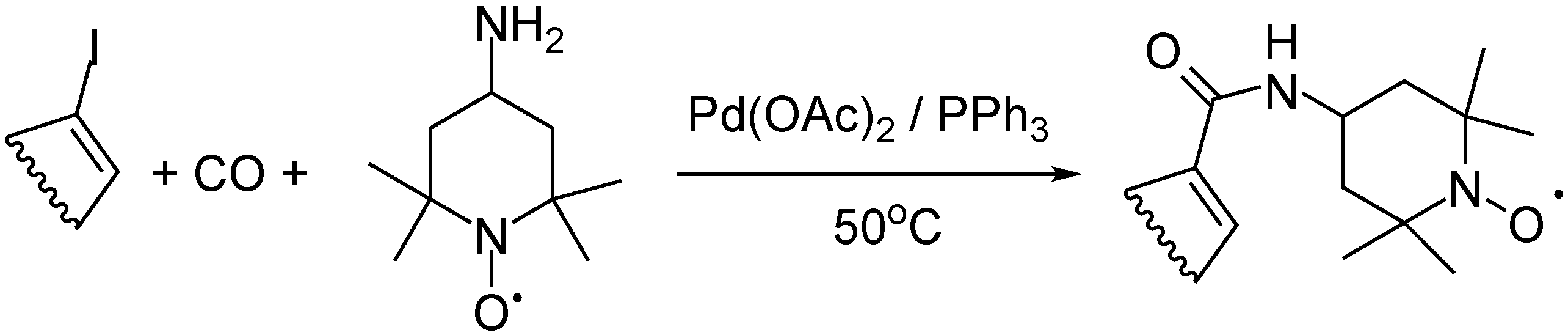

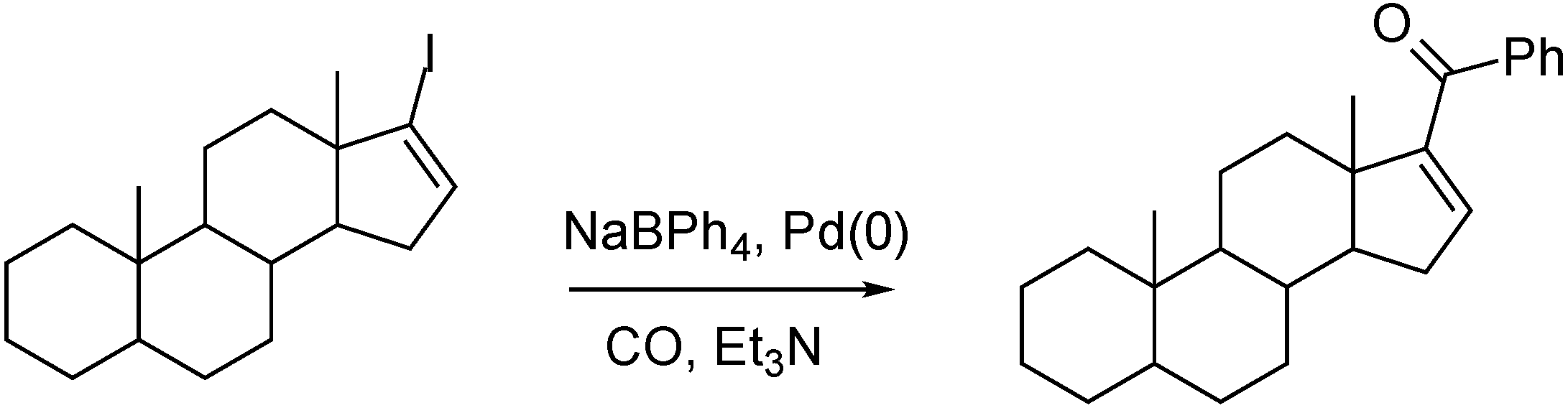
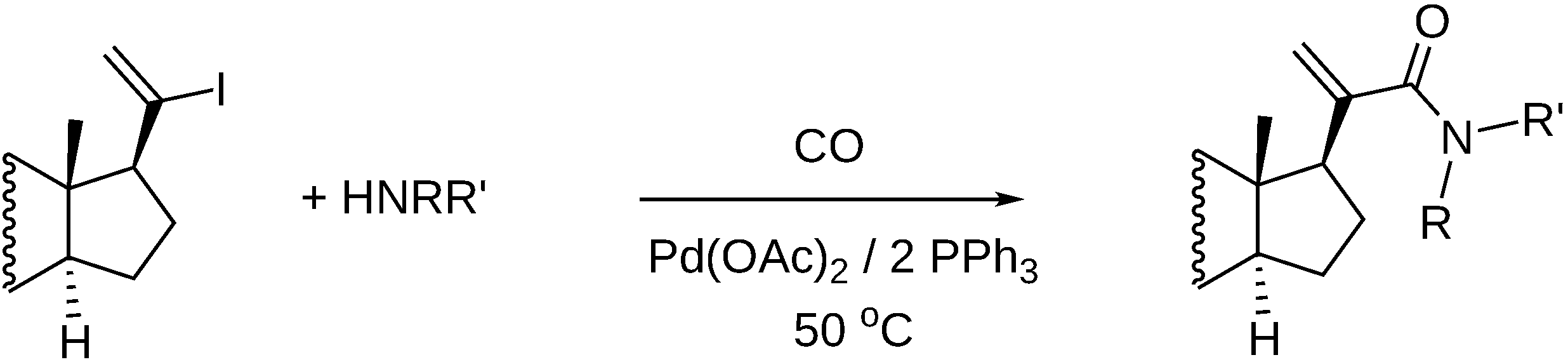
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kégl, T.R.; Mika, L.T.; Kégl, T. 27 Years of Catalytic Carbonylative Coupling Reactions in Hungary (1994–2021). Molecules 2022, 27, 460. https://doi.org/10.3390/molecules27020460
Kégl TR, Mika LT, Kégl T. 27 Years of Catalytic Carbonylative Coupling Reactions in Hungary (1994–2021). Molecules. 2022; 27(2):460. https://doi.org/10.3390/molecules27020460
Chicago/Turabian StyleKégl, Tímea R., László T. Mika, and Tamás Kégl. 2022. "27 Years of Catalytic Carbonylative Coupling Reactions in Hungary (1994–2021)" Molecules 27, no. 2: 460. https://doi.org/10.3390/molecules27020460
APA StyleKégl, T. R., Mika, L. T., & Kégl, T. (2022). 27 Years of Catalytic Carbonylative Coupling Reactions in Hungary (1994–2021). Molecules, 27(2), 460. https://doi.org/10.3390/molecules27020460





