Tunning CO2 Separation Performance of Ionic Liquids through Asymmetric Anions
Abstract
:1. Introduction
2. Results and Discussion
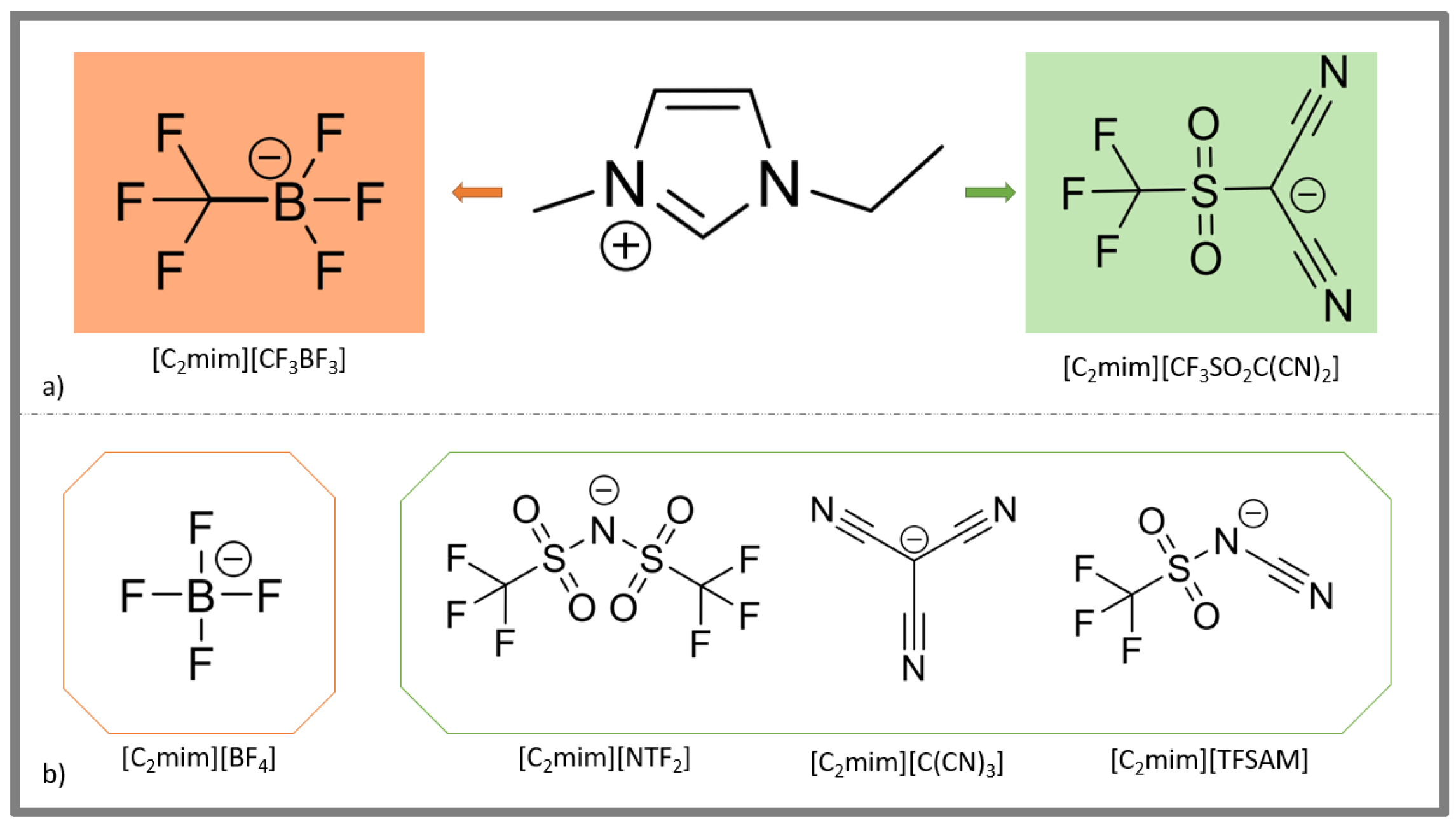
2.1. Synthesis of New ILs
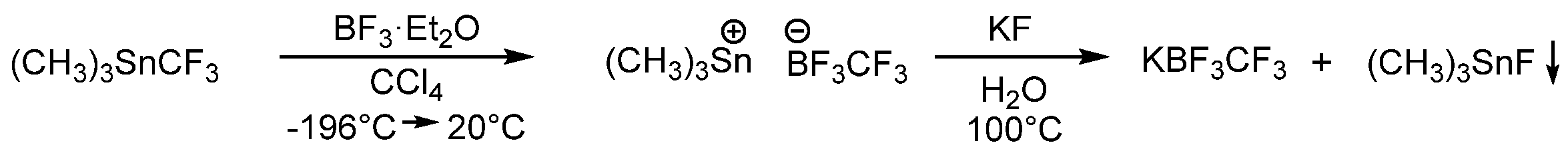
2.2. Thermal Properties of ILs
2.3. Termophysical Properties of ILs: Density, Molar Volume, and Viscosity
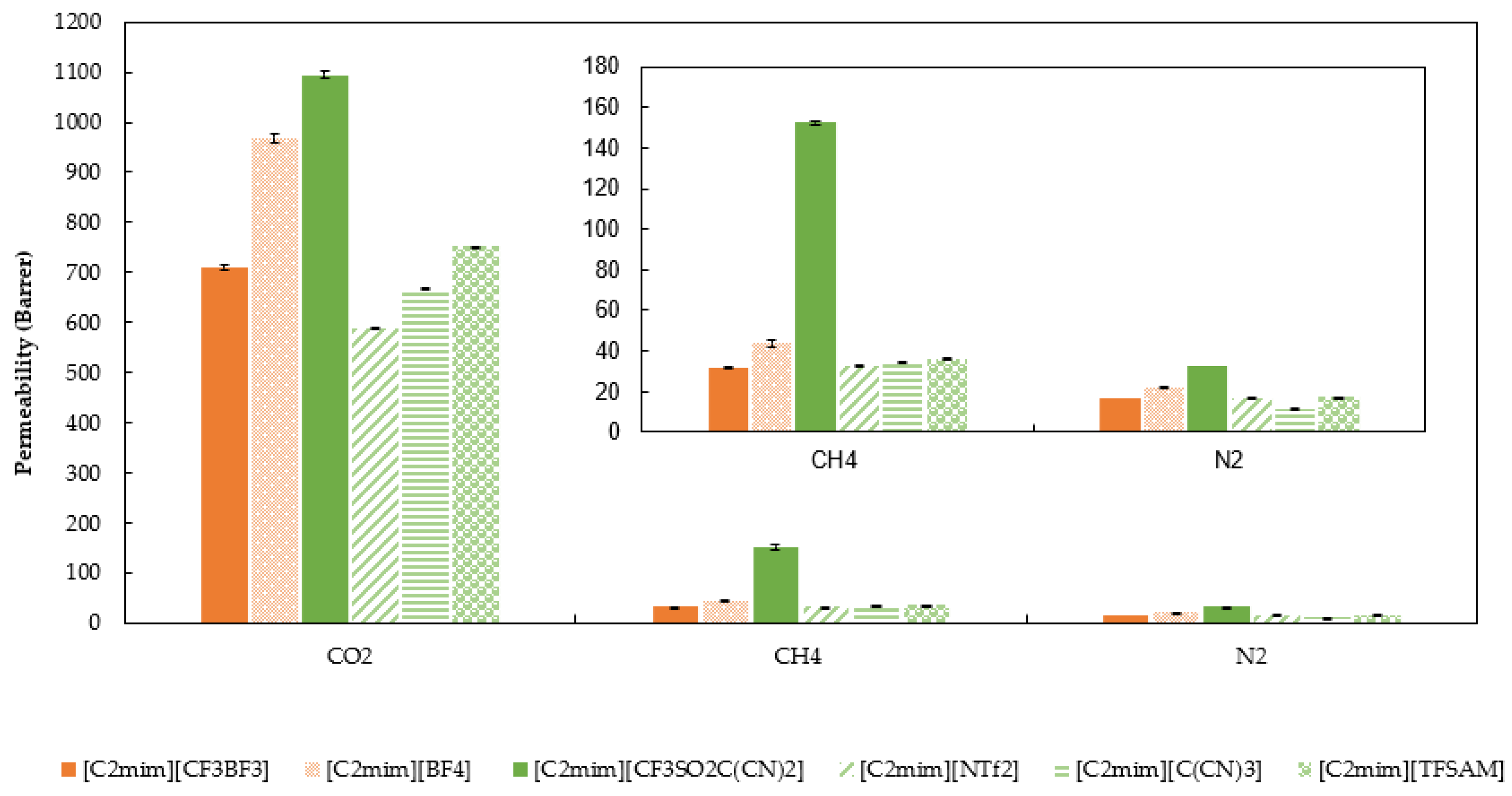
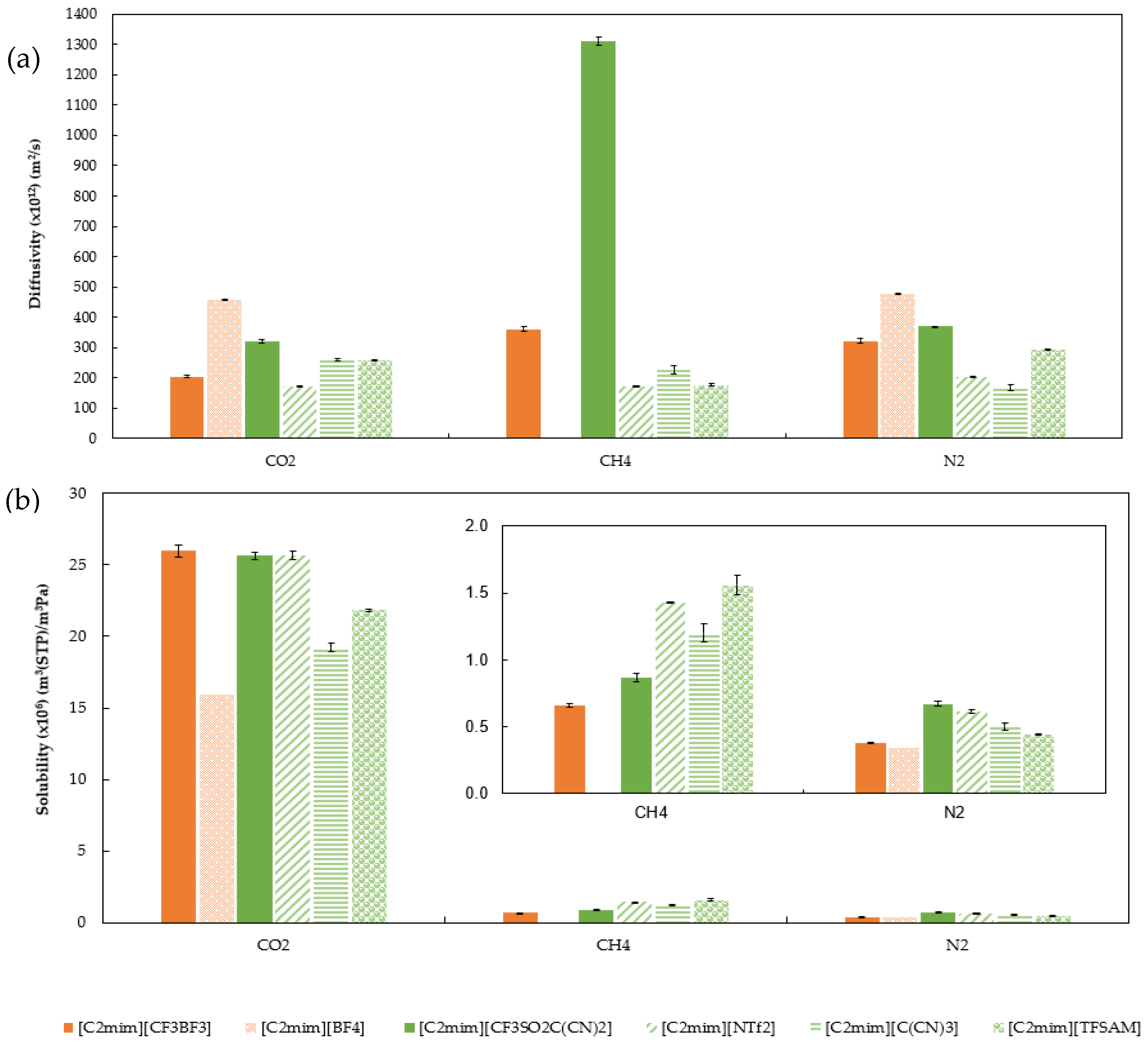
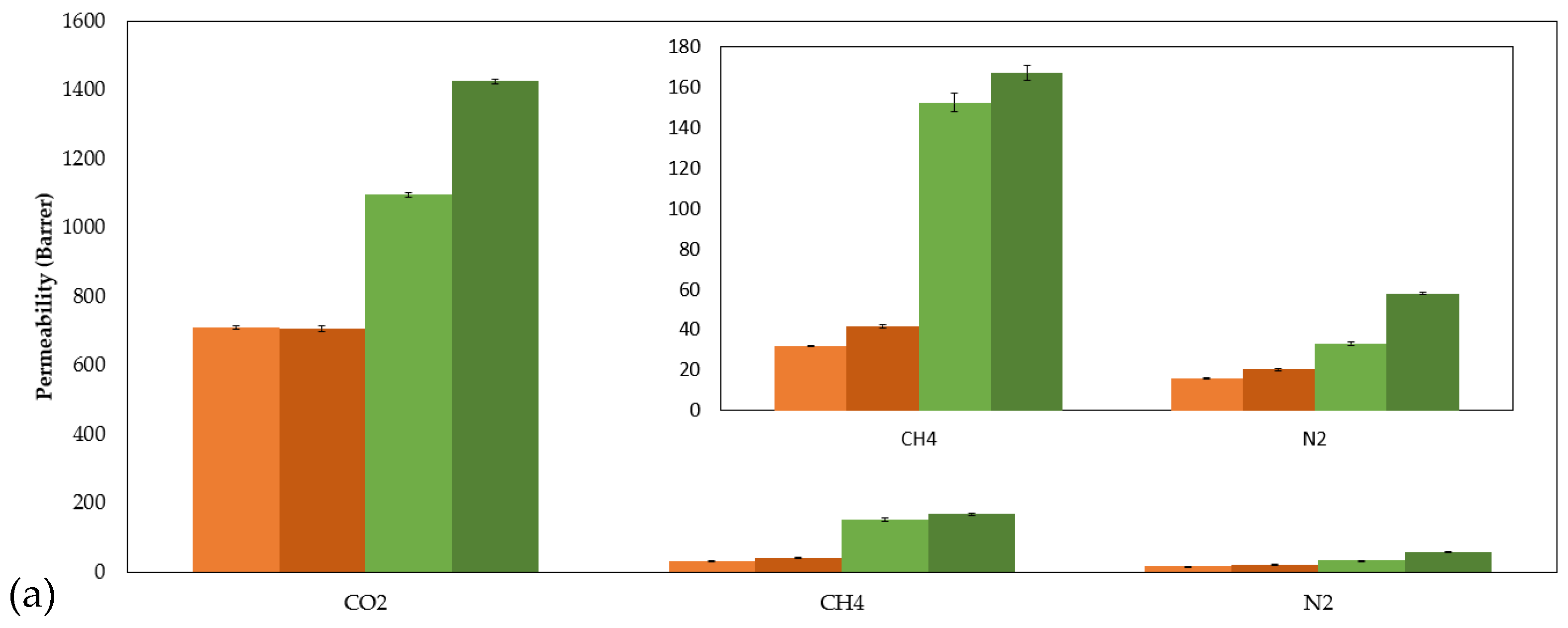
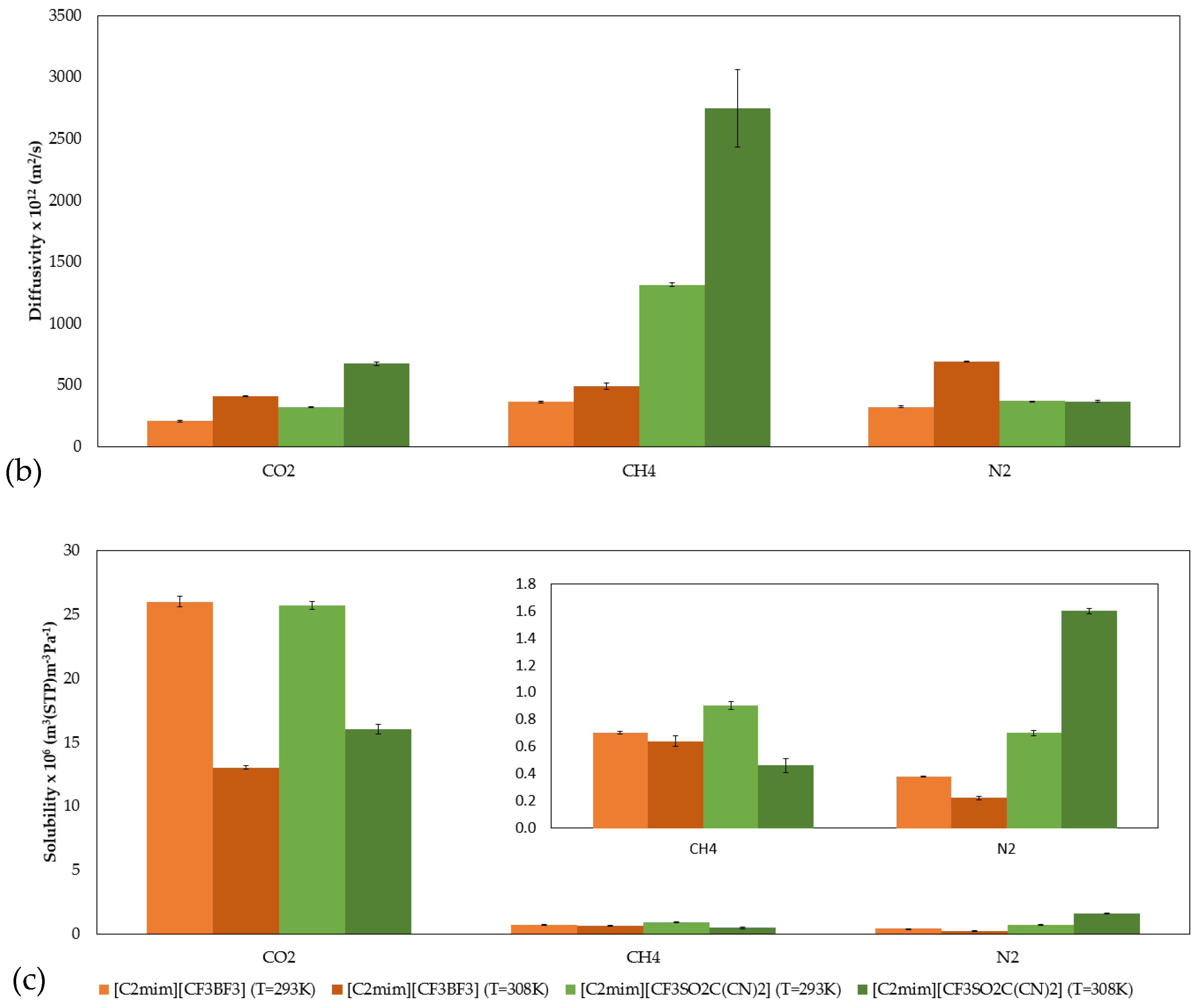
2.4. Gas Permeability, Diffusivity, and Solubility of CO2, CH4, and N2 in SILMs
2.5. Temperature Effect on Permeation Properties
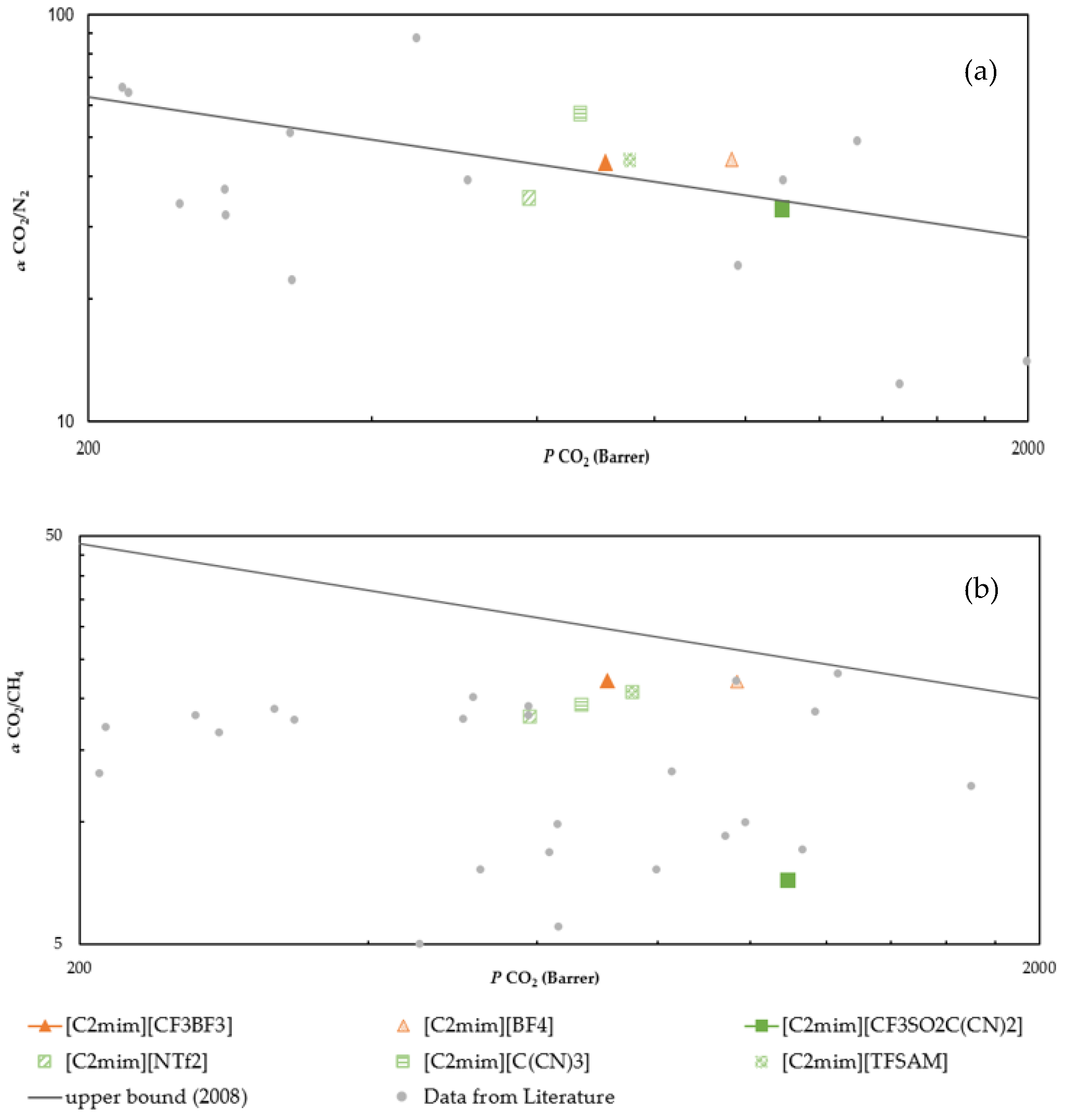
2.6. CO2 Separation Performance
2.7. Membranes Performance Comparison
3. Materials and Methods
3.1. Materials
3.2. Synthesis
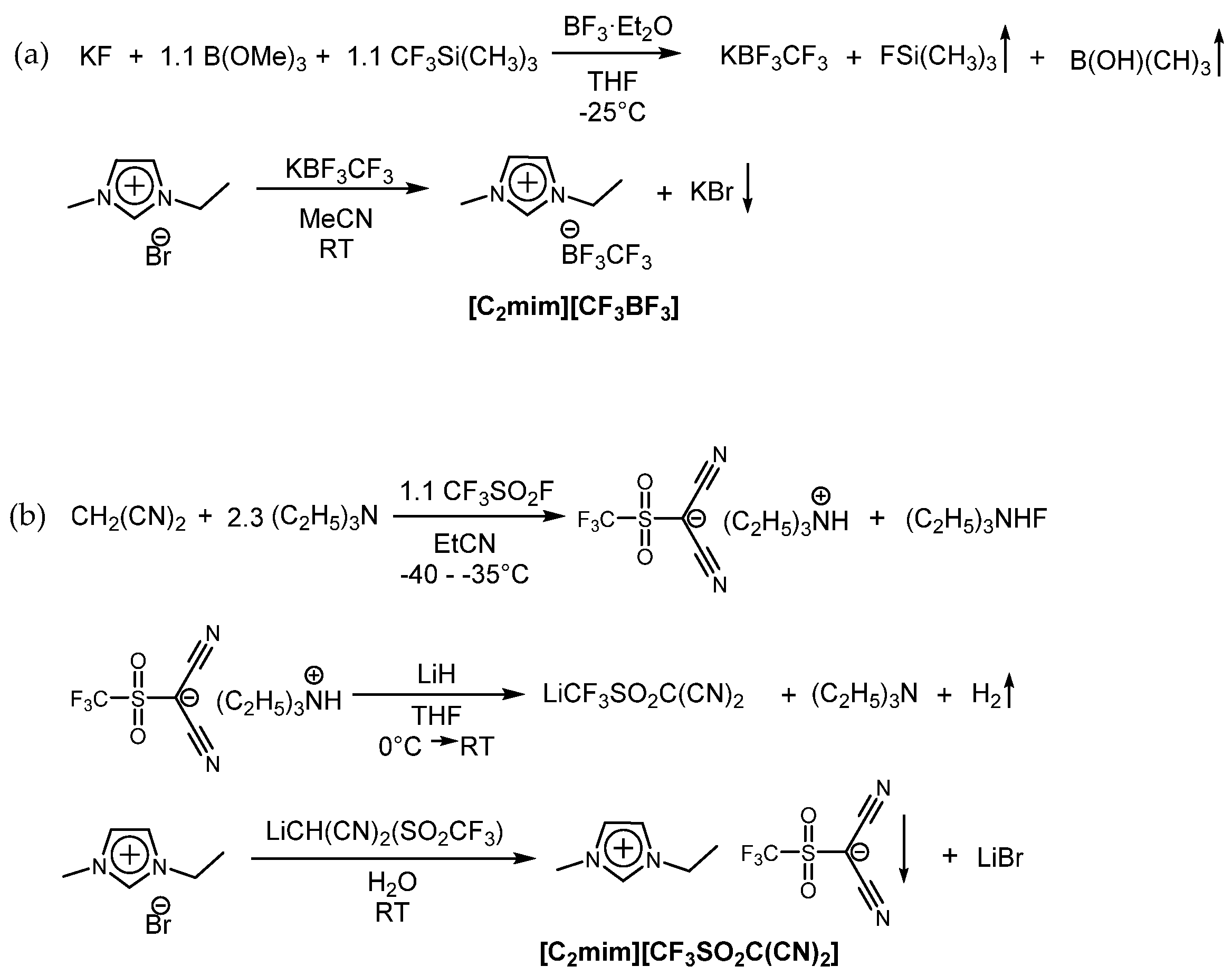
3.2.1. Potassium Trifluoro(Trifluoromethyl)Borate (KCF3BF3)
3.2.2. 1-Ethyl-3-Methyl-Imidazolium Trifluoro(Trifluoromethyl)Borate [C2mim][CF3BF3]
3.2.3. Triethylammonium Dicyano((Trifluoromethyl)Sulfonyl)Methanide [NC2C2C2H][CF3SO2C(CN)2]
3.2.4. Lithium Dicyano((Trifluoromethyl)Sulfonyl)Methanide (Li CF3SO2C(CN)2)
3.2.5. 1-Ethyl-3-Methyl-Imidazolium Dicyano((Trifluoromethyl)Sulfonyl)Methanide [C2mim][CF3SO2C(CN)2]
3.3. Analytical and Physicochemical Measurements
3.3.1. Water Content
3.3.2. Spectroscopical Properties
3.3.3. Thermal Properties
3.3.4. Thermophysical Properties
3.3.5. Supported Ionic Liquid Membranes (SILMs) Preparation
3.3.6. Gas Permeation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rebelo, L.P.N.; Canongia Lopes, J.N.; Esperança, J.M.S.S.; Filipe, E. On the Critical Temperature, Normal Boiling Point, and Vapor Pressure of Ionic Liquids. J. Phys. Chem. B 2005, 109, 6040–6043. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Aparicio, S.; Atilhan, M.; Karadas, F. Thermophysical Properties of Pure Ionic Liquids: Review of Present Situation. Ind. Eng. Chem. Res. 2010, 49, 9580–9595. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef] [PubMed]
- Shamair, Z.; Habib, N.; Gilani, M.A.; Khan, A.L. Theoretical and experimental investigation of CO2 separation from CH4 and N2 through supported ionic liquid membranes. Appl. Energy 2020, 268, 115016. [Google Scholar] [CrossRef]
- Haider, J.; Saeed, S.; Qyyum, M.A.; Kazmi, B.; Ahmad, R.; Muhammad, A.; Lee, M. Simultaneous capture of acid gases from natural gas adopting ionic liquids: Challenges, recent developments, and prospects. Renew. Sustain. Energy Rev. 2020, 123, 109771. [Google Scholar] [CrossRef]
- Bakonyi, P.; Peter, J.; Koter, S.; Mateos, R.; Kumar, G.; Koók, L.; Rózsenberszki, T.; Pientka, Z.; Kujawski, W.; Kim, S.-H.; et al. Possibilities for the biologically-assisted utilization of CO2-rich gaseous waste streams generated during membrane technological separation of biohydrogen. J. CO2 Util. 2020, 36, 231–243. [Google Scholar] [CrossRef]
- Zeng, S.; Cao, Y.; Li, P.; Liu, X.; Zhang, X. Ionic liquid–based green processes for ammonia separation and recovery. Curr. Opin. Green Sustain. Chem. 2020, 25, 100354. [Google Scholar] [CrossRef]
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Process. Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godínez, C.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Sánchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Memb. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Klingberg, P.; Wilkner, K.; Schlüter, M.; Grünauer, J.; Shishatskiy, S. Separation of Carbon Dioxide from Real Power Plant Flue Gases by Gas Permeation Using a Supported Ionic Liquid Membrane: An Investigation of Membrane Stability. Membranes 2019, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Karkhanechi, H.; Salmani, S.; Asghari, M. A Review on Gas Separation Applications of Supported Ionic Liquid Membranes. ChemBioEng Rev. 2015, 2, 290–302. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Memb. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, Y.; Deng, Y.; Zhang, S.; Wang, G. Perspectives on Ionic Liquids and Ionic Liquid Membranes for Natural Gas Sweetening. Prog. Chem. 2015, 27, 1324. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Bai, L.; Han, J.; Yang, B.; Zhang, S.; Zhang, X. Functionalized ionic liquid membranes for CO2 separation. Chem. Commun. 2018, 54, 12671–12685. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Yin, D.; Javaid, M.U.; Li, L.; Pan, C.; Tang, J.; Yu, G. Ionic Liquids-Based Membranes for Carbon Dioxide Separation. Isr. J. Chem. 2019, 59, 824–831. [Google Scholar] [CrossRef]
- García, G.; Atilhan, M.; Aparicio, S. A density functional theory insight towards the rational design of ionic liquids for SO2 capture. Phys. Chem. Chem. Phys. 2015, 17, 13559–13574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, B.R.; Senapati, S. Explaining the differential solubility of flue gas components in ionic liquids from first-principle calculations. J. Phys. Chem. B 2009, 113, 4739–4743. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion effects on gas solubility in ionic liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M. Density, Viscosity, and Refractive Index of Ionic Liquid Mixtures Containing Cyano and Amino Acid-Based Anions. J. Chem. Eng. Data 2016, 61, 83–93. [Google Scholar] [CrossRef]
- Clough, M.T.; Crick, C.R.; Gräsvik, J.; Hunt, P.A.; Niedermeyer, H.; Welton, T.; Whitaker, O.P. A physicochemical investigation of ionic liquid mixtures. Chem. Sci. 2015, 6, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Matthews, R.P.; Villar-Garcia, I.J.; Weber, C.C.; Griffith, J.; Cameron, F.; Hallett, J.P.; Hunt, P.A.; Welton, T. A structural investigation of ionic liquid mixtures. Phys. Chem. Chem. Phys. 2016, 18, 8608–8624. [Google Scholar] [CrossRef] [Green Version]
- Villar-Garcia, I.J.; Lovelock, K.R.J.; Men, S.; Licence, P. Tuning the electronic environment of cations and anions using ionic liquid mixtures. Chem. Sci. 2014, 5, 2573–2579. [Google Scholar] [CrossRef] [Green Version]
- Omar, S.; Lemus, J.; Ruiz, E.; Ferro, V.R.; Ortega, J.; Palomar, J. Ionic liquid mixtures—An analysis of their mutual miscibility. J. Phys. Chem. B 2014, 118, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Annat, G.; Forsyth, M.; MacFarlane, D.R. Ionic liquid mixtures-variations in physical properties and their origins in molecular structure. J. Phys. Chem. B 2012, 116, 8251–8258. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.J.; Castiglione, F.; Doherty, C.M.; Dolan, A.; Hill, A.J.; Hunt, P.A.; Matthews, R.P.; Mauri, M.; Mele, A.; Simonutti, R.; et al. Linking the structures, free volumes, and properties of ionic liquid mixtures. Chem. Sci. 2017, 8, 6359–6374. [Google Scholar] [CrossRef] [Green Version]
- Tomé, L.C.; Patinha, D.; Freire, C.; Rebelo, L.; Marrucho, I. CO2 separation applying ionic liquid mixtures: The effect of mixing different anions on gas permeation through supported ionic liquid membranes. RSC Adv. 2013, 3, 12220–12229. [Google Scholar] [CrossRef] [Green Version]
- Tomé, L.C.; Florindo, C.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Playing with ionic liquid mixtures to design engineered CO2 separation membranes. Phys. Chem. Chem. Phys. 2014, 16, 17172–17182. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Tome, L.C.; Lozinskaya, E.I.; Shaplov, A.S.; Vygodskii, Y.S.; Marrucho, I.M. Exploring the effect of fluorinated anions on the CO2/N-2 separation of supported ionic liquid membranes. Phys. Chem. Chem. Phys. 2017, 19, 28876–28884. [Google Scholar] [CrossRef] [Green Version]
- Scovazzo, P. Determination of the upper limits, benchmarks, and critical properties for gas separations using stabilized room temperature ionic liquid membranes (SILMs) for the purpose of guiding future research. J. Memb. Sci. 2009, 343, 199–211. [Google Scholar] [CrossRef]
- Morgan, D.; Ferguson, L.; Scovazzo, P. Diffusivities of Gases in Room-Temperature Ionic Liquids: Data and Correlations Obtained Using a Lag-Time Technique. Ind. Eng. Chem. Res. 2005, 44, 4815–4823. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kageyama, H.; Miyazaki, Y. Room temperature ionic liquids based on small aliphatic ammonium cations and asymmetric amide anions. Chem. Commun. 2002, 16, 1726–1727. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakaebe, H.; Tatsumi, K. Preparation of room temperature ionic liquids based on aliphatic onium cations and asymmetric amide anions and their electrochemical properties as a lithium battery electrolyte. J. Power Sources 2005, 146, 45–50. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Lozinskaya, E.I.; Vlasov, P.S.; Morozova, S.M.; Antonov, D.Y.; Aubert, P.-H.; Armand, M.; Vygodskii, Y.S. New family of highly conductive and low viscous ionic liquids with asymmetric 2,2,2-trifluoromethylsulfonyl-N-cyanoamide anion. Electrochim. Acta 2015, 175, 254–260. [Google Scholar] [CrossRef]
- Garlyauskayte, R.Y.; Bezdudny, A.V.; Michot, C.; Armand, M.; Yagupolskii, Y.L.; Yagupolskii, L.M. Efficient synthesis of N-(trifluoromethylsulfonyl)trifluoromethanesulfonimidoyl fluoride—The key agent in the preparation of compounds with superstrong electron-withdrawing groups and strong acidic properties. J. Chem. Soc. Perkin Trans. 1 2002, 1887–1889. [Google Scholar] [CrossRef]
- Garlyauskayte, R.Y.; Chernega, A.N.; Michot, C.; Armand, M.; Yagupolskii, Y.L.; Yagupolskii, L.M. Synthesis of new organic super acids—N-(trifluoromethylsulfonyl)imino derivatives of trifluoromethanesulfonic acid and bis(trifluoromethylsulfonyl)imide. Org. Biomol. Chem. 2005, 3, 2239–2243. [Google Scholar] [CrossRef]
- Matsumoto, H.; Terasawa, N.; Umecky, T.; Tsuzuki, S.; Sakaebe, H.; Asaka, K.; Tatsumi, K. Low Melting and Electrochemically Stable Ionic Liquids Based on Asymmetric Fluorosulfonyl(trifluoromethylsulfonyl)amide. Chem. Lett. 2008, 37, 1020–1021. [Google Scholar] [CrossRef]
- Han, H.-B.; Zhou, Y.-X.; Liu, K.; Nie, J.; Huang, X.; Armand, M.; Zhou, Z.-B. Efficient Preparation of (Fluorosulfonyl)(pentafluoroethanesulfonyl)imide and Its Alkali Salts. Chem. Lett. 2010, 39, 472–474. [Google Scholar] [CrossRef]
- Zhang, H.; Han, H.; Cheng, X.; Zheng, L.; Cheng, P.; Feng, W.; Nie, J.; Armand, M.; Huang, X.; Zhou, Z.-B. Lithium salt with a super-delocalized perfluorinated sulfonimide anion as conducting salt for lithium-ion cells: Physicochemical and electrochemical properties. J. Power Source 2015, 296, 142–149. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. Structure and properties of new ionic liquids based on alkyl- and alkenyltrifluoroborates. Chemphyschem 2005, 6, 1324–1332. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. Low-Melting, Low-Viscous, Hydrophobic Ionic Liquids: 1-Alkyl(Alkyl Ether)-3-methylimidazolium Perfluoroalkyltrifluoroborate. Chem.-A Eur. J. 2004, 10, 6581–6591. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.S.L.; Bernardes, C.E.S.; Lozinskaya, E.I.; Shaplov, A.S.; Canongia Lopes, J.N.; Marrucho, I.M. Neat ionic liquids versus ionic liquid mixtures: A combination of experimental data and molecular simulation. Phys. Chem. Chem. Phys. 2019, 21, 23305–23309. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.S.L.; Malcaite, E.; Lozinskaya, E.I.; Shaplov, A.S.; Tome, L.C.; Marrucho, I.M. Poly(ionic liquid)-Ionic Liquid Membranes with Fluorosulfonyl-Derived Anions: Characterization and Biohydrogen Separation. ACS Sustain. Chem. Eng. 2020, 8, 7087–7096. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Molander, G.A.; Hoag, B.P. Improved Synthesis of Potassium (Trifluoromethyl)trifluoroborate [K(CF3BF3)]. Organometallics 2003, 22, 3313–3315. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. Viscosity of ionic liquids: An extensive database and a new group contribution model based on a feed-forward artificial neural network. J. Chem. Inf. Model. 2014, 54, 1311–1324. [Google Scholar] [CrossRef]
- Chambers, R.D.; Clark, H.C.; Willis, C.J. Some Salts of Trifluoromethylfluoroboric Acid1. J. Am. Chem. Soc. 1960, 82, 5298–5301. [Google Scholar] [CrossRef]
- Nishida, T.; Tashiro, Y.; Yamamoto, M. Physical and electrochemical properties of 1-alkyl-3-methylimidazolium tetrafluoroborate for electrolyte. J. Fluor. Chem. 2003, 120, 135–141. [Google Scholar] [CrossRef]
- Gómez, E.; Calvar, N.; Domínguez, Á. Thermal Behaviour of Pure Ionic Liquids. In Ionic Liquids; Handy, S., Ed.; IntechOpen: Rijeka, Hrvatska, 2015. [Google Scholar]
- Jin, L.; Nairn, K.M.; Forsyth, C.M.; Seeber, A.J.; MacFarlane, D.R.; Howlett, P.C.; Forsyth, M.; Pringle, J.M. Structure and Transport Properties of a Plastic Crystal Ion Conductor: Diethyl(methyl)(isobutyl)phosphonium Hexafluorophosphate. J. Am. Chem. Soc. 2012, 134, 9688–9697. [Google Scholar] [CrossRef]
- Yoshida, Y.; Muroi, K.; Otsuka, A.; Saito, G.; Takahashi, M.; Yoko, T. 1-Ethyl-3-methylimidazolium Based Ionic Liquids Containing Cyano Groups: Synthesis, Characterization, and Crystal Structure. Inorg. Chem. 2004, 43, 1458–1462. [Google Scholar] [CrossRef]
- Saroj, A.L.; Singh, R.K.; Chandra, S. Studies on polymer electrolyte poly (vinyl) pyrrolidone (PVP) complexed with ionic liquid: Effect of complexation on thermal stability, conductivity and relaxation behaviour. Mater. Sci. Eng. B 2013, 178, 231–238. [Google Scholar] [CrossRef]
- Thermodynamics, J.C.; Parajó, J.J.; Teijeira, T.; Fernández, J.; Salgado, J.; Villanueva, M. Thermal stability of some imidazolium [NTf 2] ionic liquids: Isothermal and dynamic kinetic study through thermogravimetric procedures. J. Chem. Thermodyn. 2017, 112, 105–113. [Google Scholar] [CrossRef]
- Costa, A.J.L.; Esperança, J.M.S.S.; Marrucho, I.M.; Rebelo, L.P.N. Densities and Viscosities of 1-Ethyl-3-methylimidazolium n-Alkyl Sulfates. J. Chem. Eng. Data 2011, 56, 3433–3441. [Google Scholar] [CrossRef]
- Neves, C.M.S.S.; Kurnia, K.A.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Freire, M.G.; Rebelo, L.P.N. Systematic Study of the Thermophysical Properties of Imidazolium-Based Ionic Liquids with Cyano-Functionalized Anions. J. Phys. Chem. B 2013, 117, 10271–10283. [Google Scholar] [CrossRef] [PubMed]
- Scovazzo, P.; Havard, D.; McShea, M.; Mixon, S.; Morgan, D. Long-term, continuous mixed-gas dry fed CO2/CH4 and CO2/N2 separation performance and selectivities for room temperature ionic liquid membranes. J. Memb. Sci. 2009, 327, 41–48. [Google Scholar] [CrossRef]
- Li, P.; Paul, D.R.; Chung, T.S. High performance membranes based on ionic liquid polymers for CO2 separation from the flue gas. Green Chem. 2012, 14, 1052–1063. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.; Koval, C.; Noble, R. Bulk-Fluid Solubility and Membrane Feasibility of Rmim-Based Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2006, 45, 6279–6283. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Yáñez, M.; Alves, V.D.; Palomar, J.; Moya, C.; Gorri, D.; Tomé, L.C.; Marrucho, I.M. CO2/H2 separation through poly(ionic liquid)–ionic liquid membranes: The effect of multicomponent gas mixtures, temperature and gas feed pressure. Sep. Purif. Technol. 2021, 259, 118113. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Saeed, U.; Khan, A.L.; Gilani, M.A.; Bilad, M.R.; Khan, A.U. Supported deep eutectic liquid membranes with highly selective interaction sites for efficient CO2 separation. J. Mol. Liq. 2021, 342, 117509. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Morozova, S.M.; Lozinskaya, E.I.; Vlasov, P.S.; Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M.; Vygodskii, Y.S. Turning into poly(ionic liquid)s as a tool for polyimide modification: Synthesis, characterization and CO2 separation properties. Polym. Chem. 2016, 7, 580–591. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Polymeric membranes for CO2 separation and capture. J. Memb. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Bara, J.E.; Gin, D.L.; Noble, R.D. Effect of Anion on Gas Separation Performance of Polymer−Room-Temperature Ionic Liquid Composite Membranes. Ind. Eng. Chem. Res. 2008, 47, 9919–9924. [Google Scholar] [CrossRef]
- Tomé, L.C.; Isik, M.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Novel pyrrolidinium-based polymeric ionic liquids with cyano counter-anions: HIGH performance membrane materials for post-combustion CO2 separation. J. Memb. Sci. 2015, 483, 155–165. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquid-based membranes: Influence of polycation variation on gas transport and CO2 selectivity properties. J. Memb. Sci. 2015, 486, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.; Scovazzo, P. Solubility, Diffusivity, and Permeability of Gases in Phosphonium-Based Room Temperature Ionic Liquids: Data and Correlations. Ind. Eng. Chem. Res. 2007, 46, 1369–1374. [Google Scholar] [CrossRef]
- Mahurin, S.M.; Hillesheim, P.C.; Yeary, J.S.; Jiang, D.; Dai, S. High CO2 solubility, permeability and selectivity in ionic liquids with the tetracyanoborate anion. RSC Adv. 2012, 2, 11813–11819. [Google Scholar] [CrossRef]
- Cserjési, P.; Nemestóthy, N.; Bélafi-Bakó, K. Gas separation properties of supported liquid membranes prepared with unconventional ionic liquids. J. Membr. Sci. 2010, 349, 6–11. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Tomé, L.C.; Martinho, S.; Rebelo, L.P.N.; Marrucho, I.M. Gas permeation properties of fluorinated ionic liquids. Ind. Eng. Chem. Res. 2013, 52, 4994–5001. [Google Scholar] [CrossRef]
- Bara, J.E.; Noble, R.D.; Gin, D.L. Effect of “Free” Cation Substituent on Gas Separation Performance of Polymer−Room-Temperature Ionic Liquid Composite Membranes. Ind. Eng. Chem. Res. 2009, 48, 4607–4610. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph—Optical Spectroscopy Software. Available online: http://www.effemm2.de/spectragryph (accessed on 16 December 2021).
- Gouveia, A.S.L.; Soares, B.; Simões, S.; Antonov, D.Y.; Lozinskaya, E.I.; Saramago, B.; Shaplov, A.S.; Marrucho, I.M. Ionic Liquid with Silyl Substituted Cation: Thermophysical and CO2/N2 Permeation Properties. Isr. J. Chem. 2019, 59, 852–865. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Pyrrolidinium-based polymeric ionic liquid materials: New perspectives for CO2 separation membranes. J. Memb. Sci. 2013, 428, 260–266. [Google Scholar] [CrossRef]
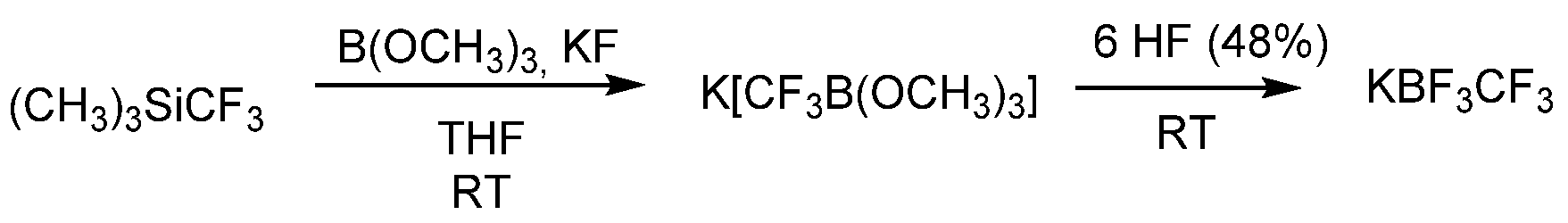
 ) and [C2mim][CF3SO2C(CN)2] (
) and [C2mim][CF3SO2C(CN)2] (  ). The errors bars are smaller than the symbols used to represent the experimental data.
). The errors bars are smaller than the symbols used to represent the experimental data.
 ) and [C2mim][CF3SO2C(CN)2] (
) and [C2mim][CF3SO2C(CN)2] (  ). The errors bars are smaller than the symbols used to represent the experimental data.
). The errors bars are smaller than the symbols used to represent the experimental data.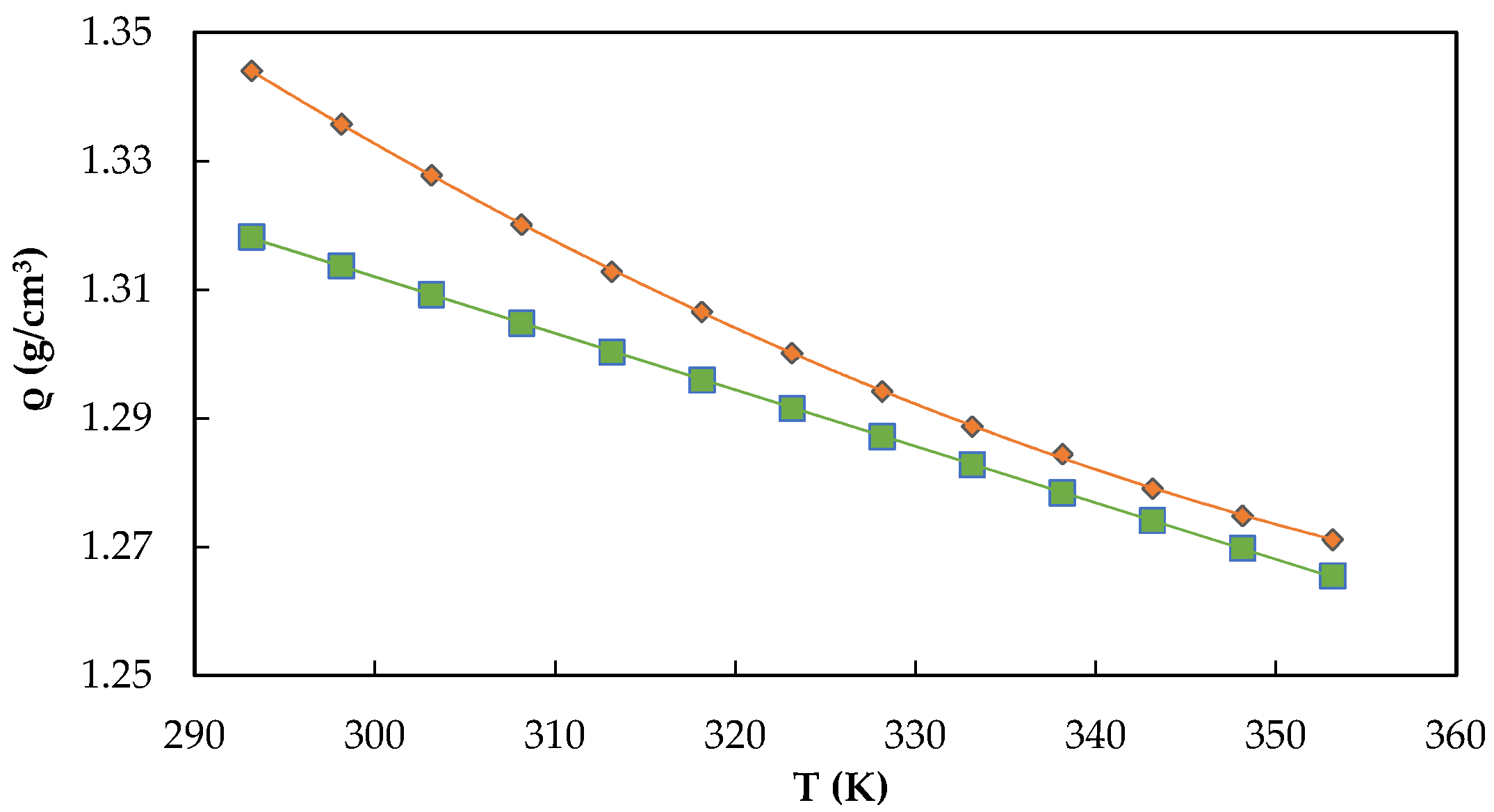
 ) and [C2mim][CF3SO2C(CN)2] (
) and [C2mim][CF3SO2C(CN)2] (  ). The errors bars marked are smaller than the symbols used to represent the experimental data.
). The errors bars marked are smaller than the symbols used to represent the experimental data.
 ) and [C2mim][CF3SO2C(CN)2] (
) and [C2mim][CF3SO2C(CN)2] (  ). The errors bars marked are smaller than the symbols used to represent the experimental data.
). The errors bars marked are smaller than the symbols used to represent the experimental data.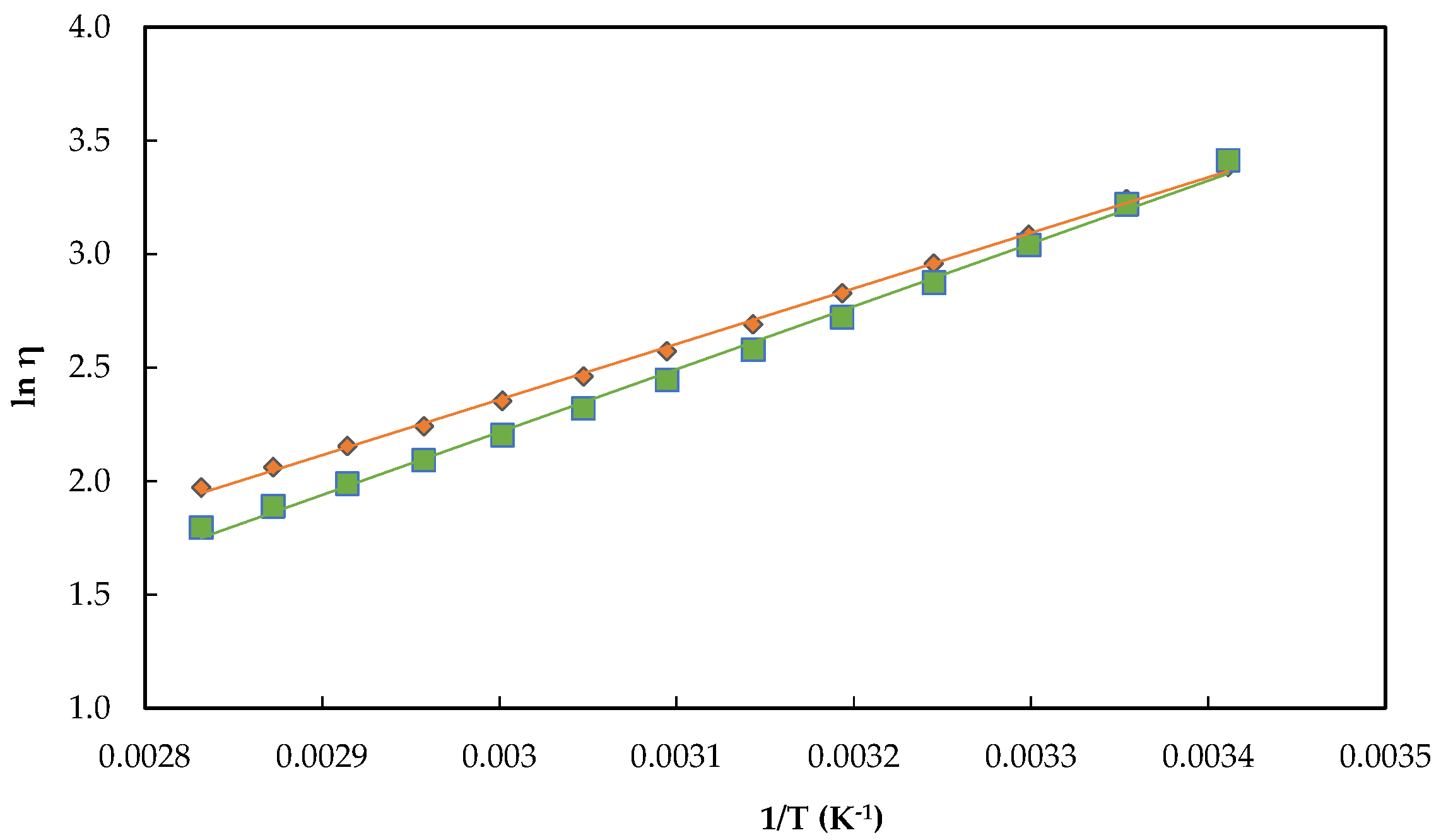
| Linear Fitting | a (g·cm−3) | b × 104 (g·cm−3·K−1) | c × 106 (g·cm−3·K−2) | R2 |
|---|---|---|---|---|
| [C2mim][CF3BF3] | 2.56 | 0.655 | 8.26 | 0.9999 |
| [C2mim][CF3SO2C(CN)2] | 1.576 | −8.79 | - | 1.000 |
| Parameter Fitting | η∞ × 103 (mPa·s) | Ea (kJ·mol−1) | R2 |
|---|---|---|---|
| [C2mim][CF3BF3] | 6.83 | 20.35 | 0.9989 |
| [C2mim][CF3SO2C(CN)2] | 2.26 | 23.02 | 0.9965 |
| Linear Fitting | wt% of Water | M (g·mol−1) | η (mPa·s) 1 | ρ (g·cm−3) 1 | Vm (cm3·mol−1) 2 |
|---|---|---|---|---|---|
| [C2mim][CF3BF3] | 0.21 | 248.09 | 29.47 | 1.344 | 384.61 |
| [C2mim][CF3SO2C(CN)2] | 0.17 | 308.28 | 30.35 | 1.318 | 233.86 |
| [C2mim][NTf2] 3 | 0.02 | 391.31 | 39.08 | 1.524 | 256.78 |
| [C2mim][N(C2F5SO2)2] 4 | 0.02 | 491.33 | 85.50 | 1.599 | 307.3 |
| [C2mim][N(CN)2] 3 | 0.09 | 177.21 | 17.95 | 1.106 | 160.24 |
| [C2mim][C(CN)3] 5 | 0.01 | 201.10 | 16.62 | 1.085 | 185.54 |
| [C2mim][TFSAM] 6 | 0.02 | 284.26 | 23.70 | 1.352 | 210.30 |
| [C2mim][BF4] 7 | 0.03 | 198.09 | 23.35 | 1.287 | 155.90 |
| SILM Membrane | |||
|---|---|---|---|
| Permeation Property | Gases | [C2mim][CF3BF3] | [C2mim][CF3SO2C(CN)2] |
| P (barrer) | CO2 | 710 ± 5 | 1095 ± 6 |
| CH4 | 32.0 ± 0.1 | 152 ± 5 | |
| N2 | 16.4 ± 0.4 | 32.8 ± 0.7 | |
| D × 1012 (m2/s) | CO2 | 205 ± 4 | 320 ± 5 |
| CH4 | 361 ± 7 | 1313 ± 14 | |
| N2 | 322 ± 8 | 370 ± 1 | |
| S × 106 (m3 (STP) m−3·Pa−1) | CO2 | 26.0 ± 0.4 | 25.7 ± 0.3 |
| CH4 | 0.66 ± 0.01 | 0.87 ± 0.03 | |
| N2 | 0.38± 0.004 | 0.67 ± 0.02 | |
| IL | T (K) | CO2/CH4 | σ 1 | CO2/N2 | σ 1 |
|---|---|---|---|---|---|
| [C2mim][CF3BF3] | 293.15 | 22.2 | 0.20 | 44.4 | 1.21 |
| 308.15 | 16.9 | 0.14 | 37.0 | 0.92 | |
| [C2mim][CF3SO2C(CN)2] | 293.15 | 7.2 | 0.28 | 33.2 | 0.85 |
| 308.15 | 8.5 | 0.29 | 24.6 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, B.F.; Nosov, D.R.; Pires, J.M.; Tyutyunov, A.A.; Lozinskaya, E.I.; Antonov, D.Y.; Shaplov, A.S.; Marrucho, I.M. Tunning CO2 Separation Performance of Ionic Liquids through Asymmetric Anions. Molecules 2022, 27, 413. https://doi.org/10.3390/molecules27020413
Soares BF, Nosov DR, Pires JM, Tyutyunov AA, Lozinskaya EI, Antonov DY, Shaplov AS, Marrucho IM. Tunning CO2 Separation Performance of Ionic Liquids through Asymmetric Anions. Molecules. 2022; 27(2):413. https://doi.org/10.3390/molecules27020413
Chicago/Turabian StyleSoares, Bruna F., Daniil R. Nosov, José M. Pires, Andrey A. Tyutyunov, Elena I. Lozinskaya, Dmitrii Y. Antonov, Alexander S. Shaplov, and Isabel M. Marrucho. 2022. "Tunning CO2 Separation Performance of Ionic Liquids through Asymmetric Anions" Molecules 27, no. 2: 413. https://doi.org/10.3390/molecules27020413
APA StyleSoares, B. F., Nosov, D. R., Pires, J. M., Tyutyunov, A. A., Lozinskaya, E. I., Antonov, D. Y., Shaplov, A. S., & Marrucho, I. M. (2022). Tunning CO2 Separation Performance of Ionic Liquids through Asymmetric Anions. Molecules, 27(2), 413. https://doi.org/10.3390/molecules27020413






