Abstract
We herein describe a general approach to 5-trifluoromethyl 1,2,4-triazoles via the [3 + 2]-cycloaddition of nitrile imines generated in situ from hydrazonyl chloride with CF3CN, utilizing 2,2,2-trifluoroacetaldehyde O-(aryl)oxime as the precursor of trifluoroacetonitrile. Various functional groups, including alkyl-substituted hydrazonyl chloride, were tolerated during cycloaddition. Furthermore, the gram-scale synthesis and common downstream transformations proved the potential synthetic relevance of this developed methodology.
1. Introduction
1,2,4-Triazoles, five-membered heterocycles with three nitrogen atoms, prevalent in both natural and synthetic molecules, find use as drugs, insecticides, and synthetic materials. Their diverse biological activities in natural products and therapeutic agents include antifungal, antibacterial, antitubercular, antiviral, anti-inflammatory, anticancer, and analgesic activities [1,2,3,4]. Some potent molecules, such as deferasirox [5], 3-triazolylphenylsulfide [6], and sitagliptin [7], contain 1,2,4-triazole as the core structural framework (Figure 1).
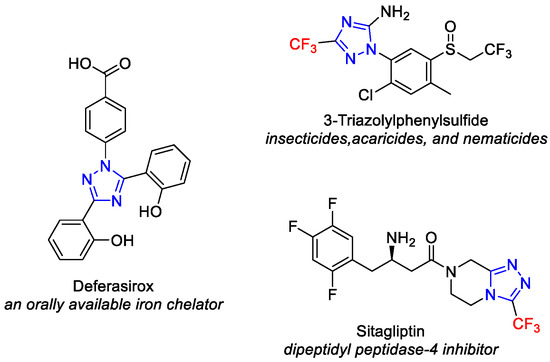
Figure 1.
Representative examples of potent molecules bearing 1,2, 4-triazole scaffold.
Among these 1,2,4-triazoles, trifluoromethylated 1,2,4-triazoles have attracted significant attention [8]. It has been well established that the introduction of trifluoromethyl into therapeutic compounds can impart beneficial effects to lipophilicity, metabolic stability, conformational preference, and bioavailability [9,10,11]. As a consequence, a broad range of protocols for the construction of trifluoromethylated 1,2,4-triazoles have been disclosed [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28].
However, current synthetic approaches for 5-trifluoromethyl 1,2,4-triazoles are scarce. Wu and co-workers reported a iodine-mediated annulation and a FeCl3-mediated cascade annulation of trifluoroacetimidoyl chlorides and hydrazones or hydrazides for the synthesis of 5-trifluoromethyl-1,2,4-triazoles (Scheme 1a) [29,30]. Wu and co-workers subsequently developed a metal-free oxidative cyclization and a copper-catalyzed intramolecular decarbonylative cyclization reaction of trifluoroacetimidohydrazides with methylhetarenes or isatins for the synthesis of 5-trifluoromethyl-1,2,4-triazoles [31,32,33] and 2-(5-trifluoromethyl-1,2,4-triazol-3-yl)aniline derivatives [34], respectively. Darehkordi and co-workers described the synthesis of 1,3-diaryl-5-(trifluoromethyl)-1H-1,2,4-triazoles via the iodine-mediated intramolecular oxidative cyclization of N-(2,2,2-trifluoro-1-(arylimino)ethyl)benzimidamide intermediates, synthesized from the reaction of N-aryl-2,2,2-trifluoroacetimidoyl chlorides and benzamide hydrochloride derivatives (Scheme 1c) [35]. More recently, the Ma research group developed a copper-catalyzed three-component reaction of aryldiazonium salts with fluorinated diazo reagents and nitriles, leading to two regiomers of trifluoromethylated N1-aryl-1,2,4-triazoles (Scheme 1d) [36]. Although these previous reports were found to be effective, they suffered from some limitations, such as utilizing transition-metal catalysis, the use of the explosiveness of diazo and diazonium reagents, the complexity of the reaction mixture, and poor regioselectivities. Therefore, there is still a need for additional methodologies to access this important chemical scaffold from readily available starting materials under mild conditions.
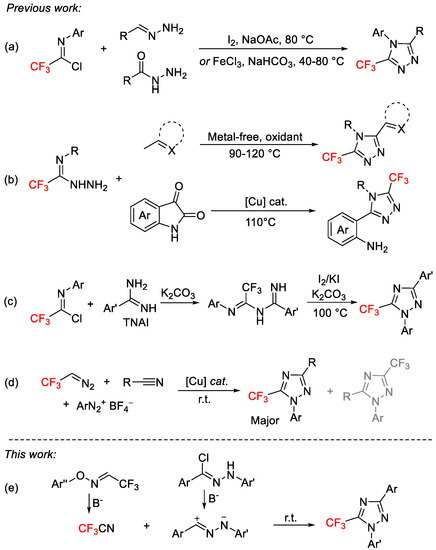
Scheme 1.
Methods for the preparation of 5-trifluoromethyl 1,2,4-triazoles.(a) FeCl3-mediated cascade annulation of trifluoroacetimidoyl chlorides (b) Copper-catalyzed intramolecular decarbonylative cyclization reaction of trifluoroacetimidohydrazides (c) Iodine-mediated intramolecular oxidative cyclization of N-(2,2,2-trifluoro-1-(arylimino)ethyl)benzimidamide (d) Copper-catalyzed three-component reaction of aryldiazonium salts with 2,2,2-trifluorodiazoethane and nitriles (e) [3 + 2]-Cycloaddition of nitrile imines with CF3CN.
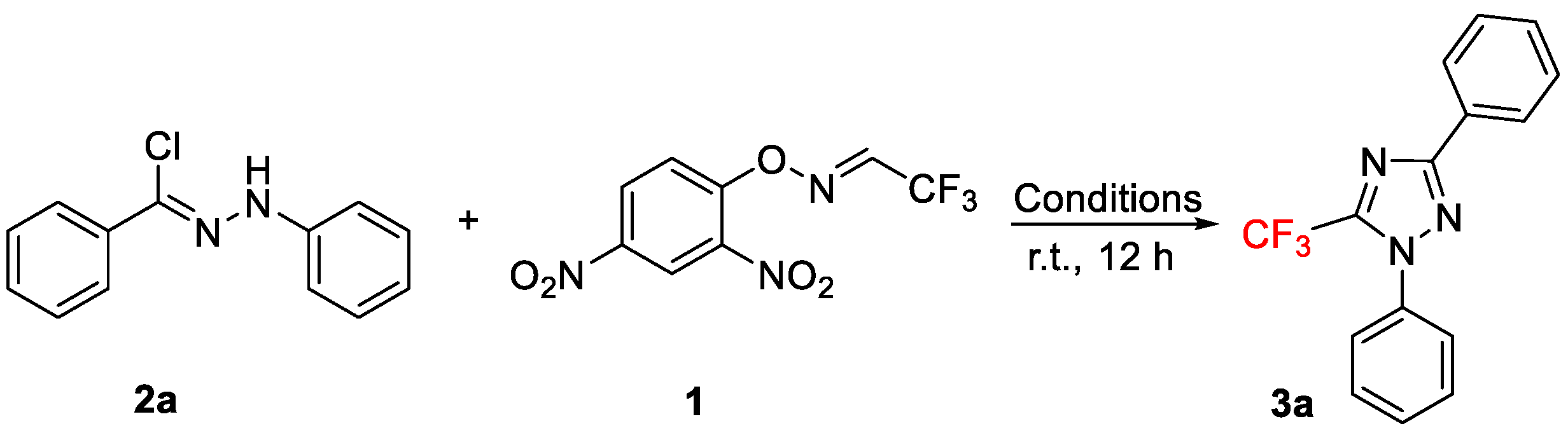
As part of our ongoing interest centered on the synthesis of trifluoromethylated heterocycles [37], we recently disclosed a novel protocol for the construction of trifluoromethylated oxadiazoles from the reaction of nitrile oxide derivatives using 2,2,2-trifluoroacetaldehyde O-(aryl)oxime as the precursor of trifluoroacetonitrile [38]. Based on these results and considering that nitrile imines, generated in situ via the treatment of the hydrazonyl halides with stoichiometric amounts of base [39], could serve as the attractive C1N2 synthons in [3 + 2] cycloaddition reactions [40,41], we now investigated the feasibility of this approach for the regioselective synthesis of 5-trifluoromethyl 1,2,4-triazoles (Scheme 1e).
2. Results
Our study commenced by choosing N-phenyl-benzohydrazonoyl chloride, 2a, and trifluoroacetaldehyde O-(2,4-dinitrophenyl) oxime, 1, as the model substrates (Table 1), using our previously reported optimized conditions for the synthesis of trifluoromethylated oxadiazoles [38]. To our delight, the reaction of 2a with 1 in a 1:2 ratio in the presence of 2.0 equiv of NEt3 afforded the desired product, 5-trifluoromethyl 1,2,4-triazole, 3a, in 3% and 8% yields in THF and DMSO, respectively (Entries 1 and 2). Among other solvents (Entries 3–6), the reaction worked best in CH2Cl2 (Entry 5), whereas in toluene, the reaction could not proceed (Entry 6). Notably, upon increasing the amount of 2a and keeping the other parameters constant, the yield of 3a was further improved (37% and 49% with 2a and 1 in 1:1 and 2:1 ratios, respectively; Entries 7 and 8). Gratifyingly, the yield of 3a was further improved to 75% by increasing the amount of NEt3 (3.0 equiv) (Entry 9). The best yield (83%) was obtained with the reaction of 2a with 1 in a 1.5:1 ratio in the presence of 3.0 equiv of NEt3 in CH2Cl2 (Entry 10).

Table 1.
Optimization of the reaction conditions a.
Subsequently, the efficacy of this developed protocol for the regioselective synthesis of 5-trifluoromethyl 1,2,4-triazoles with various hydrazonyl chlorides 2 was investigated (Figure 2). The reaction of 3-, or 4-Me-, and 4-t-Bu-substituted on the Ar moieties of hydrazonyl chlorides 2b–2d with 1 provided annulated products 3b–3d in 98%, 75%, and 60% yields, respectively. The variation in the electronic properties of the aryl substituents in the hydrazonyl chlorides had a significant effect on the reaction efficiency. The reaction of the hydrazonyl chloride possessing a strong electron-donating group such as -OMe (2e) successfully provided the corresponding triazole, 3e, with good yield (75%). On the other hand, the substrates (2f and 2g) containing strongly electron-withdrawing substituents (-CO2Me, -CF3, and –SO2N(n-Pr)2) furnished the desired triazoles (3f–3h) in moderate yields. These results are consistent with the previous observations in the synthesis of 1,2,4-triazol-3-ones by Wu and co-workers [42]. Of note, the halogen substituents, such as fluoro, and chloro, were also tested for the cycloaddition, and to our delight, good yields (56–74%) of the corresponding triazoles products, 3i–3l, were obtained. A 2-naphthyl-substituted hydrazonyl chloride also furnished the desired product, 3m, in 75% yield. Likewise, 2-thienyl substituted hydrazonyl chloride also smoothly underwent a reaction with 1, giving the desired product, 3n, in a 55% yield.
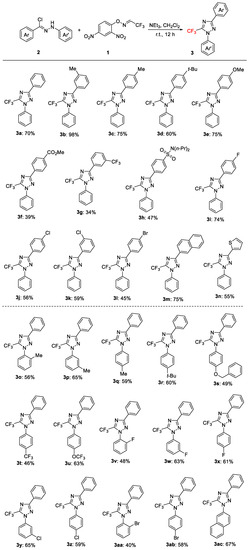
Figure 2.
Scope of the hydrazonyl chlorides. Reaction conditions: 2 (0.30 mmol), 1 (0.20 mmol), NEt3 (0.60 mmol, 3.0 equiv), CH2Cl2 (1.0 mL), r.t., 12 h, in benchtop air atmosphere. Isolated yields (calculated on basis of starting material 1).
Meanwhile, cycloaddition was tested with different substituents at the Ar′ moieties of the hydrazonyl chlorides. Under the optimized conditions, a wide range of hydrazonyl chlorides contained different functional groups, such as methyl, tert-butyl, benzyloxy, trifluoromethyl, trifluoromethoxy, fluoro, chloro, and bromo groups, which were well tolerated and afforded triazole products 3o–3ab in good yields (40–65%). 2-naphthyl-substituted hydrazonyl chloride was also efficient as a substrate to produce corresponding product 3ac in a 67% yield.
The structures of products 3a–3ac were confirmed by the IR spectroscopy, and 1H, 13C{1H}, 19F NMR spectroscopy (Supplementary Materials). Additionally, the structure of 3s was unambiguously confirmed via single-crystal X-ray diffraction analyses (Figure 3).
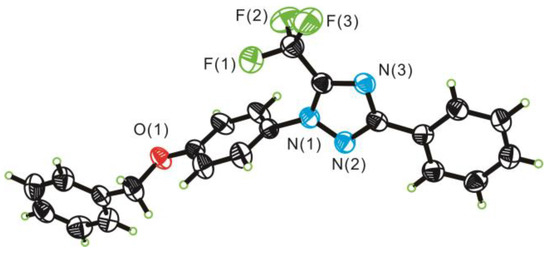
Figure 3.
ORTEP diagram of 3s with thermal ellipsoids at the 40% probability level.
Next, we attempted the cycloaddition of alkyl-substituted hydrazonyl chlorides 4 with 1 (Figure 4). Under the optimized conditions, both methyl, ethyl, and n-propyl-substituted hydrazonyl chlorides furnished the desired products, 5a–5c, in only moderate yields (22–40%), which could be a result of the inherent instability of the hydrazonyl chloride substrates with an alkyl substituent. However, ester-substituted hydrazonyl chloride 4d did not result in the desired cyclized product, 5d, under our standard or modified reaction conditions, probably due to the resulting decreased nucleophilicity of the nitrogen atom through the conjugated system from the nitrile imine intermediates.
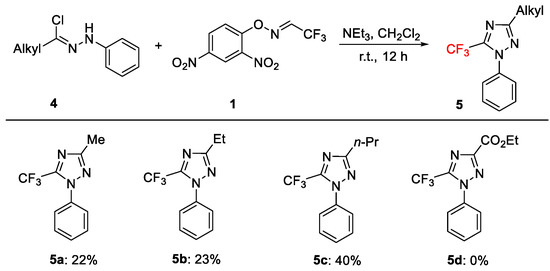
Figure 4.
Scope of alkyl-substituted hydrazonyl chlorides. Reaction conditions: 4 (0.30 mmol), 1 (0.20 mmol), NEt3 (0.60 mmol, 3.0 equiv), CH2Cl2 (1.0 mL), r.t., 12 h, in benchtop air atmosphere. Isolated yields (calculated on basis of starting material 1).
Nevertheless, a hydrazonyl chloride with a t-butyl substituent on the nitrogen atom reacted with 1 to produce the corresponding product, 5e, in a 26% yield (Scheme 2).
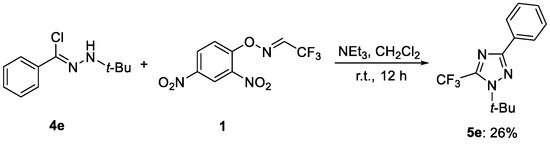
Scheme 2.
Synthesis of 5e.
To assess the scale-up of the procedure, the reaction of 2a with 1 as the representative example was investigated on a 10.0 mmol scale (Scheme 3). Under the optimized reaction conditions, the cycloaddition occurred to give 3a in a 56% (1.63 g) yield.
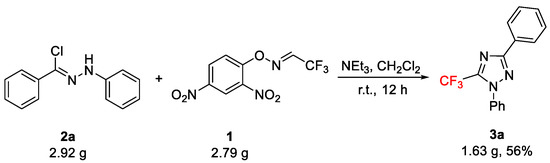
Scheme 3.
Gram-scale synthesis of 3a.
To further demonstrate the synthetic utility of the products, Heck reaction and Sonogashira coupling of bromide 3l were carried out with p-methylstyrene and phenylacetylene to afford the corresponding products, 6 and 7, in 62% and 71% yields, respectively (Scheme 4a). Finally, the bromination of the C–H bond of 3e was performed under oxidative conditions to furnish product 8 in a 74% yield (Scheme 4b).
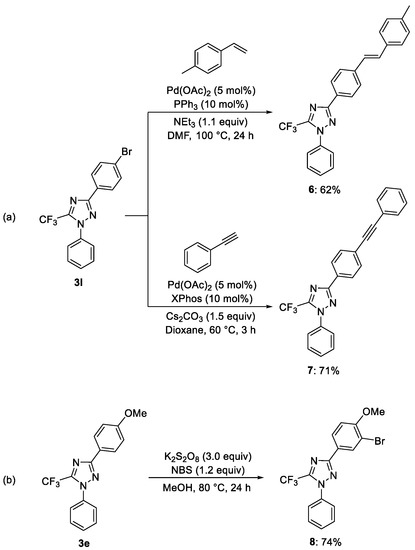
Scheme 4.
Synthetic utility. (a) Heck reaction and Sonogashira coupling with 3l (b) Bromination of 3e.
Based on the results obtained from these experiments and literature reports [38,43], a plausible mechanism for the formation of 5-trifluoromethyl 1,2,4-triazole (3) was proposed (Scheme 5). The nitrile imine generated in situ from hydrazonyl chloride 2 in the presence of a base underwent regioselective [3 + 2] cycloaddition with the in situ-generated CF3CN from 1 to generate the desired product, 3.
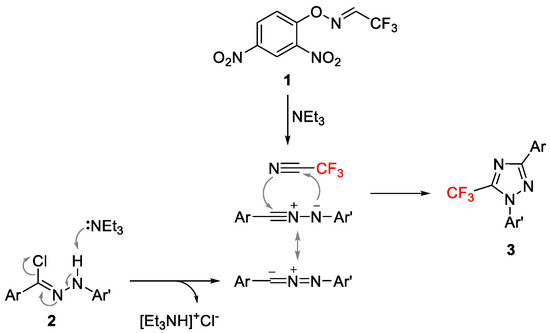
Scheme 5.
Proposed mechanism for the formation of 3.
3. Materials and Methods
1H NMR, 19F NMR, and 13C NMR spectra were recorded using a Bruker AVIII 400 spectrometer. 1H NMR and 13C NMR chemical shifts were reported in parts per million (ppm) downfield from tetramethylsilane, and 19F NMR chemical shifts were determined relative to CFCl3 as the external standard; low field was positive. Coupling constants (J) are reported in Hertz (Hz). The residual solvent peak was used as an internal reference: 1H NMR (CDCl3 δ 7.26), 13C NMR (CDCl3 δ 77.0). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. The infrared (IR) spectra were recorded using Nicolet iS50 at room temperature. HRMS were obtained from State Key Discipline Testing Center for Physical Chemistry of Fuzhou University. Trifluoroacetaldehyde O-(2,4-dinitrophenyl) oxime 1 [38], and N′-phenylacylhydrazides and hydrazonoyl chlorides [44,45] were prepared according to the published procedures. Starting materials and solvents that were received from commercial sources were used without further purification. Column chromatography purifications were performed via flash chromatography using Merck silica gel 60.
Caution: It is known that trifluoroacetonitrile is a highly toxic gas (boiling point, −64 °C) and must be handled with care. The rapid evolution of CF3CN gas occurs when this precursor reacts with base. All operations were performed in a fume hood under good conditions.
General Procedure for the Synthesis of 5-Trifluoromethyl 1,2,4-Triazoles 3
A mixture of hydrazonoyl chloride, 2 (0.30 mmol, 1.5 equiv), and trifluoroacetaldehyde O-(2,4-dinitrophenyl) oxime, 1 (54.1 mg, 0.20 mmol, 1.0 equiv), in CH2Cl2 (1.0 mL) was added to a Schlenk tube equipped with a stir bar. Then, NEt3 (60.6 mg, 83.2 μL, 0.60 mmol, 3.0 equiv) was added. The tube was immediately sealed with a Teflon cap and stirred at room temperature for 12 h. After the reaction was terminated, the solvent was removed in vacuo under reduced pressure. Product 3 was purified via flash column chromatography on silica gel with petroleum ether and CH2Cl2 as eluent.
Crystal Structure Analyses
The suitable crystals of 3s were mounted on quartz fibers and X-ray data collected on a Bruker AXS APEX diffractometer, equipped with a CCD detector at −50 ºC, using MoKα radiation (λ 0.71073 Å). The data was corrected for Lorentz and polarisation effect with the SMART suite of programs and for absorption effects with SADABS [46]. Structure solution and refinement were carried out with the SHELXTL suite of programs. The structure was solved by direct methods to locate the heavy atoms, followed by difference maps for the light non-hydrogen atoms. CCDC 2183507 contain the supplementary crystallographic data. These data can also be obtained free of charge at ccdc.cam.ac.uk/structures/ from the Cambridge Crystallographic Data Centre.
4. Conclusions
In summary, we accomplished a [3 + 2]-cycloaddition of nitrile imines with CF3CN for the synthesis of 5-trifluoromethyl 1,2,4-triazoles utilizing 2,2,2-trifluoroacetaldehyde O-(aryl)oxime as the precursor of trifluoroacetonitrile. The exclusive regioselectivity, functional group tolerance, mild reaction conditions, and efficient scalability are the important practical advantages.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27196568/s1, Experimental procedures and copies of spectroscopic characterization of all new compounds.
Author Contributions
Conceptualization, Y.Y. (Yi You) and Z.W.; Investigation, B.L., Z.Z. and Y.Y. (Yunfei Yao); Project administration, Z.W.; Supervision, Z.W.; Writing—original draft, Z.W.; Writing—review and editing, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by National Natural Science Foundation of China (21772022 and 22171124), Minjiang University, and Fuzhou University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3, 5, and 6–8 are available from the authors.
References
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in Synthetic Approach to and Antifungal Activity of Triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Wang, Y. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Sumran, G. An Insight on Medicinal Attributes of 1,2,4-Triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M. Deferasirox. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2008; pp. 1–4. [Google Scholar]
- Toriyabe, K.; Yamaguchi, M.; Itou, Y.; Kinpara, S.; Yano, H.; Takahashi, S.; Yonekura, N.; Hamaguchi, R. Preparation of 3-Triazolylphenylsulfide Compounds as Insecticides/Acaricides/Nematicides for Agricultural or Horticultural Plants. Patent WO2006043635A1, 2006. [Google Scholar]
- Plosker, G.L. Sitagliptin: A review of its use in patients with type 2 diabetes mellitus. Drugs 2014, 74, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Ostrovskii, V.A.; Trifonov, R.E. Fluorinated Triazoles and Tetrazoles. In Fluorine in Heterocyclic Chemistry; Nenajdenko, V., Ed.; Springer: Berlin, Germany, 2014; Volume 1, pp. 459–513. [Google Scholar]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Brown, H.C.; Cheng, M.T. Nucleophilic Attack on the 2,5-Bis(perfluoroalkyl)-1,3,4-oxadiazoles. I. Synthesis of 3,5-Bis(perfluoroalkyl)-1,2,4-triazoles and 4-Methyl-1,2,4,4H-triazoles1. J. Org. Chem. 1962, 27, 3240–3243. [Google Scholar] [CrossRef]
- Czollner, L.; Szilgyi, G.; Lang, J.; Janky, J. 1,2,4-Triazoles, II Synthesis of 1,5-diphenyl-3-trifluoromethyl-1H-1,2,4-triazoles. Monatsh. Chem. 1988, 119, 349–353. [Google Scholar] [CrossRef]
- Reitz, D.B.; Finkes, M.J. Reaction of 2,5-bis(trifluoromethyl)-1,3,4-oxadiazole with hydrazine. The synthesis of 4-amino-3,5-bis(trifluoromethyl)-4H-1,2,4-triazole. J. Org. Chem. 1989, 54, 1760–1762. [Google Scholar] [CrossRef]
- Reitz, D.B.; Finkes, M.J. Reaction of 2,5-bis(trifluoromethyl)-1,3,4-oxadiazole with primary amines. Synthesis of 4-substituted-3,5-bis(trifluoromethyl)-4H-1,2,4-triazoles. J. Heterocycl. Chem. 1989, 26, 225–230. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; Tipping, A.E. The Synthesis from 2.5-Dichloro-1.1.1.6.6,6-hexafluoro-3.4-diazahexa-2.4- diene of 4H-3.5-Bis(trifluoromethyl)-1.2,4-triazole and some 1- and 4-Substituted Derivatives. J. Fluor. Chem. 1990, 48, 149–152. [Google Scholar] [CrossRef]
- Uneyama, K.; Sugimoto, K. N-Substituted 2,2,2-trifluoroethanimidic acid 1-methylethylidene hydrazides as synthetic blocks for trifluoromethylated nitrogen heterocycles. J. Org. Chem. 1992, 57, 6014–6019. [Google Scholar] [CrossRef]
- Funabiki, K.; Noma, N.; Kuzuya, G.; Matsui, M.; Shibata, K. A Direct and General Synthesis of 5-Substituted 3-Trifluoromethyl-1,2,4-triazoles via the Three Component Condensation Reaction of Ethyl Trifluoroacetate, Hydrazine and Amidines. J. Chem. Res. 1999, 23, 300–301. [Google Scholar] [CrossRef]
- Buscemi, S.; Pace, A.; Pibiri, I.; Vivona, N.; Spinelli, D. Fluorinated heterocyclic compounds. An expedient route to 5-perfluoroalkyl-1,2,4-triazoles via an unusual hydrazinolysis of 5-perfluoroalkyl-1,2,4-oxadiazoles. J. Org. Chem. 2003, 68, 605–608. [Google Scholar] [CrossRef]
- Xue, H.; Twamley, B.; Shreeve, J.n.M. The first 1-alkyl-3-perfluoroalkyl-4,5- dimethyl-1,2,4-triazolium salts. J. Org. Chem. 2004, 69, 1397–1400. [Google Scholar] [CrossRef]
- Sibgatulin, D.; Bezdudny, A.; Mykhailiuk, P.; Voievoda, N.; Kondratov, I.; Volochnyuk, D.; Tolmachev, A. Novel Synthetic Approaches to (Trifluoromethyl)triazoles. Synthesis 2010, 2010, 1075–1077. [Google Scholar]
- Grünebaum, M.; Gerlitz, A.I.; Buchheit, A.; Jeschke, S.; Daniliuc, C.G.; Wiemhöfer, H.-D. Improved synthesis of perfluoroalkyl substituted 1, 3, 4-oxadiazoles as precursors for corresponding 1, 2, 4-triazoles. J. Fluor. Chem. 2016, 183, 30–35. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Wang, Y.-R.; Pan, J.-H.; He, Y.-H.; Yu, W.; Han, B. Deconstructive Oxygenation of Unstrained Cycloalkanamines. Angew. Chem. Int. Ed. 2020, 59, 3900–3904. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, T.; Liu, Y.; Liu, W.; Zhao, B.; Wang, Y.; Ge, Z. High Thermal Stability and Insensitive Fused Triazole-Triazine Trifluoromethyl-Containing Explosives (TFX). ACS Omega 2021, 6, 18591–18597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Peng, X.; Ma, J.A. Recent Advances in the Synthesis of CF3-Substituted Triazoles and Tetrazoles. Chin. J. Org. Chem. 2019, 39, 109. [Google Scholar] [CrossRef]
- Yang, H.; Lu, S.-N.; Song, Y.; Chen, Z.; Wu, X.-F. Copper-mediated [3 + 2] cycloaddition of trifluoroacetimidoyl chlorides and N -isocyanoiminotriphenylphosphorane for the synthesis of 3-trifluoromethyl-1,2,4-triazoles. Org. Chem. Front. 2021, 8, 5040–5044. [Google Scholar] [CrossRef]
- Lu, S.N.; Yang, H.; Zhang, J.; Chen, Z.; Wu, X.F. Oxidative Cyclization of Trifluoroacetimidohydrazides with D-Glucose for the Metal-Free Synthesis of 3-Trifluoromethyl-1,2,4-Triazoles. Adv. Synth. Catal. 2021, 363, 4982–4987. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Bian, C.; Wang, K.-H.; Wang, J.; Huang, D.; Su, Y.; Lv, X.; Hu, Y. Synthesis of 3-Trifluoromethyl-1,2,4-triazolines and 1,2,4-Triazoles via Tandem Addition/Cyclization of Trifluoromethyl N-Acylhydrazones with Cyanamide. J. Org. Chem. 2022, 87, 5882–5892. [Google Scholar] [CrossRef]
- Hu, S.; Yang, Z.; Chen, Z.; Wu, X.F. Metal-Free Synthesis of 5-Trifluoromethyl-1,2,4-Triazoles from Iodine-Mediated Annulation of Trifluoroacetimidoyl Chlorides and Hydrazones. Adv. Synth. Catal. 2019, 361, 4949–4954. [Google Scholar] [CrossRef]
- Du, S.; Wang, L.C.; Yang, Z.; Chen, Z.; Wu, X.F. A Convenient FeCl3-Mediated Synthesis of 5-Trifluoromethyl-1,2,4-triazoles from Trifluoroacetimidoyl Chlorides and Hydrazides. Adv. Synth. Catal. 2020, 362, 5130–5134. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, T.-H.; Chen, Z.; Wu, X.-F. Metal-free oxidative cyclization of trifluoroacetimidohydrazides with methylhetarenes: A facile access to 3-hetaryl-5-trifluoromethyl-1,2,4-triazoles. Org. Chem. Front. 2021, 8, 4490–4495. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, J.; Chen, Z.; Wu, X.F. Elemental Sulfur and Dimethyl Sulfoxide-Promoted Oxidative Cyclization of Trifluoroacetimidohydrazides with Methylhetarenes for the Synthesis of 3-Hetaryl-5-trifluoromethyl-1,2,4-triazoles. Chin. J. Chem. 2021, 39, 3443–3447. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, J.; Chen, Z.; Wu, X.F. Synthesis of 5-Trifluoromethyl-1,2,4-Triazoles via Metal-Free Annulation of Trifluoroacetimidohydrazides and Methyl Ketones. Adv. Synth. Catal. 2021, 363, 3060–3069. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Chen, Z.; Liu, L.; Wu, X.F. Copper-Catalyzed Decarbonylative Cyclization of Isatins and Trifluoroacetimidohydrazides for the Synthesis of 2-(5-Trifluoromethyl-1,2,4-triazol-3-yl)anilines. Adv. Synth. Catal. 2022, 364, 1044–1049. [Google Scholar] [CrossRef]
- Kazemi, E.; Darehkordi, A. Intramolecular oxidative cyclization of N-(2,2,2-trifluoro-1-(phenylimino)ethyl)benzimidamide. Mol. Divers. 2020, 24, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, F.G.; Ma, J.A. Cu-Catalysed Three-Component Reaction of Aryldiazonium Salts with Fluorinated Diazo Reagents and Nitriles. Adv. Synth. Catal. 2020, 362, 4432–4437. [Google Scholar] [CrossRef]
- Wu, W.; You, Y.; Weng, Z. Recent advances in the synthesis of fluoroalkylated compounds using fluoroalkyl anhydrides. Chin. Chem. Lett. 2022, 33, 4517–4530. [Google Scholar] [CrossRef]
- Lin, B.; Yao, Y.; Huang, Y.; Weng, Z. 2,2,2-Trifluoroacetaldehyde O-(Aryl)oxime: A Precursor of Trifluoroacetonitrile. Org. Lett. 2022, 24, 2055–2058. [Google Scholar] [CrossRef]
- Huisgen, R.; Seidel, M.; Wallbillich, G.; Knupfer, H. Diphenyl-nitrilimin und seine 1.3-dipolaren additionen an alkene und alkine. Tetrahedron 1962, 17, 3–29. [Google Scholar] [CrossRef]
- Deepthi, A.; Acharjee, N.; Sruthi, S.L.; Meenakshy, C.B. An overview of nitrile imine based [3 + 2] cycloadditions over half a decade. Tetrahedron 2022, 116, 132812. [Google Scholar] [CrossRef]
- Livingstone, K.; Little, G.; Jamieson, C. Recent Advances in the Generation of Nitrilium Betaine 1,3-Dipoles. Synthesis 2021, 53, 2395–2407. [Google Scholar]
- Du, S.; Wang, W.-F.; Song, Y.; Chen, Z.; Wu, X.-F. Palladium-Catalyzed Cascade Carbonylative Synthesis of 1,2,4-Triazol-3-ones from Hydrazonoyl Chlorides and NaN3. Org. Lett. 2021, 23, 974–978. [Google Scholar] [CrossRef]
- Zeng, H.; Fang, X.; Yang, Z.; Zhu, C.; Jiang, H. Regioselective Synthesis of 5-Trifluoromethylpyrazoles by [3 + 2] Cycloaddition of Nitrile Imines and 2-Bromo-3,3,3-trifluoropropene. J. Org. Chem. 2021, 86, 2810–2819. [Google Scholar] [CrossRef]
- Tirapegui, C.; Acevedo-Fuentes, W.; Dahech, P.; Torrent, C.; Barrias, P.; Rojas-Poblete, M.; Mascayano, C. Easy and rapid preparation of benzoylhydrazides and their diazene derivatives as inhibitors of 15-lipoxygenase. Bioorg. Med. Chem. Lett. 2017, 27, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Yavari, I.; Khaledian, O. A formal [3+2] cycloaddition reaction of N-methylimidazole as a masked hydrogen cyanide: Access to 1,3-disubstitued-1H-1,2,4-triazoles. Chem. Commun. 2020, 56, 9150–9153. [Google Scholar] [CrossRef] [PubMed]
- SHELXTL, version 5.03; Bruker Analytical X-ray Systems: Madison, WI, USA, 1997.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).