Forensic Toxicological Aspects of Misoprostol Use in Pharmacological Abortions
Abstract
1. Introduction
Case History
- Case 1
- Case 2
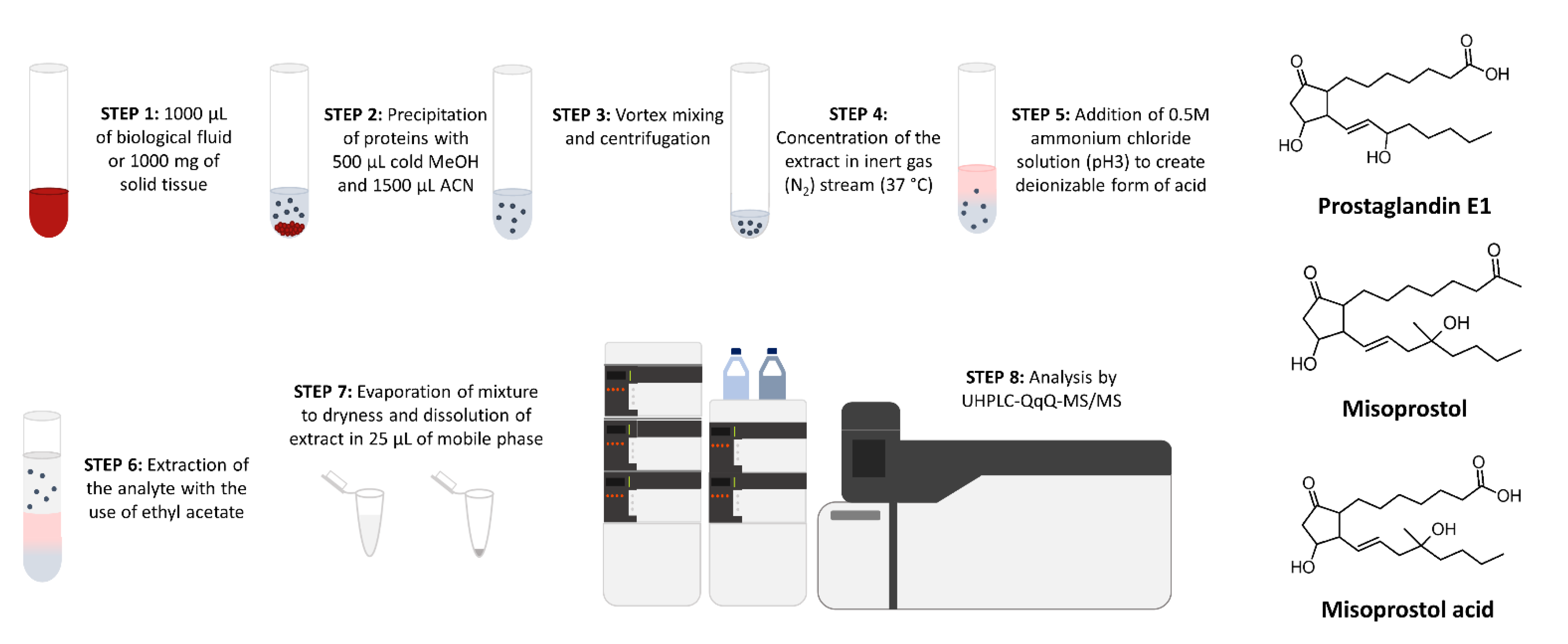
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Blank Material
2.4. Sample Preparation
2.5. Working Solutions, Calibration Curve, Quality Control Samples
2.6. Validation
3. Results
3.1. Method Development
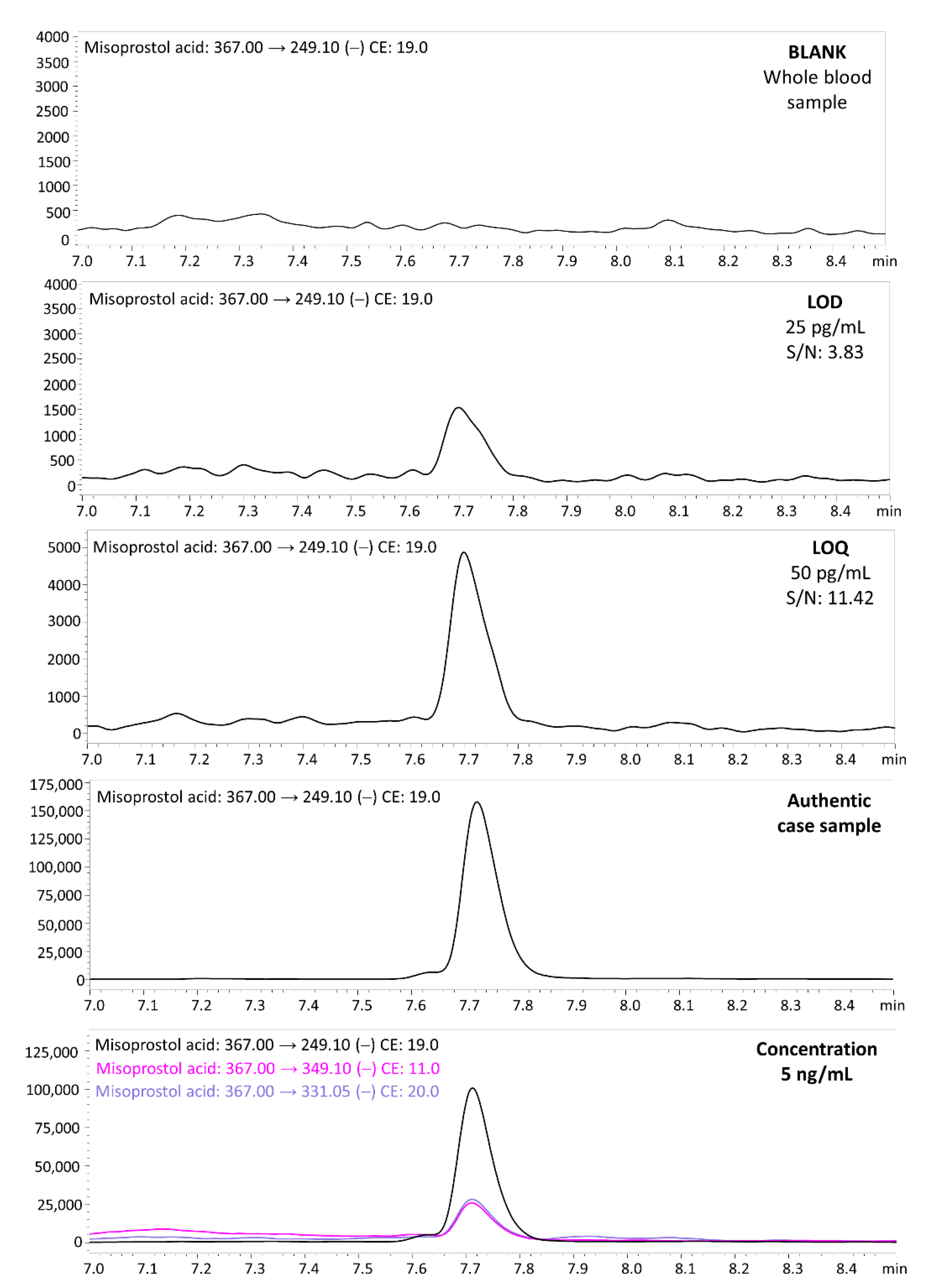
3.2. Validation Results
3.3. Autopsy, Histopatological and Toxicological Findings
- Case 1
- Case 2
4. Discussion
5. Conclusions
- Misoprostol acid passes through breastmilk;
- Average concentrations of misoprostol acid in clinical studies (biological specimens collected from women), depending on the route of administration, are in the range of 27.2─2683 pg/mL;
- Misoprostol acid is highly thermally unstable;
- Misoprostol is rapidly converted into misoprostol acid, which is rapidly eliminated from the body;
- Approximately two days after taking misoprostol (at the dose as in Case 1), misoprostol acid is not determinable in maternal blood due to progressive metabolism and/or degradation processes;
- The developed method allows detection of misoprostol acid in blood at concentrations as observed in clinical studies;
- Concentrations of misoprostol acid in postmortem materials were in range of 309─2332 pg/mL.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Unsafe Abortion: Global and Regional Estimates of the Incidence of Unsafe Abortion and Associated Mortality in 2008, 6th ed.; WHO: Geneva, Switzerland, 2008; Available online: https://www.who.int/publications/i/item/9789241501118 (accessed on 8 September 2022).
- Ganatra, B.; Gerdts, C.; Rossier, C.; Johnson, B.R., Jr.; Tunçalp, Ö.; Assifi, A.; Sedgh, G.; Singh, S.; Bankole, A.; Popinchalk, A.; et al. Global, regional, and subregional classification of abortions by safety, 2010–2014: Estimates from a Bayesian hierarchical model. Lancet 2017, 90, 2372–2381. [Google Scholar] [CrossRef]
- Bearak, J.; Popinchalk, A.; Ganatra, B.; Moller, A.B.; Tunçalp, Ö.; Beavin, C.; Kwok, L.; Alkema, L. Unintended pregnancy and abortion by income, region, and the legal status of abortion: Estimates from a comprehensive model for 1990-2019. Lancet Glob. Health 2020, 8, e1152–e1161. [Google Scholar] [CrossRef]
- Singh, S.; Maddow-Zimet, I. Facility-based treatment for medical complications resulting from unsafe pregnancy termination in the developing world, 2012: A review of evidence from 26 countries. BJOG 2016, 123, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Gomperts, R.J.; Jelinska, K.; Davies, S.; Gemzell-Danielsson, K.; Kleiverda, G. Using telemedicine for termination of pregnancy with mifepristone and misoprostol in settings where there is no access to safe services. BJOG 2008, 115, 1171–1178. [Google Scholar] [CrossRef]
- Baulieu, E.E. The Antiprogestin Steroid RU 486 and Human Fertility Control. Baulieu, E.E., Segal, S.J., Eds.; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Allen, R.; O’Brien, B.M. Uses of misoprostol in obstetrics and gynecology. Rev. Obstet. Gynecol. 2009, 2, 159–168. [Google Scholar] [PubMed]
- Zieman, M.; Fong, S.K.; Benowitz, N.L.; Banskter, D.; Darney, P.D. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet. Gynecol 1997, 90, 88–92. [Google Scholar] [CrossRef]
- Aslan, H.; Unlu, E.; Agar, M.; Ceylan, Y. Uterine rupture associated with misoprostol labor induction in women with previous cesarean delivery. Eur. J. Obstet. Gynecol. Reprod. Biol 2004, 113, 45–48. [Google Scholar] [CrossRef]
- Plaut, M.M.; Schwartz, M.L.; Lubarsky, S.L. Uterine rupture associated with the use of misoprostol in the gravid patient with a previous cesarean section. Am. J. Obstet. Gynecol 1999, 180, 1535–1542. [Google Scholar] [CrossRef]
- Nayki, U.; Taner, C.E.; Mizrak, T.; Nayki, C.; Derin, G. Uterine rupture during second trimester abortion with misoprostol. Fetal. Diagn. 2005, 20, 469–471. [Google Scholar] [CrossRef]
- Ramadan, M.K.; Darido, J.; Bazzi, Z.; Wehbe, G.; Khaza, J.; Chahine, R. First-Trimester Placenta Percreta Causing Massive Vaginal Bleeding Following the Use of Misoprostol: A Case Report. Int. J. Clinical. Case 2020, 4, 12–15. [Google Scholar]
- Cohen, A.L.; Bhatnagar, J.; Reagan, S.; Zane, S.B.; D’Angeli, M.A.; Fischer, M.; Killgore, G.; Kwan-Gett, T.S.; Blossom, D.B.; Shieh, W.J.; et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet. Gynecol 2007, 110, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Bhatnagar, J.; Guarner, J.; Reagan, S.; Hacker, J.K.; Van Meter, S.H.; Poukens, V.; Whiteman, D.B.; Iton, A.; Cheung, M.; et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N. Engl. J. Med. 2005, 353, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.; Lourenço, A.V.; Ribeirinho, A.; Ferreira, H.; Graça, L.M. Maternal death related to misoprostol overdose. Obstet. Gynecol 2007, 109, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.R.; Van Zee, A. Overdosage of misoprostol in pregnancy. Am. J. Obstet. Gynecol 1994, 171, 561–562. [Google Scholar] [CrossRef]
- Bentov, Y.; Sheiner, E.; Katz, M. Misoprostol overdose during the first trimester of pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 108–109. [Google Scholar] [CrossRef]
- Graber, D.J.; Meier, K.H. Acute misoprostol toxicity. Ann. Emerg. Med. 1991, 20, 549–551. [Google Scholar] [CrossRef]
- Jones, M.M.; Fraser, K. Misoprostol and attempted self-induction of abortion. J. R. Soc. Med. 1998, 91, 204–205. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.N.; Kim, N.S.; Park, H.J.; Park, S.; Shin, D.; Kang, H. Detection of Illegal Abortion-Induced Drugs Using Rapid and Simultaneous Method for the Determination of Abortion-Induced Compounds by LC–MS/MS. Chromatographia 2019, 82, 1365–1371. [Google Scholar] [CrossRef]
- Watzer, B.; Seyberth, H.W.; Schweer, H. Determination of misoprostol free acid in human breast milk and serum by gas chromatography/negative ion chemical ionization tandem mass spectrometry. J. Mass. Spectrom 2002, 37, 927–933. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Song, B.; Zhong, D. Determination of misoprostol acid in human plasma by liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 852, 122–127. [Google Scholar] [CrossRef]
- Vijaya Bharathi, D.; Jagadeesh, B.; Hotha, K.K.; Patil, U.; Bhushan, I. Development and validation of highly sensitive method for determination of misoprostol free acid in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry: Application to a clinical pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.S.; Ajenjo, A.C.; Dias, M.J. Validated method for the determination of misoprostol acid in whole blood by ultra-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 71, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, K.G.; Marions, L.; Rodriguez, A.; Spur, B.W.; Wong, P.Y.; Bygdeman, M. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet. Gynecol 1999, 93, 275–280. [Google Scholar] [CrossRef]
- De Oliveira Filgueira, G.C.; de Fátima Pinto Rodrigues, G.; Benzi, J.R.L.; Nardotto, G.H.B.; Marques, M.P.; Lanchote, V.L.; Cavalli, R.C. Determination of misoprostol acid in plasma samples by UPLC-MS/MS with application in a maternal-fetal pharmacokinetic study following a low misoprostol dose vaginally to induce labor. J. Pharm. Biomed. Anal. 2021, 202, 114138. [Google Scholar] [CrossRef]
- Watzer, B.; Lusthof, K.J.; Schweer, H. Abortion after deliberate Arthrotec® addition to food. Mass spectrometric detection of diclofenac, misoprostol acid, and their urinary metabolites. Int. J. Legal. Med. 2015, 129, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Wagrowski-Diehl, D.M.; Lu, Z.; Mazzeo, J.R. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 852, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination. Toxics 2022, 10, 421. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, K.N.; Kim, Y.N.; Kim, J.H.; Kim, J.Y.; Sim, S.J.; Lee, H.S. Development and validation of a selective and sensitive LC-MS/MS method for determination of misoprostol acid in human plasma: Application to bioequivalence study. Anal. Sci. Technol. 2015, 28, 17–25. [Google Scholar] [CrossRef][Green Version]
- Vogel, D.; Burkhardt, T.; Rentsch, K.; Schweer, H.; Watzer, B.; Zimmermann, R.; Von Mandach, U. Misoprostol versus methylergometrine: Pharmacokinetics in human milk. Am. J. Obstet. Gynecol. 2004, 191, 2168–2173. [Google Scholar] [CrossRef]
- Tang, O.S.; Schweer, H.; Seyberth, H.W.; Lee, S.W.; Ho, P.C. Pharmacokinetics of different routes of administration of misoprostol. Hum. Reprod 2002, 17, 332–336. [Google Scholar] [CrossRef]
- Fiala, C.; Aronsson, A.; Granath, F.; Stephansson, O.; Seyberth, H.W.; Watzer, B.; Gemzell-Danielsson, K. Pharmacokinetics of a novel oral slow-release form of misoprostol. Hum. Reprod. 2005, 20, 3414–3418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, R.U.; El-Refaey, H.; Sharma, S.; Sooranna, D.; Stafford, M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet. Gynecol. 2004, 103, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Meckstroth, K.R.; Whitaker, A.K.; Bertisch, S.; Goldberg, A.B.; Darney, P.D. Misoprostol administered by epithelial routes: Drug absorption and uterine response. Obstet. Gynecol 2006, 108, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, A.; Fiala, C.; Stephansson, O.; Granath, F.; Watzer, B.; Schweer, H.; Gemzell-Danielsson, K. Pharmacokinetic profiles up to 12 h after administration of vaginal, sublingual and slow-release oral misoprostol. Hum. Reprod 2007, 22, 1912–1918. [Google Scholar] [CrossRef]
- Lee, V.C.; Yung, S.S.; Li, R.H.; Watzer, B.; Schweer, H.; Ng, E.H.; Ho, P.C. A randomized comparison of pharmacokinetics of a single vaginal dose of dry misoprostol or misoprostol moistened with normal saline or with acetic acid. Hum. Reprod 2011, 26, 2981–2987. [Google Scholar] [CrossRef]
- Schaff, E.A.; DiCenzo, R.; Fielding, S.L. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception 2005, 71, 22–25. [Google Scholar] [CrossRef]
- Frye, L.J.; Byrne, M.E.; Winikoff, B. A crossover pharmacokinetic study of misoprostol by the oral, sublingual and buccal routes. Eur. J. Contracept. Reprod. Health Care 2016, 21, 265–268. [Google Scholar] [CrossRef]
- Khan, R.U.; El-Refaey, H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet. Gynecol 2003, 101, 968–974. [Google Scholar] [CrossRef]
- Hopson, D.L.; Ross, J. Maternal Abortifacient use for Clandestine Abortion. Acad. Forensic. Pathol 2016, 6, 663–672. [Google Scholar] [CrossRef]
- Nishibayashi, S.W.; Andrassy, R.J.; Woolley, M.M. Congenital cystic adenomatoid malformation: 1 30-year experience. J. Pediatr. Surg. 1981, 16, 704–706. [Google Scholar] [CrossRef]
- Roberts, D.J. Placental pathology, a survival guide. Arch. Pathol. Lab. Med. 2008, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
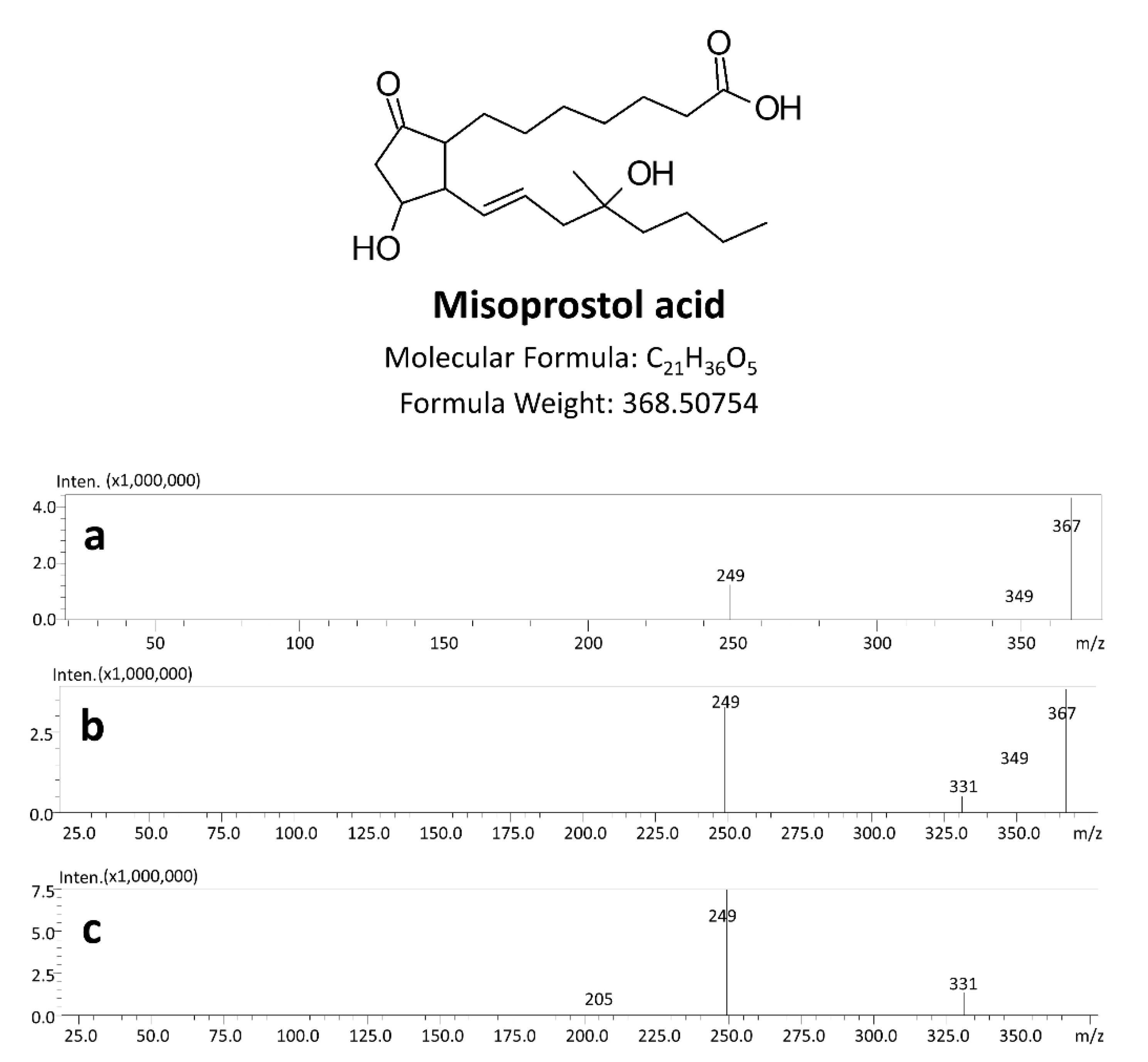
| Compounds | Retention Time (min) | Precursor Ions (m/z) | Product Ions (m/z) | Dwell Time (ms) | Q1 Pre Bias (V) | CE (V) | Q3 Pre Bias (V) |
|---|---|---|---|---|---|---|---|
| Misoprostol acid | 7.71 | 367.0 | 249.1 * | 30 | 19 | 19 | 12 |
| 349.1 | 26 | 11 | 12 | ||||
| 331.05 | 13 | 20 | 12 | ||||
| Misoprostol acid-d5 | 7.70 | 372.5 | 249.0 * | 30 | 14 | 17 | 19 |
| 354.1 | 26 | 11 | 12 | ||||
| 336.05 | 13 | 20 | 12 |
| Validation Parameters | ||||||
|---|---|---|---|---|---|---|
| Concentration Level [pg/mL] | Intraday | Interday | Recovery [%] | Matrix Effect [%] | ||
| Precision [%] | Accuracy [%] | Precision [%] | Accuracy [%] | |||
| 100 | 13.0 | 5.6 | 5.9 | 13.0 | 88.3 | −11.7 |
| 1000 | 12.2 | 2.0 | 9.7 | 13.7 | 95.0 | −5.0 |
| 10000 | 6.7 | 2.8 | 6.7 | 2.4 | 95.1 | −4.9 |
| Dose (Route) | Number of Participants | Type of Biological Sample | Mean Time of Maximum Concentration (Tmax) [Time Min] | Mean Maximum Drug Concentration (Cmax) [Pg/Ml] | Ref. |
|---|---|---|---|---|---|
| 400 µg (oral) | 45 | human plasma | 15.0 ± 4.8 | 777.74 ± 259.80 | [30] |
| 200 µg (oral) | 10 | human milk | 66.0 ± 12.0 | 7.6 ± 2.8 | [31] |
| 400 µg (sublingual) | 10 | human plasma | 26.0 ± 11.5 | 574.8 ± 250.7 | [32] |
| 400 µg (oral) | 10 | 27.5 ± 14.8 | 287.6 ± 144.3 | ||
| 400 µg (vaginal) | 10 | 72.0 ± 34.5 | 125.2 ± 53.8 | ||
| 400 µg (vaginal+water) | 10 | 75.0 ± 31.6 | 162.8 ± 57.1 | ||
| 400 µg slow release (oral) | 10 | human plasma | 54.0 ± 46.5 | 27.2 ± 14.5 | [33] |
| 800 µg slow release (oral) | 11 | 81.8 ± 111.5 | 43.5 ± 17.7 | ||
| 400 µg (oral) | 10 | 36.0 ± 12.6 | 186.2 ± 118.8 | ||
| 400 µg (oral) | 9 | human plasma | 14.2 ± 7.0 | 258.7 ± 83.8 | [34] |
| 400 µg (rectal) | 9 | 71.7 ± 23.5 | 86.8 ± 44.7 | ||
| 400 µg (vaginal) | 9 | 65.0 ± 21.2 | 210.8 ± 63.0 | ||
| 400 µg (oral) | 10 | human plasma | 34.0 ± 17.0 | 277.0 ± 124.0 | [8] |
| 400 µg (vaginal) | 10 | 80.0 ± 27.0 | 165.0 ± 86.0 | ||
| 400 µg (vaginal) | 10 | human serum | 91.5 ± 82.0 | 445.9 ± 428.7 | [35] |
| 400 µg (vaginal) | 10 | 51.0 ± 20.2 | 427.1 ± 235.5 | ||
| 400 µg (buccal) | 10 | 84.0 ± 81.9 | 264.8 ± 170.7 | ||
| 400 µg (rectal) | 10 | 19.5 ± 14.2 | 202.2 ± 195.7 | ||
| 800 µg slow release (oral) | 11 | human serum | 96.0 ± 168 | 78.8 ± 51.1 | [36] |
| 400 µg (sublingual) | 9 | 30.0 ± 0.0 | 580 ± 178.1 | ||
| 400 µg (vaginal) | 10 | 102.0 ± 72.0 | 117.7 ± 42.1 | ||
| 400 µg (vaginal) | 14 | human serum | 120 ± 90 | 262.6 ± 201.1 | [37] |
| 400 µg (vaginal) misoprostol tablets moistened with 3 mL of saline solution | 14 | 56.8 ± 45.6 | 1092.4 ± 1538.3 | ||
| 400 µg (vaginal) misoprostol tablets moistened with 3 mL of 5% acetic acid | 14 | 52.5 ± 37.16 | 703.4 ± 464.6 | ||
| 800 µg (sublingual) | 10 | human plasma | 30.0 | 1140 (817–2060) | [38] |
| 800 µg (buccal) | 8 | 30.0 | 229 (140 –1160) | ||
| 800 µg (oral) | 10 | human serum | 20.7 ± 11.16 | 2683.0 ± 1216.1 | [39] |
| 800 µg (sublingual) | 10 | 42.72 ± 24.9 | 2439.1 ± 1156.7 | ||
| 800 µg (buccal) | 10 | 78.48 ± 37.44 | 1361.1 ± 343.6 | ||
| 600 µg (rectal) | 10 | human serum | 40.5 ± 15.9 | 184.0 ± 64.5 | [40] |
| 600 µg (oral) | 10 | 18.0 ± 8.8 | 327.9 ± 102.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpot, P.; Wachełko, O.; Zawadzki, M. Forensic Toxicological Aspects of Misoprostol Use in Pharmacological Abortions. Molecules 2022, 27, 6534. https://doi.org/10.3390/molecules27196534
Szpot P, Wachełko O, Zawadzki M. Forensic Toxicological Aspects of Misoprostol Use in Pharmacological Abortions. Molecules. 2022; 27(19):6534. https://doi.org/10.3390/molecules27196534
Chicago/Turabian StyleSzpot, Paweł, Olga Wachełko, and Marcin Zawadzki. 2022. "Forensic Toxicological Aspects of Misoprostol Use in Pharmacological Abortions" Molecules 27, no. 19: 6534. https://doi.org/10.3390/molecules27196534
APA StyleSzpot, P., Wachełko, O., & Zawadzki, M. (2022). Forensic Toxicological Aspects of Misoprostol Use in Pharmacological Abortions. Molecules, 27(19), 6534. https://doi.org/10.3390/molecules27196534








