Abstract
A new series of 5-norbornene-2-carboxamide derivatives was prepared and their affinities to the 5-HT1A, 5-HT2A, and 5-HT2C receptors were evaluated and compared to a previously synthesized series of derivatives characterized by exo-N-hydroxy-5-norbornene-2,3-dicarboximidenucleus, in order to identify selective ligands for the above-mentioned subtype receptors. Arylpiperazines represents one of the most important classes of 5-HT1AR ligands, and recent research concerning new derivatives has been focused on the modification of one or more portions of such pharmacophore. The combination of structural elements (heterocyclic nucleus, propyl chain and 4-substituted piperazine), known to be critical to the affinity to 5-HT1A receptors, and the proper selection of substituents led to compounds with high specificity and affinity towards serotoninergic receptors. The most active compounds were selected for further in vivo assays to determine their functional activity. Finally, to rationalize the obtained results, molecular docking studies were performed. The results of the pharmacological studies showed that Norbo-4 and Norbo-18 were the most active and promising derivatives for the serotonin receptor considered in this study.
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is one of the major central neurotransmitters involved in many neuropsychiatric and neurological disorders, including depression, schizophrenia, migraine, pain, and Parkinson’s disease, to cite a few and, not surprisingly, its receptors have attracted interest as targets for therapeutic intervention. Early studies identified two subtypes of serotonin receptors (denoted S1 and S2) using radiolabeled ligand binding, but molecular biology and gene cloning techniques revealed that there are 14 different subtypes of receptors, divided into different families, based on sequence homology, signaling, and pharmacological criteria [1]. While 5-HT3Rs are cation-permeable ion channels, all the others are G-protein-coupled receptors (GPCRs) and are classified as rhodopsin-like receptors. From a therapeutic perspective, serotonin receptors (5-HTRs) represent an important challenge to identify novel drugs because of the numerous biological effects of the endogenous ligand. Serotonin plays a role in several physiological and pathological processes, including circadian rhythms, sexual and feeding behavior, thermoregulation, and cardiovascular function [2,3,4,5,6]. In addition, it is already known that 5-HT acts as a trophic, mitogenic, and anti-apoptotic factor for a wide range of normal and tumor cells [7,8]. One of the most prominent subtypes involved in serotonergic function is the 5-HT1A receptor, which acts as a presynaptic autoreceptor to exert a powerful inhibitory control on serotonergic neurons in Raphe nuclei. It is also expressed as a postsynaptic heteroreceptor in multiple other brain regions, such as the hippocampus, amygdala, septum, hypothalamus, basal ganglia, and brain stem, associated with different neurological disorders, as well as known to be implicated in the proliferation of human tumor cells. 5-HT1AR antagonists inhibit the growth of different prostatic tumor cell lines, such as PC-3, DU-145, and LNCaP, as well as the proliferation of PC-3 xenografted subcutaneously in athymic nude mice [9]. Concerning the 5-HT2 receptor family (5-HT2A, 5-HT2B, and 5-HT2C), 5-HT2ARs activation presents promising neurotherapeutic targets. However, extensive structural homology complicates the design of selective agents. For example, 5-HT2-type receptors share 60–70% amino acid identity, and 27–31% identity with histamine H1 receptors within structurally conserved regions. Antagonism of 5-HT2A receptors is associated with the improved efficacy of so-called atypical antipsychotics to treat schizophrenia and hallucinations. Additionally, inverse agonism of 5-HT2C receptors, represents a tool for atypical antipsychotic poly-pharmacology and could be useful for the treatment of anxiety, major depression, and schizophrenia [10]. Arylpiperazines represent one of the most important classes of molecules approved for the management of neurological disorders. Mechanistically, almost all of them act as an agonist (partial/full) of the serotonin receptor (5-HT1A). Interestingly, close inspection of their structural framework reveals that most piperazine-based FDA-approved antidepressants demonstrate the presence of piperazine as a bridge between the two aromatic/heteroaromatic flanks. A general binding conformation of different FDA-approved antidepressants in the binding pocket of 5-HT1A presents certain common features, including a salt-bridge with Asp-116, CH–π/π–π interactions, side chains of Phe361, and π–π stacking interactions with Phe-362. However, certain interactions are peculiar to specific molecules, including hydrogen bond interactions with Cys-187, Ser-199, and Asn-386. Further, detailed analysis of their bound conformation focusing on piperazine in the binding domain of serotonin receptor disclosed that the nitrogen atom of piperazine protonates at the physiological pH and forms a key salt bridge interaction with Asp116 of the binding domain of 5-HT1A. Along with other key interactions, this crucial contact stabilizes the complex of these therapeutic agents with the target. It also improves the binding affinity between the serotonin receptor and the piperazine bridge containing molecules. This key interaction also explains why piperazine moiety, a conventionally terminally attached moiety, is placed in the center of these molecules [11]. Anyway, a strong limitation in the potential use of many 5-HT1A receptor ligands as pharmacological tools is represented by their undesired high affinity for other receptors. The dopaminergic D2 receptor and α1-adrenoceptor are two other examples of receptors for which several 5-HT1A ligands show high affinity. Nevertheless, polypharmacology is considered an appropriate solution to achieve high efficacy complex therapy for neurological disorders. In fact, recent studies focusing on the search for new therapeutic agents indicates the importance of serotonin and dopamine receptors. However, it has already been demonstrated that dual- and multitarget acting compounds are useful against disorders affecting the central nervous system (CNS), which involve serotoninergic receptors and other GPCRs, such as muscarinic M4 receptors [12].
In our laboratories, there has been ongoing research to develop more selective serotoninergic ligands [13,14,15,16,17,18,19,20,21,22,23,24] to obtain novel pharmacological tools that could improve our knowledge of the signal transduction mechanism, leading to compounds with high affinity and selectivity. Previously described studies focused on the synthesis and pharmacological evaluation of a set of arylpiperazine derivatives containing an exo-N-hydroxy-5-norbornene-2,3-dicarboximide fragment nucleus. The binding data reported in these studies identified this original scaffold as an optimal structural element to enhance 5-HT1A receptor affinity [14,16].
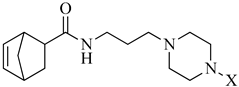
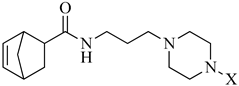
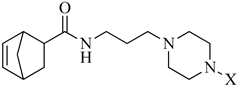
In continuation of our research program, we designed a new set of derivatives where the piperazine-N-alkyl moiety has been linked via propyl spacing unit to a 5-norbornene-2-carboxilic acid fragment as terminal part of long-chain arylpiperazines (LCAPs) (Scheme 1); this choice was made considering this scaffold as a molecular simplification of derivatives previously synthesized, embodying the exo-N-oxy-5-norbornene-2,3-dicarboximide nucleus [14,16]. Consequently, we decided to investigate how the substitution of dicarboximide moiety presents in the exo-N-oxy-5-norbornene-2,3-dicarboximide nucleus with a simple amide bond, which may influence the binding affinity/selectivity profile. The 5-norbornene-2-carboxilic acid scaffold was linked via three methylene spacing unit to the N-4-aryl-substituted piperazines and this choice was done based on our previous investigations, where, as a general trend, compounds with piperazinyl propyl chain linked to different nuclei showed good and preferential affinity for the 5-HT1AR, with respect to compounds in which the spacer is one atom shorter. Finally, the use of 5-norbornene-2-carboxilic acid as a mixture of endo/exo allowed us to investigate the influence of endo and exo stereoisomerism of synthetized compounds on the binding affinity/selectivity profile towards the serotoninergic receptors investigated. All the new compounds were tested for their functional activity or affinity to 5-HT1A, 5-HT2A, and 5-HT2C receptors and their multireceptor profiles were also evaluated in terms of functional activity for dopaminergic (D2). Moreover, compounds showing the best affinity and selectivity binding profiles towards serotoninergic receptors, have been evaluated by in vivo assay, i.e., behavioral tests, with the aim to discover novel pharmacological tools useful in treating psychiatric and neurological disorders, such as schizophrenia, depression and anxiety. Therefore, we evaluated the antipsychotic activity of the compounds in an amphetamine-induced hyperactivity test, antidepressant-like activity in the forced swim test (FST), and anxiolytic-like effects in the elevated plus-maze test (EPM). Moreover, we used additional tests, namely the spontaneous locomotor activity, rota-rod, and chimney tests, to assess potential adverse effects of the compounds. Results obtained from the tested compounds in the FST were compared with the commonly known antidepressant sertraline, and those in EPM with the clinically useful anxiolytic buspirone.
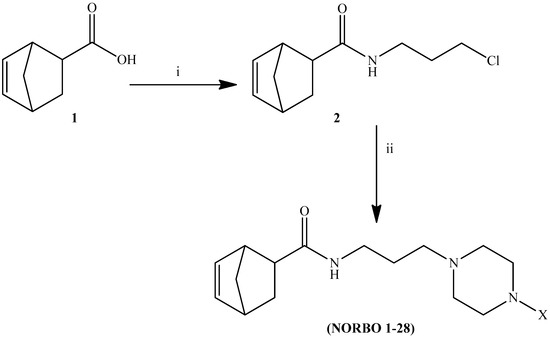
Scheme 1.
Reagents and conditions: (i) Cl(CH2)3NH2·HCl, DCC, HOBt, TEA, CH3CN, r.t., 24h; (ii) 4-X-substituted-piperazine, K2CO3, NaI, CH3CN, 70 °C, 24 h.
2. Results and Discussion
2.1. Chemistry
The general strategy for the synthesis of the target compounds (Table 1) is summarized in Scheme 1. Briefly, 5-norbornene-2-carboxilic acid reacted with 3-chloropropan-1-amine hydrochloride in acetonitrile in the presence of N,N’-dicyclohexylcarbodiimide (DCC), hydroxybenzotriazole (HOBt) and triethylamine (TEA) to give the corresponding N-(3-chloropropyl)bicyclo [2.2.1]hept-5-ene-2-carboxamide (2). Subsequent condensation of intermediate (2) with the appropriate 4-X-substituted-piperazine performed in acetonitrile (CH3CN) with potassium carbonate (K2CO3) and sodium iodide (NaI), under reflux, provided the corresponding compounds as a mixture of endo and exo isomers. The purification of the reaction mixture and the separation of the isomers were carried out by silica gel open chromatography and further crystallization from the appropriate solvent. All new compounds were characterized by mono and bidimensional NMR spectroscopy and mass spectrometry. The endo and exo stereoisomerism of the two compounds was determined by comparing their spectroscopic data with those reported in the literature for related compounds. 1H-NMR, 13C-NMR, and MS for all final compounds were consistent with the proposed structures.

Table 1.
Agonist properties of compounds Norbo 1–28 at the 5-HT1A receptor.
The olefinic protons of the endo compound of the norbornene nucleus resonate at chemical shift δ 5.97 and 6.21 while those of the exo compound resonate at δ 6.05 and 6.11. The comparison of these data with those presents in the literature allowed us to assign the endo and exo configuration to the two compounds. A further confirmation was possible thanks to the analysis of the ROESY spectrum. In the ROESY spectrum of the endo compound, there was a key correlation between the methine proton at δ 2.84 with the methylene bridge proton at δ 1.27, while in the exo compound there was a correlation between the methine proton at δ 1.96 with the olefin proton at δ 6.05 (Figure 1). These correlations together with the comparison of the data present in the literature allowed us to univocally assign the endo and exo stereoisomerism of the two compounds under examination.
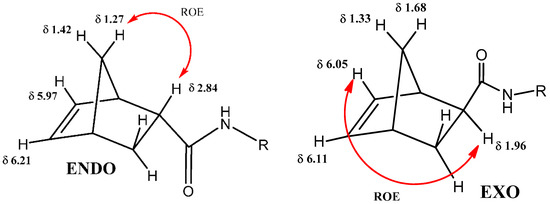
Figure 1.
Signal assignment of the ROESY spectrum for the determination endo and exo stereoisomer of norbornene derivatives.
2.2. Functional Activation of the 5-HT1A and D2 Receptors
Potencies (EC50) and efficacies (Emax) of 5-HT1A receptor activation by a series of Norbo-1-28 compounds are given in Table 1. As evidenced by the [35S]GTPγS functional assay, most compounds screened were agonists for the 5-HT1A receptor with potencies in the submicromolar and micromolar range. Compounds’ potencies differed depending on the type of substitution on the phenyl ring but did not rely too much on their stereochemistry. As revealed by one-way ANOVA, substitution of the piperazine fragment with 2,3-dimethyl- (Norbo-3, Norbo-4), 4-methoxy- (Norbo-7, Norbo-8), 2-chloro- (Norbo-9, Norbo-10) or 3-CF3-phenyl (Norbo-19, Norbo-20) moieties, enhanced the potency of 5-HT1A receptor activation by almost 10-fold for the endo isomers and 6-fold for the exo isomers on average. The EC50 values for the above-mentioned endo isomers were in the submicromolar range, i.e., 181 nM, 194 nM, 165 nM, 198 nM, and 195 nM for Norbo-4, Norbo-8, Norbo-10, and Norbo-20, respectively, while the calculated EC50 for the unsubstituted compound (Norbo-2) was 1633 nM. Potencies for the exo analogs ranged from 177.4 nM for Norbo-3 to 347.8 nM for Norbo-7, while the potency of the mother Norbo-1 was 1200 nM.
Most of the exo and endo isomer pairs did not differ in terms of potency with only two exceptions. Namely, only the endo isomer was active among 4-chlorophenyl derivatives (Norbo-11 and Norbo-12) (p < 0.001). On the other hand, the exo isomer showed greater potency among the 1,3-benzodioxole-derived compounds (Norbo-25 and Norbo-26) (p < 0.05).
In general, none of the substitutions introduced into the 4-phenylpiperazin-1-yl ring significantly increased 5-HT1A activation efficacy. However, most of the compounds still expressed higher 5-HT1A activation efficacies than the reference compound, 8-hydroxy-di-propylaminotetralin (8-OH-DPAT), classifying them as full agonists. However, the introduction of several substitutions proved detrimental for 5-HT1A activation efficacy. Namely, derivatives bearing the 2-methoxyphenyl (Norbo-5, Norbo-6), 4-chlorophenyl (Norbo-11, Norbo-12), 4-fluorophenyl (Norbo-17, Norbo-18), and 2-chlorophenyl (Norbo-9) piperazine substituents were less efficacious when compared with the unsubstituted mother compounds Norbo-1 or Norbo-2, respectively. Differences in efficacy were also evidenced among some pairs of stereoisomers. The endo isomers with monohalogenophenyl functionalities at the piperazine ring (Norbo-10, Norbo-12, Norbo-16 and Norbo-18) were characterized by higher 5-HT1A efficacies than their corresponding exo analogs.
The study also identified some inactive derivatives expressing no agonist activity for the 5-HT1A receptor. Namely, within the chlorine substituted analogs, all substitutions of the phenyl ring other than one ortho- positioned chlorine atom (Norbo-9) produced analogs devoid of 5-HT1A agonist activity. Additionally, derivatives with the furoyl (Norbo-27, Norbo-28) terminal fragment also exerted no agonist activity at the 5-HT1A receptor.
Finally, none of the compounds tested expressed no agonistic activity for the D2 receptor (Table 2). When screened for their antagonistic properties, the compounds only showed submilimolar inhibitory potencies against dopamine-induced stimulation of the D2 receptor.

Table 2.
Antagonistic activity of Norbo 1–28 compounds at the D2 receptor.
2.3. 5-HT2A and 5-HT2C Receptor Binding
All of the new compounds were tested for their affinity at 5-HT2A and 5-HT2C receptors. Some of the new synthesized derivatives showed interesting affinity values in the nanomolar range towards 5-HT2A receptors and lower affinities for 5-HT2C receptors (Table 3). Except for the outstanding 5-HT2A receptor affinity and selectivity of compound Norbo-14 (Ki = 17.93 nM with pKi = 7.75 ± 0,07) and Norbo-18 (Ki = 18.65 nM with pKi = 7.73 ± 0.11). Other interesting Ki values were those of compounds Norbo-17 (Ki = 22.66 nM with pKi =7.64 ± 0.08), Norbo-20 (Ki = 22.86 nM with pKi = 7.64 ± 0.10), Norbo-3 (Ki = 23.49 nM with pKi = 7.63 ± 0.08), and Norbo-13 (Ki = 28.81 nM with pKi = 7.54 ± 0.08). Moreover, compound Norbo-13 showed an interesting mixed 5-HT2A/5-HT2C profile with Ki values of 28.81/122 nM, whereas compounds Norbo-10, Norbo-11, and Norbo-15 presented the most attractive 5-HT2C affinity profile with a Ki value of 13, 31 and 31 nM and pKi values of 7.87 ± 0.35, 7.51 ± 0.29 and 7.50 ± 0.25, respectively. As compared to the reference 5-HT2A receptor ligand, ketanserin (pKi = 8.27 ± 0.06), one can conclude that compounds Norbo-14, Norbo-18, Norbo-17, Norbo-20, and Norbo-3 expressed satisfactory affinities to the 5-HT2A receptor. Simultaneously, the 3,4-dichloro-, 4-fluoro, 3-trifluoromethyl- and 2,3-dimethyphenyl piperazine substituents had the strongest influence on 5-HT2A receptor binding affinity. When comparing the evaluated series of arylpiperazine derivatives to 5-HT2C receptor selective ligands, such as RS-102221 (pKi = 8.25 ± 0.12), one can point to norbornene derivatives supporting the 2-chloro, 4-chloro, and 2-fluorophenylpiperazine moieties as terminal group (Norbo-10, Norbo-11, and Norbo-15) are the most promising, with their pKi values below 40 nM.

Table 3.
Affinities of compounds Norbo 1–28 for 5-HT2A and 5-HT2C receptors.
The difference in affinity observed between this new series of derivatives (Norbo 1–28) and the previously described series [14,16] characterized by analog exo-N-oxy-5-norbornene-2,3-dicarboximide nucleus linked via two or three methylene spacing unit to 4-substituted piperazines, demonstrated that a simple amide bond (Norbo 1–28) instead of a dicarboximide moiety represents a critical feature in determining differences in binding with 5-HTRs.
In fact, regarding these novel derivatives, although they have a lower affinity profile than those previously synthesized, the influence of this original scaffold associated to the appropriate substituents on the phenylpiperazine ring and heterocyclic nucleus were particularly profitable to enhance 5-HT2A and 5-HT2C receptor affinity. Anyway, in order to rationalize the differential binding affinities/activities, molecular docking studies were carried out on the complete series of derivatives.
2.4. In Vitro Evaluation of 5-HT-Evoked Contractions
Successively, the compounds Norbo-5, Norbo14, Norbo-16, Norbo-17, Norbo-18, Norbo-19, and Norbo-20 with better affinity/selectivity binding profiles towards 5-HT2A receptors were tested by in vitro assay to determine their activity on 5-HT-evoked contractions. In the rat ileum, 5-HT2A receptors are located on smooth muscles and their activation by 5-HT is known to induce contraction. Consequently, 5-HT2A antagonists depress 5-HT-induced contractions in the rat ileum [24]. According to Briejer and colleagues, we have shown that 5-HT contracted the rat ileum longitudinal muscle. In preliminary experiments we found that the neuronal blocker tetrodotoxin (0.3 µM), the muscarinic receptor antagonist atropine (1 µM), the adrenergic receptor antagonists phentolamine (10−6 M) plus propranolol (10−6 M) did not affect the contractions by 5-HT. In contrast, ketanserin (0.1 µM), at concentration that blocks 5-HT2A receptors, depressed the contractions induced by 5-HT. Collectively, these results suggest that 5-HT contracts the ileum by acting on 5-HT2A receptors located on smooth muscle while muscarinic or adrenergic receptors are not involved. Results show the potency (expressed by the IC50 value) and the efficacy (expressed by the Emax value) of the compounds under investigation in inhibiting 5-HT-induced contractions in the rat ileum (a pharmacological assay useful to detect activity towards 5-HT2A receptors). The compounds under investigation did not significantly inhibit the contractions induced by 5-HT. The rank order of efficacy was: Norbo-16 (3.51 × 10−7 M) > Norbo-5 (7.48 × 10−7 M) > Norbo-20 (7.51 × 10−7 M) > Norbo14 (9.63 × 10−7 M) > Norbo-18 (3.06 × 10−6 M) > Norbo-17 (3.25 × 10−6 M) > Norbo-19 (3.56 × 10−6 M). Concerning the potency, these compounds displayed potency approximately in the 10−7–10−6 M range, specifically, the rank order of potency was Norbo-19 (Emax = 39.00%) > Norbo-17 (Emax = 32.50%) > Norbo-18 (Emax = 25.28%) > Norbo-16 (Emax = 23.47%) > Norbo14 (Emax = 22.57%) > Norbo-20 (Emax = 21.79%) > Norbo-5 (Emax = 15.42%). Finally, none of the compounds under investigation contracted per se, the rat ileum.
2.5. Molecular Docking Studies
In order to study the ligand-receptor interactions at the molecular level and to rationalize the observed structure–activity relationships, compounds Norbo 1–28 were docked to the orthosteric sites of 5-HT1A, 5-HT2A, and 5-HT2C receptors. The visualization of molecular interactions of selected most potent compounds with the receptors under investigation is shown in Figure 1, Figure 2 and Figure 3. As the studied compounds follow the classical pharmacophore model for the aminergic G protein-coupled receptor (GPCRs) ligands [25], the electrostatic interaction between the protonatable nitrogen atom of the ligand and the conserved Asp 3.32 of the receptor is a key ligand-receptor contact for all studied complexes [26]. In the case of most ligand-receptor complexes, Trp 6.48 and Phe 6.52 are crucial residues engaged in π-π stacking contact with N-aryl moieties of the ligands, as found for many similar complexes [22,23,27,28,29]. These residues, accompanied by Phe 6.51 and His 6.55, constitute an aromatic microdomain of, i.a., serotonin and dopamine receptors [30]. The importance of these residues for binding ritanserin, an inverse agonist of serotonin 5-HT2C receptor, was confirmed by X-ray crystallography (PDB ID: 6BQH [6]) and verified by the mutation of Phe 5.47, Phe 6.44, and Trp 6.48. In addition, the serotonin receptor subtype selectivity may be governed by residues from the second extracellular loop (ecl2) [31], which serves for recognition of the “message” part of the ligand [32].
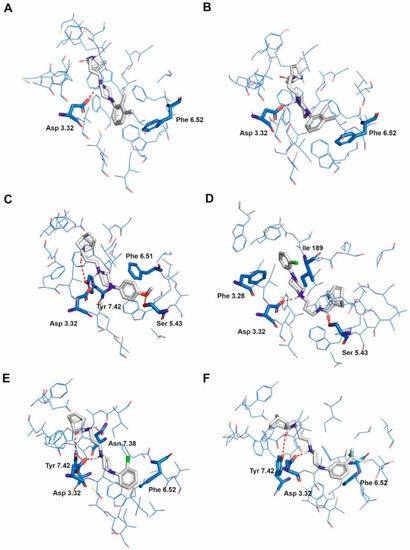
Figure 2.
Selected ligands in complex with serotonin 5-HT1A receptor: Norbo-3 (A), Norbo-4 (B), Norbo-8 (C), Norbo-9 (D), Norbo-10 (E) and Norbo-20 (F). Protein shown in wire representation with blue carbon atoms. The most important residues shown as sticks. Ligands shown as sticks with grey carbon atoms. Polar interactions shown as red dashed lines. All hydrogen atoms of the receptor and non-polar hydrogen atoms of the ligands omitted for clarity.
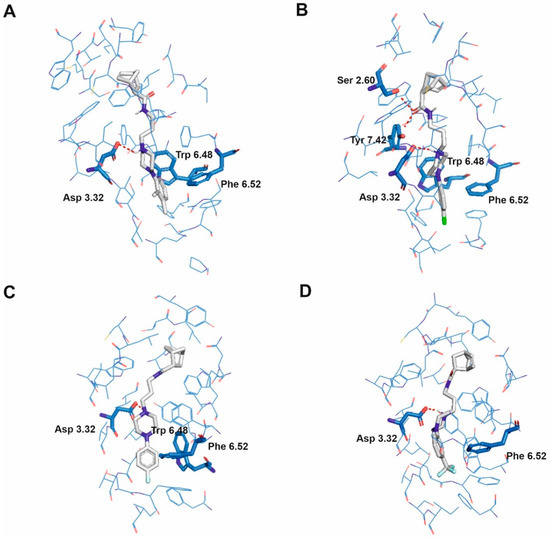
Figure 3.
Selected ligands in complex with serotonin 5-HT2A receptor: Norbo-3 (A), Norbo-14 (B), Norbo-18 (C) and Norbo-20 (D). Protein shown in wire representation with blue carbon atoms. The most important residues shown as sticks. Ligands shown as sticks with grey carbon atoms. Polar interactions shown as red dashed lines. All hydrogen atoms of the receptor and non-polar hydrogen atoms of the ligands omitted for clarity.
The mode of binding of the studied ligands is more differentiated for 5-HT1A (Figure 2) and similar for 5-HT2A (Figure 3) and 5-HT2C (Figure 4) receptors and receptors. In the case of most ligands interacting with 5-HT2A and 5-HT2C receptors, the ligands adopt an extended conformation parallel to the transmembrane helix bundle. The norbornene moiety of the ligands is directed towards the extracellular vestibule while the N-aryl group penetrates deeper into receptor aromatic microdomain which is in accordance with our previous results [22,23]. This is also in line with the data recently published by Kucwaj-Brysz et al. [33], who identified molecular docking poses of serotonin receptor ligands with the arylpiperazine moiety deeply buried in the binding pocket. Moreover, such a ligand binding mode is in agreement with the one found for ritanserin in 5-HT2C receptor (PDB ID: 6BQH [6]). In the case of 5-HT1A receptor, some ligands are able to adopt an extended conformation parallel to the transmembrane bundle similar to that described above (e.g., Norbo-3 and Norbo-4, see Figure 2A and Figure 2B, respectively), some adopt an extended conformation, but they are situated at the angle of 30–60 degrees regarding the transmembrane bundle (e.g., Norbo-8, Figure 2C, Norbo-10, Figure 2E, and Norbo-20, Figure 2F), while others adopt a bent conformation with norbornene moiety, penetrating deeper in the receptor cavity than the N-aryl group, e.g., Norbo-8 (Figure 2C).
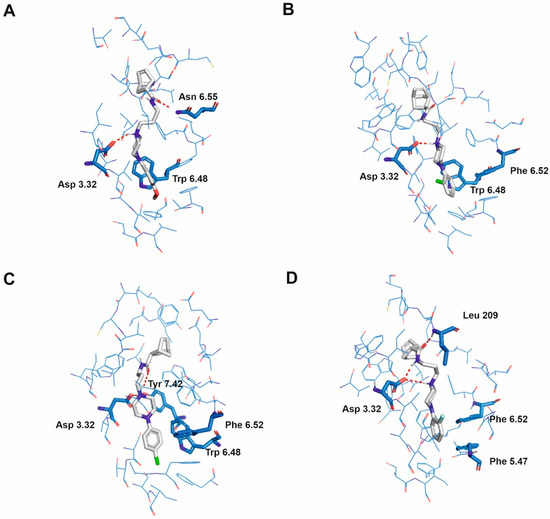
Figure 4.
Selected ligands in complex with serotonin 5-HT2A receptor: Norbo-7 (A), Norbo-10 (B), Norbo-11 (C) and Norbo-15 (D). Protein shown in wire representation with blue carbon atoms. The most important residues shown as sticks. Ligands shown as sticks with grey carbon atoms. Polar interactions shown as red dashed lines. All hydrogen atoms of the receptor and non-polar hydrogen atoms of the ligands omitted for clarity.
The affinity of the studied ligands to the serotonin receptors is mainly governed by the type of N-aryl group, as previously reported by us [22,23] and by Zagórska et al. [34] for similar series. Many potent compounds bear a halogen substituent in the aryl ring which can be rationalized by the possibility of halogen bond formation as suggested by Partyka et al. [35]. The exo/endo isomerism seems to have a less important effect as it displays no clear trend and is case-specific. This can be explained by the fact that the norbornene moiety of the ligand directs in most complexes towards the extracellular part of the receptor, constituted by flexible loops which can accommodate both isomers. However, regarding the general pharmacological profile of the compounds, the endo isomers are more beneficial, and they have been selected for animal studies (Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18, and Norbo-20). The selectivity of the studied ligands to the serotonin receptor subtypes is first governed by the residues from the ecl2. Moreover, in case of 5-HT1A receptor Phe 3.28, Ser 5.43, and Tyr 7.42 were found to be important in many ligand-receptor complexes. In summary, the performed molecular modeling study can facilitate the design of subsequent compound series with the expected serotonin receptor subtype selectivity or polypharmacology.
2.6. In Vivo Behavioral Test
Mental disorders represent a specific group of diseases since their course is miscellaneous in different patients. In fact, each patient should be treated individually, and drugs should possess multidirectional action, which increases the chances of amending the health. There is a great need to improve the treatment and dose selection for patients with mental disorders. Hence, the search for new drugs and effective therapeutic solutions is a constant problem.
Behavioral pharmacology research is the cornerstone of understanding the processes underlying the behavior of living organisms, as well as the biological basis of the behavioral, emotional and cognitive disorders that affect humans. Discoveries in this area have helped to explore the potential therapeutic effects of many substances in treating these disorders. Since biochemical abnormalities causing psychiatric disorders are not limited to single signaling pathways, a variety of screening tests are used in experimental pharmacology to reveal new potential drugs. Basic research in laboratory animals is a promising approach to study behavioral abnormalities associated with mental disorders and to identify new pharmacological therapies to address mental health challenges. Knowledge of pharmacology allows us to understand that chemicals with very specific structures and properties that, at controlled doses, can interact with the normal physiology processes to produce health-enhancing effects known as therapeutic effects. However, if dosages are insufficient or excessive, the effects will be useless or harmful (toxic), respectively [36].
Compounds Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18, and Norbo-20 were selected for further functional in vivo studies. The first part of experiments included: motor coordination and locomotor activity tests, generally accepted as basic procedures in central activity investigations of new agents [37]. Firstly, all compounds were administered at the dose of 30 mg/kg. It was observed that three of them (Norbo-4, Norbo-14 and Norbo-18) disturbed the behavior of mice in the chimney test (Figure 5A) and impaired motor coordination assessed in the rota-rod test (Figure 5B). In the locomotor activity test, the same compounds at dose of 30 mg/kg decreased spontaneous motility after 6 and 20 min (Figure 6) of observation. Similarly, Norbo-4 induced the same effect at the dose of 15 mg/kg. In the second stage of this study, we evaluated the antipsychotic ability of the new compounds. Presently, animal models of schizophrenia, commonly employed for preclinical studies of antipsychotic properties of drugs, regard mainly amphetamine and MK-801 models [38]. The first model is based on the manipulation of the dopaminergic system activity, and it may primarily respond to drugs that affect this neurotransmitter system. Many neuroleptics acting as dopaminergic antagonists reverse this effect [38]. Noteworthy, the hyperlocomotion following amphetamine is also sensitive to other classes of drugs [39]. On the other hand, several preclinical tests have pointed to the role of 5-HT2C ligands in the modulation of monoaminergic systems, including dopaminergic. Indeed, dysfunction in serotoninergic activity could contribute to the alteration of dopaminergic function seen in schizophrenia [40]. Nevertheless, in the amphetamine model, none of the tested compounds at the dose of 15 mg/kg reduced amphetamine-induced hyperactivity of mice (Figure 7). Furthermore, due to the modulation of the central serotonin neurotransmission, the new compounds may also show anxiolytic and/or antidepressant activity [41,42]. Considering this premise, as well as in vitro data obtained for the tested compounds (mixed 5-HT1A/5-HT2C affinity profile for all the compounds), we examined their antidepressant and anxiolytic potential in behavioral models commonly used in mice, i.e., FST and EPM.
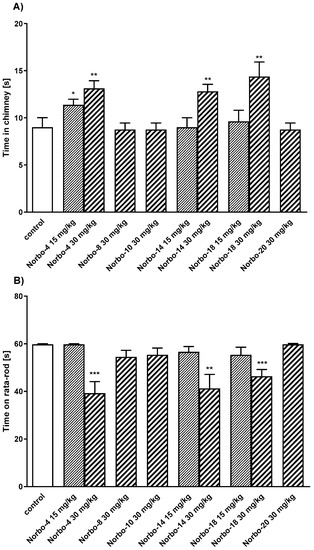
Figure 5.
The influence of the tested compounds Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18 and Norbo-20 (15 and 30 mg/kg) on motor coordination in mice evaluated in chimney (A) and rota-rod (B) tests. Investigated compounds were injected i.p. 60 min before the test. Data are expressed as mean ± SEM values of the 1 independent experiment. * p < 0.05; ** p < 0.01; *** p < 0.001 vs. control (Dunnett’s test). One-way ANOVA showed significant changes in the time in chimney after administration of the compound Norbo-4 (F(2,21) = 6.276; p < 0.01), Norbo-14 (F(2,21) = 5.615, p < 0.05 and Norbo-18 (F(2,21) = 5.381, p < 0.05) (A) and the time spent on the rota-rod for compound Norbo-4 (F(2,21) = 17.56; p < 0.0001), Norbo-14 (F(2,21) = 7.439, p < 0.01 and Norbo-18 (F(2,21) = 7.591, p < 0.01) (B). Dunnett’s post hoc test confirmed a significant increase of time in chimney after the administration of compound Norbo-4 administrated at the doses of 15 (p < 0.05) and 30 mg/kg (p < 0.01), Norbo-14 at the dose of 30 mg/kg (p < 0.01) and Norbo-18 at the dose of 30 mg/kg (p < 0.01) (A). These compounds decreased also time on rota-rod at the dose of 30 mg/kg (Norbo-4 (p < 0.001); Norbo-14 (p < 0.01) and Norbo-18 (p < 0.001)) (B).
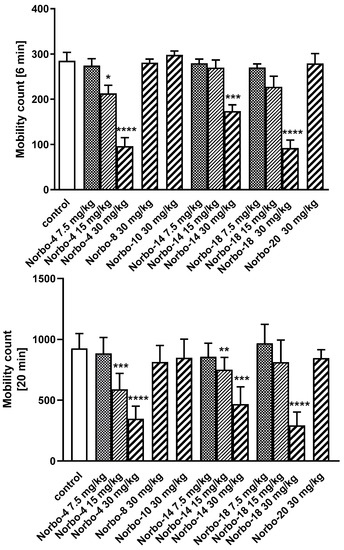
Figure 6.
The influence of the tested compounds Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18 and Norbo-20 (7.5–30 mg/kg) on the spontaneous locomotor activity of mice. Investigated compounds were injected i.p. 60 min before the test. Locomotor activity was measured after 6 and 20 min. Data are expressed as mean ± SEM values of the 1 independent experiment. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 vs. control (Dunnett’s test). One-way ANOVA showed significant changes in locomotor activity of mice in 6 min after administration of the compound Norbo-4 (F(3,24) = 22.94; p < 0.0001), Norbo-14 (F(3,24) = 13.41, p < 0.0001 and Norbo-18 (F(3,24) = 23.51, p < 0.001). Dunnett’s post hoc test confirmed a significant decrease in locomotor activity of mice after the administration of compound Norbo-4 administrated at the doses of 15 (p < 0.05) and 30 mg/kg (p < 0.0001), Norbo-14 at the dose of 30 mg/kg (p < 0.001) and Norbo-18 at the dose of 30 mg/kg (p < 0.0001) after 6 min of observation. One-way ANOVA reveled also significant changes in locomotor activity of mice in 20 min after administration of the compound Norbo-4 (F(3,24) = 39.23; p < 0.0001), Norbo-14 (F(3,24) = 22.27, p < 0.0001 and Norbo-18 (F(3,24) = 36.73, p < 0.001). Dunnett’s post hoc test confirmed a significant decrease in locomotor activity of mice after the administration of compound Norbo-4 administrated at the doses of 15 (p < 0.001) and 30 mg/kg (p < 0.0001), Norbo-14 at the doses of 15 (p < 0.01) and 30 mg/kg (p < 0.0001) and Norbo-18 at the dose of 30 mg/kg (p < 0.0001) after 20 min of observation.
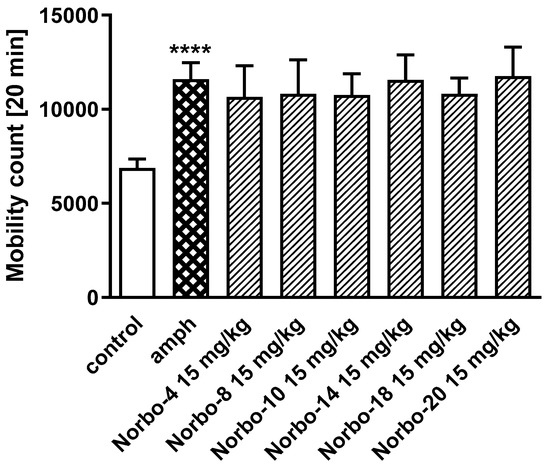
Figure 7.
The influence of the tested compounds Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18 and Norbo-20 (15 mg/kg) on the amphetamine-induced hyperactivity of mice. Compounds tested were injected 60 min and amphetamine 5 mg/kg 30 min before the test. Locomotor activity was measured for a period of 20 min. Data are expressed as mean ± SEM values of the 1 independent experiment. **** p < 0.001 vs. control. One-way ANOVA did not showed significant changes in the locomotor activity of mice [F(7, 53) = 1601; p = 0.1555]. Dunnett’s post hoc test confirmed a significant increase in locomotor activity of mice after the administration of amphetamine (5 mg/kg) (p < 0.001). However, the administered compounds had no influence on amphetamine-induced hyperactivity of mice (p > 0.05).
FST is a simple and fast test established in experimental pharmacology for detecting the antidepressant effect of tested substances [43]. The mouse placed in a beaker with water, without the possibility of escaping, initially makes intensive attempts to get out, but after a short time it gives up and adopts an attitude known as immobility. Selective serotonin reuptake inhibitors (SSRIs) are an example of antidepressants active in this test. They block the serotonin transporter (SERT) protein, which transports serotonin, and reduce its reuptake from the synaptic cleft. Due to their effectiveness, they are one of the most commonly used drugs in the treatment of depression. This group includes, among others, sertraline, which is a 5-HT1A agonist. Sertraline at the dose of 15 mg/kg used in our experiments significantly shortened the immobility time of mice. The tested compound Norbo-4 at three doses of 15, 7.5, and 3.75 mg/kg and Norbo-18 (at the doses of 15 and 7.5 mg/kg) showed an antidepressant effect similar to fluoxetine, which was manifested by a significant reduction of immobility time of mice (Figure 8). This effect should be considered specific as the tested compound did not show a negative effect on the locomotor activity of mice (in the two lowest used doses) nor induce sedation (at a dose of 15 mg/kg). This suggests that the mice were able to cope with the sedation in this stressful confined space swimming situation. The twice-as-high dose was found to inhibit the CNS so strongly that the immobility time in this group was comparable to the control group. Meanwhile, the other tested compounds, at used doses, remained inactive in this test. Anxiety and stress-related disorders represent severe mental health conditions that affect the performance of daily tasks and represent a high cost to public health. Charles Darwin’s preliminary observation that animals and humans have similar traits in expressing emotions opens the possibility to study the mechanisms of mental disorders in other mammals (mainly rodents). The animal test for assessing anxiolytic effects, EPM, is based on the test animal’s aversion to open spaces [44]. Fear induced inhibition of exploratory activity affects entries into open arms in this task as a significant increase in the percentage of time spent on the open arms and the number of entries into open arms is observed with drugs that are clinically effective anxiolytics [45]. Therefore, this model has been used to assess the anxiolytic-like activity of new putative anxiolytic compounds [46]. Buspirone (5 mg/kg), used as a reference anxiolytic drug, also prolonged the time and increased the percentage of entries into the open arms (Figure 9). As in the FST test, also in this test, the Norbo-4 compound, used in three lower doses, i.e., 15, 7.5, and 3.75 mg/kg, increased the percentage of open arm exploration time, analogous to buspirone. Additionally, the increase in the percentage of entries into the open arms was statistically significant. The remaining compounds at the used doses were inactive in this test.
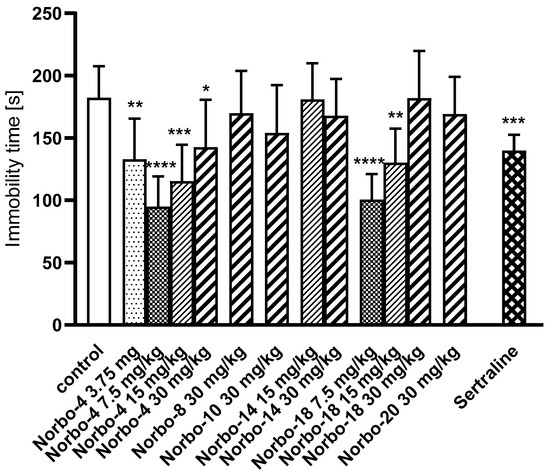
Figure 8.
The influence of the investigated compounds Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18 and Norbo-20 (3.75–30 mg/kg) and sertraline (15 mg/kg) on the total duration in the forced swim test in mice (FST). The investigated compounds were administered i.p. 60 min before the test. The values represent means ± SEM of the 1 independent experiment. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 vs. control (Dunnett’s test). One-way ANOVA showed significant changes in immobility time after administration of the compound Norbo-4 (F(4,35) = 0.386; p < 0.0001) and Norbo-18 (F(3,26) = 16.07; p < 0.0001). Dunnett’s post hoc test confirmed a significant reduction in immobility time after the administration of compounds Norbo-4 applied at the doses of 3.75 (p < 0.01), 7.5 (p < 0.0001), 15 (p < 0.001) and 30 mg/kg (p < 0.05), and Norbo-18 applied at the doses of 7.5 (p < 0.0001) and 15 mg/kg (p < 0.01). Also, sertraline (15 mg/kg) induced a significant reduction in the immobility time (p < 0.001).
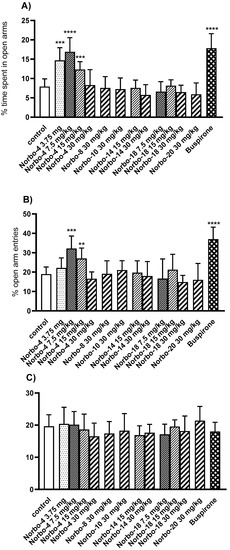
Figure 9.
The influence of the investigated compounds Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18 and Norbo-20 (3.75–30 mg/kg) on elevated plus-maze performance in mice-percentage of time spent in open arms (A), the percentage of the open arm entries (B) and total arm entries (C). Investigated compounds were injected i.p. 60 min before the test. The results are expressed as mean ± SEM of the 1 independent experiment. ** p < 0.01; *** p < 0.001; **** p < 0.0001 vs. control (Dunnett’s test). One-way ANOVA showed significant changes in percentage of time spent in open arms of EPM (Norbo-4 F(4,39) = 10.14; p < 0.001) (A) and in the percentage of open arm entries (Norbo-4 F(4,35) = 12.93; p < 0.0001) (B). There were no significant changes in the total arm entries (F(13,94) = 1.08; p = 0.3853) (C). Dunnett’s post hoc test confirmed a significant increase in time spent in open arms after the administration of compounds: Norbo-4 at the dose of 3.75 (p < 0.001), 7.5 (p < 0.0001) and 15 mg/kg (p < 0.05). This compound was also able to increase the percentage of open arm entries at the dose of 7.5 (p < 0.001) and 15 mg/kg (p < 0.01). Also, buspirone (5 mg/kg) induced a significant increase in percentage of time spent in open arms and in percentage of open arms entries (p < 0.0001).
3. Conclusions
We have described the synthesis of a new series of arylpiperazines as serotoninergic ligands (Norbo 1–28). The 4-methoxyphenyl and 2,3-dimethylphenyl piperazine derivatives supporting an 5-norbornene-2-carboxamide scaffold as terminal fragment (Norbo-4 and Norbo-8) afforded a favorable agonistic profile for 5-HT1A receptors (pEC50 = 6.74 ± 0.08 and pEC50 = 6.78 ± 0.09 respectively), whereas, besides the outstanding 5-HT2A receptor affinity and selectivity of compounds Norbo-14 (Ki = 17.93 nM) and Norbo-18 (Ki = 18.65 nM), other interesting Ki values included that of compound Norbo-20 (Ki = 22.86 nM), while compounds Norbo-10 and Norbo-11 presented the most attractive 5-HT2C affinity profile with Ki values of 13 and 31 nM, respectively.
Based on the in vitro results, the compounds Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18, and Norbo-20 were selected for further in vivo studies to investigate their functional activity.
The obtained results showed that compounds Norbo-4 and Norbo-18 exerted antidepressant-like effects. Interestingly, the compound Norbo-4 revealed significant anxiolytic properties, and in the EPM test it was almost as efficacious as buspirone. However, further pharmacological studies are necessary to determine detailed mechanism of action and clinical prospective usefulness of the new compounds.
In conclusion, data presented in this study confirm that, as obtained with the series previously synthesized [14,16], the novel synthesized compounds display a general trend of affinity towards serotoninergic receptors investigated. Molecular docking studies supported these results, highlighting some selective and additional interactions of the identified ligands with the investigated receptor subtype.
4. Materials and Methods
4.1. Synthesis
4.1.1. General Procedures
All reagents substituted piperazines and solvents were commercial products obtained from Merck (Darmstadt, Germany). Melting points, determined using a Buchi Melting Point B-540 (Flawil, Switzerland) instrument, are uncorrected and represent values obtained on recrystallized or chromatographically purified material. 1H and 13C-NMR spectra were recorded on Bruker Advanced 400 MHz spectrometer (USA). COSY, HSQC and ROESY experiments were recorded on Bruker Advanced 700 MHz spectrometer (USA). Unless otherwise stated, all spectra were recorded in CDCl3. Chemical shifts are reported in ppm. The following abbreviations are used to describe peak patterns when appropriate: s (singlet), d (doublet), t (triplet), m (multiplet), q (quartet), qt (quintet), dd (doublet of doublet), td (triplet of doublets), and bs (broad singlet). Mass spectra of the final products were recorded on a LTQ-XL mass spectrometer equipped with an HESI ion source (Thermo Fisher Scientific, Waltham, MA, USA). Elemental analyses were carried out on a Carlo Erba model 1106. Analyses indicated by the symbols of the elements were within ±0.4% of the theoretical values. All reactions were followed by thin-layer chromatography, carried out on Merck silica gel 60 F254 plates with a fluorescent indicator, and the plates were visualized with UV light (254 nm). Preparative chromatographic purifications were performed using a silica gel column (Kieselgel 60). Solutions were dried over Na2SO4 and concentrated with a Buchi R-114 rotary evaporator at low pressure.
4.1.2. Synthesis of N-(3-Chloropropyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (2)
Commercially available 5-norbornene-2-carboxilic acid (mixture of endo and exo, predominantly endo) (1.00 g; 7.24 mmol) was solubilized in acetonitrile (10 mL) and cooled to 0 °C for 30 min. N,N’-dicyclohexylcarbodiimide (DCC, 1.64 g; 7.96 mmol) and hydroxybenzotriazole (HOBt, 1.22 g; 7.96 mmol) were added and the mixture was stirred for one hour. Finally, triethylamine (TEA, 1.11 mL; 7.96 mmol) and 3-chloropropylamine hydrochloride (0.94 g; 7.24 mmol) were added and the reaction mixture was stirred at room temperature for 8 h. When the reaction was completed, it was cooled to 0 °C. Subsequently, the mixture was filtered and evaporated under reduced pressure. The resulting residue was diluted with DCM (30 mL) and washed with NaHCO3 5% and brine. The organic phase was dried over Na2SO4 and concentrated, yielding the desired intermediate 2 as a brown oil (yield: 70%), the mixture of exo/endo isomers of which was used without further purification in the next steps. 1H-NMR spectra for purified exo/endo intermediates were consistent with the proposed structures.
exo:1H-NMR (400 MHz, CDCl3) δ:1.29 (d, 1H, J = 1.9 Hz), 1.31–1.36 (m, 1H), 1.68 (d, 1H, J = 8.2 Hz), 1.88–1.91 (m, 1H), 1.97–2.03 (m, 3H), 2.90 (s, 2H), 3.39 (q, 2H, -NH-CH2-, J = 6.4 Hz), 3.56 (t, 2H, J = 6.3 Hz), 6.09 (dd, 1H, J = 2.2 Hz), 6.13(dd,1H, J = 2.2 Hz). 13C-NMR (101 MHz, CDCl3) δ: 30.64, 32.31, 37.26, 41.27, 42.76, 44.81, 46.48, 47.32, 136.08, 138.39, 176.05; ESI-MS m/z [M+H]+ calculated for C11H16ClNO 213.70, Found = 214.4
endo:1H-NMR (400 MHz, CDCl3) δ:1.29 (d, 1H, J = 3.3 Hz), 1.32–1.33 (m, 1H), 1.44 (dd, 1H, J = 8.2, 2.0 Hz), 1.91–1.99 (m, 3H), 2.84–2.88 (m, 1H), 2.92 (s, 1H), 3.13 (s, 1H), 3.33–3.37 (m, 2H, -NH-CH2-), 3.54 (t, 2H, J = 6.4 Hz), 5.95 (dd, 1H, J = 5.5, 2.7 Hz), 6.22 (dd, 1H, J = 5.5, 3.1 Hz). 13C-NMR (101 MHz, CDCl3) δ: 30.06, 32.21, 37.17, 42.82, 42.86, 44.99, 46.33, 50.20, 132.31, 138.04, 174.66; ESI-MS m/z [M+H]+ calculated for C11H16ClNO 213.70, Found = 214.5
4.1.3. General Procedure for the Synthesis of Norbornene Derivatives (Norbo-1-28)
To a solution of N-(3-chloropropyl) bicyclo[2.2.1]hept-5-ene-2-carboxamide (2, 1.00 g; 4.70 mmol) in acetonitrile, sodium iodide (NaI, 0.77 g; 5.17 mmol) was added. The mixture was heated at reflux and stirred for 30 min. T
Then, the appropriate 4-X-substitued-piperazine (4.70 mmol) and potassium carbonate (K2CO3, 0.71 g; 5.17 mmol) were added. The reaction was stirred at reflux overnight. Subsequently, the mixture was filtered, and the solvent was removed in vacuo. The resulting product was diluted with DCM (30 mL) and washed with water and brine. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by silica gel open chromatography using dichloromethane/methanol (9:1 v/v) as eluent to obtain the final compound as a mixture of endo and eso isomers. The separation of two isomers was carried out by silica gel open chromatography with diethyl ether/methanol (8:2 v/v) as eluent. The combined and evaporated product fractions were crystallized from diethyl ether or converted into the corresponding hydrochloride salt, yielding the desired products (Norbo 1–28) as white solids.
4.1.4. Synthesis of Exo-N-(3-(4-phenylpiperazin-1-yl) propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-1) and endo-N-(3-(4-phenylpiperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-2)
Following the synthetic procedure reported above, Norbo-1 and Norbo-2 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-phenylpiperazine (0.76 g; 4.70 mmol).
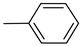
Norbo-1: Yield: 33%; mp: 120.8–121.8 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27–1.35 (m, 2H), 1.68 (d, 1H, J = 8.5 Hz), 1.78–1.81 (m, 2H), 1.90–1.92 (m, 1H), 1.96–1.98 (m, 1H), 2.59 (t, 2H, -CH2-N1-, J = 6.0 Hz), 2.73 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.89 (s, 1H), 2.93 (s, 1H), 3.27 (bs, 4H, 2CH2 pip., J = 4.5 Hz), 3.38–3.40 (m, 2H, -NH-CH2-), 6.07 (dd, 1H, J = 5.5, 3.0 Hz), 6.11(dd, 1H, J = 5.3, 3.1 Hz), 6.87–6.94 (m, 3H), 7.00 (bs, 1H, NH), 7.28–7.30 (m, 2H); 13C-NMR (101 MHz, CDCl3) δ: 25.00, 30.39, 39.25,41.54, 44.88, 46.35, 47.22, 48.98, 53.16, 57.34, 116.20, 120.16, 129.18, 135.96, 138.19, 150.84, 175.52; ESI-MS m/z [M+H]+ calculated for C21H29N3O 339.47, Found = 340.24; Anal. Calcd for C21H29N3O: C, 74.30; H, 8.61; N, 12.38. Found C, 74.52; H, 8.60; N, 12.40.
Norbo-2: Yield: 30%; mp: 113.7–114.1 °C; 1H-NMR (400 MHz, CDCl3) δ:1.26 (d, 1H, J = 8.2 Hz), 1.32–1.36 (m, 1H), 1.41 (dd, 1H, J = 8.2, 1.7 Hz), 1.66–1.73 (m, 3H), 1.87–1.94 (m, 1H), 2.47 (t, 2H, -CH2-N1-, J = 6.4 Hz), 2.62 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.81–2.86 (m, 1H), 2.89 (s, 1H), 3.13 (s, 1H), 3.21 (bs, 4H, 2CH2 pip., J = 4.7 Hz) 3.29–3.34 (m, 2H, -NH-CH2-), 5.97 (dd, 1H, J = 5.5, 2.7 Hz), 6.20 (dd, 1H, J = 5.5, 3.0 Hz), 6.57 (bs, 1H, NH), 6.85 (t, 1H, J = 7.3 Hz), 6.93 (d, 2H, J = 8.2 Hz), 7.28 (d, 2H, J = 7.7 Hz); 13C-NMR (101 MHz, CDCl3) δ: 24.94, 30.39, 39.16, 41.54, 44.86, 46.35, 47.22, 48.91, 53.13, 57.26, 116.24, 120.21, 129.19, 135.96, 138.19, 150.80, 175.57; ESI-MS m/z [M+H]+ calculated for C21H29N3O 339.47, Found = 340.24; Anal. Calcd for C21H29N3O: C, 74.30; H, 8.61; N, 12.38. Found C, 74.07; H, 8.59; N, 12.42.
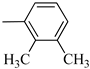
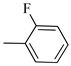
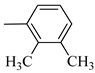
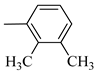
4.1.5. Synthesis of Exo-N-(3-(4-(2,3-dimethylphenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-3) and endo-N-(3-(4-(2,3-dimethylphenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-4)
Following the synthetic procedure reported above, Norbo-3 and Norbo-4 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(2,3-dimethylphenyl) piperazine (0.89 g; 4.70 mmol).
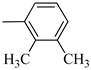
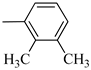
Norbo-3: Yield: 32%; mp: 109.1–111.4 °C; 1H-NMR (400 MHz, CDCl3) δ:1.29–1.36 (m, 2H), 1.69 (d, 1H, J = 8.5 Hz), 1.77–1.80 (m, 2H), 1.92–1.95 (m, 1H), 2.01–2.03 (m, 1H), 2.21 (s, 3H), 2.27 (s, 3H), 2.62 (t, 2H, -CH2-N1-, J = 6.0 Hz), 2.73 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.91–2.97 (m, 6H), 3.38–3.40 (m, 2H, -NH-CH2-), 6.10 (dd, 1H, J = 5.5, 3.2 Hz), 6.12 (dd, 1H, J = 5.5, 3.1 Hz), 6.89–6.93 (m, 2H), 7.07 (t, 1H, J = 7.0 Hz), 7.20 (bs, 1H, NH); 13C-NMR (101 MHz, CDCl3) δ: 13.89, 20.59, 24.85, 30.35, 39.41, 41.56, 44.93, 46.36, 47.25, 51.83, 53.68, 57.49, 116.55, 125.27, 125.90, 131.23, 135.98, 138.01, 138.19, 150.97, 175.49; ESI-MS m/z [M+H]+ calculated for C23H33N3O 367.53, Found = 368.27; Anal. Calcd for C23H33N3O: C, 75.16; H, 9.05; N, 11.43. Found C, 75.23; H, 9.06; N, 11.45.
Norbo-4: Yield: 38%; mp: 119.8–120.6 °C; 1H-NMR (400 MHz, CDCl3) δ:1.28 (d, 1H, J = 8.0 Hz), 1.35–1.40 (m, 1H), 1.43–1.45 (m, 1H), 1.73–1.76 (m, 2H), 1.90–1.95 (m, 1H), 2.22 (s, 3H), 2.27 (s, 3H), 2.56 (t, 2H, -CH2-N1-, J = 5.5 Hz), 2.71(bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.86–2.88 (m, 1H), 2.91 (s, 1H), 2.97 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.18 (s, 1H), 3.31 (t, 2H, -NH-CH2-, J = 5.1 Hz), 5.98 (dd, 1H, J = 5.4, 2.7 Hz), 6.20 (dd, 1H, J = 5.4, 3.1 Hz), 6.78 (bs, 1H, NH), 6.91 (d, 1H, J = 7.6 Hz), 7.07–7.11 (m, 2H) 13C-NMR (101 MHz, CDCl3) δ: 13.90, 20.59, 25.07, 29.79, 39.06, 42.67, 44.91, 46.15, 49.87, 51.79, 53.77, 57.36, 116.54, 125.23, 125.89, 131.25, 132.43, 137.51, 138.08, 151.06, 174.22; ESI-MS m/z [M+H]+ calculated for C23H33N3O 367.53, Found = 368.27; Anal. Calcd for C23H33N3O: C, 75.16; H, 9.05; N, 11.43. Found C, 75.00; H, 9.03; N, 11.39.
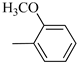
4.1.6. Synthesis of Exo-N-(3-(4-(2-methoxyphenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-5) and endo-N-(3-(4-(2-methoxyphenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-6)
Following the synthetic procedure reported above, Norbo-5 and Norbo-6 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(2-methoxyphenyl) piperazine (0.90 g; 4.70 mmol). The final isomer Norbo-5 was converted into the corresponding hydrochloride salt.
Norbo-5: Yield: 45%; mp: 194.8–195.7 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 1.15 (m, 2H), 1.61 (d, 1H, J = 8.3 Hz), 1.75–1.78 (m, 2H), 1.84–1.87 (m, 1H), 2.02–2.03 (m, 1H), 2.81 (bs, 4H, 2CH2 pip., J = 4.5 Hz), 3.00–3.14 (m, 8H), 3.44 (m, 2H, -NH-CH2-), 3.77 (s, 3H, -OCH3), 6.10 (dd, 1H, J = 5.5, 2.6 Hz), 6.12(dd,1H, J = 5.5, 2.9 Hz), 6.86–6.93 (m, 2H), 6.95–7.00 (m, 2H), 8.06 (bs, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ: 24.23, 30.28, 36.39, 40.60, 41.42, 43.49, 46.13, 47.28, 51.59, 53.96, 55.82, 112.39, 118.69, 121.29, 123.93, 136.67, 138.23, 139.78, 152.26, 175.34; ESI-MS m/z [M+H]+ calculated for C22H31N3O2 369.50, Found = 370.0; Anal. Calcd for C22H31N3O2: C, 71.51; H, 8.46; N, 11.37. Found C, 71.65; H, 8.47; N, 11.40.
Norbo-6: Yield: 31%; mp: 83.9–86.1 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27 (d, 1H, J = 8.0 Hz), 1.34 (dt, 1H, J = 6.5, 2.1 Hz), 1.41 (d, 1H, J = 6.6 Hz), 1.79–1.82 (m, 1H), 1.9–1.96 (m, 2H), 2.65 (t, 2H, -CH2-N1-, J = 5.4 Hz), 2.84–2.86 (m, 1H), 2.89 (bs, 4H, 2CH2 pip., J = 4.5 Hz), 3.18 (s, 2H), 3.23 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.31–3.35 (m, 2H, -NH-CH2-), 3.86 (s, 3H, -OCH3), 5.97 (dd, 1H, J = 5.5, 2.5 Hz), 6.20 (dd, 1H, J = 5.5, 2.6 Hz), 6.82 (bs, 1H, NH), 6.86–6.88 (m, 2H), 6.90–6.94 (m, 1H), 7.01–7.05 (m, 1H); 13C-NMR (101 MHz, CDCl3) δ: 24.70, 29.72, 38.40, 42.66, 44.85, 46.14, 49.65, 49.89, 53.26, 55.39, 56.75, 111.24, 118.34, 121.06, 123.48, 132.39, 137.56, 140.36, 152.17, 174.46; ESI-MS m/z [M+H]+ calculated for C22H31N3O2 369.50, Found = 370.3; Anal. Calcd for C22H31N3O2: C, 71.51; H, 8.46; N, 11.37. Found C, 71.36; H, 8.49; N, 11.38.
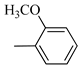
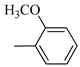
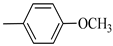
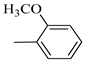
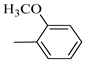
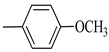
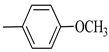
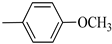
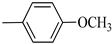
4.1.7. Synthesis of Exo-N-(3-(4-(4-methoxyphenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-7) and endo-N-(3-(4-(4-methoxyphenyl) piperazin-1-yl) propyl) bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-8)
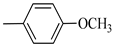
Following the synthetic procedure reported above, Norbo-7 and Norbo-8 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(4-methoxyphenyl) piperazine (0.90 g; 4.70 mmol).
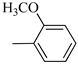
Norbo-7: Yield: 33%; mp: 119.3–120.1 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27 (d, 1H, J = 2.0 Hz), 1.32 (d, 1H, J = 8.3 Hz), 1.68 (d, 1H, J = 8.3 Hz), 1.77–1.80 (m, 2H), 1.90–1.93 (m, 1H), 1.97–1.99 (m, 1H), 2.60 (t, 2H, -CH2-N1- J = 6.1 Hz), 2.74 (bs, 4H, 2CH2 pip., J = 4.6 Hz), 2.89 (s, 1H), 2.93 (s, 1H), 3.16 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.38–3.39 (m, 2H, -NH-CH2-), 3.77 (s, 3H, -OCH3), 6.07 (dd, 1H, J = 5.3, 2.7 Hz), 6.11 (dd, 1H, J = 5.5, 2.8 Hz), 6.83 (d, 2H, J = 8.9 Hz), 6.89 (d, 2H, J = 8.9 Hz), 7.05 (bs, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ: 24.92, 30.36, 39.22, 41.55, 44.87, 46.35, 47.24, 50.39, 53.25, 55.54, 57.28, 114.49, 118.39, 135.96, 138.19, 145.16, 154.12, 175.54; ESI-MS m/z [M+H]+ calculated for C22H31N3O2 369.50, Found = 370.4; Anal. Calcd for C22H31N3O2: C, 71.51; H, 8.46; N, 11.37. Found C, 71.65; H, 8.49; N, 11.41.
Norbo-8: Yield: 36%; mp: 115.1–116.2 °C.1H-NMR (400 MHz, CDCl3) δ: 1.26 (d, 1H, J = 3.3 Hz), 1.34 (dd, 1H, J = 5.4, 3.0 Hz), 1.41 (d, 1H, J = 6.9 Hz), 1.74–1.77 (m, 1H), 1.89–1.93 (m, 2H), 2.58 (t, 2H, -CH2-N1-, J = 5.4 Hz), 2.73 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.84–2.86 (m, 1H), 2.89 (s, 2H), 3.18 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.32–3.33 (m, 2H, -NH-CH2-), 3.77 (s, 3H, -OCH3), 5.98 (dd, 1H, J = 5.5, 2.7 Hz), 6.20 (dd, 1H, J = 5.5, 2.6 Hz), 6.66 (bs, 1H, NH), 6.84 (d, 2H, J = 8.7 Hz), 6.90 (d, 2H, J = 8.7 Hz). 13C-NMR (101 MHz, CDCl3) δ: 25.16, 29.80, 38.94, 42.65, 44.89, 46.12, 49.87, 50.38, 53.37, 55.55, 57.21, 114.48, 118.33, 132.41, 137.52, 145.26, 154.05, 174.22;
ESI-MS m/z [M+H] + calculated for C22H31N3O2 369.50, Found = 370.3; Anal. Calcd for C22H31N3O2: C, 71.51; H, 8.46; N, 11.37. Found C, 71.66; H, 8.48; N, 11.38.
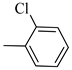
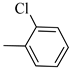
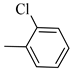
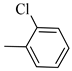
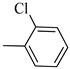
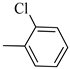
4.1.8. Synthesis of Exo-N-(3-(4-(2-chlorophenyl) piperazin-1-yl) propyl) bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-9) and endo-N-(3-(4-(2-chlorophenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-10)
Following the synthetic procedure reported above, Norbo-9 and Norbo-10 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(2-chlorophenyl) piperazine hydrochloride (1.10 g; 4.70 mmol), in the presence of two equivalents of K2CO3 (1.30 g; 9.4 mmol). The final isomer Norbo-10 was converted into the corresponding hydrochloride salt.
Norbo-9: Yield: 34%; mp: 77.6–80.0 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.32–1.34 (m, 2H), 1.69 (d, 1H, J = 8.1 Hz), 1.81 (m, 2H), 1.92–1.95 (m, 1H), 2.02 (m, 1H), 2.66 (t, 2H, -CH2-N1-, J = 6.1 Hz), 2.80 (bs, 4H, 2CH2 pip., J = 4.5 Hz), 2.91 (s, 1H), 2.95 (s, 1H), 3.17 (bs, 4H, 2CH2 pip., J = 4.5 Hz), 3.40 (m, 2H, -NH-CH2-), 6.10 (dd, 1H, J = 5.5, 2.4 Hz), 6.13 (dd,1H, J = 5.5, 2.6 Hz), 7.00–7.05 (m, 3H), 7.24 (t, 1H, J = 7.3 Hz), 7.36 (d, 1H, J = 7.6 Hz). 13C-NMR (101 MHz, CDCl3) δ: 24.87, 30.37. 39.03. 41.56, 44.89, 46.36, 47.24, 50.70, 53.27, 57.13, 119.98, 123.72, 127.68, 130.70, 134.18, 135.97, 138.02, 168.88, 175.57; ESI-MS m/z [M+H]+ calculated for C21H28ClN3O 373.92, Found = 374.2; Anal. Calcd for C21H28ClN3O: C, 67.45; H, 7.55; N, 11.24. Found C, 67.46; H, 7.57; N, 11.27.
Norbo-10: Yield: 37%; mp: 93.4–96.1 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.24–1.26 (d, 1H, J = 3.0 Hz), 1.29–1.32 (m, 2H), 1.70–1.75 (m, 1H), 1.83 (m, 2H), 2.48 (t, 2H, -CH2-N1-, J = 5.1 Hz), 2.80 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.08 (m, 1H), 3.15 (s, 1H), 3.31 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.41 (m, 1H), 3.53 (m, 2H, -NH-CH2-), 5.86 (dd, 1H, J = 5.4, 2.6 Hz), 6.10 (dd, 1H, J = 5.5, 3.0 Hz), 7.08 (t, 1H, J = 7.0 Hz), 7.18 (d, 1H, J = 7.3 Hz), 7.31 (t, 1H, J = 7.3 Hz), 7.43 (d, 1H, J = 7.3 Hz), 7.81 (bs, 1H, NH); 13C-NMR (101 MHz, CDCl3) δ: 24.26, 28.82, 36.28, 42.53, 43.84, 46.01, 48.12, 49.84, 51.65, 53.96, 121.46, 125.28, 127.98, 128.72, 130.92, 132.75, 137.38, 147.84, 173.63; ESI-MS m/z [M+H]+ calculated for C21H28ClN3O 373.92, Found = 374.0; Anal. Calcd for C21H28ClN3O: C, 67.45; H, 7.55; N, 11.24. Found C, 67.47; H, 7.56; N, 11.27.
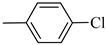
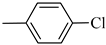
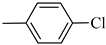
4.1.9. Synthesis of Exo-N-(3-(4-(4-chlorophenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-11) and endo-N-(3-(4-(4-chlorophenyl) piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-12)
Following the synthetic procedure reported above, Norbo-11 and Norbo-12 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(4-chlorophenyl) piperazine (0.92 g; 4.70 mmol).
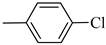
Norbo-11: Yield: 34%; mp: 144.7–146.2 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27–1.29 (m, 1H), 1.33 (d, 1H, J = 8.3 Hz), 1.68 (d, 1H, J = 8.3 Hz), 1.76–1.79 (m, 2H), 1.90–1.92 (m, 1H), 1.96–1.98 (m, 1H), 2.56 (t, 2H, -CH2-N1-, J = 6.3 Hz), 2.68 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.90 (s, 1H), 2.92 (s, 1H), 3.19 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.34–3.36 (m, 2H, -NH-CH2-), 6.05 (dd, 1H, J = 5.4, 3.0 Hz), 6.11 (dd, 1H, J = 5.5, 2.9 Hz), 6.83 (d, 2H, J = 8.9 Hz), 6.92 (bs, 1H, NH), 7.20 (d, 2H, J = 8.9 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.11, 30.39, 39.28, 41.56, 44.93, 46.38, 47.25, 49.11, 53.07, 57.32, 117.36, 124.97, 129.06, 135.95, 138.28, 149.59, 175.52; ESI-MS m/z [M+H]+ calculated for C21H28ClN3O 373.92, Found = 374.2; Anal. Calcd for C21H28ClN3O: C, 67.45; H, 7.55; N, 11.24. Found C, 67.71; H, 7.58; N, 11.28.
Norbo-12: Yield: 38%; mp: 147.8–148.9 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.26 (d, 1H, J = 8.2 Hz), 1.32 (dd, 1H, J = 8.5, 3.0 Hz), 1.41 (d, 1H, J = 6.9 Hz), 1.70–1.77 (m, 1H), 1.87–1.93 (m, 2H), 2.51 (t, 2H, -CH2-N1-, J = 5.4 Hz), 2.66 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.83–2.86 (m, 1H), 2.89 (s, 1H), 3.14 (s, 1H), 3.21 (bs, 4H, 2CH2 pip., J = 4.7 Hz) 3.30–3.33 (m, 2H, -NH-CH2-), 5.97 (dd, 1H, J = 5.2, 2.5 Hz), 6.20 (dd,1H, J = 5.5, 3.1 Hz), 6.48 (bs, 1H, NH), 6.83 (d, 2H, J = 8.8 Hz), 7.20 (d, 2H, J = 8.8 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.42, 29.84, 38.93, 42.68, 44.93, 46.14, 49.06, 49.91, 53.16, 57.17, 117.32, 124.87, 129.04, 132.43, 137.60, 149.64, 174.24; ESI-MS m/z [M+H]+ calculated for C21H28ClN3O 373.92, Found = 374.1; Anal. Calcd for C21H28ClN3O: C, 67.45; H, 7.55; N, 11.24. Found C, 67.46; H, 7.56; N, 11.23.
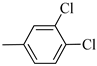
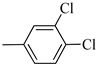
4.1.10. Synthesis of Exo-N-(3-(4-(3,4-dichlorophenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-13) and endo-N-(3-(4-(3,4-dichlorophenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-14)
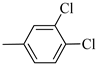
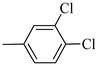
Following the synthetic procedure reported above, Norbo-13 and Norbo-14 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(3,4-dichlorophenyl) piperazine (1.09 g; 4.70 mmol).
Norbo-13: Yield: 32%; mp: 123.6–124.7 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.28–1.35 (m, 2H), 1.67 (d, 1H, J = 8.1 Hz), 1.79–1.82 (m, 2H), 1.89–1.92 (m, 1H), 1.96–1.99 (m, 1H), 2.62 (t, 2H, -CH2-N1-, J = 6.5 Hz), 2.74 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.91(s, 2H), 3.26 (bs, 4H, 2CH2 pip., J = 4.7 Hz) 3.36–3.40 (m, 2H, -NH-CH2-), 6.06 (dd, 1H, J = 5.5, 2.6 Hz), 6.11(dd, 1H, J = 5.4, 2.8 Hz), 6.72 (dd, 1H, J = 8.9, 2.1 Hz), 6.83 (bs, 1H, NH), 6.95 (d, 1H, J = 8.7 Hz), 7.27 (d, 1H, J = 8.0 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.02, 30.42, 38.75, 41.54, 44.84, 46.36, 47.23, 48.29, 52.71, 56.87, 115.54, 117.57, 130.56, 132.89, 135.47, 138.26, 152.11, 175.71; ESI-MS m/z [M+H]+ calculated for C21H27Cl2N3O 408.36, Found = 408.0; Anal. Calcd for C21H27Cl2N3O: C, 61.76; H, 6.66; N, 10.29. Found C, 61.51; H, 6.64; N, 10.24.
Norbo-14: Yield: 42%; mp: 99.1–101.3 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27 (d, 1H, J = 8.1 Hz), 1.32 (dd, 1H, J = 8.3, 3.0 Hz), 1.43 (d, 1H, J = 6.5 Hz), 1.73–1.77 (m, 1H), 1.89–1.91 (m, 2H), 2.56 (t, 2H, -CH2-N1-, J = 5.5 Hz), 2.71 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.84–2.86 (m, 1H), 2.90 (s, 1H), 3.14 (s, 1H), 3.26 (bs, 4H, 2CH2 pip., J = 4.7 Hz) 3.30–3.32 (m, 2H, -NH-CH2-), 5.98 (dd, 1H, J = 5.4, 2.7 Hz), 6.22 (dd, 1H, J = 5.4, 3.0 Hz), 6.43 (bs, 1H, NH), 6.73 (d, 1H, J = 8.8 Hz), 6.96 (s, 1H), 7.27 (d, 1H, J = 8.7 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.32, 29.81, 38.49, 42.66, 44.89, 46.12, 48.31, 49.91, 52.81, 56.74, 115.46, 117.49, 130.52, 132.35, 135.37, 137.62, 154.37, 174.31; ESI-MS m/z [M+H]+ calculated for C21H27Cl2N3O 408.36, Found = 408.4; Anal. Calcd for C21H27Cl2N3O: C, 61.76; H, 6.66; N, 10.29. Found C, 62.00; H, 6.68; N, 10.33.
4.1.11. Synthesis of Exo-N-(3-(4-(2-fluorophenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-15) and endo.-N-(3-(4-(2-fluorophenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-16)
Following the synthetic procedure reported above, Norbo-15 and Norbo-16 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(2-fluorophenyl) piperazine (0.85 g; 4.70 mmol).
Norbo-15: Yield: 33% mp: 82.8–84.1 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.29–1.43 (m, 2H), 1.68 (d, 1H, J = 8.2 Hz), 1.79 -1.85 (m, 2H), 1.91–1.94 (m, 1H), 2.00–2.02 (m, 1H), 2.68 (t, 2H, -CH2-N1-, J = 6.4 Hz), 2.83 (bs, 4H, 2CH2 pip., J = 4.9 Hz), 2.90 (s, 1H), 2.94 (s, 1H), 3.23 (bs, 4H, 2CH2 pip., J = 4.8 Hz), 3.39–3.41 (m, 2H, -NH-CH2-), 6.09 (dd, 1H, J = 5.4, 2.6 Hz), 6.12 (dd, 1H, J = 5.7, 2.9 Hz), 6.94–6.99 (m, 2H), 7.01–7.07 (m, 3H); 13C-NMR (101 MHz, CDCl3) δ: 24.77, 30.39, 38.32, 41.56, 44.85, 46.35, 47.24, 49.79, 53.11, 56.97, 116.10, 119.00, 123.06, 124.53, 135.97, 138.18, 154.46, 156.90, 177.52; ESI-MS m/z [M+H]+ calculated for C21H28FN3O 357.46, Found = 358.2; Anal. Calcd for C21H28FN3O: C, 70.56; H, 7.90; N, 11.76. Found C, 70.77; H, 7.87; N, 11.72.
Norbo-16: Yield: 44%; mp: 88.5–89.1 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.28 (d, 1H, J = 8.2 Hz), 1.34–1.37 (m, 1H), 1.43 (d, 1H, J = 6.2 Hz), 1.74–1.77 (m, 1H), 1.89–1.95 (m, 2H), 2.57 (t, 2H, -CH2-N1-, J = 5.4 Hz), 2.75 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.85–2.88 (m, 1H), 2.90 (s, 1H), 3.17 (s, 1H), 3.20 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.30–3.35 (m, 2H, -NH-CH2-), 5.98 (dd, 1H, J = 5.5, 2.7 Hz), 6.20(dd, 1H, J = 5.7, 3.2 Hz), 6.62 (bs, 1H, NH), 6.93–6.97 (m, 2H), 7.01–7.09 (m, 2H); 13C-NMR (101 MHz, CDCl3) δ: 25.12, 29.80, 38.76, 42.67, 44.90, 46.13, 49.89, 50.08, 53.27, 57.04, 116.07, 118.95, 122.86, 124.49, 132.40, 137.55, 154.49, 156.93, 174.25; ESI-MS m/z [M+H]+ calculated for C21H28FN3O 357.46, Found = 358.2; Anal. Calcd for C21H28FN3O: C, 70.56; H, 7.90; N, 11.76. Found C, 70.27; H, 7.88; N, 11.71.
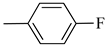
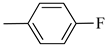
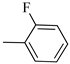
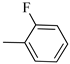
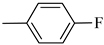
4.1.12. Synthesis of Exo-N-(3-(4-(4-fluorophenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-17) and endo.-N-(3-(4-(4-fluorophenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-18)
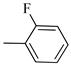
Following the synthetic procedure reported above, Norbo-17 and Norbo-18 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(4-fluorophenyl) piperazine (0.85 g; 4.70 mmol).
Norbo-17: Yield: 32%; mp: 105.5–106.2 °C. 1H-NMR (400 MHz, CDCl3) δ: 1.28 (d, 1H, J = 8.1 Hz), 1.33 (d, 1H, J = 7.9 Hz), 1.68 (d, 1H, J = 8.3 Hz), 1.79–1.81 (m, 2H), 1.90–1.93 (m, 1H), 1.98–2.00 (m, 1H), 2.63 (t, 2H, -CH2-N1-, J = 6.0 Hz), 2.75 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.90 (s, 1H), 2.93 (s, 1H), 3.20 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.37–3.41 (m, 2H, -NH-CH2-), 6.07 (dd, 1H, J = 5.2, 2.6 Hz), 6.12 (dd, 1H, J = 5.4, 2.8 Hz), 6.88 (d, 2H, J = 8.2 Hz), 6.95 (d, 2H, J = 8.2 Hz), 6.99 (bs, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ: 25.00, 30.38, 39.00, 41.55, 44.87, 46.35, 47.23, 49.87, 53.10, 57.07, 115.53, 118.07, 135.93, 138.21, 148.54, 175.57; ESI-MS m/z [M+H]+ calculated for C21H28FN3O 357.46, Found = 358.2; Anal. Calcd for C21H28FN3O: C, 70.56; H, 7.90; N, 11.76. Found C, 70.41; H, 7.91; N, 11.74.
Norbo-18: Yield: 37%; mp: 112.7–113.5 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.26 (d, 1H, J = 8.1 Hz), 1.33 (dd, 1H, J = 8.2, 3.1 Hz), 1.42 (d, 1H, J = 6.6 Hz), 1.73–1.76 (m, 1H), 1.88–1.94 (m, 2H), 2.53 (t, 2H, -CH2-N1-, J = 6.0 Hz), 2.70 (bs, 4H, 2CH2 pip., J = 4.5 Hz), 2.83–2.86 (m, 1H), 2.90 (s, 1H), 3.15 (s, 1H), 3.19 (bs, 4H, 2CH2 pip., J = 4.5 Hz), 3.30–3.34 (m, 2H, -NH-CH2-), 5.98 (dd, 1H, J = 5.4, 2.9 Hz), 6.21(dd, 1H, J = 5.4, 2.5 Hz), 6.54 (bs, 1H, NH), 6.88 (d, 2H, J = 8.2 Hz), 6.95 (d, 2H, J = 8.2 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.28, 29.80, 38.80, 42.65, 44.89, 46.12, 49.88, 49.91, 53.23, 57.04, 115.49, 117.95, 132.39, 137.56, 147.58, 156.16, 174.23; ESI-MS m/z [M+H]+ calculated for C21H28FN3O 357.46, Found = 358.2; Anal. Calcd for C21H28FN3O: C, 70.56; H, 7.90; N, 11.76. Found C, 70.53; H, 7.86; N, 11.75.
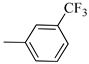
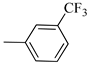
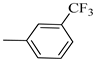
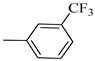
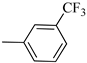
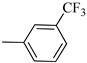
4.1.13. Synthesis of Exo-N-(3-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-19) and endo-N-(3-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-20)
Following the synthetic procedure reported above, Norbo-19 and Norbo-20 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(3-(trifluoromethyl) phenyl) piperazine (1.08 g; 4.70 mmol). The final isomer, Norbo-19, was converted into the corresponding hydrochloride salt.
Norbo-19: Yield: 35%; mp: 164.7–166.0 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 1.13 (m, 2H), 1.61 (d, 1H, J = 8.1 Hz), 1.74–1.79 (m, 2H), 1.83–1.86 (m, 1H), 2.00–2.04 (m, 1H), 2.81 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.09–3.11 (m, 8H), 3.51–3.54 (m, 2H, -NH-CH2-), 6.11 (dd, 1H, J = 5.5, 2.7 Hz), 6.12 (dd, 1H, J = 5.9, 2.9 Hz), 7.13 (d, 1H, J = 7.3 Hz), 7.25–7.28 (m, 2H), 7.43 (t, 1H, J = 8.0 Hz), 8.04 (bs, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ: 24.22, 30.29, 36.40, 41.42, 43.50, 45.28, 46.13, 47.28, 50.88, 53.89, 112.10, 116.21, 119.70, 130.13, 130.64, 136.66, 138.24, 150.29, 175.35; ESI-MS m/z [M+H]+ calculated for C22H28F3N3O 407.47, Found = 408.2; Anal. Calcd for C22H28F3N3O: C, 64.85; H, 6.93; N, 10.31. Found C, 64.59; H, 6.95; N, 10.35.
Norbo-20: Yield: 38%; mp. 79.4–81.7 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27 (d, 1H, J = 8.1 Hz), 1.32 (dd, 1H, J = 8.2, 2.7 Hz), 1.42 (d, 1H, J = 6.5 Hz), 1.74–1.77 (m, 1H), 1.89–1.94 (m, 2H), 2.56 (t, 2H, -CH2-N1-, J = 5.5 Hz), 2.72 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 2.83–2.86 (m, 1H), 2.90 (s, 1H), 3.14 (s, 1H), 3.32 (bs, 6H), 5.98 (dd, 1H, J = 5.4, 2.9 Hz), 6.21(dd, 1H, J = 5.5, 3.1 Hz), 6.46 (bs, 1H, NH), 7.05–7.11 (m, 2H), 7.25 (d, 1H, J = 7.3 Hz), 7.34–7.37 (m, 1H); 13C-NMR (101 MHz, CDCl3) δ: 25.33, 29.81, 38.60, 42.66, 44.89, 46.13, 48.36, 49.90, 52.96, 56.86, 112.38, 116.12, 118.83, 129.31, 129.63, 132.36, 137.60, 150.94, 174.28; ESI-MS m/z [M+H]+ calculated for C22H28F3N3O 407.47, Found = 408.3; Anal. Calcd for C22H28F3N3O: C, 64.85; H, 6.93; N, 10.31. Found C, 65.10; H, 6.53; N, 10.28.
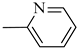
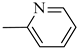
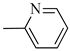
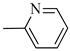
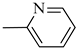
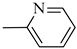
4.1.14. Synthesis of Exo-N-(3-(4-(pyridin-2-yl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-21) endo-N-(3-(4-(pyridin-2-yl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-22)
Following the synthetic procedure reported above, Norbo-21 and Norbo-22 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(pyridin-2-yl) piperazine (0.77 g; 4.70 mmol).
Yield: 44%; mp: 97.1–98.3 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27–1.35 (m, 2H), 1.69–1.75 (m, 3H), 1.90–1.96 (m, 2H), 2.51 (t, 2H, -CH2-N1-, J = 6.1 Hz), 2.59 (bs, 4H, 2CH2 pip., J = 4.9 Hz), 2.89 (s, 1H), 2.93 (s, 1H), 3.36–3.39 (m, 2H, -NH-CH2-), 3.55 (bs, 4H, 2CH2 pip., J = 4.9 Hz) 6.06 (dd, 1H, J = 5.3, 2.9 Hz), 6.10 (dd, 1H, J = 5.5, 2.7 Hz), 6.64–6.66 (m, 2H), 6.95 (bs, 1H, NH), 7.47 (t, 1H, J = 7.1 Hz), 8.20 (d, 1H, J = 5.5 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.00, 29.76, 38.62, 42.66, 44.68, 44.88, 46.12, 49.89, 52.90, 57.03, 107.16, 113.80, 132.40, 137.57, 137.62, 148.01, 159.04, 174.33; ESI-MS m/z [M+H]+ calculated for C20H28N4O 340.46, Found = 341.4; Anal. Calcd for C20H28N4O: C, 70.56; H, 8.29; N, 16.46. Found C, 70.27; H, 8.26; N, 16.39.
Norbo-22: Yield: 45%; mp: 105.3–107.0 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.26 (d, 1H, J = 8.1 Hz), 1.33 (d, 1H, J = 8.3), 1.41 (d, 1H, J = 6.8 Hz), 1.72–1.76 (m, 1H), 1.88–1.93 (m, 2H), 2.52 (t, 2H, -CH2-N1-, J = 5.7 Hz), 2.59 (bs, 4H, 2CH2 pip., J = 4.9 Hz), 2.83–2.85 (m, 1H), 2.89 (s, 1H), 3.14 (s, 1H), 3.31–3.33 (m, 2H, -NH-CH2-), 3.62 (bs, 4H, 2CH2 pip., J = 4.9 Hz), 5.98 (dd, 1H, J = 5.5, 2.9 Hz), 6.20 (dd, 1H, J = 5.4, 3.2 Hz), 6.64–6.66 (m, 3H), 7.47 (t, 1H, J = 7.3 Hz), 8.20 (d, 1H, J = 5.8 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.18, 29.79, 38.93, 42.65, 44.65, 44.92, 46.11, 49.88, 53.05, 57.30, 107.10, 113.63, 132.41, 137.53, 147.97, 159.18, 174.21; ESI-MS m/z [M+H]+ calculated for C20H28N4O 340.46, Found = 341.2; Anal. Calcd for C20H28N4O: C, 70.56; H, 8.29; N, 16.46. Found C, 70.55; H, 8.27; N, 16.44.
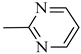
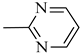
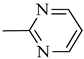
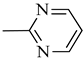
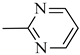
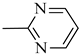
4.1.15. Synthesis of Exo-N-(3-(4-(pyrimidin-2-yl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-23) and endo-N-(3-(4-(pyrimidin-2-yl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-24)
Following the synthetic procedure reported above, Norbo-23 and Norbo-24 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 2-(piperazin-1-yl) pyrimidine (0.77 g; 4.70 mmol).
Norbo-23: Yield: 43%; mp: 107.6–108.8 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.29–1.35 (m, 2H), 1.69 (d, 1H, J = 8.0 Hz), 1.79–1.82 (m, 2H), 1.89–1.92 (m, 1H), 1.98–2.00 (m, 1H), 2.60–2.64 (m, 6H), 2.90 (s, 1H), 2.93 (s, 1H), 3.37–3.41 (m, 2H, -NH-CH2-), 3.92 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 6.09 (dd, 1H, J = 5.5, 2.7 Hz), 6.11 (dd, 1H, J = 5.5, 2.9 Hz), 6.51 (t, 1H, J = 8.0 Hz), 6.98 (bs, 1H, NH), 8.30 (d, 2H, J = 7.6 Hz); 13C-NMR (101 MHz, CDCl3) δ: 24.93, 30.48, 39.01, 41.54, 43.15, 44.86, 46.38, 47.16, 52.96, 57.25, 110.33, 136.05, 138.14, 157.77, 161.44, 175.65; ESI-MS m/z [M+H]+ calculated for C19H27N5O 341.45, Found = 342.2; Anal. Calcd for C19H27N5O: C, 66.83; H, 7.97; N, 20.51. Found C, 66.62; H, 7.99; N, 20.44.
Norbo-24: Yield: 35%; mp: 116.5–118.9 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27 (d, 1H, J = 8.2 Hz), 1.34 (dd, 1H, J = 8.3, 2.7 Hz), 1.42 (d, 1H, J = 6.5 Hz), 1.74–1.79 (m, 1H), 1.89–1.95 (m, 2H), 2.54 (t, 2H, -CH2-N1-, J = 5.7 Hz), 2.63 (bs, 4H, 2CH2 pip., J = 5.0 Hz), 2.84–2.87 (m, 1H), 2.90 (s, 1H), 3.16 (s, 1H), 3.31–3.35 (m, 2H, -NH-CH2-), 3.93 (bs, 4H, 2CH2 pip., J = 5.0 Hz), 5.99 (dd, 1H, J = 5.6, 3.1 Hz), 6.20 (dd, 1H, J = 5.7, 3.2 Hz), 6.51 (t, 1H, J = 8.0 Hz), 6.66 (bs, 1H, NH), 8.31 (d, 2H, J = 7.6 Hz); 13C-NMR (101 MHz, CDCl3) δ: 25.06, 29.77, 38.72, 42.66, 43.13, 44.90, 46.13, 49.90, 53.04, 57.16, 110.28, 132.41, 137.56, 157.75, 161.44, 172.98; ESI-MS m/z [M+H]+ calculated for C19H27N5O 341.45, Found = 342.2; Anal. Calcd for C19H27N5O: C, 66.83; H, 7.97; N, 20.51. Found C, 66.76; H, 7.96; N, 20.46.
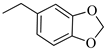
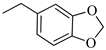
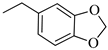
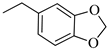
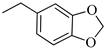
4.1.16. Synthesis of Exo-N-(3-(4-(benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-25) and endo-N-(3-(4-(benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-26)
Following the synthetic procedure reported above, Norbo-25 and Norbo-26 were synthetized starting from 2 (1.00 g; 4.70 mmol) and 1-(benzo[d][1,3] dioxol-5-ylmethyl)piperazine (1.03 g; 4.70 mmol). The final isomer Norbo-25 was converted into the corresponding hydrochloride salt.
Norbo-25: Yield: 33%; mp: 215.5–217.5 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27–1.35 (m, 2H), 1.66–1.71 (m, 3H), 1.90–1.93 (m, 1H), 1.97–2.01 (m, 1H), 2.50–2.57 (m, 6H), 2.91 (s, 2H), 3.33–3.35 (m, 2H, -NH-CH2-), 3.44 (s, 2H, -CH2-), 3.48 (bs, 4H, 2CH2 pip., J = 5.1 Hz), 5.94 (s, 2H, -OCH2), 6.10 (dd, 1H, J = 5.5, 2.9 Hz), 6.15 (dd, 1H, J = 5.5, 3.0 Hz), 6.73 (bs, 2H), 6.83 (s, 1H), 7.18 (bs, 1H, NH); 13C-NMR (101 MHz, CDCl3) δ: 24.73, 30.31, 38.71, 41.57, 44.89, 46.34, 47.25, 52.41, 53.06, 56.52, 62.54, 100.91, 107.90, 109.42, 122.24, 135.99, 138.71, 146.72, 147.76, 175.44; ESI-MS m/z [M+H]+ calculated for C23H31N3O3 397.51, Found = 398.1; Anal. Calcd for C23H31N3O3: C, 69.49; H, 7.86; N, 10.57. Found C, 69.35; H, 7.83; N, 10.54.
Norbo-26: Yield: 34%; mp: 74.9–78.8 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.27–1.34 (m, 2H), 1.43 (d, 1H, J = 6.8 Hz), 1.63–1.66 (m, 1H), 1.86–1.91 (m, 2H), 2.45–2.50 (m, 6H), 2.8–2.84 (m, 1H), 2.90 (s, 1H), 3.14 (s, 1H), 3.26–3.28 (m, 2H, -NH-CH2-), 3.43 (s, 2H, -CH2-), 3.48 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 5.93 (s, 2H, -OCH2), 5.96 (dd, 1H, J = 5.9, 2.8 Hz), 6.19 (dd, 1H, J = 5.8, 3.1 Hz), 6.73 (bs, 2H), 6.83 (s, 1H), 7.18 (bs, 1H, NH); 13C-NMR (101 MHz, CDCl3) δ: 25.09, 29.81, 39.31, 42.65, 44.88, 46.09, 49.81, 52.68, 53.28, 57.47, 62.65, 100.87, 107.84, 109.47, 122.24, 131.59, 132.47, 137.42, 146.63, 147.61, 174.09; ESI-MS m/z [M+H]+ calculated for C23H31N3O3 397.51, Found = 398.0; Anal. Calcd for C23H31N3O3: C, 69.49; H, 7.86; N, 10.57. Found C, 69.35; H, 7.87; N, 10.56.
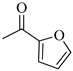
4.1.17. Synthesis of Exo- N-(3-(4-(furan-2-carbonyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-27) and endo-N-(3-(4-(furan-2-carbonyl)piperazin-1-yl)propyl)bicyclo[2.2.1]hept-5-ene-2-carboxamide (Norbo-28)
Following the synthetic procedure reported above, Norbo-27 and Norbo-28 were synthetized starting from 2 (1.00 g; 4.70 mmol) and furan-2-yl(piperazin-1-yl)-methanone (0.85 g; 4.70 mmol). The final isomers Norbo-27 and Norbo-28 were converted into the corresponding hydrochloride salts.
Norbo-27: Yield: 33%; mp: 211.5–214.2 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 1.13–1.17 (m, 2H), 1.61 (d, 1H, J = 8.0 Hz), 1.74–1.76 (m, 2H), 1.82–1.85 (m, 1H), 2.00–2.03 (m, 1H), 2.80 (bs, 4H, 2CH2 pip., J = 4.7 Hz), 3.05–3.11 (m, 8H), 3.47–3.49 (m, 2H, -NH-CH2-), 6.11 (dd, 1H, J = 5.7, 2.8 Hz), 6.12 (dd, 1H, J = 6.0, 3.0 Hz), 6.65 (t, 1H, J = 8.1 Hz), 7.09 (d, 1H, J = 8.1 Hz), 7.87 (d, 1H, J = 7.3 Hz), 8.04 (bs, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ: 24.20, 30.28, 36.32, 40.60, 41.42, 43.49, 46.13, 47.28, 51.11, 54.09, 111.97, 117.03, 136.66, 138.23, 145.74, 146.54, 158.63, 175.31; ESI-MS m/z [M+H]+ calculated for C20H27N3O3 357.45, Found = 358.2; Anal. Calcd for C20H27N3O3: C, 67.20; H, 7.61; N, 11.76. Found C, 67.18; H, 7.59; N, 11.73.
Norbo-28: Yield: 38%; mp: 194.3–197.1 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 1.22–1.31 (m, 2H), 1.68–1.75 (m, 3H), 1.76–1.79 (m, 1H), 2.76–2.79 (m, 6H), 3.00–3.08 (m, 6H), 3.13 (s, 1H), 3.46–3.49 (m, 2H, -NH-CH2-), 5.82 (dd, 1H, J = 5.5, 2.7 Hz), 6.08 (dd, 1H, J = 5.6, 3.2 Hz), 6.64 (t, 1H, J = 8.1 Hz), 7.08 (d, 1H, J = 8.3 Hz), 7.78 (bs, 1H, NH), 7.87 (d, 1H, J = 7.3 Hz). 13C-NMR (101 MHz, DMSO-d6) δ: 24.25, 28.73, 36.15, 40.52, 42.50, 43.78, 46.01, 49.84, 51.06, 54.06, 112.00, 117.07, 132.71, 137.42, 145.78, 146.59, 158.60, 173.60; ESI-MS m/z [M+H]+ calculated for C20H27N3O3 357.45, Found = 358.2; Anal. Calcd for C20H27N3O3: C, 67.20; H, 7.61; N, 11.76. Found C, 67.06; H, 7.64; N, 11.79.
4.2. In Vitro Receptor Assays
4.2.1. Functional 5-HT1A Receptor Assay
Male Sprague-Dawley rats were decapitated, with their brains removed and placed on ice. Hippocampi were dissected and homogenized with a glass homogenizer in 30 vol. ice-cold TED buffer (50 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiotheritol, pH 7.4). Next, the homogenate was centrifuged at 21,000× g for 30 min at 4 °C. The pellet was suspended in 30 vol. TED buffer (pH 7.4) and incubated in a water bath for 10 min at 37 °C to remove endogenous serotonin. The suspension was centrifuged again at 21,000× g for 30 min at 4 °C. The pellet was resuspended in 30 vol. TED buffer (pH 7.4) and the centrifugation step was repeated. The final pellet was suspended in 10 vol. 50 mM Tris-HCl (pH 7.4) and stored at −80 °C until use. In the agonist mode, 15 μg/mL of hippocampus homogenate was incubated in triplicate with 0.8 nM [35S]GTPγS (guanosine-5′-(γ-thio)-triphosphate) in a assay buffer (50 mM Tris-HCl, pH = 7.4, 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid tetrasodium salt (EGTA), 3 mM MgCl2, 100 mM NaCl, 30 µM guanosine diphosphate (GDP)) in the presence of increasing concentrations of the tested compounds (10−10–10−5 M). Compounds were dissolved in ethanol, so that the concentration of ethanol in the assay did not exceed 0.75%. Non-specific binding was determined with 100 µM of unlabeled GTPγS. The reaction mixture was incubated for 90 min. at 37 °C in a volume of 250 µL. Next, 96-well Unifilter® Plates (Perkin Elmer, Waltham, MA, USA) were presoaked for 1 h with 50 mM Tris-HCl (pH = 7.4) before harvesting. The reaction was terminated by vacuum filtration onto filter plates with the FilterMate Harvester® (Perkin Elmer, Waltham, MA, USA). The samples were then rapidly washed with 2 mL of 50 mM Tris-HCl (pH = 7.4) buffer. Filter plates were dried for 2 h at 50 °C. After drying, 45 µL of EcoScint-20 scintillant (Perkin Elmer, Waltham, MA, USA) was added to every well. Radioactivity was counted in a Trilux MicroBeta2 counter (Perkin Elmer, Waltham, MA, USA). Data were analyzed with GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). Curves were fitted with a one-site non-linear regression model. Efficacy (Emax) and potency (EC50) were calculated from the Cheng-Prusoff equation and expressed as means ± SEM.
4.2.2. 5-HT2A Competition Binding Assay
Male Sprague-Dawley (SD) rats were decapitated, their brains removed and placed on ice. Frontal cortices were homogenized with a glass homogenizer in 30 vol. ice-cold homogenization buffer (50 mM Tris-HCl, 1 mM EDTA, 5 mM MgCl2, pH 7.4). Next, the homogenate was centrifuged at 20,000× g for 15 min at 4 °C. The pellet was suspended in 30 vol. 50 mM Tris-HCl (pH 7.4) and incubated in a water bath for 15 min. at 37 °C to remove endogenous serotonin. The suspension was again centrifuged at 20,000× g for 15 min at 4 °C. The pellet was resuspended in 10 vol. 50 mM Tris-HCl (pH 7.4) and the centrifugation step was repeated. The final pellet was suspended in 10 vol. 50 mM Tris-HCl (pH 7.4) and stored at −80 °C. For the 5-HT2A assay frontal cortex homogenates (160 µg protein/mL) were incubated in triplicate with 1 nM [3H]ketanserin for 60 min. at 36 °C in a 50 mM Tris-HCl (pH 7.4) buffer containing 0.1% ascorbate, 3 mM CaCl2 and 10 µM pargyline) and increasing concentrations (10−11 M–10−5 M) of the compound of interest. Non-specific binding was determined in the presence of 10 μM mianserin. After incubation, the reaction mixture was deposited onto UniFilter-96 GF/B plates with the aid of a FilterMate-96 Harvester. Filter plates were presoaked beforehand with 0.4% PEI for 1 h. Next, each filter well was washed with 1.75 mL of 50 mM Tris-HCl (pH 7.4) and left to dry on a heating block set to 50 °C for 2 h. Then, 45 µL of Microscint-20 scintillation fluid was added to each filter well and left to equilibrate overnight. Filter-bound radioactivity was counted in a MicroBeta2 Microplate Counter. Binding curves were fitted with one site non-linear regression. Affinity was presented as the inhibitory constant (pKi and Ki ± SEM) from two or three separate experiments.
4.2.3. 5-HT2C Competition Binding Assay
The 5-HT2C assay was performed in HEK 239 cells stably transfected with the human 5-HT2C receptor (ChemiSCREEN™ Human 5-HT2C Receptor Membrane Preparation, Merck). Membranes (5 μg/well final concentration) were mixed with 6 nM [3H]mesulergine in a 50 mM Tris-HCl (pH 7.4) buffer containing 5 mM MgCl2, 1 mM CaCl2. Compounds were dissolved in 50% DMSO and added to the reaction mixture at 10 concentrations equally spaced on a log scale (10−10–10−4.5 M) and incubated for 2 h at 25 °C. The total DMSO concentration was 5%. Non-specific binding was determined in the presence of 10 μM mianserin. After incubation, the reaction mixture was deposited onto UniFilter-96 GF/B plates with the aid of a FilterMate-96 Harvester. Filter plates were presoaked beforehand with 0.33% polyethyleneimine (PEI) and 0.5% bovine serum albumin (BSA) for 30 min. Next, each filter well was washed with 1.75 mL of 50 mM Tris-HCl (pH 7.4) buffer supplemented with 500 mM NaCl to wash out the unbound ligands. Filters were and left to dry overnight at room temperature (RT). When completely dry, 45 µL of Microscint-20 scintillation fluid was added to each filter well and allowed to equilibrate for 30 min. Filter-bound radioactivity was counted in a MicroBeta2 Microplate Counter. Binding curves were fitted with a one site non-linear regression model. Affinity was calculated using the result of the Cheng–Prusoff equation (pKi ± SEM and Ki, 95% CI) from two separate experiments.
4.2.4. Functional D2 Receptor Assay
Male Sprague-Dawley rats were decapitated, with their brains removed and placed on ice. Striatal tissue was dissected and homogenized with a glass homogenizer in 30 vol. ice-cold TED buffer (50 mM Tris-HCl, 1 mM EDTA, 1 mM dithiothreitol, pH 7.4). Next, the homogenate was centrifuged at 48,000× g for 15 min at 4 °C. The pellet was suspended in 30 vol. TED buffer (pH 7.4) and incubated in a water bath for 10 min. at 37 °C to remove endogenous ligands. The suspension was centrifuged again at 48,000× g for 15 min at 4 °C. The pellet was resuspended in 30 vol. TED buffer (pH 7.4) and the centrifugation step was repeated. The final pellet was suspended in 10 vol. 50 mM Tris-HCl (pH 7.4) and stored at −80 °C until use. For the D2 receptor antagonist [35S]GTPγS assay, 15μg/mL of striatal homogenate was incubated in triplicate with 0.8 nM [35S]GTPγS (final concentration: 0.08 nM) in an assay buffer (50 mM Tris-HCl, pH = 7.4, 1 mM EGTA, 3 mM MgCl2, 100 mM NaCl, 0.1 mM dithiothreitol, 500 µM ascorbic acid, 20 µM GDP and 40 µM dopamine) in the presence of increasing concentrations of the tested compounds (10−8−10−3.5 M). Serial dilutions of compounds were dissolved in 7.5% ethanol. Dopamine was dissolved in 50 mM Tris buffer (pH = 7.4) supplemented with 500 µM ascorbic acid to prevent oxidation. The effect on basal G-protein activation threshold was determined in assay buffer deprived of dopamine. The final ethanol concentration in the assay was 0.75%. Non-specific binding was determined with 100 µM of unlabeled GTPγS. The reaction mixture was incubated for 60 min. at 30 °C at a volume of 250 µL. Next, 96-well Unifilter® Plates (Perkin Elmer, Waltham, MA, USA) were presoaked for 1 h with 50 mM Tris-HCl (pH = 7.4) before harvesting. The reaction was terminated by vacuum filtration onto filter plates with the FilterMate Harvester® (Perkin Elmer, Waltham, MA, USA). Samples were then rapidly washed with 2 mL of 50 mM Tris-HCl (pH = 7.4) buffer. Filter plates were dried overnight at RT. After drying, 45 µL of EcoScint-20 scintillant (Perkin Elmer, Waltham, MA, USA) was added to the wells. Radioactivity was counted in a Trilux MicroBeta2 counter (Perkin Elmer, Waltham, MA, USA). Data were analyzed with GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). Curves were fitted with a one-site non-linear regression model. Inhibitory potency (pIC50) was expressed as means ± SEM. IC50 values were expressed in µM and presented as means ±95% CI from two separate experiments.
4.3. Computational Methods
4.3.1. Compound Preparation
The investigated compounds Norbo1-28 were modeled using LigPrep module [47] of Schrödinger suite of software, v. 2019-4 as previously reported [22,23]. In order to find the protonation state, Epik module [48] of Schrödinger suite of software, v. 2019-4 was used.
4.3.2. Receptor Structures
X-ray structures of respective receptors in inactive conformations were used: serotonin 5-HT2A receptor in complex with an antagonist risperidone (PDB ID: 6A93 [49]) and serotonin 5-HT2C receptor in complex with an inverse agonist ritanserin (PDB ID: 6BQH [50]) as previously reported [22,23]. In the case of serotonin 5-HT1A receptor, the cro-EM structure of the receptor in active conformation in complex with a partial agonist aripiprazole (PDB ID: 7E2Z [51]) was used. The structures of the receptors were preprocessed using the Protein Preparation Wizard of Maestro Release 2019.4 [52] as previously reported [22,23]. The Yasara Structure v. 20.12.24 [53] tool for loop modelling was used to build receptors extracellular loops if necessary.
4.3.3. Molecular Docking
Standard Precision (SP) method of molecular docking with Glide [54] from Schrödinger release 2019-4 was applied. The grid files were obtained based on co-crystallized ligands. The selected receptors hydroxyl groups in the active sites were made flexible as previously reported [22,23]. Hence, 200 poses were generated in the case of each ligand-receptor complex. The poses were then filtered to ensure the interaction of the conserved Asp3.32 (Ballesteros-Weinstein numbering [55]) of the receptor with a protonatable nitrogen atom of the ligand. The final poses were identified by consideration of Glide docking scores and based on the visual inspection. Maestro Release 2019.4 [52] and PyMol 2.0.4 [56] software were used for visualization of molecular modeling results.
4.4. In Vivo Behavioral Tests
4.4.1. General Procedures
The experiments were carried out, in the Experimental Medicine Center of Medical University of Lublin, on six-week-old naive male Swiss mice, weighing 24–30 g. The mice were housed in cages, five individuals per cage in an environmentally controlled rooms (ambient temperature 22 ± 1 °C; relative humidity 50–60%; 12 h light/dark cycle, lights on at 8:00). Standard laboratory food(LSM-Agropol-Motycz, Lublin, Poland) and filtered water were available ad libitum. All the experimental procedures were carried out, according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and to the European Community Council Directive for the Care and Use of Laboratory Animals of 22 September 2010 (2010/63/EU) and approved by the Local Ethics Committee for Animal Experimentation in Lublin. The investigated compounds (Norbo-4, Norbo-8, Norbo-10, Norbo-14, Norbo-18 and Norbo-20) in all tests were administered intraperitoneally (i.p.), dissolved in dimethyl sulfoxide (DMSO, final concentration of 0.1%) and then diluted by aqueous solution of 0.5% methylcellulose (tylose) and injected 60 min before the tests. Other drugs (amphetamine (amph), buspirone, sertraline) were administered i.p. diluted in saline, and injected30 (amph) or 60 min (buspirone, sertraline) before the tests. All compounds were given in a volume of 10 mL/kg to mice. The control animals received an equivalent volume of the solvent at the respective time before the tests. All the experiments were conducted in the light phase between 09.00 a.m. and 14.00 p.m. The experiments were performed by an observer unaware of the treatment administered.
4.4.2. Spontaneous Locomotor Activity
The locomotor activity of mice was measured using an animal activity meter Opto-Varimex-4 Auto-Track (Columbus Instruments, Columbus, OH, USA). This automatic device consists of eight transparent cages with a lid, set of four infrared emitters (each emitter has 16 laser beams) and eight detectors monitoring animal movements. To assess the spontaneous activity of mice, the compounds or vehicle (as a control) were administered 60 min before the test. In another set of experiments, the influence of tested compounds on amph-induced hyperactivity in mice was evaluated. The study was conducted in the same apparatus, but each mouse received amph (5 mg/kg, s. c.) 30 min after injection of vehicle or tested compounds. The animals were placed in the cage individually, 50 min after the administration of the tested compounds, for a period of 10 min for acclimatization. After this time, their activity was noted after 6 min (corresponded with the time duration of the FST and near (5 min) to the EPM test, respectively) and after 20 min, to observe the dynamics of changes. The distance travelled in centimeters [cm] was measured. The cages were cleaned up with 10% ethanol after each mouse.
4.4.3. Motor Coordination
The effects of investigated compounds on motor coordination were evaluated in the rota-rod [57] and chimney [58] tests. In the first test, motor impairments were measured, defined as the inability to keep balance on a rotating rod (at constant speed of 18 rpm) for 1 min. In the second test, motor impairments were assessed by mouse inability to climb up the tube backwards (3 cm in inner diameter, 25 cm long) within 60 s. Before the tests, the animals were trained once a day for 3 days. The animals able to stay on the rotating rod or to leave the chimney for 60 s were approved for experiments.
4.4.4. FST (Porsolt’s Test) in Mice
The experiment was carried out according to the method of Porsolt et al. [59]. Mice were individually placed in a glass cylinder (25 cm high; 10 cm in diameter) containing water maintained at 23–25 °C, and were left there for 6 min. The total duration of immobility was recorded during the last 4 min of a 6-min test session. A mouse was regarded as immobile when it remained floating on the water, making only small movements to keep its head above the water.
4.4.5. EPM Test
The EPM studies were carried out on mice according to the method of Lister [60]. The EPM apparatus was made of plexiglass and consisted of two open (30 × 5 cm) and two enclosed (30 × 5 × 15 cm) arms. The arms extended from a central platform of 5 × 5 cm. The apparatus was mounted on a plexiglass base, raising it 38.5 cm above the floor, and illuminated by a red light. The test consisted of placing a mouse in the center of the apparatus (facing an open arm) and allowing it to freely explore. The number of entries into the open arms and the time spent in these arms were scored for a 5 min test period. An entry was defined as placing all four paws within the boundaries of the arm. The following measures were obtained from the test: the total number of arm entries; the percentage of arm entries into the open arms; and the time spent in the open arms expressed as a percentage of the time spent in both the open and closed arms. Anxiolytic activity was indicated by increases in the time spent in open arms and in the number of open arm entries. The total number of entries into either type of arm was used additionally as a measure of overall motor activity.
4.4.6. Statistical Analysis
The results were calculated by the one-way analysis of variance ANOVA, followed by Dunnett’s post-hoc test. The results are presented as mean ± standard errors (SEM). The level of p < 0.05 was considered as statistically significant. All the figures were prepared by the GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego, CA, USA).
4.5. Ex Vivo Assays
4.5.1. General Procedures
Male rats (Sprague-Dawley, 160–200 g; Harlan Laboratories, S. Pietro al Natisone, Italy) were manipulated and cared for in strict compliance with the principles of laboratory animal care (NIH publication n° 86–23, revised 1985) and the Italian D.L. no. 116 of 27 January 1992 and associated guidelines in the European Communities Council Directive of 24 November 1986 (86/609/ECC). Animal housing complied with recent pharmacological guidance [61]. All animals weighing 160–200 g were used after a 1-week acclimation period (temperature 23 ± 2 °C; humidity 60%, free access to water and standard food).
4.5.2. Ileum Preparation and Evaluation of 5-HT-Evoked Contractions
Rats were asphyxiated using CO2 and segments (1–1.5 cm) of ileum were removed, flushed of luminal contents, and placed in Krebs solution (119 mM NaCl, 4.75 mM KCl, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl2, 1.5 mM MgSO4, and 11 mM Glucose). The segments were prepared as previously described [62]: the segments were set up in such a way as to record contractions mainly from the longitudinal axis, in an organ bath containing 20 mL of Krebs solution, bubbled with 95% O2 and 5% CO2 and maintained at 37 °C. The tissues were connected to an isotonic transducer (load: 0.5 g), connected to PowerLab system (Ugo Basile, Comerio, Italy). Ileal segments were equilibrated for 60 min [63] followed by three repeated additions of submaximal concentration of 5-HT (10−5 M) in order to record stable control contractions. To evaluate the inhibitory activity, the responses were observed in the presence of increasing concentrations (10−8–10−5 M). In preliminary experiments, the effect of 5-HT was observed in the presence of the neuronal blocker tetrodotoxin (0.3 µM), the muscarinic receptor antagonist atropine (1 µM), the adrenergic receptor antagonists phentolamine (10−6 M) plus propranolol (10−6 M) and the 5-HT2A antagonist ketanserin (0.1 µM). The contact time for each concentration was 10 min. The compounds were dissolved in DMSO. DMSO (<0.01%) did not modify 5-HT-induced contractions. Results are expressed as mean (SEM). The concentration of the compounds that produced 50% inhibition of 5-HT-induced contractions (IC50) or maximal inhibitory effect (Emax) were used to characterize compounds potency and efficacy, respectively. The IC50 and Emax values were calculated with the aid of a computer program (Graphpad Prism 5).
Author Contributions
Conceptualization, F.F. (Ferdinando Fiorino) and A.C.; methodology, J.H.K., R.S. and G.A.; software, A.A.K.; validation, B.S, E.M. and E.K.; formal analysis, A.L., M.O. and M.B.-Z.; investigation, R.C. and A.B.; resources, G.C. and F.F.(Francesco Frecentese); data curation, P.L., M.C. and B.S.; writing—original draft preparation, F.F. (Ferdinando Fiorino); writing—review and editing, F.F. (Ferdinando Fiorino), A.C.; visualization, E.G.-T.; supervision, V.S.; project administration, E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All the experimental procedures were carried out, according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and to the European Community Council Directive for the Care and Use of Laboratory Animals of 22 September 2010 (2010/63/EU) and approved by the Local Ethics Committee for Animal Experi-mentation in Lublin and in strict compliance with the principles of laboratory animal care (NIH publication n° 86–23, revised 1985) and the Italian D.L. no.116 of 27 January 1992 and associated guidelines in the European Communities Council Directive of 24 November 1986 (86/609/ECC).
Acknowledgments
The NMR spectral data were provided by Centro di Ricerca Interdipartimentale di Analisi Strumentale, Università degli Studi di Napoli Federico II. The assistance of the staff is gratefully appreciated. Calculations were partially performed under a computational grant by the Interdisciplinary Center for Mathematical and Computational Modeling (ICM), Warsaw, Poland, grant number G85-948 (to A.A.K.).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all compounds are available from the authors.
References
- Newman-Tancredi, A.; Depoortère, Y.R.; Kleven, M.S.; Kolaczkowski, M.; Zimmer, L. Translating biased agonists from molecules to medications: Serotonin 5-HT1A receptor functional selectivity for CNS disorders. Pharm. Therap. 2022, 229, 107937. [Google Scholar] [CrossRef]
- De Vry, J. 5-HT1A receptor agonists: Recent developments and controversial issues. Psychopharmacology 1995, 121, 1–26. [Google Scholar] [CrossRef]
- File, S.E. Recent developments in anxiety, stress, and depression. Pharmacol. Biochem. Behav. 1996, 54, 3–12. [Google Scholar] [CrossRef]
- Neckelmann, D.; Bjorkum, A.A.; Bjorvatn, B.; Ursin, R. Sleep and EEG power spectral effects of the 5-HT1A agonist/antagonist NAN 190 alone and in combination with citalopram. Behav. Brain Res. 1996, 75, 159–168. [Google Scholar] [CrossRef]
- Peroutka, S.J. Serotonin Receptor Subtypes. CNS Drugs 1995, 4, 18–28. [Google Scholar] [CrossRef]
- Saxena, P.R. Serotonin receptors: Subtypes, functional responses and therapeutic relevance. Pharmacol. Ther. 1995, 66, 339–368. [Google Scholar] [CrossRef]
- Cowen, D.S.; Sowers, R.S.; Manning, D.R. Activation of a mitogen-activated protein kinase (ERK2) by the 5-hydroxytryptamine 1A receptor is sensitive not only to inhibitors of phosphatidylinositol 3-kinase, but to an inhibitor of phosphatidylcholine hydrolysis. J. Biol. Chem. 1996, 271, 22297–22300. [Google Scholar] [CrossRef]
- Adayev, T.; El-Sherif, Y.; Barua, M.; Penington, N.J.; Banerjee, P. Agonist Stimulation of the Serotonin1A Receptor Causes Suppression of Anoxia-Induced Apoptosis via Mitogen-Activated Protein Kinase in Neuronal HN2-5 Cells. J. Neurochem. 2001, 72, 1489–1496. [Google Scholar] [CrossRef]
- Corvino, A.; Fiorino, F.; Severino, B.; Saccone, I.; Frecentese, F.; Perissutti, E.; Di Vaio, P.; Caliendo, G.; Magli, E. The Role of 5-HT1A Receptor in Cancer as a New Opportunity in Medicinal Chemistry. Curr. Med. Chem. 2018, 25, 3214–3227. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.A.; Mukherjee, M.; McGlynn, P.R.; Cui, M.; Kohut, J.S.; Booth, G.R. A new class of 5-HT2A/5-HT2C receptor inverse agonists: Synthesis, molecular modeling, in vitro and in vivo pharmacology of novel 2-aminotetralins. Br. J. Pharmacol. 2022, 179, 2610–2630. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sahu, B.; Pathania, S.; Singh, P.K.; Akhtar, M.J.; Kumar, B. Piperazine, a Key Substructure for Antidepressants: Its Role in Developments and Structure-Activity Relationships. ChemMedChem 2021, 16, 1878–1901. [Google Scholar] [CrossRef] [PubMed]
- Kucwaj-Brysz, K.; Kurczab, R.; Żesławska, E.; Lubelska, A.; Marć, M.A.; Latacz, G.; Satała, G.; Nitek, W.; Kieć-Kononowicz, K.; Handzlik, J. The role of aryl-topology in balancing between selective and dual 5-HT7R/5-HT1A actions of 3,5-substituted hydantoins. MedChemComm 2018, 9, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, G.; Fiorino, F.; Grieco, P.; Perissutti, E.; Santagada, V.; Severino, B.; Bruni, G.; Romeo, M.R. Synthesis of new 1,2,3-benzotriazin-4-one-arylpiperazine derivatives as 5-HT1A serotonin receptor ligands. Bioorg. Med. Chem. 2000, 8, 533–538. [Google Scholar] [CrossRef]
- Fiorino, F.; Perissutti, E.; Severino, B.; Santagada, V.; Cirillo, D.; Terracciano, S.; Massarelli, P.; Bruni, G.; Collavoli, E.; Renner, A.C.; et al. New 5-Hydroxytryptamine1A Receptor Ligands Containing a Norbornene Nucleus: Synthesis and in Vitro Pharmacological Evaluation. J. Med. Chem. 2005, 48, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Severino, B.; De Angelis, F.; Perissutti, E.; Frecentese, F.; Massarelli, P.; Bruni, G.; Collavoli, E.; Santagada, V.; Caliendo, G. Synthesis and in-vitro pharmacological evaluation of new 5-HT1A receptor ligands containing a benzotriazinone nucleus. Arch. Pharm. 2008, 341, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Severino, B.; De Angelis, F.; Perissutti, E.; Magli, E.; Frecentese, F.; Esposito, A.; Massarelli, P.; Nencini, C.; Viti, B.; et al. Synthesis and in vitro pharmacological evaluation of a new series of 5-HT1A 5-HT2A and 5-HT2C receptor ligands containing a norbornene nucleus. Die Pharm. 2009, 64, 555–564. [Google Scholar]
- Fiorino, F.; Severino, B.; De Angelis, F.; Perissutti, E.; Magli, E.; Frecentese, F.; Esposito, A.; Massarelli, P.; Nencini, C.; Santagada, V.; et al. New 5-HT1A receptor ligands containing a N′-cyanoisonicotinamidine nucleus: Synthesis and in vitro pharmacological evaluation. Bioorg. Med. Chem. Lett. 2010, 20, 2978–2982. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Severino, B.; Magli, E.; Perissutti, E.; Frecentese, F.; Esposito, A.; Incisivo, G.M.; Ciano, A.; Massarelli, P.; Nencini, C.; et al. New potent 5-HT2A receptor ligands containing an N′-cyanopicolinamidine nucleus: Synthesis and in vitro pharmacological evaluation. Eur. J. Med. Chem. 2012, 47, 520–529. [Google Scholar] [CrossRef]
- Fiorino, F.; Magli, E.; Severino, B.; Corvino, A.; Ciano, A.; Perissutti, E.; Frecentese, F.; Massarelli, P.; Nencini, C.; Santagada, V.; et al. Synthesis and In Vitro Pharmacological Evaluation of Novel 2-Hydroxypropyl-4-arylpiperazine Derivatives as Serotoninergic Ligands. Arch. Der Pharm. 2014, 347, 698–706. [Google Scholar] [CrossRef]
- Fiorino, F.; Ciano, A.; Magli, E.; Severino, B.; Corvino, A.; Perissutti, E.; Frecentese, F.; Di Vaio, P.; Izzo, A.A.; Capasso, R.; et al. Synthesis, in vitro and in vivo pharmacological evaluation of serotoninergic ligands containing an isonicotinic nucleus. Eur. J. Med. Chem. 2016, 110, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Magli, E.; Kedzierska, E.; Ciano, A.; Corvino, A.; Severino, B.; Perissutti, E.; Frecentese, F.; Di Vaio, P.; Saccone, I.; et al. New 5-HT 1A, 5HT 2A and 5HT 2C receptor ligands containing a picolinic nucleus: Synthesis, in vitro and in vivo pharmacological evaluation. Bioorg. Med. Chem. 2017, 25, 5820–5837. [Google Scholar] [CrossRef] [PubMed]
- Magli, E.; Kędzierska, E.; Kaczor, A.A.; Severino, B.; Corvino, A.; Perissutti, E.; Frecentese, F.; Saccone, I.; Massarelli, P.; Gibuła-Tarłowska, E.; et al. Synthesis, docking studies, and pharmacological evaluation of 5HT2C ligands containing the N’-cyanoisonicotinamidine or N’-cyanopicolinamidine nucleus. Arch. Pharm. 2019, 352, 1800373. [Google Scholar] [CrossRef] [PubMed]
- Magli, E.; Kędzierska, E.; Kaczor, A.A.; Bielenica, A.; Severino, B.; Gibuła-Tarłowska, E.; Kotlinska, J.H.; Corvino, A.; Sparaco, R.; Esposito, G.; et al. Synthesis, docking studies, and pharmacological evaluation of 2-hydroxypropyl-4-arylpiperazine derivatives as serotoninergic ligands. Arch. Pharm. 2021, 354, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, M.R.; Magli, E.; Caliendo, G.; Sparaco, R.; Massarelli, P.; D’Esposito, V.; Migliaccio, T.; Mosca, G.; Fiorino, F.; Formisano, P. Serotoninergic receptor ligands improve Tamoxifen effectiveness on breast cancer cells. BMC Cancer 2022, 22, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Rutkowska, E.; Bartuzi, D.; Targowska-Duda, K.M.; Matosiuk, D.; Selent, J. Computational methods for studying G protein-coupled receptors (GPCRs). Methods Cell Biol. 2016, 132, 359–399. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, D.; Kaczor, A.A.; Targowska-Duda, K.M.; Matosiuk, D. Recent Advances and Applications of Molecular Docking to G Protein-Coupled Receptors. Molecules 2017, 22, 340. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Silva, A.G.; Loza, M.I.; Kolb, P.; Castro, M.; Poso, A. Structure-Based Virtual Screening for Dopamine D2Receptor Ligands as Potential Antipsychotics. ChemMedChem 2016, 11, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Kondej, M.; Wróbel, T.M.; Silva, A.G.; Stępnicki, P.; Koszła, O.; Kędzierska, E.; Bartyzel, A.; Biała, G.; Matosiuk, D.; Loza, M.I.; et al. Synthesis, pharmacological and structural studies of 5-substituted-3-(1-arylmethyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles as multi-target ligands of aminergic GPCRs. Eur. J. Med. Chem. 2019, 180, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Targowska-Duda, K.M.; Silva, A.G.; Kondej, M.; Biała, G.; Castro, M. N-(2-Hydroxyphenyl)-1-[3-(2-oxo-2,3-dihydro-1H- benzimidazol-1-yl)propyl]piperidine-4-Carboxamide (D2AAK4), a Multi-Target Ligand of Aminergic GPCRs, as a Potential Antipsychotic. Biomolecules 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Bueschbell, B.; Barreto, C.A.V.; Preto, A.J.; Schiedel, A.C.; Moreira, I.S. A Complete Assessment of Dopamine Receptor- Ligand Interactions through Computational Methods. Molecules 2019, 24, 1196. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, Y.; Ma, J.; Wu, H.; Wacker, D.; Katritch, V.; Han, G.W.; Liu, W.; Huang, X.-P.; Vardy, E.; et al. Structural Basis for Molecular Recognition at Serotonin Receptors. Science 2013, 340, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-X.; Cheng, T.; Li, W.-H.; Liu, G.-X.; Zhu, W.-L.; Tang, Y. Computational insights into the subtype selectivity and “message-address-efficacy” mechanisms of opioid receptors through JDTic binding and unbinding. Acta Pharmacol. Sin. 2017, 39, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Kucwaj-Brysz, K.; Dela, A.; Podlewska, S.; Bednarski, M.; Siwek, A.; Satała, G.; Czarnota, K.; Handzlik, J.; Kieć-Kononowicz, K. The Structural Determinants for α1-Adrenergic/Serotonin Receptors Activity among Phenylpiperazine-Hydantoin Derivatives. Molecules 2021, 26, 7025. [Google Scholar] [CrossRef]
- Zagórska, A.; Kolaczkowski, M.; Bucki, A.; Siwek, A.; Kazek, G.; Satała, G.; Bojarski, A.; Partyka, A.; Wesołowska, A.; Pawłowski, M. Structure–activity relationships and molecular studies of novel arylpiperazinylalkyl purine-2,4-diones and purine-2,4,8-triones with antidepressant and anxiolytic-like activity. Eur. J. Med. Chem. 2015, 97, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Kurczab, R.; Canale, V.; Satała, G.; Marciniec, K.; Pasierb, A.; Jastrzębska-Więsek, M.; Pawłowski, M.; Wesołowska, A.; Bojarski, A.J.; et al. The impact of the halogen bonding on D 2 and 5-HT 1A /5-HT 7 receptor activity of azinesulfonamides of 4-[(2-ethyl)piperidinyl-1-yl]phenylpiperazines with antipsychotic and antidepressant properties. Bioorg. Med. Chem. 2017, 25, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M. History of Pharmacology—From Antiquity to the Twentieth Century. In Pharmacology, Principles and Practice; Academic Press: London, UK, 2009; pp. 1–7. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays; Vogel, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; p. 565. [Google Scholar]
- Gobira, P.H.; Ropke, J.; Aguiar, D.C.; Crippa, J.A.; Moreira, F.A. Animal models for predicting the efficacy and side effects of antipsychotic drugs. Rev. Bras. Psiquiatr. 2013, 35, S132–S139. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.G.; Fone, K.C.F. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Meltzer, H.Y. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999, 21, 106S–115S. [Google Scholar] [CrossRef][Green Version]
- Tomic, M.; Ignjatovic, D.; Tovilovic, G.; Andric, D.; Roglic, G.; Kostic-Rajacic, S. Two new phenylpiperazines with atypical antipsychotic potential. Bioorg. Med. Chem. Lett. 2007, 17, 5749–5753. [Google Scholar] [CrossRef]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Partyka, A.; Jarosz, J.; Wasik, A.; Jastrzębska-Więsek, M.; Zagórska, A. Novel tricyclic[2,1-f] theophylline derivatives of LCAP with activity in mouse models of affective disorders. J. Pharm. Pharmacol. 2014, 66, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Chopin, P.; Briley, M. The benzodiazepine antagonist flumazenil blocks the effects of CCK receptor agonists and antagonists in the elevated plus-maze. Psychopharmacology 1993, 110, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Harada, K.; Aota, M.; Inoue, T.; Matsuda, R.; Mihara, T.; Yamaji, T.; Ishibashi, K.; Matsuoka, N. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: A comparison with diazepam and buspirone. Eur. J. Pharmacol. 2006, 553, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2019-4: LigPrep, Schrödinger, LLC: New York, NY, USA, 2019.
- Schrödinger Release 2019-4: Epik, Schrödinger, LLC: New York, NY, USA, 2019.
- Kimura, K.; Asada, H.; Inoue, A.; Kadji, F.M.N.; Im, D.; Mori, C.; Arakawa, T.; Hirata, K.; Nomura, Y.; Nomura, N.; et al. Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nat. Struct. Mol. Biol. 2019, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; McCorvy, J.D.; Harpsøe, K.; Lansu, K.; Yuan, S.; Popov, P.; Qu, L.; Pu, M.; Che, T.; Nikolajsen, L.F.; et al. 5-HT2C Receptor Structures Reveal the Structural Basis of GPCR Polypharmacology. Cell 2018, 172, 719–730.e14. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.-D.; Yen, H.-Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef]
- Schrödinger Release 2019-4: BioLuminate, Schrödinger, LLC: New York, NY, USA, 2019.
- Ozvoldik, K.; Stockner, T.; Rammner, B.; Krieger, E. Assembly of Biomolecular Gigastructures and Visualization with the Vulkan Graphics API. J. Chem. Inf. Model. 2021, 61, 5293–5303. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. In Methods in Neurosciences; Sealfon, S.C., Ed.; Receptor Molecular Biology; Academic Press: Cambridge, MA, USA, 1995; Volume 25, pp. 366–428. [Google Scholar]
- The PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC: New York, NY, USA, 2019.
- Gross, F.; Tripod, J.; Meier, R. Pharmacological characteristics of the soporific doriden. Schweiz. Med. Wochenschr. 1955, 85, 305–309. [Google Scholar]
- Boissier, J.R.; Tardy, J.; Diverres, J.C. Psychopharmacological profile of a new dopaminergic agonist RU 24213. Med. Exp. 1960, 3, 81–84. [Google Scholar]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.; Izzo, A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. J. Cereb. Blood Flow Metab. 2015, 172, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- Javadi-Paydar, M.; Zakeri, M.; Norouzi, A.; Rastegar, H.; Mirazi, N.; Dehpour, A.R. Involvement of nitric oxide in granisetron improving effect on scopolamine-induced memory impairment in mice. Brain Res. 2012, 1429, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Briejer, M.R.; Mathis, C.; Schuurkes, J.A.J. 5-HT receptor types in the rat ileum longitudinal muscle: Focus on 5-HT2 receptors mediating contraction. Neurogastroenterol. Motil. 1997, 9, 231–237. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).