Chemical Profile of Cyperus laevigatus and Its Protective Effects against Thioacetamide-Induced Hepatorenal Toxicity in Rats
Abstract
1. Introduction
2. Results
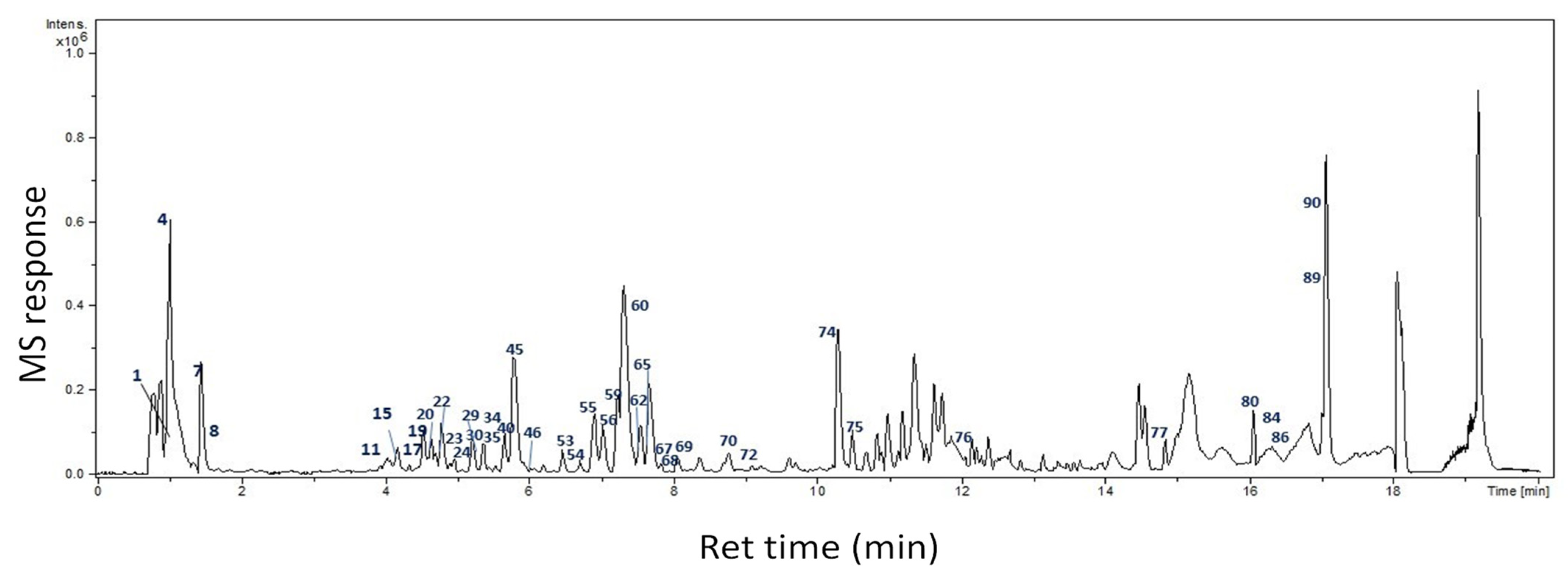
2.1. Identification of Bioactive Compounds
2.1.1. Organic Acids
| Peak No | Name | MF | Rt | [M−H]− m/z | Diff (ppm) | Ms2 | Class | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Gluconic acid | C6H12O7 | 0.968 | 195.0500 | 5.1 | 161, 129, 89, 75 | Organic acid | |
| 2 | Tetrahydroxypentanoic acid | C5H10O6 | 0.980 | 165.0405 | 5.3 | 147, 99, 87, 75 | Organic acid | [5] |
| 3 | Hexose | C6H12O6 | 0.988 | 179.0562 | −0.5 | 161, 131, 113, 85 | Sugar | |
| 4 | Malic acid | C4H6O5 | 1.033 | 133.0145 | −1.8 | 115, 89 | Organic acid | |
| 5 | Malic acid isomer | C4H6O5 | 1.308 | 133.0141 | 0.8 | 115 | Organic acid | |
| 6 | Fumaric acid | C4H4O4 | 1.346 | 115.0037 | 0.3 | 69 | Organic acid | |
| 7 | Quinic acid | C7H12O6 | 1.438 | 191.0566 | −2.5 | 111, 87 | Organic acid | |
| 8 | Citric acid/Isocitric acid | C6H8O7 | 1.514 | 191.0208 | −5.7 | 155, 129, 111 | Organic acid | |
| 9 | O-Caffeoylquinic acid | C16H18O9 | 3.479 | 353.0858 | 5.7 | 191 | Phenolic acid | |
| 10 | Unknown | C14H24O10 | 4.300 | 351.1297 | 3.5 | 113, 101, 89 | Unknown glycoside | |
| 11 | O-Caffeoylquinic acid isomer | C16H18O9 | 4.028 | 353.0877 | −0.3 | 191 | Phenolic acid | |
| 12 | O-Coumaroylhexose | C15H18O8 | 4.120 | 325.0932 | −1.0 | 191, 179 | Phenolic acid | |
| 13 | Asperuloside | C18H22O11 | 4.202 | 413.1659 | −1.4 | 353, 345, 267, 249 | Iridoid | |
| 14 | Hydroxybenzoic acid | C7H6O3 | 4.134 | 137.0244 | 1.2 | 93 | Phenolic acid | |
| 15 | Feruloyl quinic acid | C17H20O9 | 4.148 | 367.1035 | 2.0 | 193 | Phenolic acid | |
| 16 | Feruloyl-O-hexoside | C16H20O9 | 4.337 | 355.1035 | −0.2 | 163 | Phenolic acid | |
| 17 | Caffeic acid | C9H8O4 | 4.422 | 179.0350 | −9.2 | 135 | Phenolic acid | |
| 18 | O-Syringoyl quinic acid | C16H20O10 | 4.464 | 371.0988 | −1.2 | 323, 305, 121 | Phenolic acid | |
| 19 | Coumaroyl quinic acid | C16H18O8 | 4.543 | 337.0935 | −1.8 | 191, 173 | Phenolic acid | |
| 20 | Syringic acid | C9H10O5 | 4.598 | 197.0460 | −2.5 | 182, 167 | Phenolic acid | |
| 21 | Leptosidin-O-dipentoside (Tetrahydroxy-methoxy-aurone-O-di-pentoside) | C26H28O14 | 4.557 | 563.1411 | −0.8 | nd | Aurone | [21] |
| 22 | Feruloylquinic acid isomer | C17H20O9 | 4.763 | 367.1035 | −2.3 | 193, 173 | Phenolic acid | [5] |
| 23 | Luteolin di-O-hexoside | C27H30O16 | 4.852 | 609.1459 | 0.4 | 447 | Flavone | [22] |
| 24 | Luteolin- O-hexoside-O-glucuronide | C27H28O17 | 4.900 | 623.1236 | 2.9 | 461 | Flavone | |
| 25 | O-Syringoylquinic acid O-(Hydroxydimethoxy- benzoyl)-quinic acid | C16H20O10 | 4.952 | 371.0984 | 2.5 | 353, 327, 249, 231, 121 | Phenolic acid | [5] |
| 26 | Dicaffeoylquinic acid | C25H24O12 | 4.918 | 515.1213 | −3.4 | Nd | Phenolic acid | [5] |
| 27 | O-(Hydroxydimethoxybenzoyl)-quinic acid isomer | C16H20O10 | 4.969 | 371.0978 | 1.7 | 249 | Phenolic acid | |
| 28 | O-Coumaroylglycerol | C12H14O5 | 5.097 | 237.0768 | 2.1 | 145 | Phenolic acid | |
| 29 | O-Caffeoyl-O-syringoylquinic acid | C25H26O13 | 5.193 | 533.1301 | −1.6 | 469, 443, 255 | Phenolic acid | |
| 30 | Tetrahydroxy-dimethoxyflavone- di-O-hexoside | C29H34O18 | 5.364 | 669.1657 | 2.3 | 623, 619, 507, 427 | Flavone | |
| 31 | Tetrahydroxy-methoxyflavone- dihexoside | C28H32O17 | 5.282 | 639.1567 | 0.3 | 593 | Flavone | |
| 32 | 5-Hydroxy-4′,7-dimethoxy-6,8-dimethyl-2′,5′-flavanonequinone (Scaberin) | C19H18O7 | 5.357 | 357.0980 | −2.5 | 163, 119 | Flavanone quinone | [6] |
| 33 | Hydroxycinnamic acid | C9H8O3 | 5.388 | 163.0403 | −1.2 | 119 | Phenolic acid | [23] |
| 34 | Dihydroxy-dimethoxy-methylaurone | C18H16O6 | 5.411 | 327.0874 | −1.6 | 283, 163 | Aurone | [24] |
| 35 | Tetrahydroxy-dimethoxyflavone- di-O-hexoside | C29H34O18 | 5.415 | 669.1674 | −0.3 | 619, 507, 427 | Flavone | |
| 36 | Hydroxycinnamic acid isomer | C9H8O3 | 5.532 | 163.0405 | −2.4 | 119 | Phenolic acid | [23] |
| 37 | Luteolin-O-deoxyhexoside -O-glucuronide | C27H28O16 | 5.473 | 607.1305 | −0.1 | 493, 436, 284 | Flavone | |
| 38 | Hydroxyoctanoic acid-O-hexoside | C14H26O8 | 5.432 | 321.1555 | 5.2 | 158, 114 | Fatty acid | |
| 39 | Hydroxycinnamoyl-O-malic acid | C13H12O7 | 5.515 | 279.0510 | −2.2 | 163 | Phenolic acid | |
| 40 | Tetrahydroxyflavone-O-pentosyl hexoside (Luteolin-O-pentosyl hexoside) | C36H36O7 | 5.649 | 579.2388 | −1.9 | 493, 285, 284 | Flavone | |
| 41 | Tetrahydroxyaurone-O-glucuronide (Aureusidin 6-O-glucuronide) | C21H18O12 | 5.748 | 461.0725 | −2.9 | 285, 283 | Aurone | [24] |
| 42 | Tetrahydroxyaurone-O-glucuronide isomer | C21H18O12 | 5.763 | 461.0736 | −2.2 | 285 | Aurone | |
| 43 | Aureusidin (Tetrahydroxyaurone) | C15H10O6 | 5.820 | 285.0405 | −2.6 | Nd | Aurone | [22] |
| 44 | Tetrahydroxymethoxyflavone-O-glucuronide | C22H20O13 | 5.903 | 491.0831 | −2.0 | 315 | Flavone | |
| 45 | Pentahydroxymethoxyflavone-O-glucuronide | C22H20O14 | 5.910 | 507.0780 | −2.7 | 462, 331 | Flavone | |
| 46 | Ferulic acid (Hydroxymethoxycinnamic acid) | C10H10O4 | 5.937 | 193.0514 | −3.8 | 178, 134 | Phenolic acid | [5] |
| 47 | Hydroxydimethoxycinna-mic acid isomer | C11H12O5 | 5.947 | 223.0612 | −2.2 | nd | Phenolic acid | |
| 48 | Tetrahydroxymethoxyflavone-O-glucuronide isomer | C22H20O13 | 5.995 | 491.0831 | −1.5 | 315, 175 | Flavone | |
| 49 | Hydroxymethoxycinnamic acid isomer | C10H10O4 | 5.999 | 193.0506 | −2.5 | 178, 134 | Phenolic acid | [5] |
| 50 | Luteolin-O-glucuronide (Tetrahydroxy-flavone- O-glucuronide) | C21H18O12 | 6.016 | 461.0742 | −3.5 | 325, 285 | Flavone | [22] |
| 51 | Dicaffeoylquinic acid isomer | C25H24O12 | 6.164 | 515.1195 | −1.1 | 353 | Phenolic acid | [5] |
| 52 | Tetrahydroxy-methylaurone | C16H12O6 | 6.449 | 299.0561 | −4.8 | 284, 267 | Aurone | [22,23,24] |
| 53 | Luteolin-5-methyl ether (Trihydroxymethoxyflavone) | C16H12O6 | 6.507 | 299.0561 | −5.3 | 283, 267, 238, 147 | Flavone | [24] |
| 54 | Luteolin methyl ether glucuronide (Trihydroxymethoxyflavone glucuronide) | C22H20O12 | 6.579 | 475.0889 | −1.5 | 299 | Flavone | [24] |
| 55 | Tricin (Trihydroxy-dimethoxyflavone) | C17H14O7 | 6.713 | 329.0681 | −4.3 | 261 | Flavone | [22] |
| 56 | Tricin (7-O-deoxhexosyl-O-glucuronide) | C29H32O17 | 7.035 | 651.1575 | −1.3 | 329 | Flavone | |
| 57 | Luteolin-O-deoxyhexoside | C21H20O10 | 7.104 | 431.1003 | −4.4 | 285 | Flavone | |
| 58 | Trihydroxyflavone-O-glucuronide (Apigenin glucuronide) | C21H18O11 | 7.189 | 445.0786 | −2.2 | 269, 175 | [14] | |
| 59 | Trihydroxyflavone-O-glucuronide isomer | C21H18O11 | 7.257 | 445.0776 | −5.0 | 269, 175 | Flavone | |
| 60 | Tricin-7-O-deoxyhexosyl-O-hexoside | C29H34O16 | 7.346 | 637.1774 | −3.1 | 593, 447, 329 | Flavone | |
| 61 | Tricin-7-O-hexosyl sulfate | C23H24O15S | 7.397 | 571.0768 | −0.8 | 329, 255, 241, 175 | Flavone | [25] |
| 62 | Tricin-7-O-hexosyl sulfate isomer | C23H24O15S | 7.455 | 571.0767 | −1.8 | 329, 271 | Flavone | [24] |
| 63 | Tetrahydroxy-methoxyflavone-O-sulfate (Methoxyluteolin-O-sulfate) | C16H12O10S | 7.575 | 395.0078 | −4.9 | 315, 225, 209, 167 | Flavone | |
| 64 | Tricin-7-O-glucuronide | C23H22O13 | 7.616 | 505.0988 | 1.2 | 329 | Flavone | [23] |
| 65 | Luteolin methyl ether-O-glucuronide isomer | C22H20O12 | 7.647 | 475.0905 | −1.2 | 299, 177, 175 | Flavone | |
| 66 | Tetrahydroxyaurone-O-hexoside | C21H20O11 | 7.831 | 447.0954 | −4.7 | 411, 285, 233 | Aurone | [14] |
| 67 | Tetrahydroxyflavone-O-hexoside (Luteolin-O-hexoside) | C21H20O11 | 7.875 | 447.0933 | 1.1 | 285, 255 | Flavone | [26] |
| 68 | Dihydroxymethoxyaurone | C16H12O5 | 8.053 | 283.0631 | −6.6 | 268, 240 | Aurone | |
| 69 | Dihydroxymethoxyflavone | C16H12O5 | 8.104 | 283.0626 | −4.9 | 269, 147 | Flavone | |
| 70 | Pentahydroxyflavone (Tricetin) | C15H10O7 | 8.128 | 301.0360 | −2.2 | nd | Flavone | [23,27] |
| 71 | Trihydroxy-methoxy-methylaurone | C17H14O6 | 8.803 | 313.0718 | −0.2 | 298, 267 | Aurone | |
| 72 | Hydroxy-dodecenedioic acid | C12H20O5 | 9.201 | 243.1250 | −4.8 | 225, 199 | Fatty acid | |
| 73 | Dihydroxy-decenoic acid | C10H18O4 | 10.200 | 201.1120 | 6.2 | 183, 139 | Fatty acid | |
| 74 | Tetrahydroxyflavone (Luteolin) | C15H10O6 | 10.251 | 285.0405 | −0.3 | nd | Flavone | |
| 75 | Trihydroxy-methoxy-flavonol (Isorhamnetin) | C16H12O7 | 10.389 | 315.0518 | −2.4 | 300, 271, 175 | Flavonol | [22] |
| 76 | Hydroxyoctadecenedioic acid | C18H32O5 | 11.953 | 327.2196 | −6.0 | 309, 239 | Fatty acid | |
| 77 | Hydroxyoctadecadienoic acid | C18H32O3 | 14.767 | 295.2292 | −4.4 | 277, 237 | Fatty acid | |
| 78 | Hydroxytetradecanoic acid | C14H28O3 | 15.827 | 243.1963 | 1.1 | nd | Fatty acid | |
| 79 | Hydroxydocosanoic acid | C22H44O3 | 15.878 | 355.3211 | 1.8 | nd | Fatty acid | |
| 80 | Hydroxytetracosenoic acid | C24H46O3 | 16.003 | 381.3364 | 2.8 | nd | Fatty acid | |
| 81 | Hydroxypentadecanoic acid | C15H30O3 | 16.409 | 257.2124 | −0.6 | nd | Fatty acid | |
| 82 | Hydroxyhexadecenoic acid | C16H30O3 | 16.413 | 269.2131 | −3.2 | 225 | Fatty acid | |
| 83 | Hydroxyeicosanoic acid | C20H40O3 | 16.478 | 327.2894 | 3.2 | nd | Fatty acid | |
| 84 | Eicosanoic acid (Arachidic acid) | C20H40O2 | 16.491 | 311.2957 | −0.5 | nd | Fatty acid | |
| 85 | Hexacosanoic acid | C26H52O2 | 16.642 | 395.3886 | 2.0 | nd | Fatty acid | |
| 86 | Hydroxytetracosanoic acid | C24H48O3 | 16.666 | 383.3531 | 1.3 | nd | Fatty acid | |
| 87 | Octadecanoic acid | C18H36O2 | 16.734 | 283.2626 | 5.9 | nd | Fatty acid | |
| 88 | Octadecatrienoic acid | C18H30O2 | 16.799 | 277.2186 | −4.6 | nd | Fatty acid | |
| 89 | Trihydroxy octadecenoic acid | C18H34O5 | 16.876 | 329.2326 | 2.3 | nd | Fatty acid | |
| 90 | Hydroxypalmitic acid | C16H32O3 | 17.049 | 271.2270 | 3.3 | 225 | Fatty acid | |
| 91 | Hexadecenoic acid | C16H30O2 | 17.244 | 253.2173 | −3.8 | nd | Fatty acid | |
| 92 | Docosanedioic acid | C22H42O4 | 17.368 | 369.3004 | 1.4 | nd | Fatty acid | |
| 93 | Pentacosanedioic acid | C25H48O4 | 17.479 | 411.3470 | 2.4 | nd | Fatty acid | |
| 94 | Eicosanedioic acid | C20H38O4 | 17.660 | 341.2685 | 3.4 | nd | Fatty acid |
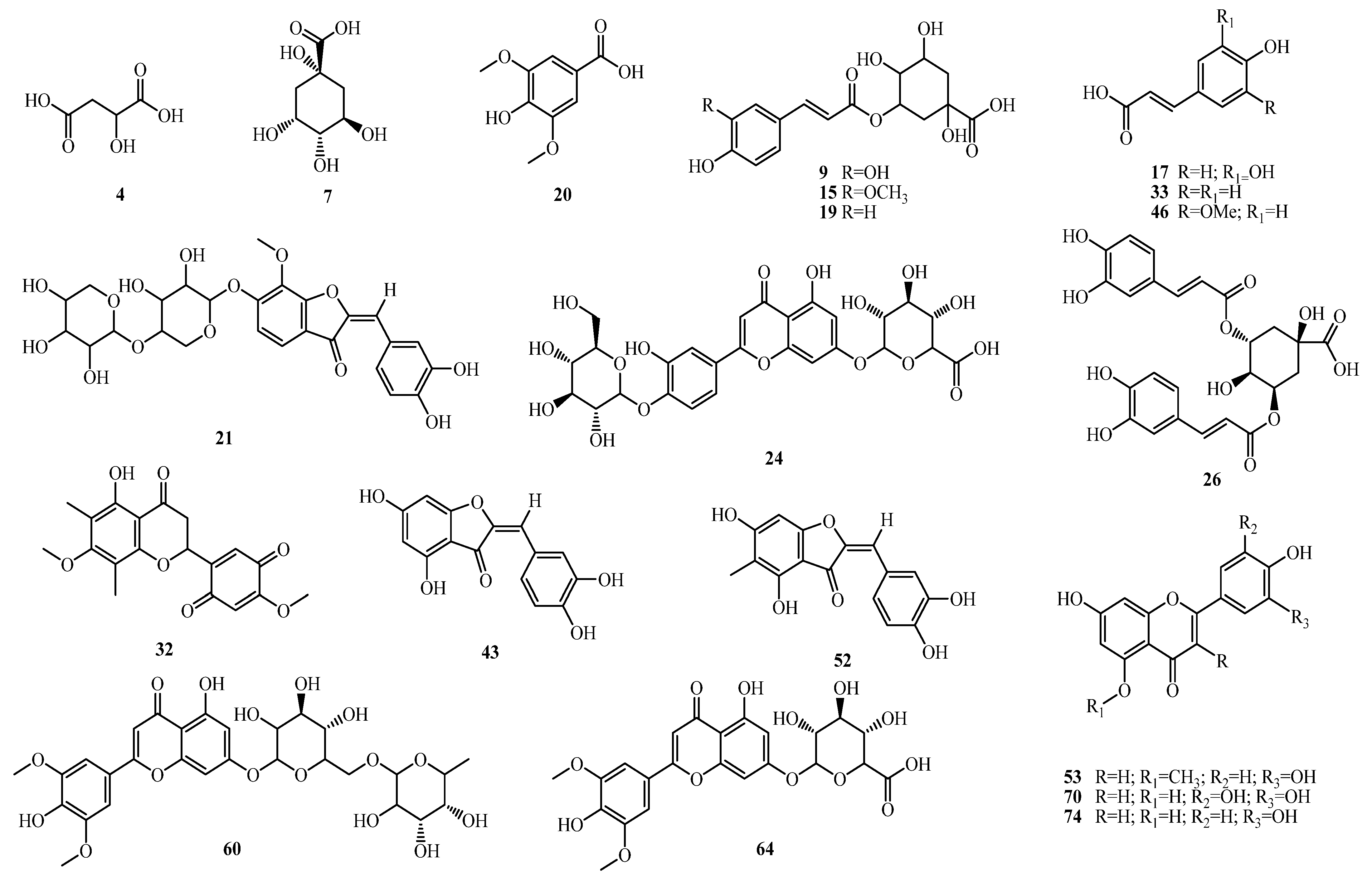
2.1.2. Phenolic Acids
2.1.3. Flavonoids
2.1.4. Aurones
2.1.5. Fatty Acids
2.2. Thioacetamide-Induced Hepatorenal Toxicity Results
2.2.1. Body Weight
2.2.2. Biochemical Indicators of Hepatic and Renal Function
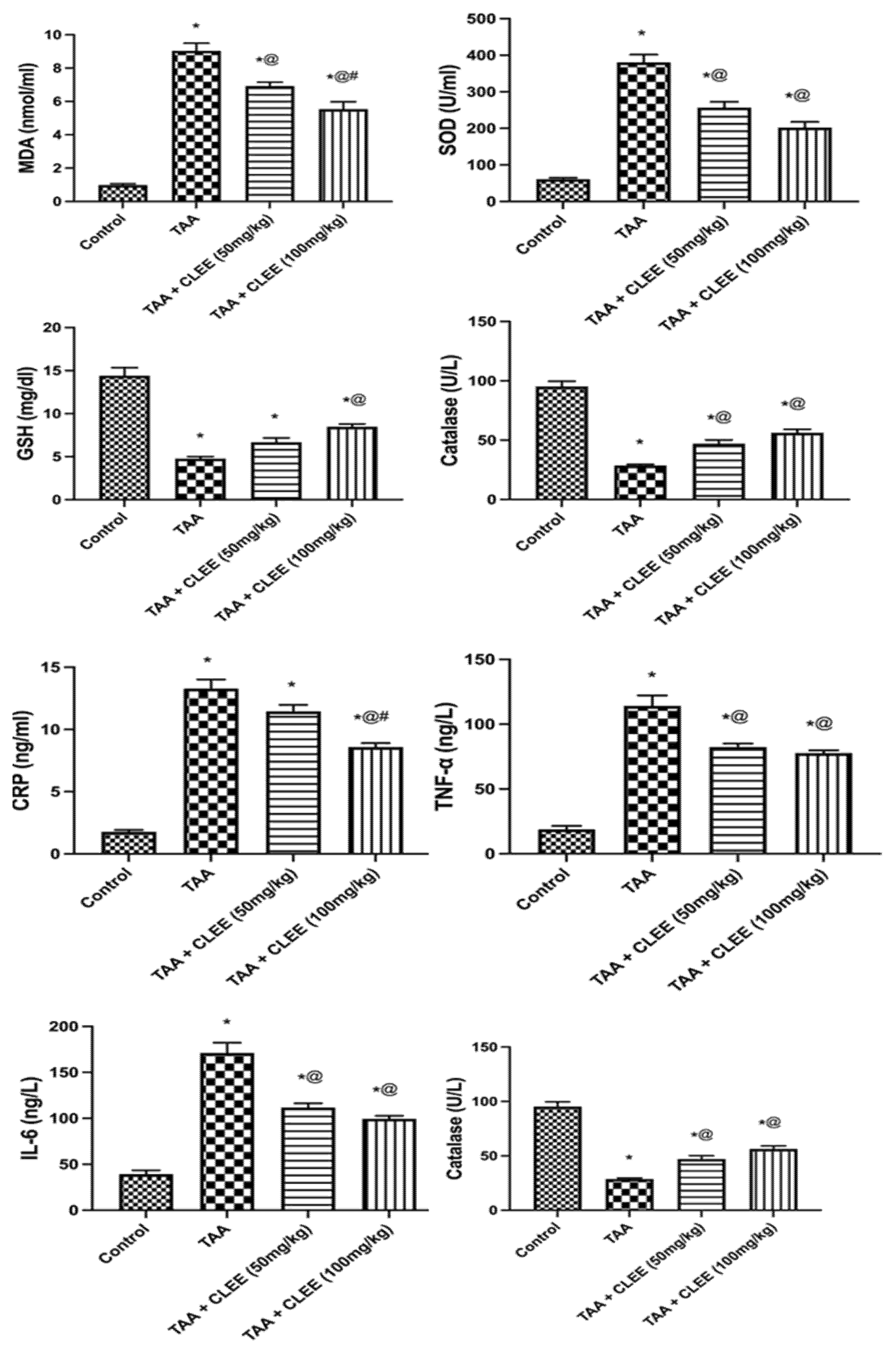
2.2.3. Plasma Oxidative Stress and Inflammatory Markers
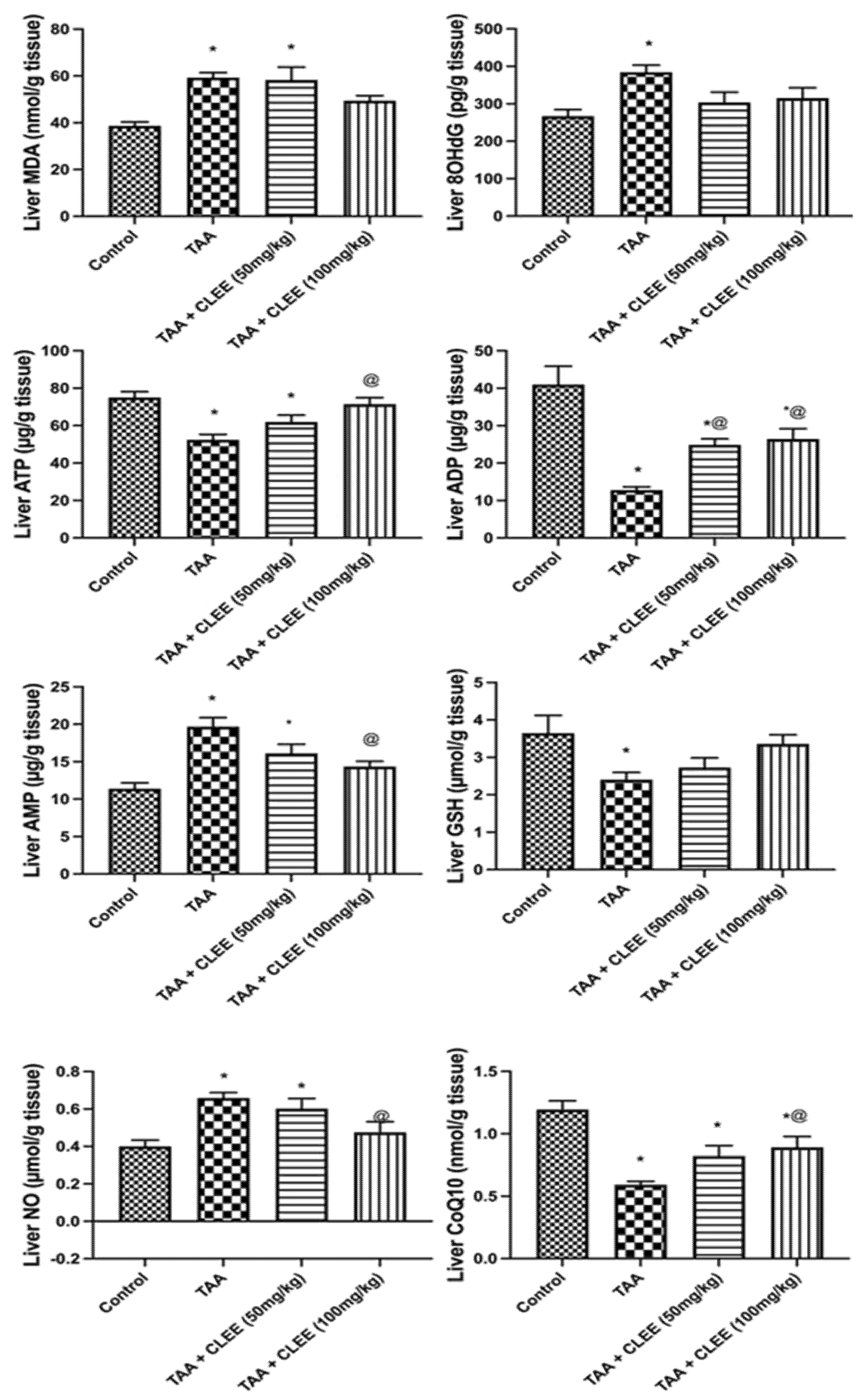
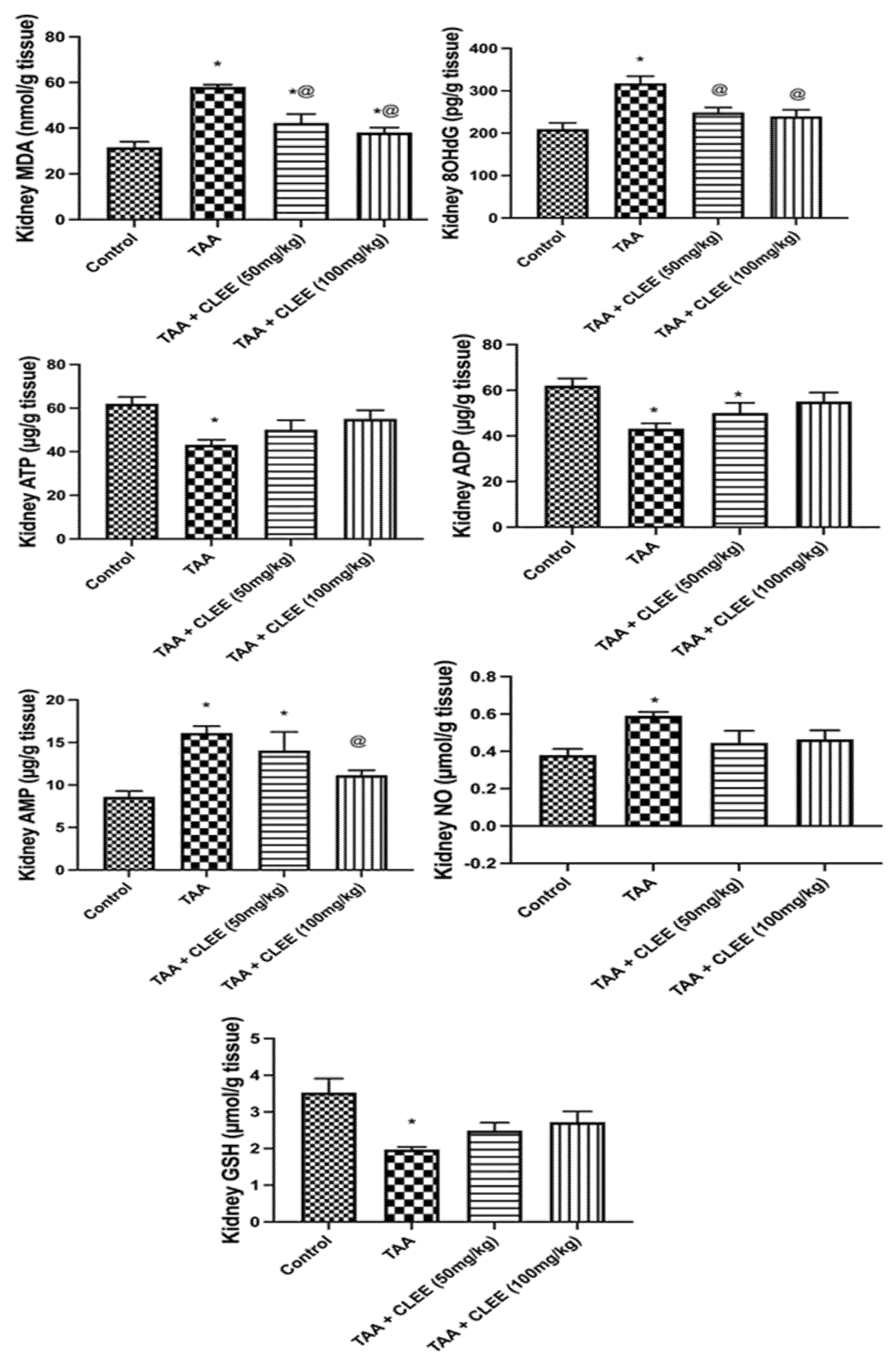
2.2.4. Hepatic and Renal Oxidative Stress and Energy Parameters
2.2.5. Histopathological Results
- Liver
- Kidney
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extract Preparation
4.3. High-Resolution Ultra-Performance Liquid Chromatography-Mass Spectrometry Analysis (UPLC-ESI–qTOF-MS)
4.4. Thioacetamide-Induced Hepatorenaltoxicity Experiments
4.4.1. Animals, Ethical Statement, and Acute Toxicological Studies
4.4.2. Grouping and Experimental Design
4.4.3. Blood Samples Collection and Tissue Homogenates
4.4.4. Liver and Kidney Function
4.4.5. Plasma Oxidative Stress and Inflammatory Markers
4.4.6. Hepatic and Renal Oxidative Stress and Energy Parameters
4.4.7. Histopathologic Investigation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Park, J.U.; Kang, J.H.; Rahman, M.A.A.; Hussain, A.; Cho, J.S.; Lee, Y.I. Gastroprotective effects of plants extracts on gastric mucosal injury in experimental sprague-dawley rats. Biomed. Res. Int. 2019, 2019, 8759708. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, S.; Dastmalchi, S.; Javadzadeh, Y. Gastroprotective effects of herbal medicines (roots). Int. J. Food Prop. 2018, 21, 902–920. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abdallah, H.M.I.; Farrag, A.R.H.; Riciputi, Y.; Pasini, F.; Taher, R.F.; Raslan, M.A.; Paré, P.W.; Hegazy, M.-E.F. Artichoke phenolics confer protection against acute kidney injury. Rev. Bras. Farmacogn. 2020, 30, 34–42. [Google Scholar] [CrossRef]
- Saha, P.; Talukdar, A.D.; Nath, R.; Sarker, S.D.; Nahar, L.; Sahu, J.; Choudhury, M.D. Role of natural phenolics in hepatoprotection: A mechanistic review and analysis of regulatory network of associated genes. Front. Pharmacol. 2019, 10, 509. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Farrag, A.R.H.; Ayoub, I.M.; Mahdy, K.A.; Taher, R.F.; Gendy, A.E.-N.G.; Mohamed, T.A.; Al-Rejaie, S.S.; Ei-Amier, Y.A.; Abd-EIGawad, A.M. UPLC-qTOF-MS phytochemical profile and antiulcer potential of Cyperus conglomeratus Rottb. alcoholic extract. Molecules 2020, 25, 4234. [Google Scholar] [CrossRef] [PubMed]
- Farrag, A.R.H.; Abdallah, H.M.I.; Khattab, A.R.; Elshamy, A.I.; Gendy, A.; Mohamed, T.A.; Farag, M.A.; Efferth, T.; Hegazy, M.F. Antiulcer activity of Cyperus alternifolius in relation to its UPLC-MS metabolite fingerprint: A mechanistic study. Phytomedicine 2019, 62, 152970. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; El-Shazly, M.; Yassine, Y.M.; El-bana, M.A.; Farrag, A.R.H.; Nassar, M.; Singab, A.N.B.; Noji, M.; Umeyama, A. Phenolic constituents, anti-inflammatory and antidiabetic activities of Cyperus laevigatus L. Polym. J. 2017, 9, 828–833. [Google Scholar] [CrossRef]
- Kumar, S.S.; Mishra, S. Hepatoprotective activity of rhizomes of Cyperus rotundus Linn against carbon tetrachloride-induced hepatotoxicity. Indian J. Pharm. Sci. 2005, 67, 84–88. [Google Scholar]
- Thebtaranonth, C.; Thebtaranonth, Y.; Wanauppathamkul, S.; Yuthavong, Y. Antimalarial sesquiterpenes from tubers of Cyperus rotundus: Structure of 10,12-peroxycalamenene, a sesquiterpene endoperoxide. Phytochemistry 1995, 40, 125–128. [Google Scholar] [CrossRef]
- Raut, N.A.; Gaikwad, N.J. Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 2006, 77, 585–588. [Google Scholar] [CrossRef]
- Abdel-Razik, A.F.; Nassar, M.I.; El-Khrisy, E.D.; Dawidar, A.A.; Mabry, T.J. New prenylflavans from Cyperus conglomeratus. Fitoterapia 2005, 76, 762–764. [Google Scholar] [CrossRef]
- Seabra, R.M.; Moreira, M.M.; Costa, M.A.C.; Paul, M.I. 6,3′,4′-Trihydroxy-4-methoxy-5-methylaurone from Cyperus capitatus. Phytochemistry 1995, 40, 1579–1580. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.W.; Yu, C.Y.; Lu, Y.; Chang, Y.; Zou, Z.M. Norcyperone, a novel skeleton norsesquiterpene from Cyperus rotundus L. Molecules 2008, 13, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Gamal, M.A.; Hani, K.; Sameh, E.-S.; Sabrin, I.R.M. A review: Compounds isolated from Cyperus species (part I): Phenolics and nitrogenous. Internat. J. Pharmacog. Phytochem. Res. 2015, 7(1), 51–67. [Google Scholar]
- El Gendy, A.E.-N.G.; Abd El-Gawad, A.M.; Taher, R.F.; El-Khrisy, E.-D.A.; Omer, E.A.; Elshamy, A.I. Essential oils constituents of aerial parts of Cyperus capitatus L. and Cyperus difformis L. grown wild in Egypt. J. Essent. Oil Bear. Plants 2017, 20, 1659–1665. [Google Scholar] [CrossRef]
- Nassar, M.I.; Yassine, Y.M.; Elshamy, A.I.; El-Beih, A.A.; El-Shazly, M.; Singab, A.N.B. Essential oil and antimicrobial activity of aerial parts of Cyperus leavigatus L. (family: Cyperaceae). J. Essent. Oil Bear. Plants 2015, 18, 416–422. [Google Scholar] [CrossRef]
- Ichimura, R.; Mizukami, S.; Takahashi, M.; Taniai, E.; Kemmochi, S.; Mitsumori, K.; Shibutani, M. Disruption of Smad-dependent signaling for growth of GST-P-positive lesions from the early stage in a rat two-stage hepatocarcinogenesis model. Toxicol. Appl. Pharmacol. 2010, 246, 128–140. [Google Scholar] [CrossRef]
- Mladenović, D.; Hrnčić, D.; Rašić-Marković, A.; Stanojlović, O. Behavioral and electroencephalographic manifestations of thioacetamide-induced encephalopathy: Possible mechanisms of neurotoxic effects. Arch. Biol. Sci. 2012, 64, 829–841. [Google Scholar] [CrossRef]
- Ansil, P.N.; Nitha, A.; Prabha, S.P.; Wills, P.J.; Jazaira, V.; Latha, M.S. Protective effect of Amorphophallus campanulatus (Roxb.) Blume. tuber against thioacetamide induced oxidative stress in rats. Asian Pac. J. Trop. Med. 2011, 4, 870–877. [Google Scholar] [CrossRef]
- Elkady, W.M.; Ayoub, I.M.; Abdel-Mottaleb, Y.; Elshafie, M.F.; Wink, M. Euryops pectinatus L. Flower extract inhibits p-glycoprotein and reverses multi-drug resistance in cancer cells: A mechanistic study. Molecules 2020, 25, 647. [Google Scholar]
- Bhatt, S.; Sthapak, J.; Singh, K. New aurone from the leaves of Cyperus scariosus. Fitoterapia 1984, 55, 370–371. [Google Scholar]
- El-Habashy, I.; Mansour, R.M.A.; Zahran, M.A.; El-Hadidi, M.N.; Saleh, N.A.M. Leaf flavonoids of Cyperus species in Egypt. Biochem. Syst. Ecol. 1989, 17, 191–195. [Google Scholar] [CrossRef]
- Seabra, R.M.; Silva, A.M.S.; Andrade, P.B.; Manuela Moreira, M. Methylaurones from Cyperus capitatus. Phytochemistry 1998, 48, 1429–1432. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A.; Wilson, K.L. Flavonoids in leaves and inflorescences of australian cyperaceae. Phytochemistry 1985, 24, 751–766. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A.; Wilson, K.L. Flavonoids in leaves and inflorescences of australian Cyperus species. Phytochemistry 1982, 21, 2491–2507. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative metabolite fingerprinting of four different cinnamon species analyzed via UPLC-MS and GC-MS and chemometric tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef]
- Babiaka, S.B.; Moumbock, A.F.A.; Günther, S.; Ntie-Kang, F. Natural products in Cyperus rotundus L. (Cyperaceae): An update of the chemistry and pharmacological activities. RSC Adv. 2021, 11, 15060–15077. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Kořínek, M.; El-Shazly, M.; Wetterauer, B.; El-Beshbishy, H.A.; Hwang, T.-L.; Chen, B.-H.; Chang, F.R.; Wink, M.; Singab, A.N.B.; et al. Anti-allergic, anti-inflammatory, and anti-hyperglycemic activity of Chasmanthe aethiopica leaf extract and its profiling using LC/MS and GLC/MS. Plants 2021, 10, 1118. [Google Scholar] [CrossRef]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural characterization of flavonoid glycosides from leaves of wheat (Triticum aestivum L.) using LC/MS/MS profiling of the target compounds. J. Mass Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef]
- Harborne, J.B.; Marby, H.; Marby, T. The Flavonoids; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Yogalakshmi, B.; Viswanathan, P.; Anuradha, C.V. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Poli, G. Pathogenesis of liver fibrosis: Role of oxidative stress. Mol. Asp. Med. 2000, 21, 49–98. [Google Scholar] [CrossRef]
- Reeves, H.L.; Friedman, S.L. Activation of hepatic stellate cells—A key issue in liver fibrosis. Front. Biosci. J. Virtual Libr. 2002, 7, d808–d826. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free. Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Jovanovic, J.M.; Nikolić, R.S.; Kocic, G.; Krstic, N.; Krsmanovic, M.M. Glutathione protects liver and kidney tissue from cadmium- and lead-provoked lipid peroxidation. J. Serb. Chem. Soc. 2013, 78, 197–207. [Google Scholar] [CrossRef]
- Samimi, F.; Baazm, M.; Eftekhar, E.; Rajabi, S.; Goodarzi, M.T.; Jalali Mashayekhi, F. Possible antioxidant mechanism of coenzyme Q10 in diabetes: Impact on Sirt1/Nrf2 signaling pathways. Res. Pharm. Sci. 2019, 14, 524–533. [Google Scholar]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Jain, N.K.; Singhai, A.K. Protective effects of Phyllanthus acidus (L.) Skeels leaf extracts on acetaminophen and thioacetamide induced hepatic injuries in Wistar rats. Asian Pac. J. Trop. Med. 2011, 4, 470–474. [Google Scholar] [CrossRef]
- Abd Elhameed, M.F.; Salama, A.A.A.; Attia, T.M.; Elbatran, S.A.; Ismaeil, I.E.K.; Hassan, A. Protective effects of Moringa oleifera extract on isoniazid and rifampicin induced hepatotoxicity in rats: Involvment of adiponectin and tumor necrosis factor-α. Egypt. J. Vet. Sci. 2018, 49, 25–34. [Google Scholar] [CrossRef]
- Spinella, R.; Sawhney, R.; Jalan, R. Albumin in chronic liver disease: Structure, functions and therapeutic implications. Hepatol. Int. 2016, 10, 124–132. [Google Scholar] [CrossRef]
- Guo, C.; Liang, F.; Shah Masood, W.; Yan, X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014, 725, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, N.; Tsutsui, H.; Wen, J.; Kang, D.; Ikeuchi, M.; Ide, T.; Hayashidani, S.; Shiomi, T.; Kubota, T.; Hamasaki, N.; et al. Oxidative stress mediates tumor necrosis factor-α-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003, 107, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Porowski, D.; Wirkowska, A.; Hryniewiecka, E.; Wyzgał, J.; Pacholczyk, M.; Pączek, L. Liver failure impairs the intrahepatic elimination of interleukin-6, tumor necrosis factor-alpha, hepatocyte growth factor, and transforming growth factor-beta. BioMed Res. Int. 2015, 2015, 934065. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lei, C.-T.; Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Kerner, A.; Avizohar, O.; Sella, R.; Bartha, P.; Zinder, O.; Markiewicz, W.; Levy, Y.; Brook, G.J.; Aronson, D. Association between elevated liver enzymes and c-reactive protein. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 193–197. [Google Scholar] [CrossRef]
- Chadipiralla, K.; Reddanna, P.; Chinta, R.M.; Reddy, P.V.B. Thioacetamide-induced fulminant hepatic failure induces cerebral mitochondrial dysfunction by altering the electron transport chain complexes. Neurochem. Res. 2012, 37, 59–68. [Google Scholar] [CrossRef]
- Shehab, N.G.; Abu-Gharbieh, E.; Bayoumi, F.A. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complement. Altern. Med. 2015, 15, 401. [Google Scholar] [CrossRef]
- Abdel Shakour, Z.T.; El-Akad, R.H.; Elshamy, A.I.; El Gendy, A.E.-N.G.; Wessjohann, L.A.; Farag, M.A. Dissection of Moringa oleifera leaf metabolome in context of its different extracts, origin and in relationship to its biological effects as analysed using molecular networking and chemometrics. Food Chem. 2023, 399, 133948. [Google Scholar] [CrossRef]
- Sekher Pannala, A.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.L.; Pan, M.-h.; Ho, C.-T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the Citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Seydi, E.; Salimi, A.; Rasekh, H.R.; Mohsenifar, Z.; Pourahmad, J. Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: Involvement of ros-mediated mitochondrial targeting. Nutr. Cancer 2018, 70, 594–604. [Google Scholar] [CrossRef]
- Seki, N.; Toh, U.; Kawaguchi, K.; Ninomiya, M.; Koketsu, M.; Watanabe, K.; Aoki, M.; Fujii, T.; Nakamura, A.; Akagi, Y.; et al. Tricin inhibits proliferation of human hepatic stellate cells in vitro by blocking tyrosine phosphorylation of PDGF receptor and its signaling pathways. J. Cell. Biochem. 2012, 113, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Malvicini, M.; Gutiérrez-Moraga, A.; Rodríguez, M.M.; Gomez-Bustillo, S.; Salazar, L.; Sunkel, C.; Nozal, L.; Salgado, A.; Hidalgo, M.; López-Casas, P.P.; et al. A tricin derivative from Deschampsia antarctica Desv. Inhibits colorectal carcinoma growth and liver metastasis through the induction of a specific immune response. Mol. Cancer Ther. 2018, 17, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xi, Y.; Jiang, W. Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 2125–2135. [Google Scholar] [CrossRef]
- Vargas, F.; Romecín, P.A.; García-Guillén, A.I.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in kidney health and disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Gerin, F.; Erman, H.; Erboğa, M.; Sener, U.; Yilmaz, A.; Seyhan, H.; Gurel, A. The effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation 2016, 39, 1377–1386. [Google Scholar] [CrossRef]
- Cheng, Q.-L.; Li, Y.-W.; Yang, C.-F.; Zhong, Y.; He, H.; Zhu, F.-C.; Li, L. Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and FoxO1 pathways in rats and L-02 cells. Chem.-Biol. Interact. 2018, 291, 180–189. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Yin, M.-C. Renal protective effects of extracts from guava fruit (Psidium guajava L.) in diabetic mice. Plant Foods Hum. Nutr. 2012, 67, 303–308. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical profile of Launaea nudicaulis ethanolic extract and its antidiabetic effect in streptozotocin-induced rats. Molecules 2021, 26, 1000. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.M.; Ammar, N.M.; Kamel, R.; Esatbeyoglu, T.; Hassan, H.A. β-Sitosterol glucoside-loaded nanosystem ameliorates insulin resistance and oxidative stress in streptozotocin-induced diabetic rats. Antioxidants 2022, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, I.H.; Ispiroglu, M.; Tuzcu, M.; Orhan, C.; Ulas, M.; Demirel, U.; Yalniz, M.; Özercan, I.H.; Ilhan, N.; Sahin, K. Pistacia terebinthus coffee protects against thioacetamide-induced liver injury in rats. Acta Med. 2015, 58, 56–61. [Google Scholar]
- Hammam, A.M.M.; Elmotayam, A.K.; Elnattat, W.M.; Ali, G.A.; Madbouly, A.E.M.; El Khatteb, R.M.; Abd Elhamied, M.F.; Ali, A.H.; Qasim, S.; Ahmed, S.R. Assessment of Ferula hermonis Boiss fertility effects in immature female rats supported by quantification of ferutinin via HPLC and molecular docking. J. Ethnopharmacol. 2022, 289, 115062. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]



| Weeks | Initial | 1st | 2nd | 3rd | 4th | 5th | 6th | |
|---|---|---|---|---|---|---|---|---|
| Groups | ||||||||
| Control | 145.33 ± 2.512 | 182.33 ± 4.958 | 220.67 ± 5.226 | 252.33 ± 3.621 | 273.67 ± 5.038 | 287.83 ± 3.728 | 275.5 ± 7.535 | |
| TAA | 151.67 ± 3.073 | 173.17 ± 4.483 * | 178.33 ± 4.295 * | 203 ± 6.088 * | 195.83 ± 4.915 * | 209.83 ± 3.42 * | 201.67 ± 4.072 * | |
| TAA + CLEE (50 mg/kg) | 157 ± 2.251 | 195.83 ± 3.516 | 211.83 ± 11.923 | 223.67 ± 9.821 * | 221.17 ± 10.199 * | 227.17 ± 14.885 * | 240 ± 10.724 * | |
| TAA + CLEE (100 mg/kg) | 150.83 ± 3.745 | 187 ± 11.204 | 195.83 ± 11.677 | 207.33 ± 11.67 | 213.33 ± 11.029 * | 224.17 ± 9.411 * | 224.83 ± 5.036 *@ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, I.M.; El-Baset, M.A.; Elghonemy, M.M.; Bashandy, S.A.E.; Ibrahim, F.A.A.; Ahmed-Farid, O.A.H.; El Gendy, A.E.-N.G.; Afifi, S.M.; Esatbeyoglu, T.; Farrag, A.R.H.; et al. Chemical Profile of Cyperus laevigatus and Its Protective Effects against Thioacetamide-Induced Hepatorenal Toxicity in Rats. Molecules 2022, 27, 6470. https://doi.org/10.3390/molecules27196470
Ayoub IM, El-Baset MA, Elghonemy MM, Bashandy SAE, Ibrahim FAA, Ahmed-Farid OAH, El Gendy AE-NG, Afifi SM, Esatbeyoglu T, Farrag ARH, et al. Chemical Profile of Cyperus laevigatus and Its Protective Effects against Thioacetamide-Induced Hepatorenal Toxicity in Rats. Molecules. 2022; 27(19):6470. https://doi.org/10.3390/molecules27196470
Chicago/Turabian StyleAyoub, Iriny M., Marawan A. El-Baset, Mai M. Elghonemy, Samir A. E. Bashandy, Fatma A. A. Ibrahim, Omar A. H. Ahmed-Farid, Abd El-Nasser G. El Gendy, Sherif M. Afifi, Tuba Esatbeyoglu, Abdel Razik H. Farrag, and et al. 2022. "Chemical Profile of Cyperus laevigatus and Its Protective Effects against Thioacetamide-Induced Hepatorenal Toxicity in Rats" Molecules 27, no. 19: 6470. https://doi.org/10.3390/molecules27196470
APA StyleAyoub, I. M., El-Baset, M. A., Elghonemy, M. M., Bashandy, S. A. E., Ibrahim, F. A. A., Ahmed-Farid, O. A. H., El Gendy, A. E.-N. G., Afifi, S. M., Esatbeyoglu, T., Farrag, A. R. H., Farag, M. A., & Elshamy, A. I. (2022). Chemical Profile of Cyperus laevigatus and Its Protective Effects against Thioacetamide-Induced Hepatorenal Toxicity in Rats. Molecules, 27(19), 6470. https://doi.org/10.3390/molecules27196470











