Evaluation of Coriolis Micro Air Sampling to Detect Volatile and Semi-Volatile Organic Compounds
Abstract
1. Introduction
2. Results
2.1. Comparison of the Two Sampling Techniques
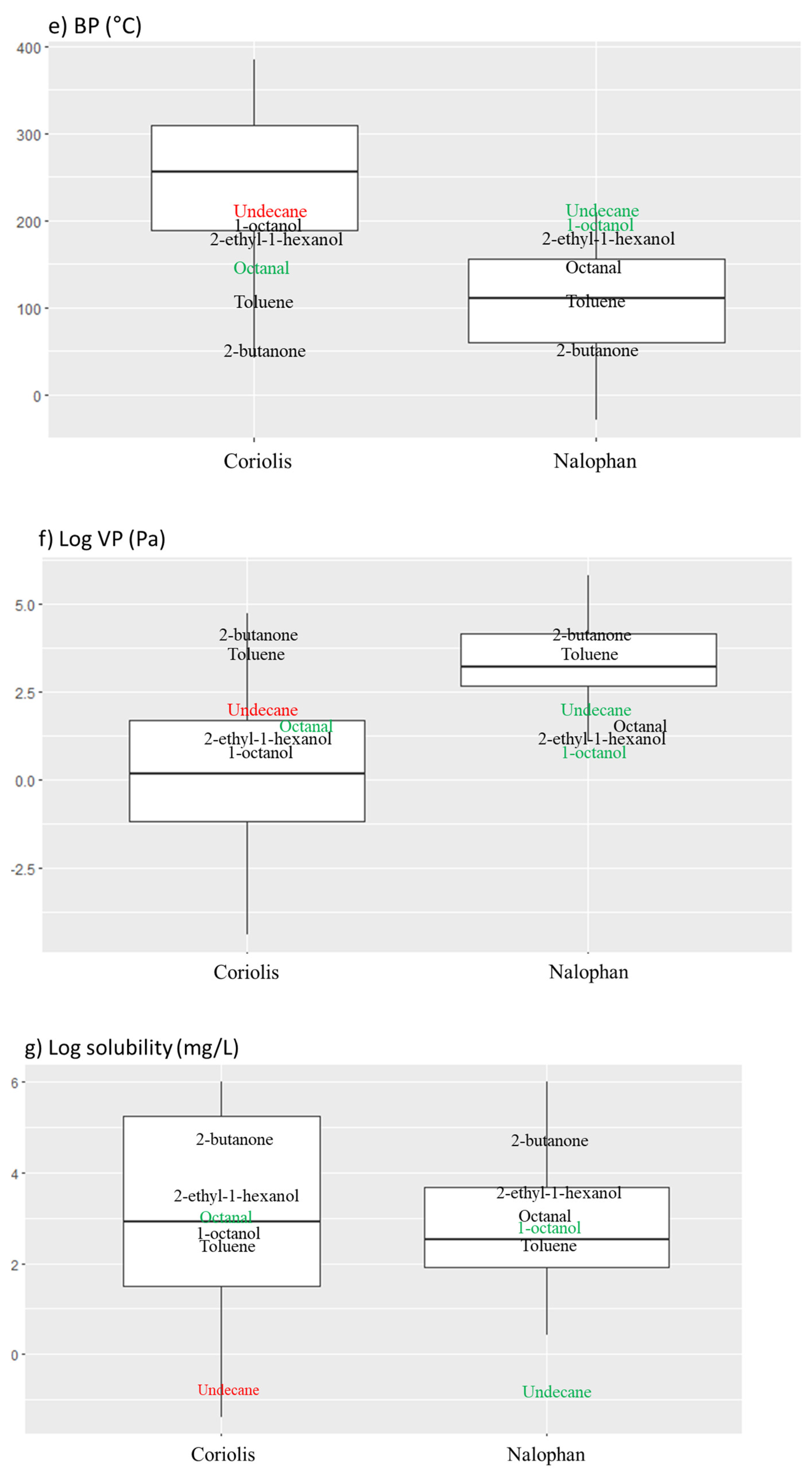
2.2. Compounds Properties
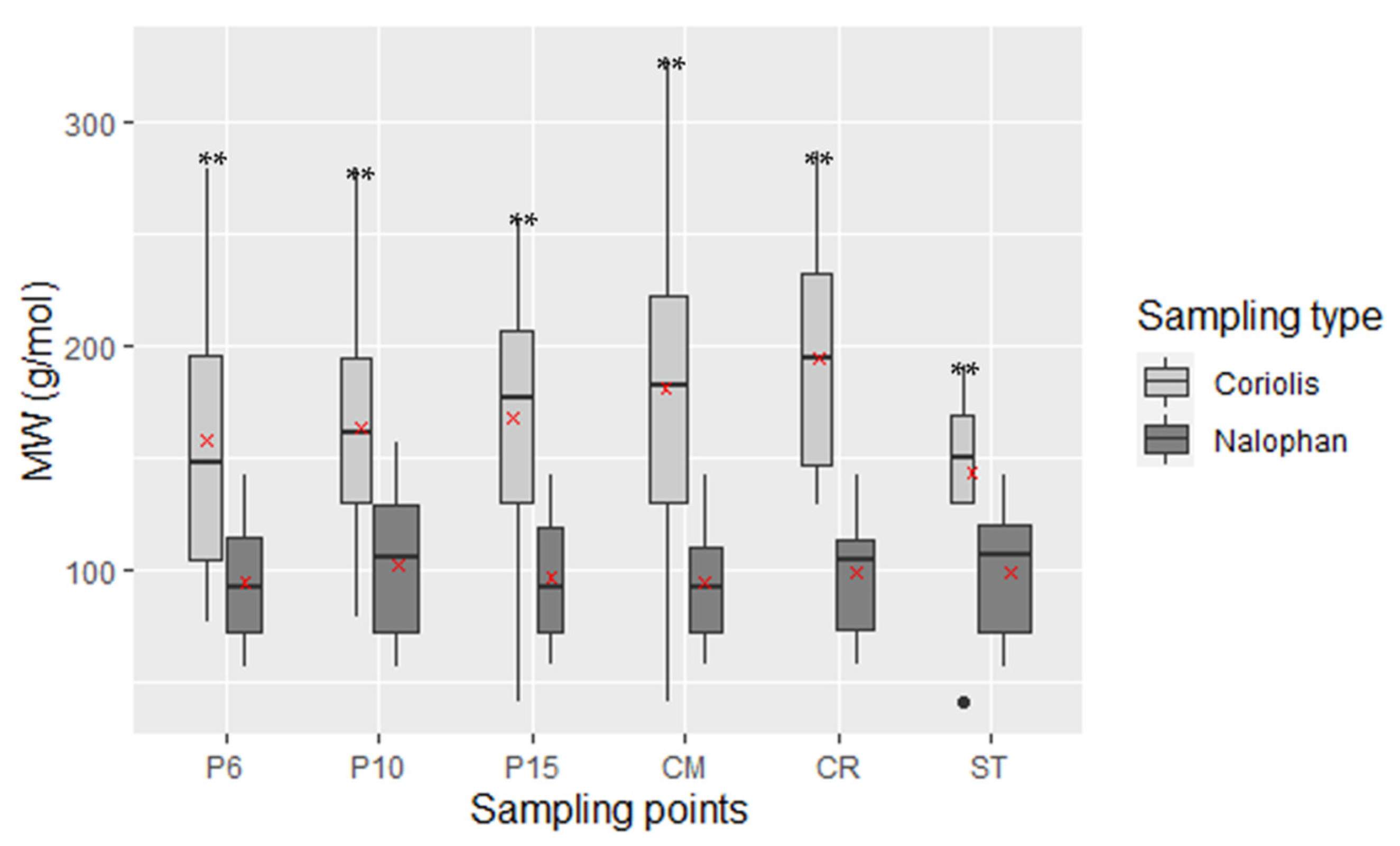
2.2.1. Molecular Weight
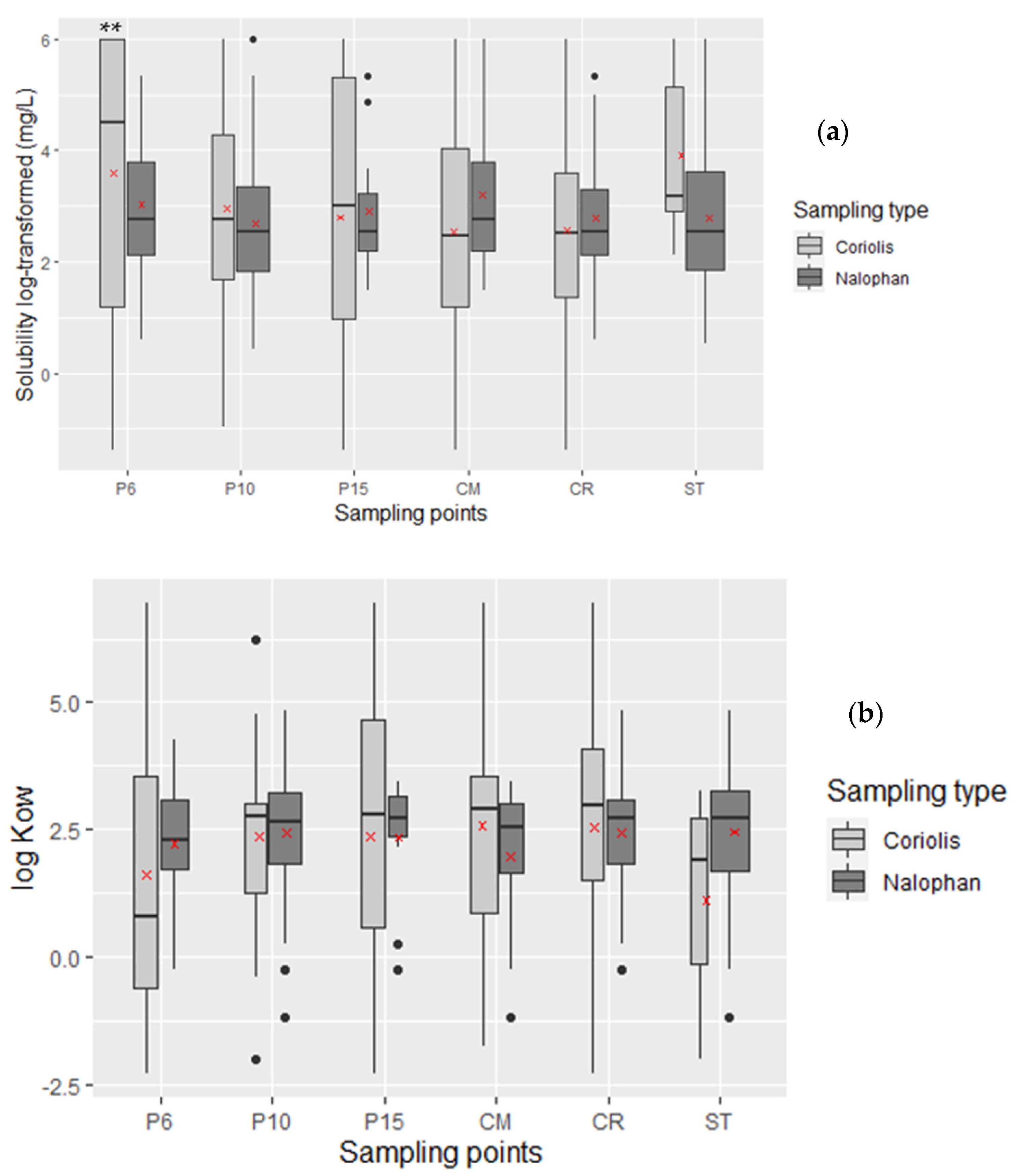
2.2.2. Behavior in Water Compartment
- Solubility in Water at 25 °C
- The Octanol/Water Partition Coefficient (log Kow)
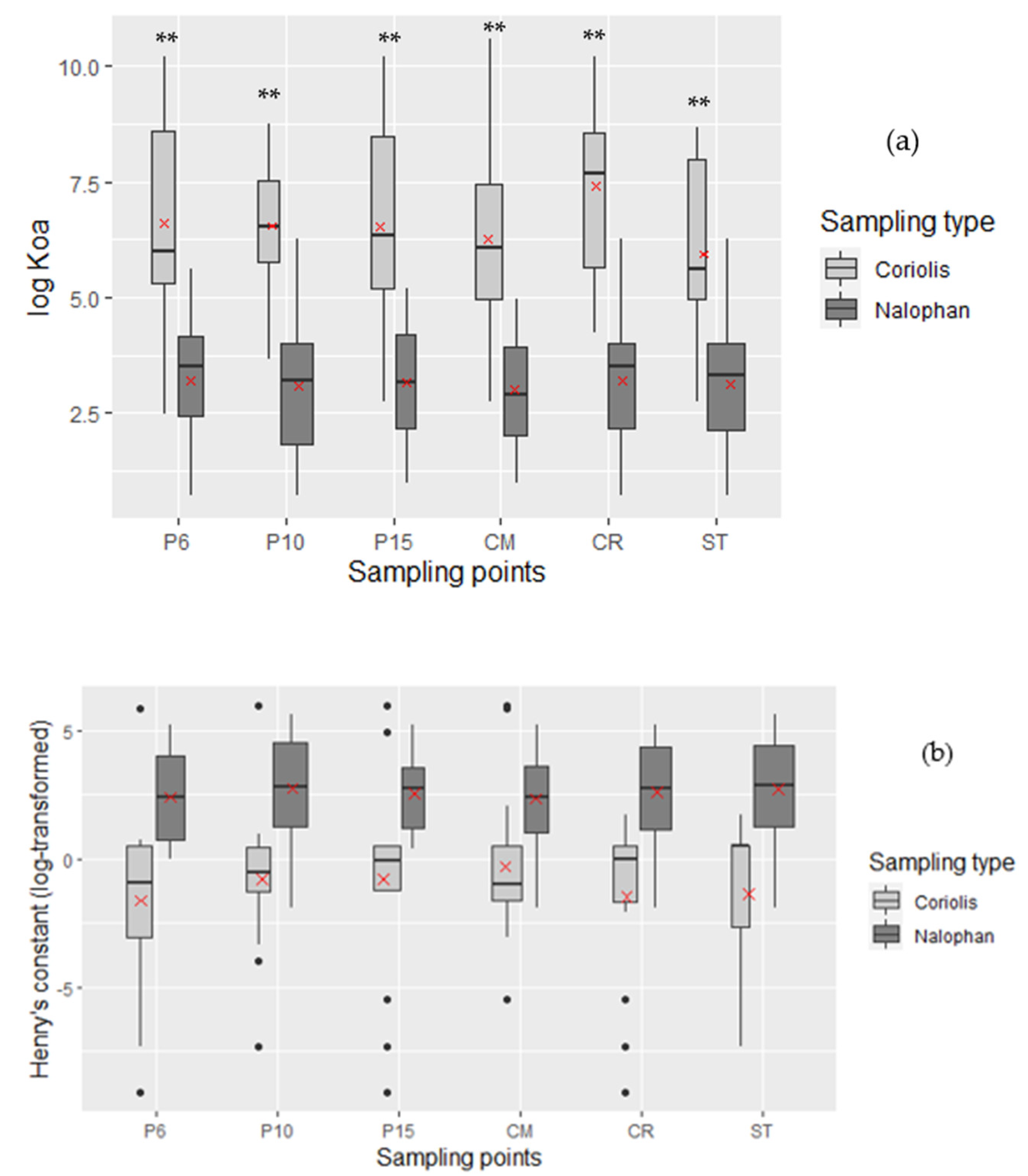
2.2.3. Behavior in Air Compartment
- The Octanol/Air Partition Coefficient (log Koa)
- Henry’s Constant
- Boiling Point (BP)
- Vapor Pressure (VP)
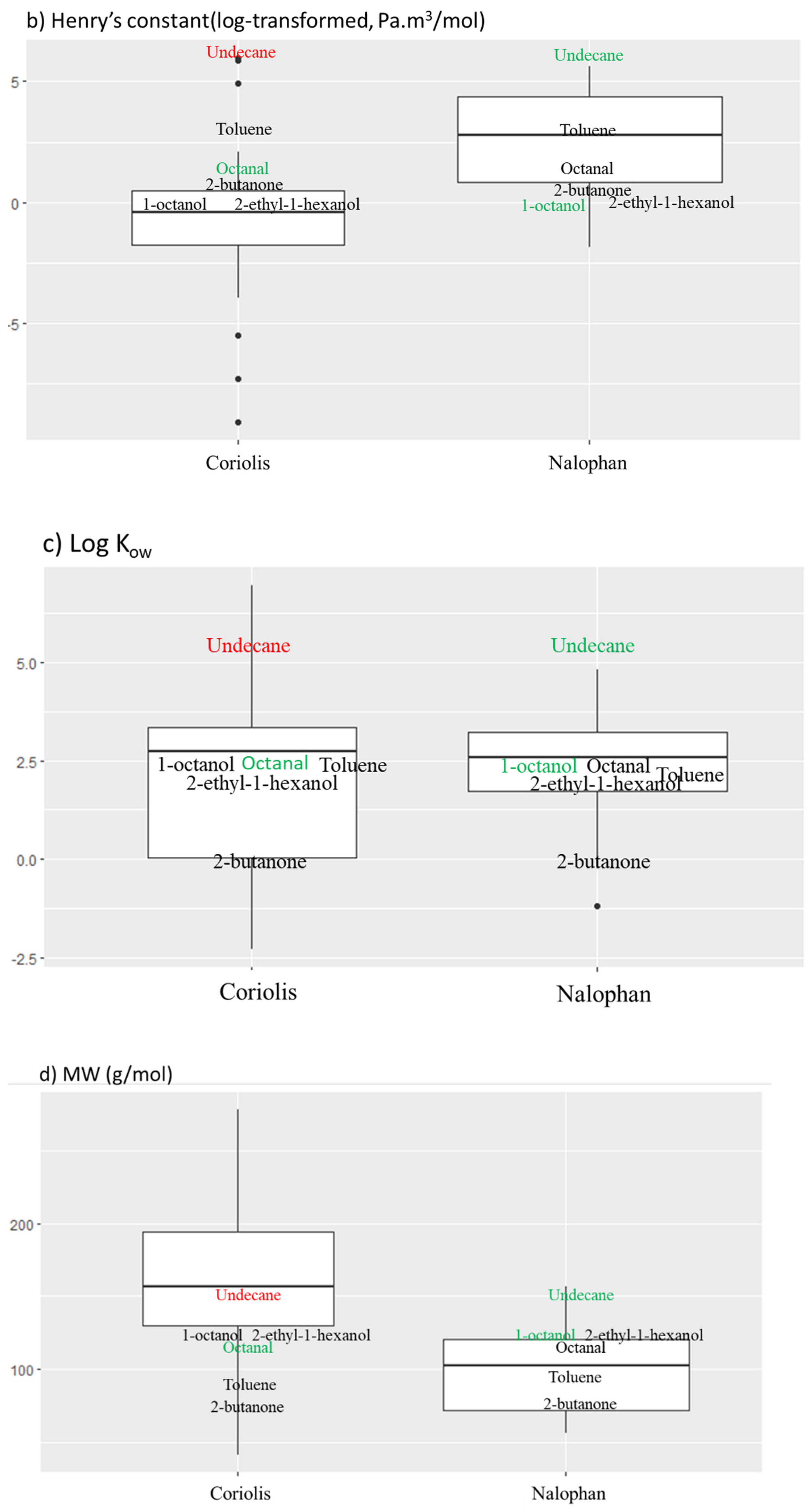
2.3. Sampling in Controlled Air
3. Discussion
4. Materials and Methods
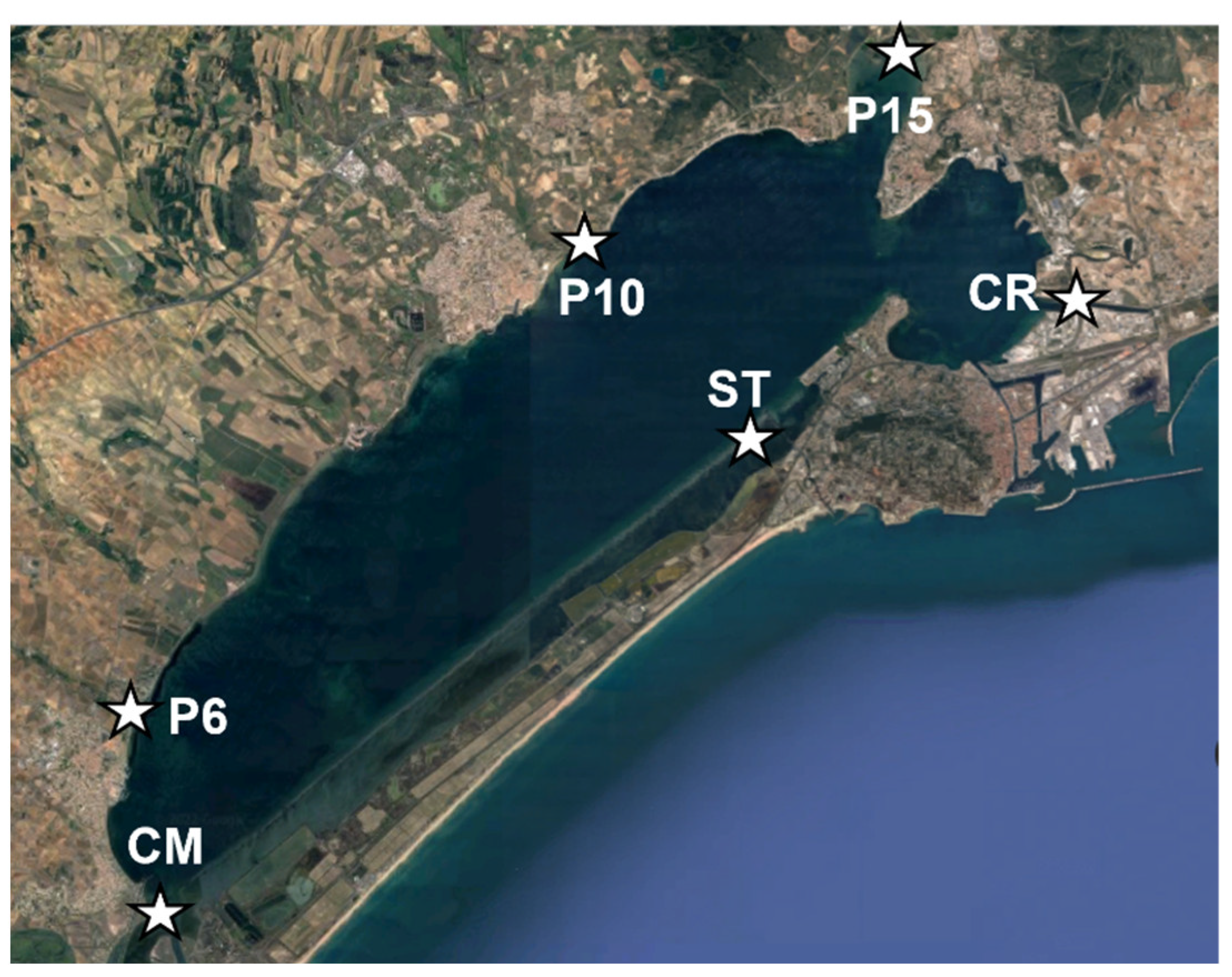
4.1. Sampling Points
4.2. Samples Collection and Analyses
4.3. Estimated Data
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution (accessed on 1 August 2022).
- Khreis, H.; Cirach, M.; Mueller, N.; de Hoogh, K.; Hoek, G.; Nieuwenhuijsen, M.J.; Rojas-Rueda, D. Outdoor Air Pollution and the Burden of Childhood Asthma across Europe. Eur. Respir. J. 2019, 54, 1802194. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental Pollution and Kidney Diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A.; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor Air Pollution and Cancer: An Overview of the Current Evidence and Public Health Recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary Health Effects of Air Pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef]
- US EPA Organization. What Is the Definition of VOC? Available online: https://www.epa.gov/air-emissions-inventories/what-definition-voc (accessed on 9 November 2021).
- Raffy, G.; Mercier, F.; Blanchard, O.; Derbez, M.; Dassonville, C.; Bonvallot, N.; Glorennec, P.; Le Bot, B. Semi-Volatile Organic Compounds in the Air and Dust of 30 French Schools: A Pilot Study. Indoor Air 2017, 27, 114–127. [Google Scholar] [CrossRef]
- Jia, L.; Chu, J.; Li, J.; Ren, J.; Huang, P.; Li, D. Formaldehyde and VOC Emissions from Plywood Panels Bonded with Bio-Oil Phenolic Resins. Environ. Pollut. 2020, 264, 114819. [Google Scholar] [CrossRef]
- Zhang, T.; Li, G.; Yu, Y.; Ji, Y.; An, T. Atmospheric Diffusion Profiles and Health Risks of Typical VOC: Numerical Modelling Study. J. Clean. Prod. 2020, 275, 122982. [Google Scholar] [CrossRef]
- Sazakli, E.; Leotsinidis, M. Odor Nuisance and Health Risk Assessment of VOC Emissions from a Rendering Plant. Air Qual. Atmos. Health 2021, 14, 301–312. [Google Scholar] [CrossRef]
- Mo, Z.; Lu, S.; Shao, M. Volatile Organic Compound (VOC) Emissions and Health Risk Assessment in Paint and Coatings Industry in the Yangtze River Delta, China. Environ. Pollut. 2021, 269, 115740. [Google Scholar] [CrossRef]
- Jantunen, M.; Jaakkola, J.J.K.; Krzyżanowski, M. (Eds.) Assessment of Exposure to Indoor Air Pollutants; WHO Regional Publications; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 1997. [Google Scholar]
- US EPA. Technical Overview of Volatile Organic Compounds. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds#8 (accessed on 27 August 2021).
- OMS. Health Effects of Particulate Matter; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- Arh, G.; Klasinc, L.; Veber, M.; Pompe, M. Calibration of Mass Selective Detector in Non-Target Analysis of Volatile Organic Compounds in the Air. J. Chromatogr. A 2011, 1218, 1538–1543. [Google Scholar] [CrossRef]
- Woolfenden, E. Sorbent-Based Sampling Methods for Volatile and Semi-Volatile Organic Compounds in Air. J. Chromatogr. A 2010, 1217, 2674–2684. [Google Scholar] [CrossRef]
- Krupa, S.V.; Legge, A.H. Passive Sampling of Ambient, Gaseous Air Pollutants: An Assessment from an Ecological Perspective. Environ. Pollut. 2000, 107, 31–45. [Google Scholar] [CrossRef]
- Seethapathy, S.; Górecki, T.; Li, X. Passive Sampling in Environmental Analysis. J. Chromatogr. A 2008, 1184, 234–253. [Google Scholar] [CrossRef]
- Crump, D. Application of Diffusive Samplers. In Organic Indoor Air Pollutants; John Wiley & Sons, Ltd.: New York, NY, USA, 2009; pp. 47–63. [Google Scholar] [CrossRef]
- Schieweck, A. Very Volatile Organic Compounds (VVOC) as Emissions from Wooden Materials and in Indoor Air of New Prefabricated Wooden Houses. Build. Environ. 2021, 190, 107537. [Google Scholar] [CrossRef]
- Woolfenden, E. Sorbent-Based Sampling Methods for Volatile and Semi-Volatile Organic Compounds in Air. Part 2. Sorbent Selection and Other Aspects of Optimizing Air Monitoring Methods. J. Chromatogr. A 2010, 1217, 2685–2694. [Google Scholar] [CrossRef]
- McDonald, J.D.; Zielinska, B.; Fujita, E.M.; Sagebiel, J.C.; Chow, J.C.; Watson, J.G. Fine Particle and Gaseous Emission Rates from Residential Wood Combustion. Environ. Sci. Technol. 2000, 34, 2080–2091. [Google Scholar] [CrossRef]
- Ibáñez, M.; Sancho, J.V.; Hernández, F.; McMillan, D.; Rao, R. Rapid Non-Target Screening of Organic Pollutants in Water by Ultraperformance Liquid Chromatography Coupled to Time-of-Light Mass Spectrometry. TrAC Trends Anal. Chem. 2008, 27, 481–489. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Gennaro, M.C.; Marengo, E. Contaminants in Water: Non-Target UHPLC/MS Analysis. Environ. Chem. Lett. 2016, 14, 51–65. [Google Scholar] [CrossRef]
- Ruff, M.; Mueller, M.S.; Loos, M.; Singer, H.P. Quantitative Target and Systematic Non-Target Analysis of Polar Organic Micro-Pollutants along the River Rhine Using High-Resolution Mass-Spectrometry – Identification of Unknown Sources and Compounds. Water Res. 2015, 87, 145–154. [Google Scholar] [CrossRef]
- Picó, Y.; El-Sheikh, M.A.; Alfarhan, A.H.; Barceló, D. Target vs Non-Target Analysis to Determine Pesticide Residues in Fruits from Saudi Arabia and Influence in Potential Risk Associated with Exposure. Food Chem. Toxicol. 2018, 111, 53–63. [Google Scholar] [CrossRef]
- Angeles, L.F.; Halwatura, L.M.; Antle, J.P.; Simpson, S.; Jaraula, C.M.B.; Aga, D.S. In Silico Supported Nontarget Analysis of Contaminants of Emerging Concern: Increasing Confidence in Unknown Identification in Wastewater and Surface Waters. ACS EST Water 2021, 1, 1765–1775. [Google Scholar] [CrossRef]
- Pourchet, M.; Debrauwer, L.; Klanova, J.; Price, E.J.; Covaci, A.; Caballero-Casero, N.; Oberacher, H.; Lamoree, M.; Damont, A.; Fenaille, F.; et al. Suspect and Non-Targeted Screening of Chemicals of Emerging Concern for Human Biomonitoring, Environmental Health Studies and Support to Risk Assessment: From Promises to Challenges and Harmonisation Issues. Environ. Int. 2020, 139, 105545. [Google Scholar] [CrossRef]
- Wilczyńska-piliszek, A.J.; Piliszek, S.; Falandysz, J. Estimation of K OA Values of 209 Polychlorinated Trans -Azobenzenes by PM6 and DFT Methods. J. Environ. Sci. Health Part B 2012, 47, 562–570. [Google Scholar] [CrossRef]
- Xu, S.; Kropscott, B. Octanol/Air Partition Coefficients of Volatile Methylsiloxanes and Their Temperature Dependence. J. Chem. Eng. Data 2013, 58, 136–142. [Google Scholar] [CrossRef]
- Collège National d’Experts en Environnement de l’Industrie Chimique. D’Experts en E. de l’Industrie C. Les Propriétés Environnementales Des Substances. 2010. Available online: https://docplayer.fr/56425504-Les-proprietes-environnementales-des-substances.html (accessed on 6 January 2022).
- Tissier, C.; Morvan, C.; Bocquené, G. Les Substances Prioritaires De La Directive Cadre Sur L’eau (DCE); Institut Ifremer: Brest, France, 2005; Volume 92. [Google Scholar]
- Shaw, P.E. Review of Quantitative Analyses of Citrus Essential Oils. J. Agric. Food Chem. 1979, 27, 246–257. [Google Scholar] [CrossRef]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor Chemistry of Lemon-Lime Carbonated Beverages. J. Agric. Food Chem. 2015, 63, 112–119. [Google Scholar] [CrossRef]
- Jang, M.; Kamens, R.M. Atmospheric Secondary Aerosol Formation by Heterogeneous Reactions of Aldehydes in the Presence of a Sulfuric Acid Aerosol Catalyst. Environ. Sci. Technol. 2001, 35, 4758–4766. [Google Scholar] [CrossRef]
- Lee, A.K.Y.; Li, Y.J.; Lau, A.P.S.; Chan, C.K. A Re-Evaluation on the Atmospheric Significance of Octanal Vapor Uptake by Acidic Particles: Roles of Particle Acidity and Gas-Phase Octanal Concentration. Aerosol Sci. Technol. 2008, 42, 992–1000. [Google Scholar] [CrossRef][Green Version]
- International Agency for Research on Cancer (IARC). Pyridine (IARC Summary & Evaluation, Volume 77, 2000). Available online: https://inchem.org/documents/iarc/vol77/77-16.html (accessed on 6 January 2022).
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Monedeiro, F.; Buszewski, B. Effects of Growth Conditions and Cultivability on the Content of Cyclitols in Medicago Sativa. Int. J. Environ. Sci. Technol. 2021, 18, 33–48. [Google Scholar] [CrossRef]
- Zalat, O.A.; Elsayed, M.A. A Study on Microwave Removal of Pyridine from Wastewater. J. Environ. Chem. Eng. 2013, 1, 137–143. [Google Scholar] [CrossRef]
- Lataye, D.H.; Mishra, I.M.; Mall, I.D. Removal of Pyridine from Aqueous Solution by Adsorption on Bagasse Fly Ash. Ind. Eng. Chem. Res. 2006, 45, 3934–3943. [Google Scholar] [CrossRef]
- USEPA. List of Lists: Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act. 2021, 131. Available online: https://www.epa.gov/epcra/consolidated-list-lists-under-epcracerclacaa-ss112r-april-2022-version (accessed on 6 January 2022).
- Wang, X.; Wei, Y.; Zhang, H.; Bao, L.; He, M.; Yuan, S. Understanding the Properties of Methyl Vinyl Ketone and Methacrolein at the Air-Water Interface: Adsorption, Heterogeneous Reaction and Environmental Impact Analysis. Chemosphere 2021, 283, 131183. [Google Scholar] [CrossRef] [PubMed]
- Biesenthal, T.A.; Shepson, P.B. Observations of Anthropogenic Inputs of the Isoprene Oxidation Products Methyl Vinyl Ketone and Methacrolein to the Atmosphere. Geophys. Res. Lett. 1997, 24, 1375–1378. [Google Scholar] [CrossRef]
- ECHA. Substance Information—ECHA. Available online: https://echa.europa.eu/fr/substance-information/-/substanceinfo/100.001.055 (accessed on 6 January 2022).
- Ramírez, N.; Cuadras, A.; Rovira, E.; Borrull, F.; Marcé, R.M. Chronic Risk Assessment of Exposure to Volatile Organic Compounds in the Atmosphere near the Largest Mediterranean Industrial Site. Environ. Int. 2012, 39, 200–209. [Google Scholar] [CrossRef]
- Li, K.; Liang, T.; Wang, L. Risk Assessment of Atmospheric Heavy Metals Exposure in Baotou, a Typical Industrial City in Northern China. Environ. Geochem. Health 2016, 38, 843–853. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Bao, L.-J.; Huang, C.-L.; Li, S.-M.; Zeng, E.Y. A Comprehensive Risk Assessment of Human Inhalation Exposure to Atmospheric Halogenated Flame Retardants and Organophosphate Esters in an Urban Zone. Environ. Pollut. 2019, 252, 1902–1909. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Taghavi, M.; Moslem, A.; Neshat, A.A.; Lari Najafi, M.; Alahabadi, A.; Ahmadi, E.; Ebrahimi aval, H.; Asour, A.A.; Rezaei, H.; et al. Ecological and Health Risk Assessment of Exposure to Atmospheric Heavy Metals. Ecotoxicol. Environ. Saf. 2019, 184, 109622. [Google Scholar] [CrossRef]
- Guida, Y.; Pozo, K.; de Carvalho, G.O.; Capella, R.; Targino, A.C.; Torres, J.P.M.; Meire, R.O. Occurrence of Pyrethroids in the Atmosphere of Urban Areas of Southeastern Brazil: Inhalation Exposure and Health Risk Assessment. Environ. Pollut. 2021, 290, 118020. [Google Scholar] [CrossRef]
- Skjevrak, I.; Brede, C.; Steffensen, I.-L.; Mikalsen, A.; Alexander, J.; Fjeldal, P.; Herikstad, H. Non-Targeted Multi-Component Analytical Surveillance of Plastic Food Contact Materials: Identification of Substances Not Included in EU Positive Lists and Their Risk Assessment. Food Addit. Contam. 2005, 22, 1012–1022. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Sun, X.; Ma, X.; Song, J.; Sui, H.; Debrah, A.A. Non-Targeted Analysis and Risk Assessment of Non-Volatile Compounds in Polyamide Food Contact Materials. Food Chem. 2021, 345, 128625. [Google Scholar] [CrossRef]
- McCord, J.P.; Groff, L.C.; Sobus, J.R. Quantitative Non-Targeted Analysis: Bridging the Gap between Contaminant Discovery and Risk Characterization. Environ. Int. 2022, 158, 107011. [Google Scholar] [CrossRef]
- SMBT. SAGE Des Bassins Versants de La Lagune de Thau et de l’étang d’Ingril; SMBT: Sète, France, 2016. [Google Scholar]
- DREAL. Réseau de Suivi de la Lagune de Thau—DREAL Occitanie. Available online: https://www.occitanie.developpement-durable.gouv.fr/reseau-de-suivi-de-la-lagune-de-thau-r1326.html (accessed on 13 June 2022).
- Berrou, K.; Dunyach-Remy, C.; Lavigne, J.-P.; Roig, B.; Cadiere, A. Comparison of Stir Bar Sorptive Extraction and Solid Phase Microextraction of Volatile and Semi-Volatile Metabolite Profile of Staphylococcus Aureus. Molecules 2020, 25, 55. [Google Scholar] [CrossRef]
- US EPA. Estimation Programs Interface SuiteTM for Microsoft® Windows; version 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2021.
- Bunn, A.; Korpela, M. Crossdating in DplR. 2014, 12. Available online: https://mran.microsoft.com/snapshot/2019-04-29/web/packages/dplR/vignettes/xdate-dplR.pdf (accessed on 6 January 2022).
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]



| Molecules | Field | Laboratory | ||
|---|---|---|---|---|
| Coriolis Micro | Nalophan® Bags | Coriolis Micro | Nalophan® Bags | |
| 1-octanol | yes | no | yes | yes |
| 2-butanone | no | yes | no | yes |
| 2-ethyl-1-hexanol | yes | yes | yes | yes |
| Octanal | no | yes | yes | yes |
| Toluene | no | yes | no | yes |
| Undecane | yes | no | no | yes |
| Stages | Description | Nalophan® Bag | Coriolis Micro |
|---|---|---|---|
| Sampling | Instruments | Laboratory-made 40 L Nalophan® bag + lung chamber (NF X 43-104) | Coriolis micro (Bertin instruments, France) |
| Quantity of air sampled | 40 L | 12,000 L | |
| Extraction | Recovery unit | 18 L are pumped through AirToxic (Perkin Elmer, Waltham, MA, USA) multi-bed sorbent tubes | 15 mL of distilled water in an amber glass vial. SBSE a with 10 mm Twister (Gerstel, Germany) magnetic stir bars coated with polydimethylsiloxane (PDMS) (0.5 mm film thickness) or ethylene glycol Silicone (EGS) *. The method was an adaptation of the Berrou et al., 2020 study [55] |
| Flow rate | 100 mL/min | - | |
| Agitation-duration | - | 600 rpm during 2 h | |
| Desorption | Instruments | Turbomatrix, PerkinElmer, USA | Thermal desorption unit (TDU) and cooled injection system (CIS) (Gerstel, Germany) |
| Steps-Temperature-duration | 250 °C for 15 min | TDU: 220 °C for 5 min CIS: −10 °C for 1 min ramped to 270 °C at a heating rate of 12 °C per second and held for 2.5 min in spitless mode | |
| Analysis | Instruments | Clarus 680, Perkin Elmer, USA | Agilent 7890B gas chromatograph |
| Columns | Elite-5-ms (60 m × 0.25 mm × 1 µm, Perkin Elmer, USA) | ZB-5MSplus fused silica capillary column (30 m × 0.25 mm × 0.25 µm, Phenomenex, Torrance, CA, USA) | |
| Gas-flow rate | Helium-constant pressure of 30 psi | Helium-0.8 mL/min | |
| Oven program | 9 min at 40 °C, a ramp at 15 °C/min until 90 °C hold 4 min, then a ramp at 10 °C/min until 250 °C hold 15 min | 4 min at 40 °C, a ramp at 6 °C/min until 300 °C, hold 1 min | |
| Detection | Instruments | SQ8T model mass spectrometer (Perkin Elmer, USA) | 5977A mass spectrometer (Agilent, Santa Clara, CA, USA) |
| Mode | Electronic impact at 70 eV | Electronic impact at 70 eV | |
| Acquired mass | 20 to 350 amu | 33 to 350 amu | |
| Analysis type | Scan mode | Scan mode | |
| Identification | NIST library | NIST library |
| CAS | Substances | Family | Molecular Weight (g/mol) | Concentration (mg/m3) | References 1 |
|---|---|---|---|---|---|
| 78-93-3 | 2-butanone | Ketone | 72.11 | 3.59 | VWR (25643.294) |
| 108-88-3 | Toluene | Aromatic | 92.14 | 1.95 | Supelco (1.00849.2500) |
| 124-13-0 | Octanal | Aldehyde | 128.21 | 2.76 | Acros (199481000) |
| 104-76-7 | 2-ethyl-1-hexanol | Alcohol | 130.23 | 2.81 | Acros (118530010) |
| 111-87-5 | 1-octanol | Alcohol | 130.23 | 3.69 | Fluka (74852) |
| 1120-21-4 | Undecane | Alkane | 156.31 | 2.48 | Acros (140665000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courtier, A.; Roig, B.; Cariou, S.; Cadiere, A.; Bayle, S. Evaluation of Coriolis Micro Air Sampling to Detect Volatile and Semi-Volatile Organic Compounds. Molecules 2022, 27, 6462. https://doi.org/10.3390/molecules27196462
Courtier A, Roig B, Cariou S, Cadiere A, Bayle S. Evaluation of Coriolis Micro Air Sampling to Detect Volatile and Semi-Volatile Organic Compounds. Molecules. 2022; 27(19):6462. https://doi.org/10.3390/molecules27196462
Chicago/Turabian StyleCourtier, Audrey, Benoit Roig, Stephane Cariou, Axelle Cadiere, and Sandrine Bayle. 2022. "Evaluation of Coriolis Micro Air Sampling to Detect Volatile and Semi-Volatile Organic Compounds" Molecules 27, no. 19: 6462. https://doi.org/10.3390/molecules27196462
APA StyleCourtier, A., Roig, B., Cariou, S., Cadiere, A., & Bayle, S. (2022). Evaluation of Coriolis Micro Air Sampling to Detect Volatile and Semi-Volatile Organic Compounds. Molecules, 27(19), 6462. https://doi.org/10.3390/molecules27196462






