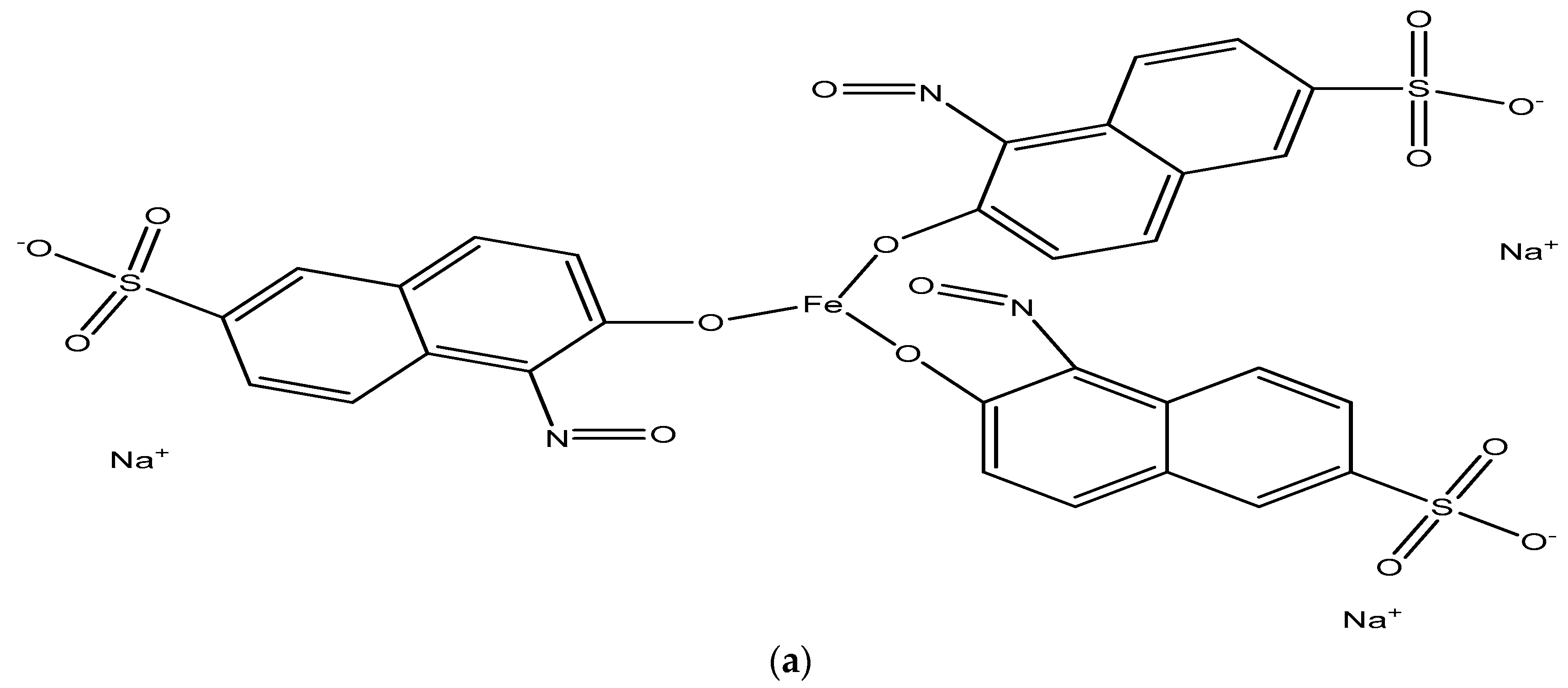
Mixed Micellar Solubilization of Naphthol Green B Followed by Its Removal from Synthetic Effluent by Micellar-Enhanced Ultrafiltration under Optimized Conditions
Abstract
1. Introduction
2. Results and Discussion
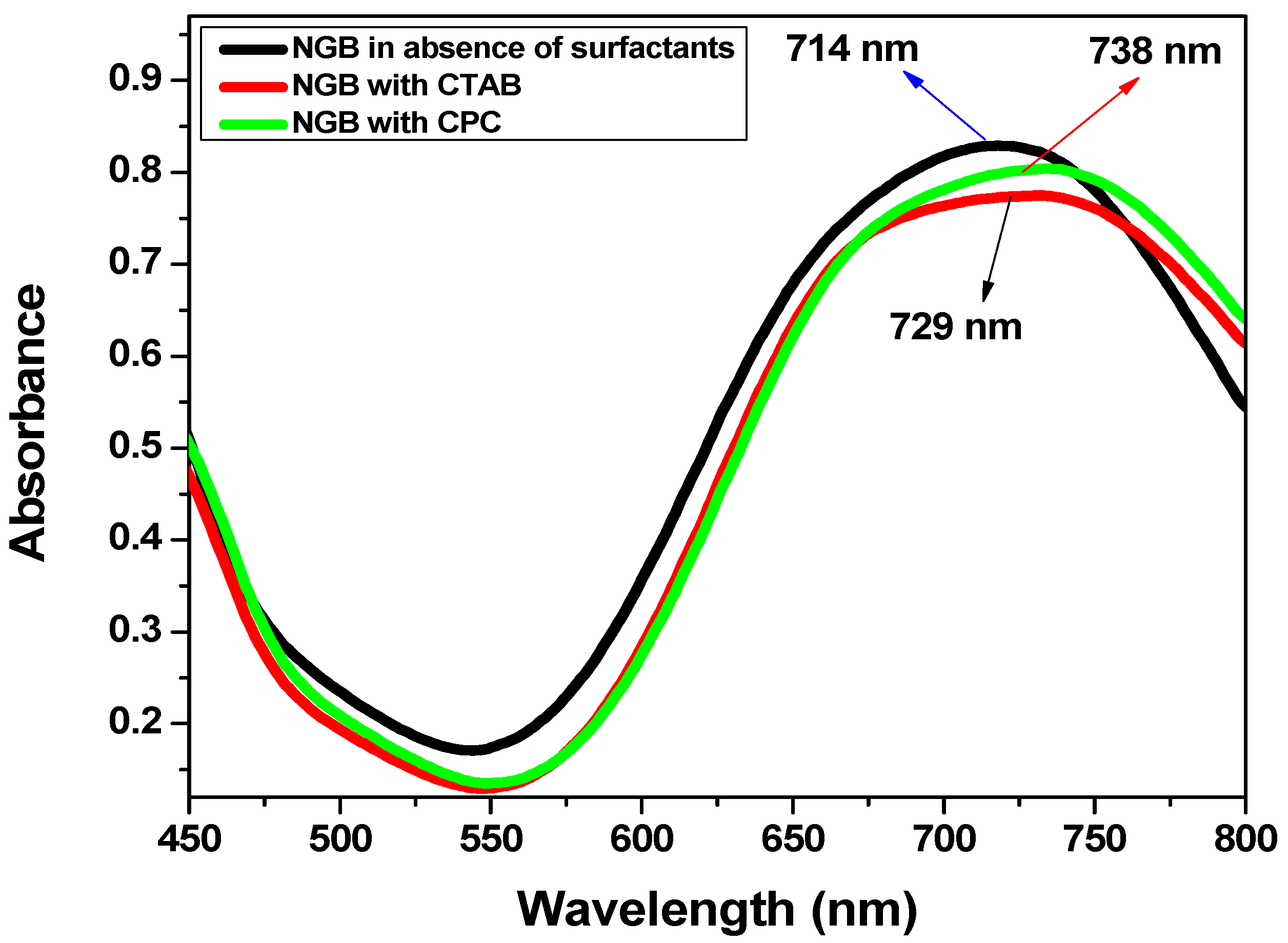
2.1. Absorption Spectroscopic Study
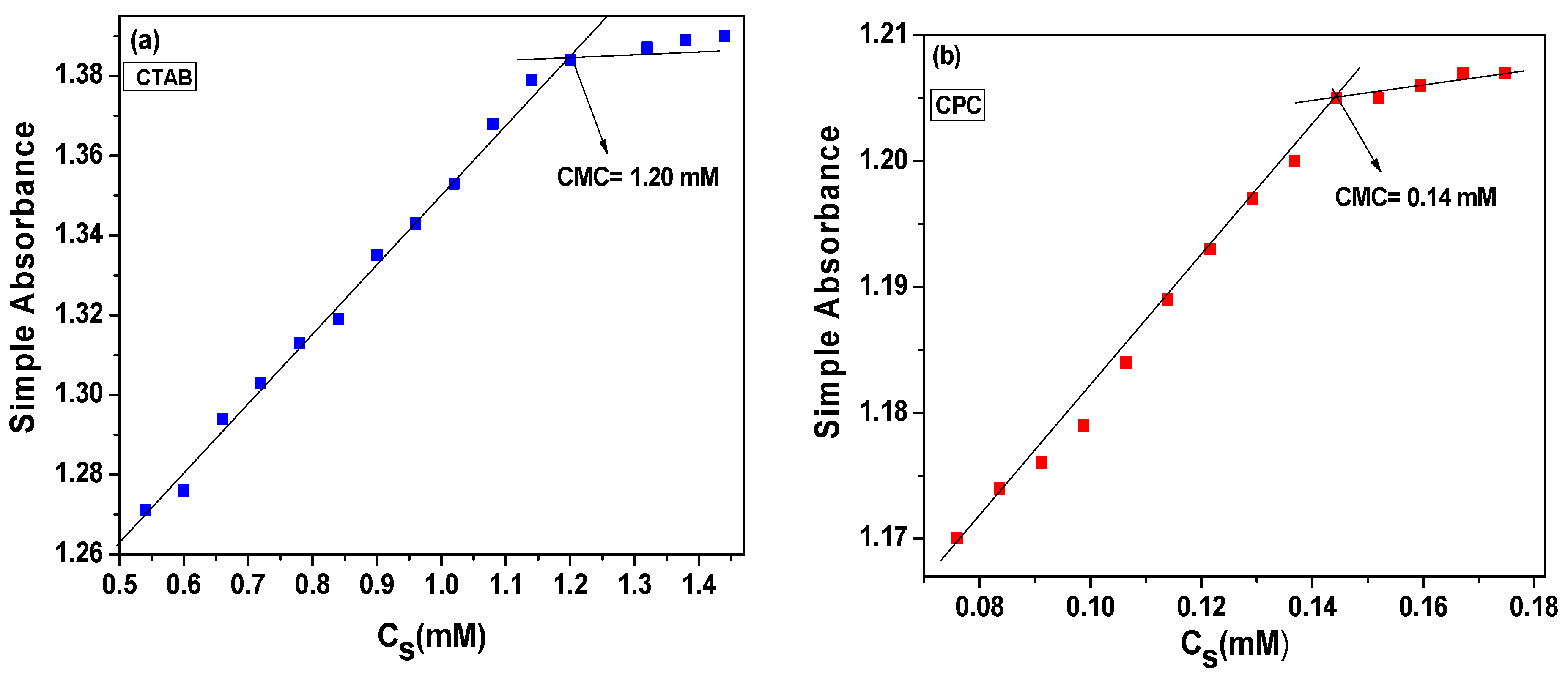
2.1.1. Interaction of NGB with CTAB and CPC
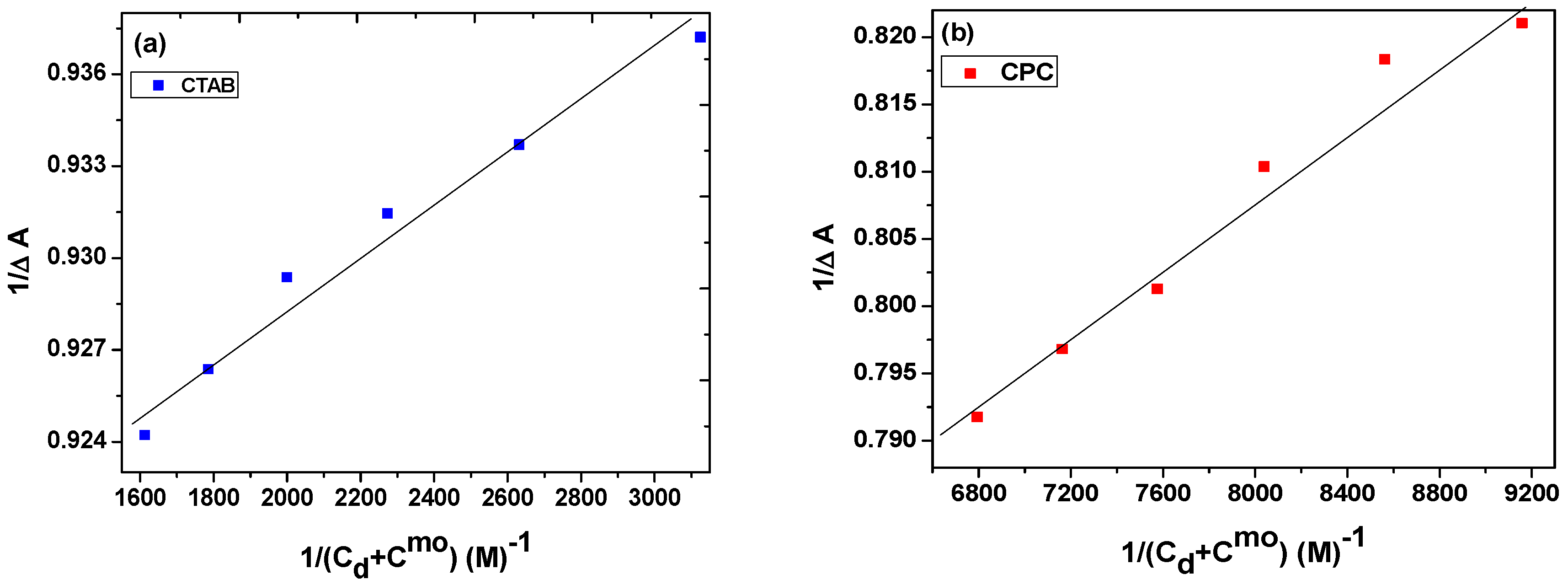
2.1.2. Water–Micelle Partitioning of NGB
2.1.3. Effects of TX-100 on the Partitioning of NGB
2.2. Mechanism of Micellar-Enhanced Ultrafiltration of NGB by CTAB and CPC
2.2.1. Calculation of Rejection Percentage (R%) and Permeate Flux (J)
Effect of Concentration of Surfactants
Effect of Electrolyte Concentration
Transmembrane Pressure
RPM
Effect of pH
Comparison among Effects of Various Factors
3. Parameter Calculated
3.1. Partition Constant and Gibbs Energy of Partition
3.2. Permeate Flux
3.3. Rejection Percentage
4. Materials and Methods
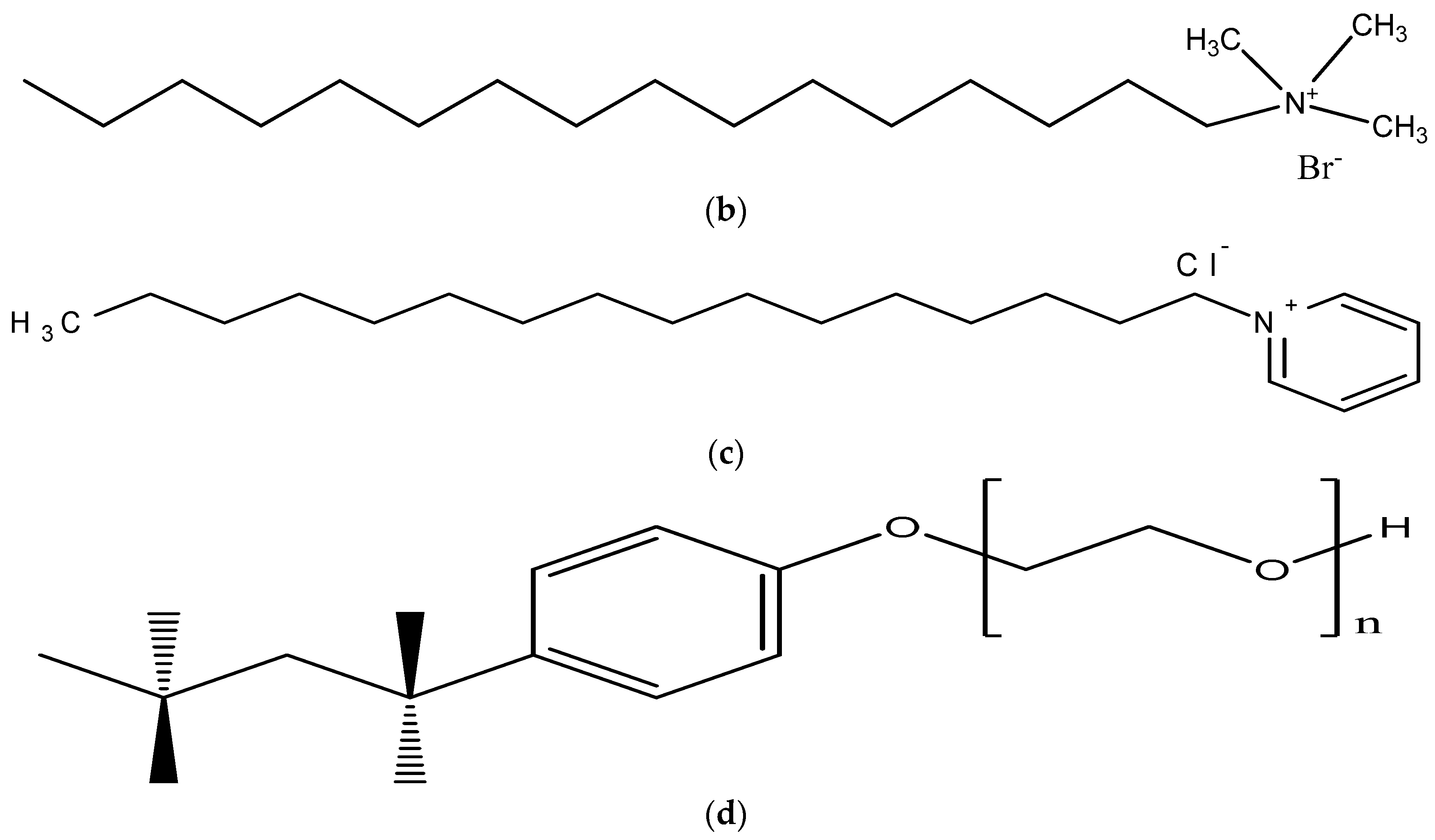
4.1. Materials Used
4.2. Experimental Procedure
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Clean Laccase immobilized nanobiocatalysts (graphene oxide-zeolite nanocomposites): From production to detailed biocatalytic degradation of organic pollutant. Appl. Catal. B Environ. 2020, 268, 118443. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, M.; Alinia Asli, M.; Mahmoodi, N.M. Carbon nanotube based metal-organic framework nanocomposites: Synthesis and their photocatalytic activity for decolorization of colored wastewater. Inorg. Chim. Acta 2019, 487, 169–176. [Google Scholar] [CrossRef]
- Mao, G.; Han, Y.; Liu, X.; Crittenden, J.; Huang, N.; Ahmad, U.M. Technology status and trends of industrial wastewater treatment: A patent analysis. Chemosphere 2022, 288, 132483. [Google Scholar] [CrossRef] [PubMed]
- Birwal, P.; Deshmukh, G.; Priyanka, S.S.; Saurabh, S. Advanced technologies for dairy effluent treatment. J. Food Nutr. Popul. Health 2017, 1, 7. [Google Scholar]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef] [PubMed]
- Yusaf, A.; Usman, M.; Mansha, A.; Saeed, M.; Ahmad, M.; Siddiq, M. Micellar-enhanced ultrafiltration (MEUF) for removal of rhodamine B (RhB) from aqueous system. J. Dispers. Sci. Technol. 2022, 43, 366-348. [Google Scholar] [CrossRef]
- Alruwaili, S.F.; Alsohaimi, I.H.; El-Sayed, M.Y.; Hassan, H.M.A.; Aldawsari, A.M.; Alshahrani, A.A.; Alraddadi, T.S. Antifouling efficiency and high-flux ultrafiltration membrane comprising sulfonated poly (ether sulfone) and TNTs-g-PSPA nanofiller. J. Taiwan Inst. Chem. Eng. 2021, 129, 350–360. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Hassan, H.M.A.; Alsohaimi, I.H.; Chen, Q.; Melhi, S.; Younes, A.A.; El-Shwiniy, W.H. Fabrication of sulfonated polyethersulfone ultrafiltration membranes with an excellent antifouling performance by impregnating with polysulfopropyl acrylate coated ZnO nanoparticles. Environ. Technol. Innov. 2022, 25, 102210. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alsuhybani, M.; Algamdi, M.S.; Alquppani, D.; Mashhour, I.; Alshammari, M.S.; Alsohaimi, I.H.; Alraddadi, T.S. Evaluating the performance of chitosan and chitosan-palm membrane for water treatment: Preparation, characterization and purification study. J. Taibah Univ. Sci. 2021, 15, 77–86. [Google Scholar] [CrossRef]
- Aryanti, N.; Sandria, F.K.I.; Putriadi, R.H.; Wardhani, D.H. Evaluation of micellar-enhanced ultrafiltration (MEUF) membrane for dye removal of synthetic remazol dye wastewater. Eng. J. 2017, 21, 23–35. [Google Scholar] [CrossRef]
- Ankita, A. A Brief Review of Micellar Enhanced Ultrafiltration (Meuf) Techniques for Treatment of Wastewater in India. J. Water Eng. Manag. 2020, 1, 14–30. [Google Scholar] [CrossRef]
- Schwarze, M. Micellar-enhanced ultrafiltration (MEUF)–state of the art. Environ. Sci. Water Res. Technol. 2017, 3, 598–624. [Google Scholar] [CrossRef]
- Sarkar, B. Micellar enhanced ultrafiltration in the treatment of dye wastewater: Fundamentals, state-of-the-art and future perspectives. Groundw. Sustain. Dev. 2022, 17, 100730. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Investigating the properties of cetyltrimethylammonium bromide/hydroxylated graphene quantum dots thin film for potential optical detection of heavy metal ions. Materials 2020, 13, 2591. [Google Scholar] [CrossRef]
- Muñoz-Basagoiti, J.; Perez-Zsolt, D.; León, R.; Blanc, V.; Raïch-Regué, D.; Cano-Sarabia, M.; Trinité, B.; Pradenas, E.; Blanco, J.; Gispert, J. Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro. J. Dent. Res. 2021, 100, 1265–1272. [Google Scholar] [CrossRef]
- Costa, A.L.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Pina, J.; Seixas de Melo, J.S. Insights into the photophysics and supramolecular organization of Congo red in solution and the solid state. ChemPhysChem 2017, 18, 564–575. [Google Scholar] [CrossRef]
- Wang, J.; Lin, C.Y.; Moore, C.; Jhunjhunwala, A.; Jokerst, J.V. Switchable Photoacoustic Intensity of Methylene Blue via Sodium Dodecyl Sulfate Micellization. Langmuir ACS J. Surf. Colloids 2018, 34, 359–365. [Google Scholar] [CrossRef]
- Ali, M.; Usman, M.; Shah, A.; Rehman, A. Encapsulation of ethyl violet by anionic-cationic mixed micellar solution: Spectroscopic and conductometric studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127423. [Google Scholar] [CrossRef]
- Petcu, A.R.; Rogozea, E.A.; Lazar, C.A.; Olteanu, N.L.; Meghea, A.; Mihaly, M. Specific interactions within micelle microenvironment in different charged dye/surfactant systems. Arab. J. Chem. 2016, 9, 9–17. [Google Scholar] [CrossRef]
- Ganguly, B.; Nath, R.K. Spectral studies of pinacyanol chloride in sodium alkyl sulfate. Chem. Mat. Res. 2012, 2, 13–23. [Google Scholar]
- Al-Thabaiti, S.A.; Aazam, E.S.; Khan, Z.; Bashir, O. Aggregation of Congo red with surfactants and Ag-nanoparticles in an aqueous solution. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2016, 156, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Taj Muhammad, M.; Khan, M.N. Study of electrolytic effect on the interaction between anionic surfactant and methylene blue using spectrophotometric and conductivity methods. J. Mol. Liq. 2017, 234, 309–314. [Google Scholar] [CrossRef]
- Göktürk, S.; Keskin, G.; Talman, R.Y.C.; Çakır, N. Spectroscopic and conductometric studies on the interactions of thionine with anionic and nonionic surfactants. Color. Technol. 2017, 133, 362–368. [Google Scholar] [CrossRef]
- Muntaha, S.-T.; Khan, M.N. Natural surfactant extracted from Sapindus mukurossi as an eco-friendly alternate to synthetic surfactant—A dye surfactant interaction study. J. Clean. Prod. 2015, 93, 145–150. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Maleki, R.; Matin, A.A.; Nikkhahi, Y. Spectrophotometric study of anionic azo-dye light yellow (X6G) interaction with surfactants and its micellar solubilization in cationic surfactant micelles. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2008, 69, 1183–1187. [Google Scholar] [CrossRef]
- Younas, N.; Rashid, M.A.; Usman, M.; Nazir, S.; Noor, S.; Basit, A.; Jamil, M. Solubilization of Ni Imidazole Complex in Micellar Media of Anionic Surfactants, Sodium Dodecyl Sulfate and Sodium Stearate. J. Surfactants Deterg. 2017, 20, 1311–1320. [Google Scholar] [CrossRef]
- Mallick, S.; Arathi, A.S.; Koner, A.L. Customized tuning of aggregation-induced emission of a napthalimide dye by surfactants and cyclodextrin. J. Colloid Interface Sci. 2017, 499, 46–53. [Google Scholar] [CrossRef]
- Ali, A.; Alam, M.; Farooq, U.; Uzair, S. Effect of the nature of counterion on the micellar properties of cationic surfactants: A conductometric study. Phys. Chem. Liq. 2018, 56, 528–543. [Google Scholar] [CrossRef]
- Ali, A.; Uzair, S.; Malik, N.A.; Ali, M. Study of interaction between cationic surfactants and cresol red dye by electrical conductivity and spectroscopy methods. J. Mol. Liq. 2014, 196, 395–403. [Google Scholar] [CrossRef]
- Lu, S.; Wu, J.; Somasundaran, P. Micellar evolution in mixed nonionic/anionic surfactant systems. J. Colloid Interface Sci. 2012, 367, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Obradović, S.; Poša, M. The influence of the structure of selected Brij and Tween homologues on the thermodynamic stability of their binary mixed micelles. J. Chem. Thermodyn. 2017, 110, 41–50. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, G.; Kansal, S.K.; Chaudhary, G.R.; Mehta, S.K. (Cationic + nonionic) mixed surfactant aggregates for solubilisation of curcumin. J. Chem. Thermodyn. 2016, 93, 115–122. [Google Scholar] [CrossRef]
- Bergström, L.M. A theoretical investigation of the influence of the second critical micelle concentration on the solu-bilization capacity of surfactant micelles. AIP Adv. 2018, 8, 055136. [Google Scholar] [CrossRef]
- Vinarov, Z.; Dobreva, P.; Tcholakova, S. Effect of surfactant molecular structure on Progesterone solubili-zation. J. Drug Deliv. Sci. Technol. 2018, 43, 44–49. [Google Scholar] [CrossRef]
- Abdel-Rahem, R. The influence of hydrophobic counterions on micellar growth of ionic surfactants. Adv. Colloid Interface Sci. 2008, 141, 24–36. [Google Scholar] [CrossRef]
- Zhao, Y.; Haward, S.J.; Shen, A.Q. Rheological characterizations of wormlike micellar solutions containing cationic surfactant and anionic hydrotropic salt. J. Rheol. 2015, 59, 1229–1259. [Google Scholar] [CrossRef]
- Iqbal, J.; Kim, H.J.; Yang, J.S.; Baek, K.; Yang, J.W. Removal of arsenic from groundwater by micellar-enhanced ultrafiltration (MEUF). Chemosphere 2007, 66, 970–976. [Google Scholar] [CrossRef]
- Verma, R.K.; Sankhla, M.S.; Rathod, N.V.; Sonone, S.S.; Parihar, K.; Singh, G.K. Eradication of Fatal Textile Industrial Dyes by Wastewater Treatment. Biointerface Res. Appl. Chem. 2021, 12, 567–587. [Google Scholar] [CrossRef]
- Meena, P.L.; Surela, A.K.; Poswal, K.; Saini, J.K.; Chhachhia, L.K. Biogenic synthesis of Bi2O3 nanoparticles using Cassia fistula plant pod extract for the effective degradation of organic dyes in aqueous medium. Biomass Convers. Biorefin. 2022, 1–17. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Zeng, G.; Shi, L.; Gu, Y.; Shi, Y.; Tang, B.; Li, X. Removal of Cd(II) by MEUF-FF with anionic-nonionic mixture at low concentration. Sep. Purif. Technol. 2018, 207, 199–205. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H. Heavy metals removal from aqueous solution through micellar enhanced ultrafiltration: A review. Environ. Eng. Res. 2018, 24, 363–375. [Google Scholar] [CrossRef]
- Xu, K.; Zeng, G.-M.; Huang, J.-H.; Wu, J.-Y.; Fang, Y.-Y.; Huang, G.; Li, J.; Xi, B.; Liu, H. Removal of Cd2+ from synthetic wastewater using micellar-enhanced ultrafiltration with hollow fiber membrane. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 140–146. [Google Scholar] [CrossRef]
- Saeed, R.; Usman, M.; Mansha, A.; Rasool, N.; Naqvi, S.A.R.; Zahoor, A.F.; Rahman, H.M.A.; Rana, U.A.; Al-Zahrani, E. Partitioning of structurally related thiophene derivatives between solvent and micellar media of anionic surfactant sodium dodecyl sulphate. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 51–60. [Google Scholar] [CrossRef]
- Kawamura, H.; Manabe, M.; Miyamoto, Y.; Fujita, Y.; Tokunaga, S. Partition coefficients of homologous. omega.-phenylalkanols between water and sodium dodecyl sulfate micelles. J. Phys. Chem. 1989, 93, 4. [Google Scholar] [CrossRef]
- Landaburu-Aguirre, J.; Pongrácz, E.; Perämäki, P.; Keiski, R.L. Micellar-enhanced ultrafiltration for the removal of cadmium and zinc: Use of response surface methodology to improve understanding of process performance and optimisation. J. Hazard. Mater. 2010, 180, 524–534. [Google Scholar] [CrossRef]
- Fazeli, S.; Sohrabi, B.; Tehrani-Bagha, A.R. The study of Sunset Yellow anionic dye interaction with gemini and conventional cationic surfactants in aqueous solution. Dye. Pigment. 2012, 95, 768–775. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef]





| [TX-100] (mM) | Kc × 10−4 (dm3 mol−1) | Kx × 10−5 | ΔGp (kJ mol−1) |
|---|---|---|---|
| 0 | 1.67 | 9.30 | −34.05 |
| 0.09 | 7.15 | 39.7 | −37.64 |
| 0.11 | 1.01 | 56.3 | −38.51 |
| 0.13 | 16.1 | 89.3 | −39.65 |
| 0.15 | 4.36 | 24.2 | −36.42 |
| 0.17 | 3.22 | 17.9 | −35.67 |
| [TX-100] (mM) | Kc × 10−4 (dm3 mol−1) | Kx × 10−5 | ΔGp (kJ mol−1) |
|---|---|---|---|
| 0 | 7.1 | 39.4 | −37.62 |
| 0.09 | 401 | 2230 | −47.62 |
| 0.11 | 45.7 | 25.4 | −47.94 |
| 0.13 | 57 | 317 | −42.78 |
| 0.15 | 17.7 | 98.1 | −39.88 |
| 0.17 | 1.12 | 6.24 | −33.06 |
| Sr. No. | CS (mM) | CF (mM) | Cp (μM) | R % | t (h) | Vp (ml) | J (L/hm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | |||
| 1 | 0 | 0 | 0.02 | 9.26 | 1.63 | 53.71 | 29.92 | 0.184 | 0.154 | 50 | 1669 | 1025 |
| 2 | 0.94 | 0.15 | 0.02 | 1.69 | 1.56 | 91.52 | 92.17 | 0.155 | 0.138 | 50 | 712 | 996 |
| 3 | 0.96 | 0.17 | 0.02 | 1.63 | 1.56 | 91.85 | 92.17 | 0.150 | 0.124 | 50 | 684 | 996 |
| 4 | 0.98 | 0.19 | 0.02 | 1.56 | 1.43 | 92.17 | 92.82 | 0.143 | 0.121 | 50 | 649 | 988 |
| 5 | 1.0 | 0.21 | 0.02 | 1.53 | 1.30 | 92.34 | 93.48 | 0.138 | 0.119 | 50 | 626 | 959 |
| 6 | 1.02 | 0.24 | 0.02 | 1.34 | 1.27 | 93.31 | 93.64 | 0.140 | 0.120 | 50 | 595 | 864 |
| 7 | 1.04 | 0.25 | 0.02 | 1.30 | 1.24 | 93.48 | 93.80 | 0.136 | 0.116 | 50 | 584 | 773 |
| Sr. No. | [NaCl] (mM) | CF (mM) | Cp (μM) | R % | t (h) | Vp (ml) | J (L/hm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | |||
| 1 | 20 | 0.03 | 16.3 | 1.50 | 94.57 | 95.00 | 0.141 | 0.14 | 50 | 50 | 844 | 834 |
| 2 | 40 | 0.03 | 15.3 | 1.37 | 94.91 | 95.43 | 0.161 | 0.15 | 50 | 50 | 739 | 768 |
| 3 | 60 | 0.03 | 14.6 | 1.24 | 95.12 | 95.87 | 0.183 | 0.16 | 50 | 50 | 652 | 714 |
| 4 | 80 | 0.03 | 14.2 | 0.78 | 95.27 | 97.39 | 0.205 | 0.17 | 50 | 50 | 583 | 691 |
| 5 | 100 | 0.03 | 13.8 | 0.48 | 95.39 | 98.37 | 0.207 | 0.18 | 50 | 50 | 576 | 649 |
| 6 | 120 | 0.03 | 13.5 | 0.58 | 95.51 | 98.04 | 0.216 | 0.20 | 50 | 50 | 552 | 596 |
| Sr. No. | Pressure (Bar) | CF (mM) | Cp (μM) | R % | t (h) | Vp (ml) | J (L/hm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | |||
| 1 | 5 | 0.03 | 1.27 | 1.34 | 95.76 | 95.54 | 0.45 | 0.60 | 50 | 50 | 265 | 199 |
| 2 | 10 | 0.03 | 1.34 | 1.43 | 95.54 | 95.21 | 0.33 | 0.30 | 50 | 50 | 356 | 398 |
| 3 | 15 | 0.03 | 1.43 | 1.53 | 95.21 | 94.89 | 0.23 | 0.18 | 50 | 50 | 512 | 652 |
| 4 | 20 | 0.03 | 1.53 | 1.56 | 94.89 | 94.78 | 0.20 | 0.13 | 50 | 50 | 579 | 897 |
| 5 | 25 | 0.03 | 1.56 | 1.60 | 94.78 | 94.67 | 0.12 | 0.11 | 50 | 50 | 981 | 1025 |
| 6 | 30 | 0.03 | 1.60 | 1.63 | 94.67 | 94.56 | 0.08 | 0.08 | 50 | 50 | 1372 | 1426 |
| Sr. No. | RPM | CF (mM) | Cp (μM) | R % | t (h) | Vp (ml) | J (L/hm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | |||
| 1 | 10 | 0.03 | 1.24 | 1.04 | 95.87 | 96.52 | 0.158 | 0.115 | 50 | 50 | 1194 | 1040 |
| 2 | 20 | 0.03 | 1.37 | 1.27 | 95.43 | 95.76 | 0.141 | 0.143 | 50 | 50 | 1141 | 834 |
| 3 | 30 | 0.03 | 1.43 | 1.30 | 95.21 | 95.65 | 0.118 | 0.157 | 50 | 50 | 1116 | 759 |
| 4 | 40 | 0.03 | 1.56 | 1.43 | 94.78 | 95.21 | 0.107 | 0.192 | 50 | 50 | 1008 | 622 |
| 5 | 50 | 0.03 | 1.60 | 1.50 | 94.67 | 95.00 | 0.104 | 0.205 | 50 | 50 | 844 | 581 |
| 6 | 60 | 0.03 | 1.60 | 1.56 | 94.67 | 94.78 | 0.100 | 0.217 | 50 | 50 | 755 | 549 |
| Sr. No | pH | CF (mM) | Cp (μM) | R % | t (h) | Vp (ml) | J (L/hm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | CTAB | CPC | |||
| 1 | 4 | 0.03 | 2.27 | 1.21 | 99.07 | 96.98 | 0.157 | 0.235 | 50 | 50 | 761 | 305 |
| 2 | 7 | 0.03 | 1.37 | 0.94 | 99.54 | 97.63 | 0.256 | 0.250 | 50 | 50 | 466 | 287 |
| 3 | 10 | 0.03 | 0.68 | 0.58 | 99.77 | 98.53 | 0.358 | 0.368 | 50 | 50 | 333 | 194 |
| Chemicals/Materials | Nature | Molecular Weight g/mol | Source |
|---|---|---|---|
| Naphthol Green B | Anionic | 878.4 | Sigma–Aldrich |
| Cetyl trimethyl ammonium bromide (CTAB) | Cationic | 364.44 | Daejung, Korea |
| Cetyl pyridinium chloride (CPC) | Cationic | 339.99 | Daejung, Korea |
| Membranes | Regenerated cellulose of 10,000 MWCO | - | Amicon Bioseparations EMD Millipore Corporation |
| Ultrafiltration stirred cell | Pressure-controlled | - | Amicon 8400 Millipore, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusaf, A.; Usman, M.; Siddiq, M.; Bakhtiar, M.; Mansha, A.; Shaukat, S.; Rehman, H.F. Mixed Micellar Solubilization of Naphthol Green B Followed by Its Removal from Synthetic Effluent by Micellar-Enhanced Ultrafiltration under Optimized Conditions. Molecules 2022, 27, 6436. https://doi.org/10.3390/molecules27196436
Yusaf A, Usman M, Siddiq M, Bakhtiar M, Mansha A, Shaukat S, Rehman HF. Mixed Micellar Solubilization of Naphthol Green B Followed by Its Removal from Synthetic Effluent by Micellar-Enhanced Ultrafiltration under Optimized Conditions. Molecules. 2022; 27(19):6436. https://doi.org/10.3390/molecules27196436
Chicago/Turabian StyleYusaf, Amnah, Muhammad Usman, Muhammad Siddiq, Manahil Bakhtiar, Asim Mansha, Saadia Shaukat, and Hafiza Fatima Rehman. 2022. "Mixed Micellar Solubilization of Naphthol Green B Followed by Its Removal from Synthetic Effluent by Micellar-Enhanced Ultrafiltration under Optimized Conditions" Molecules 27, no. 19: 6436. https://doi.org/10.3390/molecules27196436
APA StyleYusaf, A., Usman, M., Siddiq, M., Bakhtiar, M., Mansha, A., Shaukat, S., & Rehman, H. F. (2022). Mixed Micellar Solubilization of Naphthol Green B Followed by Its Removal from Synthetic Effluent by Micellar-Enhanced Ultrafiltration under Optimized Conditions. Molecules, 27(19), 6436. https://doi.org/10.3390/molecules27196436









