Quantification of Chemical Groups and Quantitative HPLC Fingerprint of Poria cocos (Schw.) Wolf
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantitative Analysis of Chemical Groups and Method Validation
2.2. Determination of TS, WSP, and AP in PC
2.3. HPLC Fingerprint for Alcohol Extract of PC
2.3.1. HPLC Fingerprint and Method Validation in PC
2.3.2. Analysis of HPLC Fingerprint and Similarity for Alcohol Extract of PC
2.4. Determination of Six Main Components in PC by QAMS
Determination of Six Main Components in an Alcohol Extract of PC and Method Validation
2.5. Principal Component Analysis
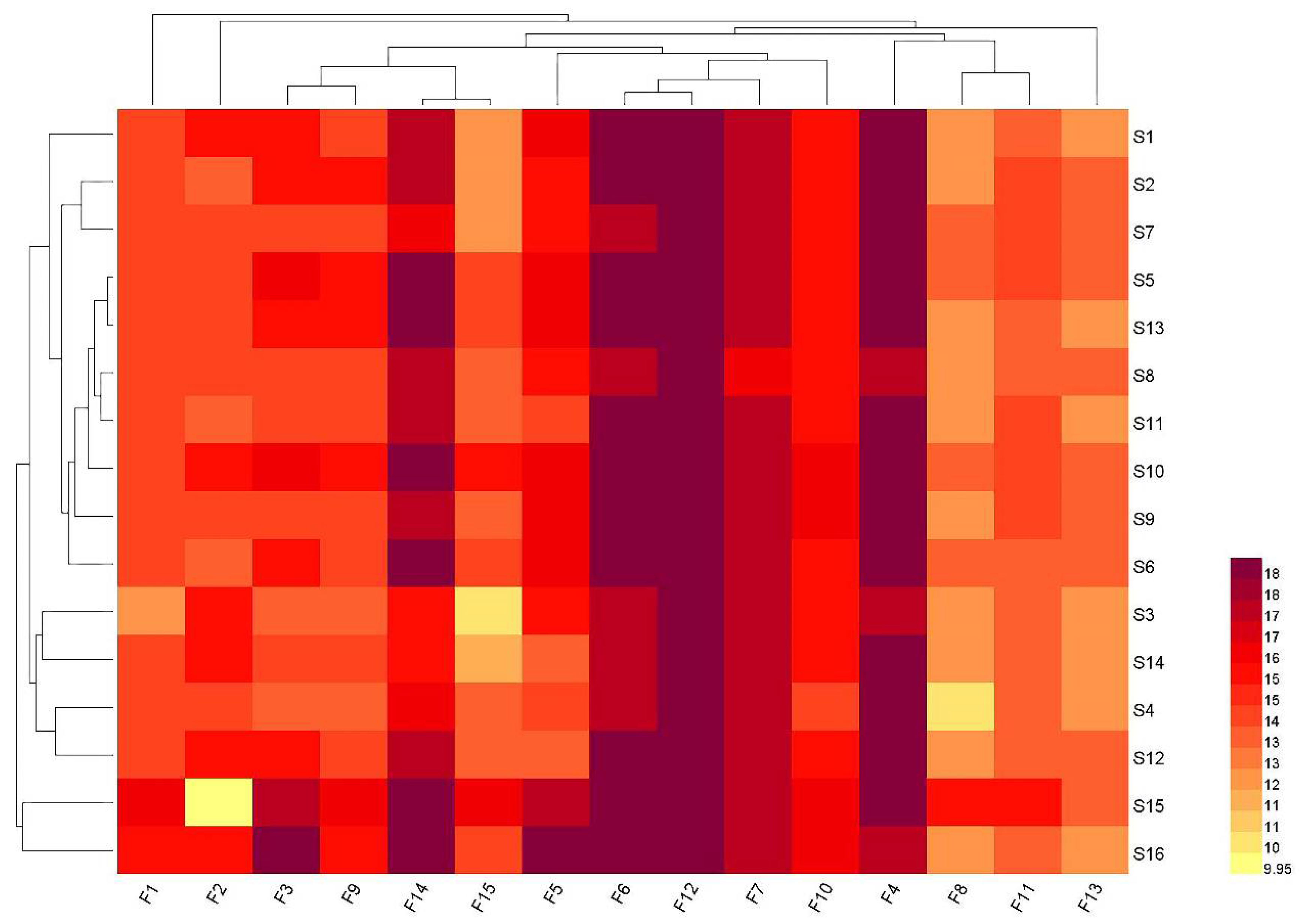
2.6. Heatmap Hierarchical Clustering Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Samples and Samples Collection
3.3. Sample Preparation
3.4. Preparation of Standard Solution
3.5. Establishment of a Method for the Determination of Chemical Groups (TS, WSP, and AP)
3.5.1. Method Validation of UV–Vis Spectrophotometry Analyses
3.5.2. Determination Methods for TS, WSP, and AP
3.6. Establishment of HPLC Fingerprinting for the Total TS
3.6.1. Establishment of HPLC Fingerprinting and Method Validation
3.6.2. HPLC Fingerprinting and Similarity Evaluation of Alcohol Extract of PC
3.7. QAMS for Alcohol Extract of PC
3.7.1. Preparation of Standard Solution and Sample Preparation
3.7.2. QAMS Validation, Durability, and System Suitability Test
3.7.3. Establishment of QAMS
3.8. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rios, J.L.; Andujar, I.; Recio, M.C.; Giner, R.M. Lanostanoids from fungi: A group of potential anticancer compounds. J. Nat. Prod. 2012, 75, 2016–2044. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, S.; Moon, E.; Park, H.J.; Park, H.B.; Kim, K.H. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorg. Chem. 2016, 70, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, L.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 263–296. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Zhao, J.; Xiao, X.; Li, W.; Zang, L.; Yu, J.; Liu, H.; Niu, X. Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE−/− mice by inhibiting inflammation. Phytother. Res. 2020, 35, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zheng, Y.; Sun, Y.; Liu, S.; Pi, Z.; Song, F.; Liu, Z. A targeted strategy for analyzing untargeted mass spectral data to identify lanostane–type triterpene acids in Poria cocos by integrating a scientific information system and liquid chromatography–tandem mass spectrometry combined with ion mobility spectrometry. Anal. Chim. Acta 2018, 1033, 87–99. [Google Scholar] [CrossRef]
- Nie, A.; Chao, Y.; Zhang, X.; Jia, W.; Zhou, Z.; Zhu, C. Phytochemistry and Pharmacological Activities of Wolfiporia cocos (F.A. Wolf) Ryvarden & Gilb. Front. Pharmacol. 2020, 11, 505249. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Y.; Ge, J.-C.; Wang, L.; Peng, D.-Y.; Yu, N.-J.; Zhang, Y.; Jiang, Y.-H.; Luo, J.-P.; Chen, W.-D. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr. Polym. 2021, 263, 117979. [Google Scholar] [CrossRef]
- Zhang, C.W.; Zhang, Y.; Su, S.; Cheng, L. Advances in studies on herbology, chemical constituents and pharmacological action of Poria cocos. HuBei Agric. Sci. 2021, 60, 9–14. [Google Scholar] [CrossRef]
- Han, X.J.; Luo, J.G.; Lu, Y.Y.; Kong, L.Y. Identification of triterpenoids in Poria cocos by HPLC-DAD-ESI-MSn. Pharm. Clin. Res. 2009, 17, 290–293. [Google Scholar] [CrossRef]
- Kang, A.; Guo, J.R.; Xie, T.; Shan, J.J.; Di, L.Q. Analysis of chemical constituents in Poria cocos by UPLC-LTQ-Orbitrap mass spectrometry. J. Nanjing Univ. Tradit. Chin. Med. 2014, 30, 561–565. [Google Scholar] [CrossRef]
- Kruszewski, B.; Obiedziński, M.W. Multivariate analysis of essential elements in raw cocoa and processed chocolate mass materials from three different manufacturers. LWT 2018, 98, 113–123. [Google Scholar] [CrossRef]
- Yildiz, O.; Gurkan, H.; Sahingil, D.; Degirmenci, A.; Kemal, M.E.; Kolayli, S.; Hayaloglu, A.A. Floral authentication of some monofloral honeys based on volatile composition and physicochemical parameters. Eur. Food Res. Technol. 2022, 248, 2145–2155. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, F.; Han, M.; Zhang, L.; Luan, S.; Li, W.; Deng, H.; Lan, Z.; Wu, Z.; Luo, X.; et al. Qualitative analysis of the roots of Salvia miltiorrhiza and Salvia yunnanensis based on NIR, UHPLC and LC–MS-MS. J. Pharm. Biomed. Anal. 2019, 170, 295–304. [Google Scholar] [CrossRef]
- Rivera-Pérez, A.; Romero-González, R.; Frenich, A.G. Fingerprinting based on gas chromatography-Orbitrap high-resolution mass spectrometry and chemometrics to reveal geographical origin, processing, and volatile markers for thyme authentication. Food Chem. 2022, 393, 133377. [Google Scholar] [CrossRef]
- Wroniak, M.; Raczyk, M.; Kruszewski, B.; Symoniuk, E.; Dach, D. Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants 2021, 10, 1637. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.; Pang, X.; Sun, G. Entirely control the quality consistency of Rong’e Yishen oral liquid by both quantified profiling and quantitative analysis of multi-components by single marker method. J. Pharm. Biomed. Anal. 2020, 193, 113719. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Jia, X.-H.; Zhu, S.; Komatsu, K.; Wang, X.; Cai, S.-Q. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta 2015, 134, 587–595. [Google Scholar] [CrossRef]
- Su, C.; Li, C.; Sun, K.; Li, W.; Liu, R. Quantitative analysis of bioactive components in walnut leaves by UHPLC-Q-Orbitrap HRMS combined with QAMS. Food Chem. 2020, 331, 127180. [Google Scholar] [CrossRef]
- Lan, L.; Sun, W.; Chang, Q.; Sun, G. Comprehensive evaluation of Licorice extract by five-dimensional quantitative profiling. J. Chromatogr. A 2021, 1644, 462105. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, J.; Zhang, H.; Fan, X. Investigating chemical features of Panax notoginseng based on integrating HPLC fingerprinting and determination of multiconstituents by single reference standard. J. Ginseng Res. 2017, 42, 334–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Xi, J.; Pan, C.; Xu, Z.; Xia, W.; Zhang, W. Multiple-fingerprint analysis of Poria cocos polysaccharide by HPLC combined with chemometrics methods. J. Pharm. Biomed. Anal. 2021, 198, 114012. [Google Scholar] [CrossRef] [PubMed]
- Gabetti, E.; Sgorbini, B.; Stilo, F.; Bicchi, C.; Rubiolo, P.; Chialva, F.; Reichenbach, S.E.; Bongiovanni, V.; Cordero, C.; Cavallero, A. Chemical fingerprinting strategies based on comprehensive two-dimensional gas chromatography combined with gas chromatography-olfactometry to capture the unique signature of Piemonte peppermint essential oil (Mentha x piperita var Italo-Mitcham). J. Chromatogr. A 2021, 1645, 462101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gong, D.; Lan, L.; Zheng, Z.; Pang, X.; Guo, P.; Sun, G. Comprehensive evaluation of Loblolly fruit by high performance liquid chromatography four wavelength fusion fingerprint combined with gas chromatography fingerprinting and antioxidant activity analysis. J. Chromatogr. A 2022, 1665, 462819. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, R.; Yong, L.; Gong, Y.; Ding, L.; Xin, Y.; Wang, H.; Xue, Y.; Qing, L. Determination of nine nucleosides in Rhizoma Paridis by quantitative analysis of multi-components via a single marker method. J. Sep. Sci. 2021, 44, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Cheng, J.; Li, C.; Li, S.; Tian, Y.; Wang, T.; Fu, Y. Quality evaluation of Acanthopanax senticosus via quantitative analysis of multiple components by single marker and multivariate data analysis. J. Pharm. Biomed. Anal. 2021, 201, 114090. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Sun, G.; Guo, Y. Quality evaluation of powdered poppy capsule extractive by systematic quantified fingerprint method combined with quantitative analysis of multi-components by single marker method. J. Pharm. Biomed. Anal. 2020, 185, 113247. [Google Scholar] [CrossRef]
- Wu, K.; Guo, C.; Yang, B.; Wu, X.; Wang, W. Antihepatotoxic benefits of Poria cocos polysaccharides on acetaminophen-lesioned livers in vivo and in vitro. J. Cell. Biochem. 2018, 120, 7482–7488. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zhang, G.; Peng, X. Poria cocos polysaccharides attenuate chronic nonbacterial prostatitis by targeting the gut microbiota: Comparative study of Poria cocos polysaccharides and finasteride in treating chronic prostatitis. Int. J. Biol. Macromol. 2021, 189, 346–355. [Google Scholar] [CrossRef]
- Qin, L.; Huang, D.; Huang, J.; Qin, F.; Huang, H. Poria cocos integrated analysis and finding reveal anti-liver cancer targets and mechanisms of pachyman (polysaccharides). Front. Pharmacol. 2021, 12, 742349. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2018, 23, 4–20. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, L.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Immunomodulatory Activity of Carboxymethyl Pachymaran on Immunosuppressed Mice Induced by Cyclophosphamide. Molecules 2021, 26, 5733. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, Z.; Xu, J.; Liang, X.; Zhao, Q. Poricoic acid A induces apoptosis and autophagy in ovarian cancer via modulating the mTOR/p70s6k signaling axis. Braz. J. Med Biol. Res. 2021, 54. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Chen, N.; Li, Y.-J.; Fan, Q.-L.; Yu, T.-F.; Wang, K.-X.; Dong, B.-T.; Fan, E.-Y.; Yuan, P.-L.; Hu, G.-W.; et al. Glucose fed-batch integrated dissolved oxygen control strategy enhanced polysaccharide, total triterpenoids and inotodiol production in fermentation of a newly isolated Inonotus obliquus strain. Process Biochem. 2018, 66, 1–6. [Google Scholar] [CrossRef]
- Hua, H.; Yi, Y.; Li, H.; Chen, C. Monitoring the extraction process of acidic polysaccharides in Poria cocos by near infrared spectroscopy combined with chemometrics. Infrared Phys. Technol. 2020, 113, 103613. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Wang, H.; Wan, X. Protective Effect of Poria cocos Polysaccharides on Fecal Peritonitis-Induced Sepsis in Mice Through Inhibition of Oxidative Stress, Inflammation, Apoptosis, and Reduction of Treg Cells. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Gui, Y.; Sun, L.; Liu, R.; Luo, J. Pachymic acid inhibits inflammation and cell apoptosis in lipopolysaccharide (LPS)-induced rat model with pneumonia by regulating NF-κB and MAPK pathways. Allergol. Et Immunopathol. 2021, 49, 87–93. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Lin, C.-H.; Shih, C.-C. Antidiabetic and Antihyperlipidemic Properties of a Triterpenoid Compound, Dehydroeburicoic Acid, from Antrodia camphorata In Vitro and in Streptozotocin-Induced Mice. J. Agric. Food Chem. 2015, 63, 10140–10151. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Chu, Z.; Ni, T.; Ou, S.; Dai, X.; Zhang, X.; Liu, Y. Poria Acid, Triterpenoids Extracted from Poria cocos, Inhibits the Invasion and Metastasis of Gastric Cancer Cells. Molecules 2022, 27, 3629. [Google Scholar] [CrossRef]
- Wu, K.; Fan, J.; Huang, X.; Wu, X.; Guo, C. Hepatoprotective effects exerted by Poria cocos polysaccharides against acetaminophen-induced liver injury in mice. Int. J. Biol. Macromol. 2018, 114, 137–142. [Google Scholar] [CrossRef]
- Yi, Z.H.; Zheng, Y.M.; Xu, X.Y.; Fu, S.Q. Determination of total triterpenes from Poria cocos (schw.) wolf by spectrophotometry. Lishizhen Med. Mater. Med. Res. 2005, 847–848. [Google Scholar] [CrossRef]
- Li, J.; Han, X.H.; Li, Z.H. Isolation and determination of pachyman in Poria cocos (Schw.) Wolf. Chin. J. Mod. Appl. Pharm. 2000, 17, 49–50. [Google Scholar] [CrossRef]
- Deng, Y.Y. Study on extraction and pharmacological effects of alkali soluble polysaccharide in Poria cocos. Hubei Univ. Chin. Med. 2012. [Google Scholar] [CrossRef]



| NO. | Content (mg/mg) % | ||
|---|---|---|---|
| TS | WSP | AP | |
| S1 | 1.53% | 1.23% | 69.86% |
| S2 | 2.73% | 0.98% | 80.65% |
| S3 | 3.31% | 2.60% | 72.40% |
| S4 | 2.15% | 1.15% | 75.44% |
| S5 | 4.22% | 1.59% | 70.24% |
| S6 | 4.87% | 1.08% | 63.51% |
| S7 | 3.30% | 1.11% | 84.46% |
| S8 | 2.26% | 0.68% | 63.63% |
| S9 | 2.78% | 1.68% | 68.46% |
| S10 | 3.20% | 1.17% | 68.71% |
| S11 | 1.47% | 2.21% | 88.78% |
| S12 | 3.41% | 1.19% | 61.48% |
| S13 | 2.59% | 1.03% | 58.43% |
| S14 | 1.50% | 0.66% | 50.81% |
| S15 | 5.64% | 1.10% | 72.65% |
| S16 | 5.87% | 0.48% | 58.43% |
| Analytes | Regression Equation | R2 | Linear Range (µg/mL) | LODs (µg/mL) | LOQs (µg/mL) | Precision (RSD%, n = 6) | Repeatability (RSD%, n = 6) | Stability (RSD%, n = 6) | Recovery (%, n = 6) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Average | RSD% | |||||||||
| PAB | y = 22976x − 10056 | 0.9986 | 0.61–19.46 | 0.44 | 1.46 | 2.47 | 3.18 | 2.25 | 101.78 | 4.08 |
| DTA | y = 25606x − 25520 | 0.9968 | 0.62–36.86 | 0.58 | 2.30 | 1.43 | 1.40 | 1.76 | 103.46 | 1.80 |
| PAC | y = 35737x − 18430 | 0.9994 | 0.60–38.40 | 0.32 | 2.24 | 3.87 | 3.58 | 3.62 | 105.52 | 3.82 |
| EA (Internal reference) | y = 18451x − 15077 | 0.9973 | 1.10–36.35 | 1.10 | 2.21 | 1.00 | 1.00 | 1.00 | 100.98 | 4.30 |
| DPA | y = 28364x + 16934 | 0.9993 | 0.68–34.00 | 0.34 | 0.68 | 3.31 | 3.79 | 3.39 | 98.23 | 4.50 |
| DEA | y = 29463x − 384.58 | 0.9996 | 0.40–10.75 | 0.30 | 0.74 | 2.38 | 1.97 | 3.93 | 101.73 | 2.67 |
| Sample | EA | PAB | DTA | PAC | DPA | DEA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESM | ESM | QAMS | r E/Q | ESM | QAMS | r E/Q | ESM | QAMS | r E/Q | ESM | QAMS | r E/Q | ESM | QAMS | r E/Q | |
| S1 | 0.233 | 0.055 | 0.052 | 1.051 | 0.308 | 0.331 | 0.931 | 0.197 | 0.201 | 0.980 | 0.268 | 0.241 | 1.110 | 0.005 | 0.004 | 1.041 |
| S2 | 0.170 | 0.055 | 0.052 | 1.053 | 0.230 | 0.241 | 0.954 | 0.160 | 0.161 | 0.993 | 0.231 | 0.209 | 1.102 | 0.005 | 0.005 | 1.038 |
| S3 | 0.198 | - | - | - | 0.222 | 0.232 | 0.957 | 0.150 | 0.150 | 0.998 | 0.217 | 0.198 | 1.099 | - | - | - |
| S4 | 0.168 | 0.044 | 0.040 | 1.104 | 0.315 | 0.339 | 0.930 | 0.144 | 0.143 | 1.001 | 0.214 | 0.195 | 1.098 | 0.007 | 0.006 | 1.022 |
| S5 | 0.191 | 0.083 | 0.083 | 0.989 | 0.265 | 0.281 | 0.942 | 0.198 | 0.202 | 0.980 | 0.248 | 0.224 | 1.106 | 0.014 | 0.014 | 0.999 |
| S6 | 0.170 | 0.061 | 0.060 | 1.031 | 0.241 | 0.254 | 0.950 | 0.172 | 0.174 | 0.989 | 0.215 | 0.196 | 1.098 | 0.021 | 0.021 | 0.994 |
| S7 | 0.161 | 0.047 | 0.043 | 1.087 | 0.247 | 0.261 | 0.947 | 0.153 | 0.153 | 0.997 | 0.219 | 0.199 | 1.099 | 0.003 | 0.003 | 1.062 |
| S8 | 0.155 | 0.048 | 0.044 | 1.084 | 0.193 | 0.198 | 0.972 | 0.134 | 0.134 | 1.006 | 0.199 | 0.182 | 1.093 | 0.008 | 0.008 | 1.014 |
| S9 | 0.208 | 0.051 | 0.048 | 1.067 | 0.273 | 0.290 | 0.940 | 0.204 | 0.209 | 0.979 | 0.266 | 0.239 | 1.109 | 0.010 | 0.010 | 1.007 |
| S10 | 0.276 | 0.068 | 0.067 | 1.014 | 0.381 | 0.415 | 0.919 | 0.272 | 0.281 | 0.966 | 0.347 | 0.310 | 1.121 | 0.025 | 0.025 | 0.992 |
| S11 | 0.164 | 0.046 | 0.042 | 1.095 | 0.250 | 0.264 | 0.946 | 0.157 | 0.158 | 0.994 | 0.203 | 0.186 | 1.095 | 0.008 | 0.008 | 1.013 |
| S12 | 0.189 | 0.046 | 0.043 | 1.090 | 0.257 | 0.272 | 0.944 | 0.157 | 0.158 | 0.994 | 0.199 | 0.182 | 1.094 | 0.010 | 0.010 | 1.007 |
| S13 | 0.170 | 0.049 | 0.046 | 1.075 | 0.262 | 0.277 | 0.943 | 0.169 | 0.171 | 0.989 | 0.231 | 0.209 | 1.102 | 0.014 | 0.014 | 1.000 |
| S14 | 0.184 | 0.041 | 0.037 | 1.124 | 0.251 | 0.266 | 0.946 | 0.144 | 0.144 | 1.001 | 0.211 | 0.192 | 1.097 | - | - | - |
| S15 | 0.222 | 0.135 | 0.143 | 0.946 | 0.356 | 0.385 | 0.923 | 0.251 | 0.259 | 0.969 | 0.250 | 0.226 | 1.106 | 0.074 | 0.075 | 0.985 |
| S16 | 0.255 | 0.305 | 0.335 | 0.911 | 0.189 | 0.194 | 0.974 | 0.455 | 0.479 | 0.951 | 0.349 | 0.311 | 1.121 | 0.017 | 0.017 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Huang, X.-L.; Jiang, Z.-M.; Li, X.-F.; Qi, Y.; Yu, J.; Yang, X.-X.; Zhang, M. Quantification of Chemical Groups and Quantitative HPLC Fingerprint of Poria cocos (Schw.) Wolf. Molecules 2022, 27, 6383. https://doi.org/10.3390/molecules27196383
Yang Y, Huang X-L, Jiang Z-M, Li X-F, Qi Y, Yu J, Yang X-X, Zhang M. Quantification of Chemical Groups and Quantitative HPLC Fingerprint of Poria cocos (Schw.) Wolf. Molecules. 2022; 27(19):6383. https://doi.org/10.3390/molecules27196383
Chicago/Turabian StyleYang, Yu, Xing-Lin Huang, Zhong-Min Jiang, Xue-Fang Li, Yan Qi, Jie Yu, Xing-Xin Yang, and Mei Zhang. 2022. "Quantification of Chemical Groups and Quantitative HPLC Fingerprint of Poria cocos (Schw.) Wolf" Molecules 27, no. 19: 6383. https://doi.org/10.3390/molecules27196383
APA StyleYang, Y., Huang, X.-L., Jiang, Z.-M., Li, X.-F., Qi, Y., Yu, J., Yang, X.-X., & Zhang, M. (2022). Quantification of Chemical Groups and Quantitative HPLC Fingerprint of Poria cocos (Schw.) Wolf. Molecules, 27(19), 6383. https://doi.org/10.3390/molecules27196383




