Extracts Prepared from Feed Supplements Containing Wood Lignans Improve Intestinal Health by Strengthening Barrier Integrity and Reducing Inflammation
Abstract
:1. Introduction
2. Results
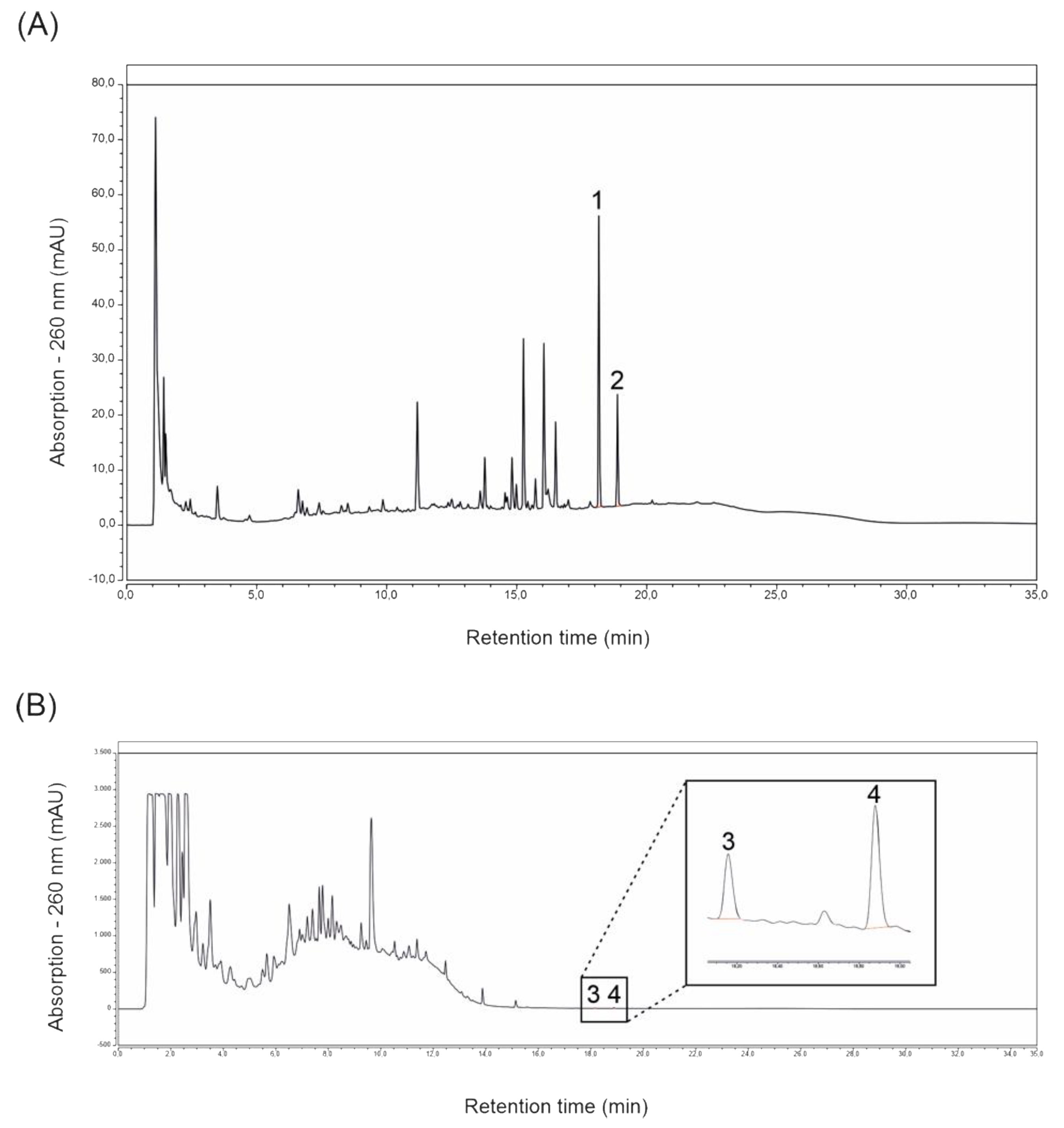
2.1. Magnolol and Honokiol Concentrations of Extracts Prepared from ROI and Protect
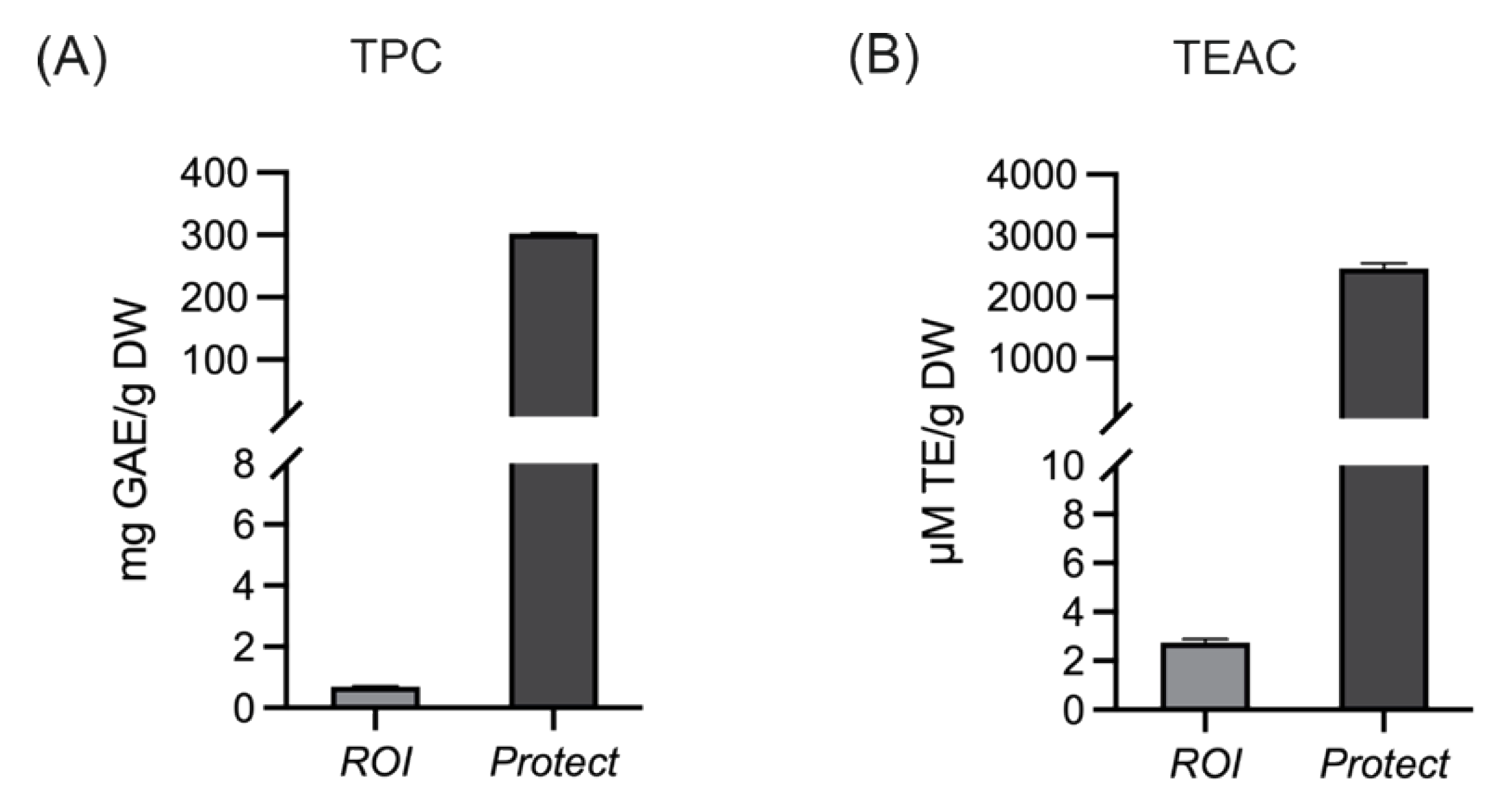
2.2. Total Phenolic Content and the Antioxidant Capacity of Extracts Prepared from ROI and Protect
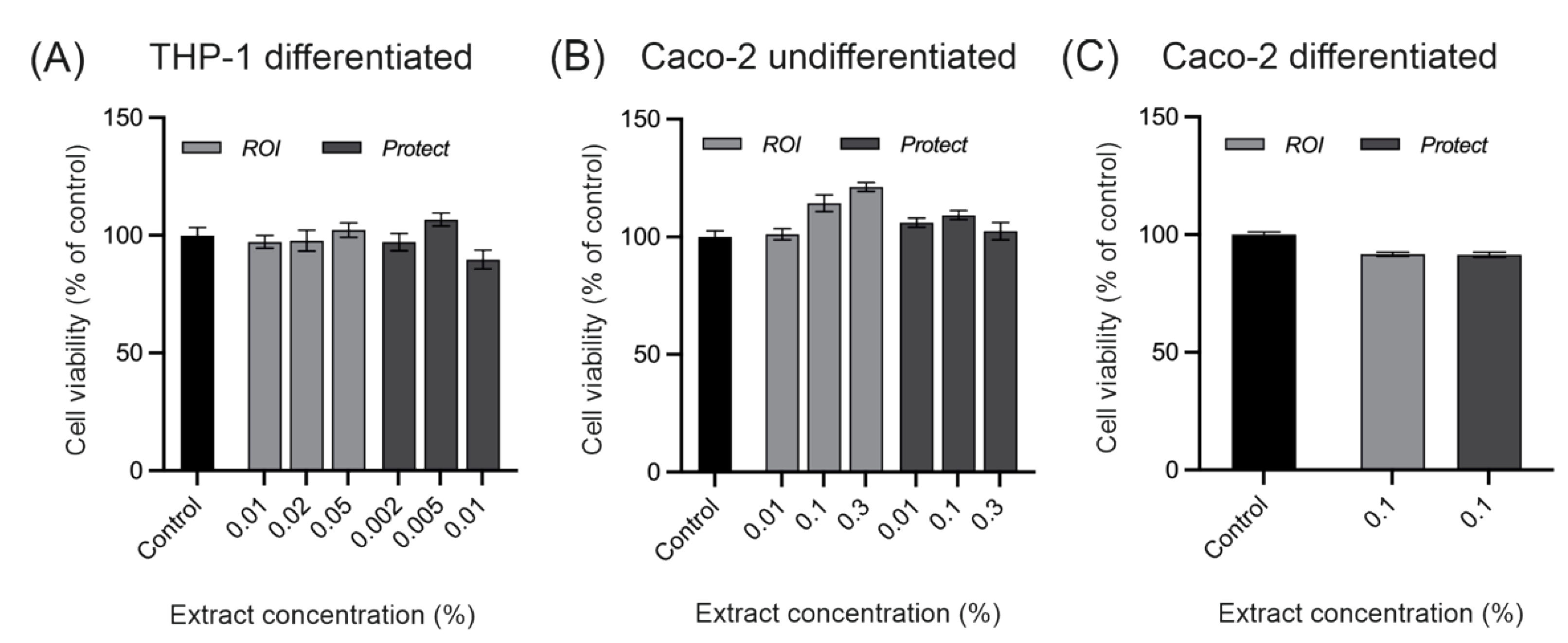
2.3. Cytotoxicity of extracts prepared from ROI and Protect
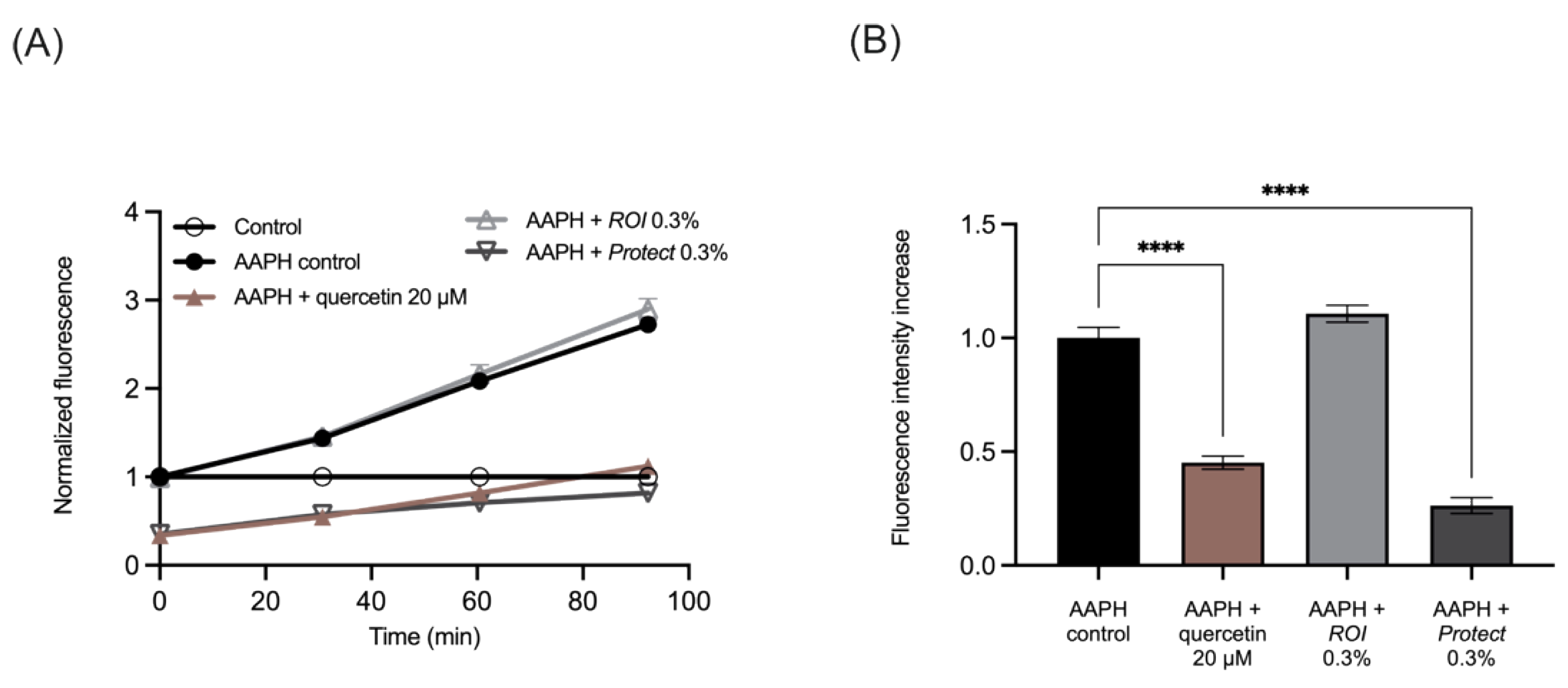
2.4. Protect Extract Reduces Intracellular ROS Production
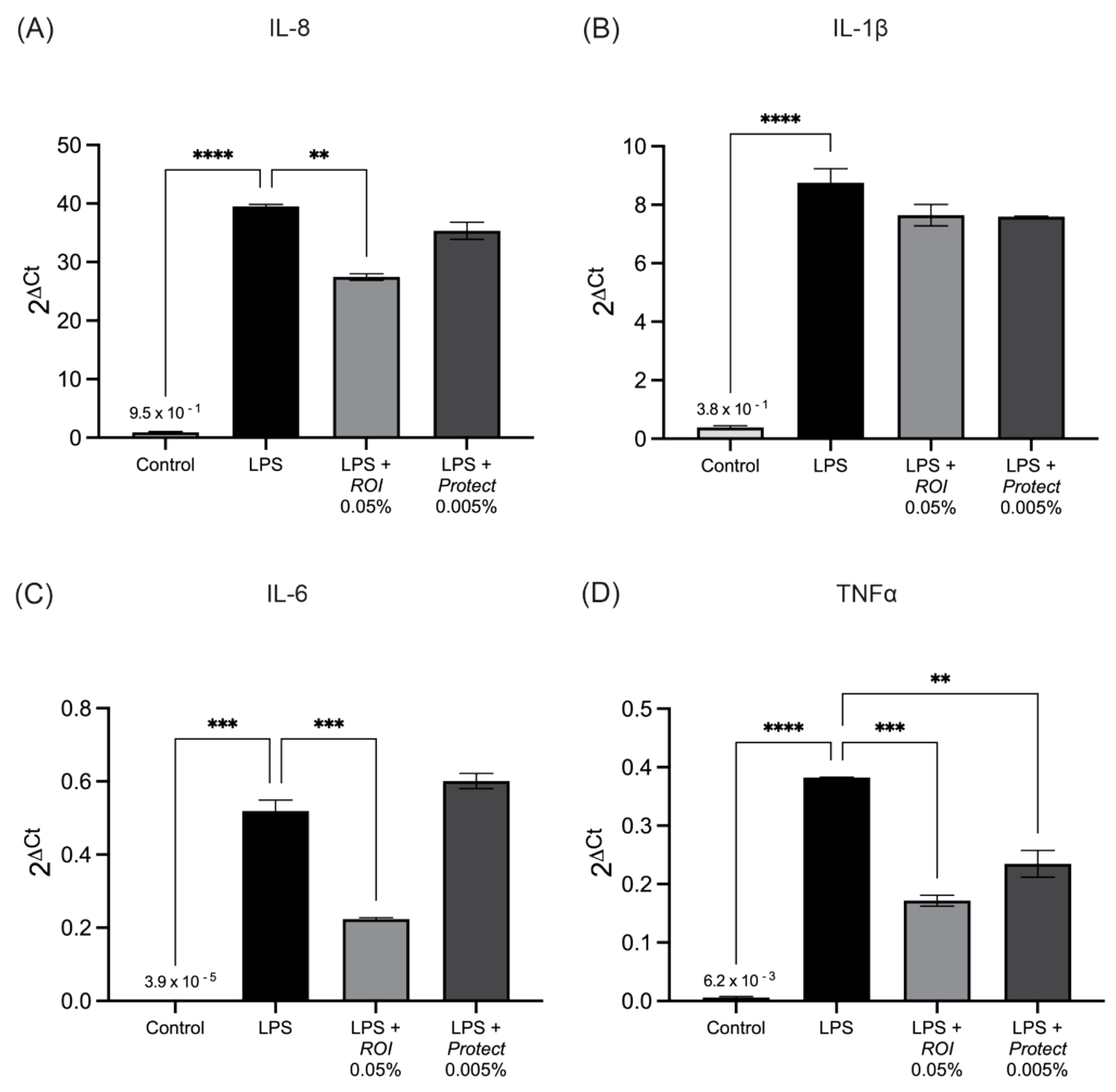
2.5. ROI and Protect Extracts Decrease the Expression Levels and Secretion of Pro-Inflammatory Cytokines
2.6. Protect and ROI Extracts Stabilize Caco-2 Epithelial Monolayers
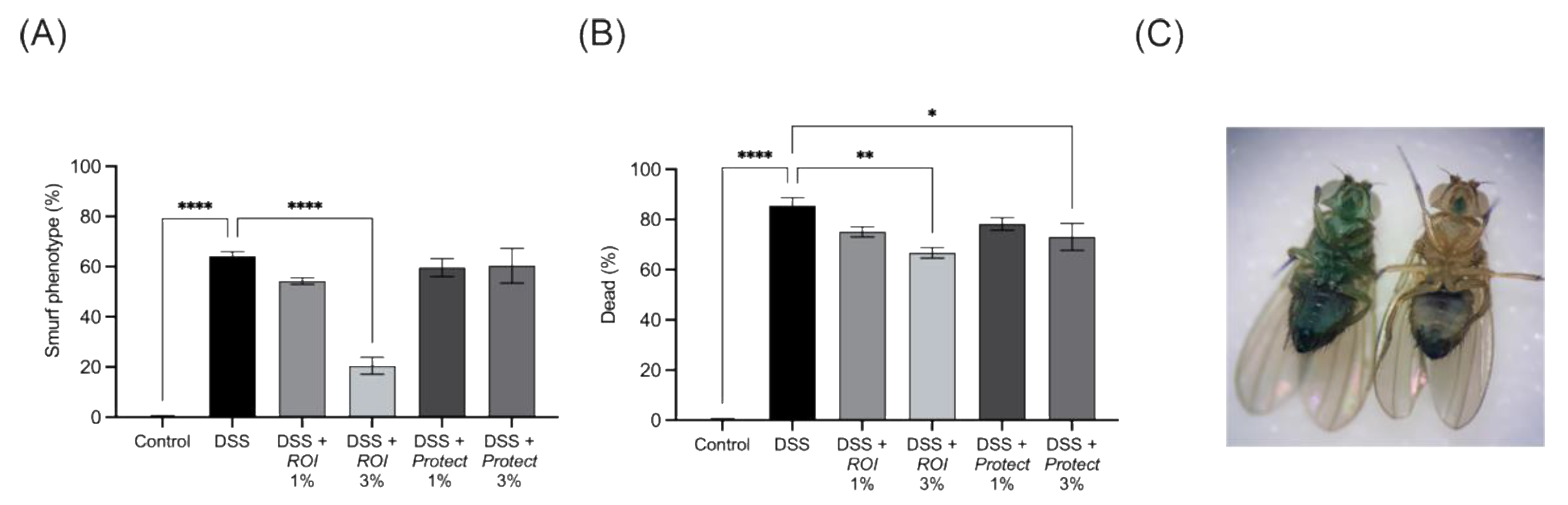
2.7. Protect and ROI Extracts Improve Intestinal Integrity and Reduce Mortality Rates of D. melanogaster
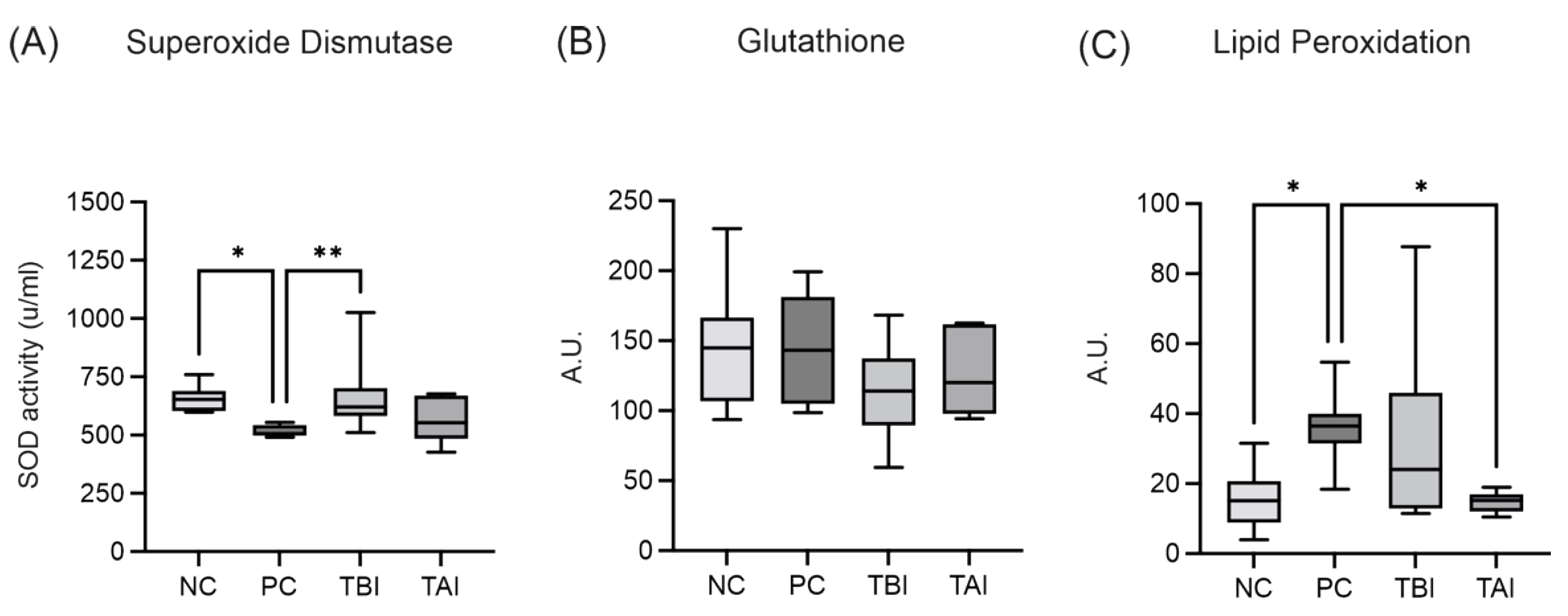
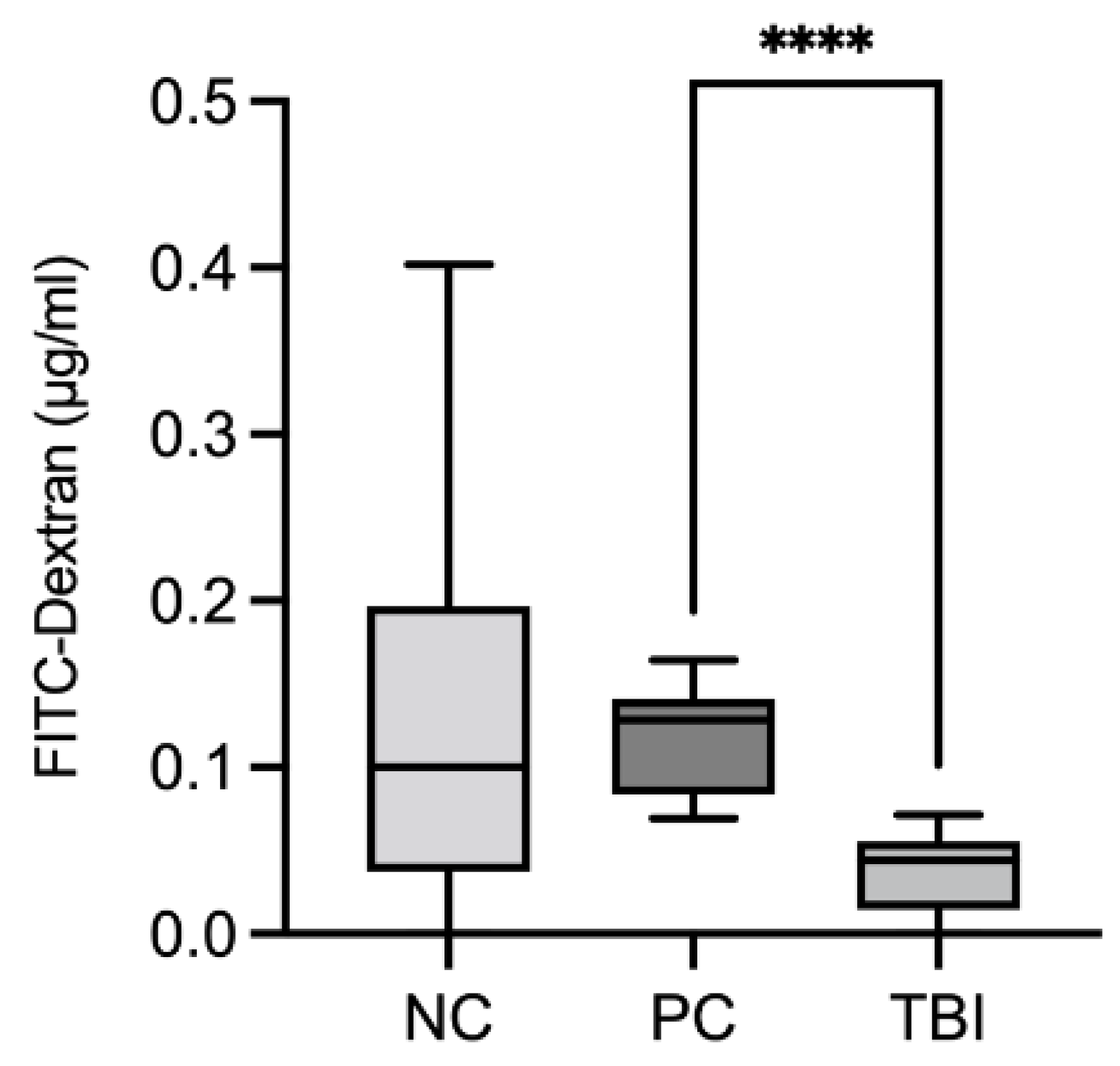
2.8. Impact of the Feed Supplement Protect on Piglets under Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Extract Preparation
4.2. Determination of the Main Lignans Contained in ROI and Protect Extracts via HPLC
4.3. Determination of TPC and TEAC
4.4. Cell Culture
4.5. Determination of Cell Viability in THP-1 and Caco-2 Cells
4.6. Determination of Antioxidant Capacity in Caco-2 Cells
4.7. Determination of Inflammatory Gene Expression and Cytokine Concentration in THP-1 Cells
4.8. Determination of Intestinal Barrier Integrity in Caco-2 Cells
4.9. Determination of Intestinal Barrier Function in D. melanogaster
4.9.1. w1118 D. Melanogaster Rearing Conditions and Synchronization
4.9.2. Intestinal Barrier Challenge
4.10. Piglet Feeding Study Experimental Design
Oxidative Injury and Intestinal Permeability Assessment
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad Khan, M.S.; Ahmad, I. Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. [Google Scholar]
- Seca, A.M.L.; Pinto, D.C.G.A. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S.; et al. Ginseng Extract Ameliorates the Negative Physiological Effects of Heat Stress by Supporting Heat Shock Response and Improving Intestinal Barrier Integrity: Evidence from Studies with Heat-Stressed Caco-2 Cells, C. Elegans and Growing Broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential Oil and Aromatic Plants as Feed Additives in Non-Ruminant Nutrition: A Review. J. Anim Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chowdhury, M.A.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of Phytogenic Products as Feed Additives for Swine and Poultry1. J. Anim Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary Lignans: Physiology and Potential for Cardiovascular Disease Risk Reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A Potential Source of Food, Feed and Fiber. Crit Rev. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Di, Y.; Jones, J.; Mansell, K.; Whiting, S.; Fowler, S.; Thorpe, L.; Billinsky, J.; Viveky, N.; Cheng, P.C.; Almousa, A.; et al. Influence of Flaxseed Lignan Supplementation to Older Adults on Biochemical and Functional Outcome Measures of Inflammation. J. Am. Coll Nutr. 2017, 36, 646–653. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An Update on Bioactive Plant Lignans. Nat. Prod. Rep. 2005, 22, 696. [Google Scholar] [CrossRef]
- Bylund, A.; Saarinen, N.; Zhang, J.; Bergh, A.; Widmark, A.; Johansson, A.; Lundin, E.; Adlercreutz, H.; Hallmans, G.; Stattin, P.; et al. Anticancer Effects of a Plant Lignan 7-Hydroxymatairesinol on a Prostate Cancer Model In Vivo. Exp. Biol. Med. 2005, 230, 217–223. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Shouman, S.A.; Elkhoely, A.; Attia, Y.M.; Elsesy, M.S.; el Senousy, A.S.; Choucry, M.A.; el Gayed, S.H.; el Sayed, A.A.; Sattar, E.A.; et al. Anticancer Potentiality of Lignan Rich Fraction of Six Flaxseed Cultivars. Sci. Rep. 2018, 8, 544. [Google Scholar] [CrossRef]
- Zhao, M.; Zheng, Y.-H.; Zhao, Q.-Y.; Zheng, W.; Yang, J.-H.; Pei, H.-Y.; Liu, L.; Liu, K.-J.; Xue, L.-L.; Deng, D.-X.; et al. Synthesis and Evaluation of New Compounds Bearing 3-(4-Aminopiperidin-1-Yl)Methyl Magnolol Scaffold as Anticancer Agents for the Treatment of Non-Small Cell Lung Cancer via Targeting Autophagy. Eur. J. Med. Chem. 2021, 209, 112922. [Google Scholar] [CrossRef]
- Kangas, L.; Saarinen, N.; Mutanen, M.; Ahotupa, M.; Hirsinummi, R.; Unkila, M.; Perälä, M.; Soininen, P.; Laatikainen, R.; Korte, H.; et al. Antioxidant and Antitumor Effects of Hydroxymatairesinol (HM-3000, HMR), a Lignan Isolated from the Knots of Spruce. Eur. J. Cancer Prev. 2002, 11, 48–57. [Google Scholar]
- Saarinen, N.M.; Warri, A.; Makela, S.I.; Eckerman, C.; Reunanen, M.; Ahotupa, M.; Salmi, S.M.; Franke, A.A.; Kangas, L.; Santti, R. Hydroxymatairesinol, a Novel Enterolactone Precursor with Antitumor Properties from Coniferous Tree (Picea Abies). Nutr. Cancer 2000, 36, 207–216. [Google Scholar] [CrossRef]
- Cho, M.K.; Jang, Y.P.; Kim, Y.C.; Kim, S.G. Arctigenin, a Phenylpropanoid Dibenzylbutyrolactone Lignan, Inhibits MAP Kinases and AP-1 Activation via Potent MKK Inhibition: The Role in TNF-α Inhibition. Int. Immunopharmacol. 2004, 4, 1419–1429. [Google Scholar] [CrossRef]
- Küpeli, E.; Erdemoğlu, N.; Yeşilada, E.; Şener, B. Anti-Inflammatory and Antinociceptive Activity of Taxoids and Lignans from the Heartwood of Taxus Baccata L. J. Ethnopharmacol. 2003, 89, 265–270. [Google Scholar] [CrossRef]
- Dong, D.-D.; Li, H.; Jiang, K.; Qu, S.-J.; Tang, W.; Tan, C.-H.; Li, Y.-M. Diverse Lignans with Anti-Inflammatory Activity from Urceola Rosea. Fitoterapia 2019, 134, 96–100. [Google Scholar] [CrossRef]
- Kim, T.-W.; Shin, J.-S.; Chung, K.-S.; Lee, Y.-G.; Baek, N.-I.; Lee, K.-T. Anti-Inflammatory Mechanisms of Koreanaside A, a Lignan Isolated from the Flower of Forsythia Koreana, against LPS-Induced Macrophage Activation and DSS-Induced Colitis Mice: The Crucial Role of AP-1, NF-ΚB, and JAK/STAT Signaling. Cells 2019, 8, 1163. [Google Scholar] [CrossRef]
- Polat Kose, L.; Gulcin, İ. Evaluation of the Antioxid.dant and Antiradical Properties of Some Phyto and Mammalian Lignans. Molecules 2021, 26, 7099. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bao, Y.; Zhang, W.; Ying, X.; Stien, D. Four Lignans from Portulaca Oleracea L. and Its Antioxidant Activities. Nat. Prod. Res. 2020, 34, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xue, Y.; Chen, S.; Zhu, H.; Zhang, J.; Li, X.-N.; Wang, J.; Liu, J.; Qi, C.; Du, G.; et al. Antioxidant Lignans and Neolignans from Acorus Tatarinowii. Sci. Rep. 2016, 6, 22909. [Google Scholar] [CrossRef] [PubMed]
- He, W.-J.; Chu, H.-B.; Zhang, Y.-M.; Han, H.-J.; Yan, H.; Zeng, G.-Z.; Fu, Z.-H.; Olubanke, O.; Tan, N.-H. Antimicrobial, Cytotoxic Lignans and Terpenoids from the Twigs of Pseudolarix Kaempferi. Planta Med. 2011, 77, 1924–1931. [Google Scholar] [CrossRef]
- Erdemoglu, N.; Sener, B.; Choudhary, M.I. Bioactivity of Lignans from Taxus Baccata. Z. Für Nat. C 2004, 59, 494–498. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. Natural Lignans Honokiol and Magnolol as Potential Anticarcinogenic and Anticancer Agents. A Comprehensive Mechanistic Review. Nutr. Cancer 2022, 74, 761–778. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Du, E.; Jin, F.; Zheng, C.; Fan, Q.; Zhao, N.; Guo, W.; Zhang, W.; Huang, S.; et al. Effects of Magnolol on Egg Production, Egg Quality, Antioxidant Capacity, and Intestinal Health of Laying Hens in the Late Phase of the Laying Cycle. Poult Sci. 2021, 100, 835–843. [Google Scholar] [CrossRef]
- Deng, Y.; Han, X.; Tang, S.; Li, C.; Xiao, W.; Tan, Z. Magnolol and Honokiol Attenuate Apoptosis of Enterotoxigenic Escherichia Coli-Induced Intestinal Epithelium by Maintaining Secretion and Absorption Homeostasis and Protecting Mucosal Integrity. Med. Sci. Monit. 2018, 24, 3348–3356. [Google Scholar] [CrossRef]
- Han, X.; Pang, Y.; Liu, S.; Tan, Z.; Tang, S.; Zhou, C.; Wang, M.; Xiao, W. Antidiarrhea and Antioxidant Activities of Honokiol Extract from Magnoliae Officinalis Cortex in Mice. Trop. J. Pharm. Res. 2014, 13, 1643. [Google Scholar] [CrossRef]
- Taira, J.; Ikemoto, T.; Mimura, K.; Hagi, A.; Murakami, A.; Makino, K. Effective Inhibition of Hydroxyl Radicals by Hydroxylated Biphenyl Compounds. Free Radic Res. Commun. 1993, 19, s71–s77. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, J.; Han, L.; Jin, Z.; Wang, J.; Li, X.; Man, S.; Liu, C.; Gao, W. Protective Effect of Magnolol on Oxaliplatin-induced Intestinal Injury in Mice. Phytother. Res. 2019, 33, 1161–1172. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and Human Health: A Review. Crit Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin Analysis of Chestnut Bark Samples (Castanea Sativa Mill.) by HPLC-DAD–MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Reggi, S.; Giromini, C.; Dell’Anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In Vitro Digestion of Chestnut and Quebracho Tannin Extracts: Antimicrobial Effect, Antioxidant Capacity and Cytomodulatory Activity in Swine Intestinal IPEC-J2 Cells. Animals 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Eddouks, M. The Promising Role of Plant Tannins as Bioactive Antidiabetic Agents. Curr Med. Chem 2019, 26, 4852–4884. [Google Scholar] [CrossRef] [PubMed]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and Quebracho Tannins in Pig Nutrition: The Effects on Performance and Intestinal Health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef]
- Girard, M.; Bee, G. Invited Review: Tannins as a Potential Alternative to Antibiotics to Prevent Coliform Diarrhea in Weaned Pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, K.; Zhao, J.S.; Deng, W. Effects of Chestnut Tannins on Intestinal Morphology, Barrier Function, pro-Inflammatory Cytokine Expression, Microflora and Antioxidant Capacity in Heat-Stressed Broilers. J. Anim Physiol. Anim Nutr. 2018, 102, 717–726. [Google Scholar] [CrossRef]
- Amcheslavsky, A.; Jiang, J.; Ip, Y.T. Tissue Damage-Induced Intestinal Stem Cell Division in Drosophila. Cell Stem Cell 2009, 4, 49–61. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Kruk, I.; Kładna, A.; Lichszteld, K.; Michalska, T. Scavenging Effects of Phenolic Compounds on Reactive Oxygen Species. Biopolymers 2007, 86, 222–230. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional Profiling of the LPS Induced NF-KappaB Response in Macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef]
- Auwerx, J. The Human Leukemia Cell Line, THP-1: A Multifacetted Model for the Study of Monocyte-Macrophage Differentiation. Experientia 1991, 47, 22–31. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Lee, S.; Hong, S.; Park, J.; Park, E.; Kim, J.; Park, S.; et al. Anti-Inflammatory Effects of Magnolol and Honokiol Are Mediated through Inhibition of the Downstream Pathway of MEKK-1 in NF-ΚB Activation Signaling. Planta Med. 2005, 71, 338–343. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J.; Zhan, S.; Lu, X. Honokiol Inhibits Endoplasmic Reticulum Stress-associated Lipopolysaccharide-Induced Inflammation and Apoptosis in Bovine Endometrial Epithelial Cells. Exp. Ther. Med. 2021, 22, 1476. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Y.; Zhang, M.; Gao, L.; Fang, C.; Zhou, C. Magnolol Inhibits LPS-Induced Inflammatory Response in Uterine Epithelial Cells. Inflammation 2013, 36, 997–1003. [Google Scholar] [CrossRef]
- Mao, S.-H.; Feng, D.-D.; Wang, X.; Zhi, Y.-H.; Lei, S.; Xing, X.; Jiang, R.-L.; Wu, J.-N. Magnolol Protects against Acute Gastrointestinal Injury in Sepsis by Down-Regulating Regulated on Activation, Normal T-Cell Expressed and Secreted. World J. Clin. Cases 2021, 9, 10451–10463. [Google Scholar] [CrossRef]
- Song, D.; Zhao, J.; Deng, W.; Liao, Y.; Hong, X.; Hou, J. Tannic Acid Inhibits NLRP3 Inflammasome-Mediated IL-1β Production via Blocking NF-ΚB Signaling in Macrophages. Biochem. Biophys. Res. Commun. 2018, 503, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, S.; Xia, H.; Lou, H.; Zhu, F.; Sun, L. Anti-Inflammatory Activities and Potential Mechanisms of Phenolic Acids Isolated from Salvia Miltiorrhiza f. Alba Roots in THP-1 Macrophages. J. Ethnopharmacol. 2018, 222, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Tannic Acid Modulates NFκB Signaling Pathway and Skin Inflammation in NC/Nga Mice through PPARγ Expression. Cytokine 2015, 76, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric Acid Supplementation Prevents Systemic Inflammation-induced Memory Impairment and Amyloidogenesis via Inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Capo, F.; Wilson, A.; di Cara, F. The Intestine of Drosophila Melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans. Microorganisms 2019, 7, 336. [Google Scholar] [CrossRef]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T.; Jones, D.L.; Walker, D.W. Modulation of Longevity and Tissue Homeostasis by the Drosophila PGC-1 Homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal Barrier Dysfunction Links Metabolic and Inflammatory Markers of Aging to Death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Liu, S.; Zhuang, Y.; Yang, H.; Li, Y.; Chen, S.; Wang, L.; Yin, L.; Yao, Y.; et al. Tannic Acid Modulates Intestinal Barrier Functions Associated with Intestinal Morphology, Antioxidative Activity, and Intestinal Tight Junction in a Diquat-Induced Mouse Model. RSC Adv. 2019, 9, 31988–31998. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Zhao, N.; Du, E.; Jin, F.; Fan, Q.; Guo, W.; Huang, S.; Wei, J. Effects of Magnolol and Honokiol Blend on Performance, Egg Quality, Hepatic Lipid Metabolism, and Intestinal Morphology of Hens at Late Laying Cycle. Animal 2022, 16, 100532. [Google Scholar] [CrossRef]
- Du, E.; Fan, Q.; Zhao, N.; Zhang, W.; Wei, J.; Chen, F.; Huang, S.; Guo, W. Supplemental Magnolol Improves the Antioxidant Capacity and Intestinal Health of Broiler Chickens. Anim. Sci. J. 2021, 92, e13665. [Google Scholar] [CrossRef]
- Xu, T.; Ma, X.; Zhou, X.; Qian, M.; Yang, Z.; Cao, P.; Han, X. Coated Tannin Supplementation Improves Growth Performance, Nutrients Digestibility, and Intestinal Function in Weaned Piglets. J. Anim Sci. 2022, 100, skac088. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Yan, H.; et al. Tannic Acid Prevents Post-Weaning Diarrhea by Improving Intestinal Barrier Integrity and Function in Weaned Piglets. J. Anim Sci. Biotechnol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Jacobson, M.; Lindberg, R.; Jonasson, R.; Fellström, C.; Jensen Waern, M. Consecutive Pathological and Immunological Alterations during Experimentally Induced Swine Dysentery—A Study Performed by Repeated Endoscopy and Biopsy Samplings through an Intestinal Cannula. Res. Vet. Sci. 2007, 82, 287–298. [Google Scholar] [CrossRef]
- Burrough, E.R. Swine Dysentery. Vet. Pathol. 2017, 54, 22–31. [Google Scholar] [CrossRef]
- Panah, F.M.; Lauridsen, C.; Højberg, O.; Nielsen, T.S. Etiology of Colitis-Complex Diarrhea in Growing Pigs: A Review. Animals 2021, 11, 2151. [Google Scholar] [CrossRef]
- Helm, E.T.; Lin, S.J.; Gabler, N.K.; Burrough, E.R. Brachyspira Hyodysenteriae Infection Reduces Digestive Function but Not Intestinal Integrity in Growing Pigs While Disease Onset Can Be Mitigated by Reducing Insoluble Fiber. Front. Vet. Sci. 2020, 7, 587926. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Peng, S.; Liu, C.; Lv, T.; Liao, L.; Li, Y.; Wang, Y.; Fan, Z.; Wu, W.; et al. Magnolol Additive Improves Growth Performance of Linwu Ducklings by Modulating Antioxidative Status. PLoS ONE 2021, 16, e0259896. [Google Scholar] [CrossRef]
- Xie, Q.; Xie, K.; Yi, J.; Song, Z.; Zhang, H.; He, X. The Effects of Magnolol Supplementation on Growth Performance, Meat Quality, Oxidative Capacity, and Intestinal Microbiota in Broilers. Poult Sci. 2022, 101, 101722. [Google Scholar] [CrossRef]
- Basu, T.; Panja, S.; Shendge, A.K.; Das, A.; Mandal, N. A Natural Antioxidant, Tannic Acid Mitigates Iron-Overload Induced Hepatotoxicity in Swiss Albino Mice through ROS Regulation. Env. Toxicol. 2018, 33, 603–618. [Google Scholar] [CrossRef]
- Günther, I.; Rimbach, G.; Nevermann, S.; Neuhauser, C.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Ipharraguerre, I.R.; Weghuber, J.; Lüersen, K. Avens Root (Geum Urbanum L.) Extract Discovered by Target-Based Screening Exhibits Antidiabetic Activity in the Hen’s Egg Test Model and Drosophila Melanogaster. Front. Pharm. 2021, 12, 3687. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 Monolayers in Experimental and Theoretical Predictions of Drug Transport. Adv. Drug Deliv Rev. 2012, 64, 280–289. [Google Scholar] [CrossRef]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 Cell Models in Food-Intestine Interaction Study: Current Applications and Future Trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 Cell-Based Quantitative Antioxidant Activity Assay for Antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Piegholdt, S.; Rimbach, G.; Wagner, A.E. The Phytoestrogen Prunetin Affects Body Composition and Improves Fitness and Lifespan in Male Drosophila Melanogaster. FASEB J. 2016, 30, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Leser, T.D.; Møller, K.; Jensen, T.K.; Jorsal, S.E. Specific Detection of Serpulina Hyodysenteriae and Potentially Pathogenic Weakly β-Haemolytic Porcine Intestinal Spirochetes by Polymerase Chain Reaction Targeting 23S RDNA. Mol. Cell Probes 1997, 11, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, R.A.; Harris, D.L.; Kinyon, J.M. Autoclaved Liquid Medium for Propagation of Treponema Hyodysenteriae. J. Clin. Microbiol. 1986, 24, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Y.; Woollard, A.C.S.; Wolff, S.P. Lipid Hydroperoxide Measurement by Oxidation of Fe2+ in the Presence of Xylenol Orange. Comparison with the TBA Assay and an Iodometric Method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of Pyrogallol Autoxidation and Determination of Superoxide Dismutase Enzyme Activity. Bioelectrochemistry Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Vicuña, E.A.; Kuttappan, V.A.; Tellez, G.; Hernandez-Velasco, X.; Seeber-Galarza, R.; Latorre, J.D.; Faulkner, O.B.; Wolfenden, A.D.; Hargis, B.M.; Bielke, L.R. Dose Titration of FITC-D for Optimal Measurement of Enteric Inflammation in Broiler Chicks. Poult Sci. 2015, 94, 1353–1359. [Google Scholar] [CrossRef]


| Genes | Forward Primer Sequence (5’–3’) | Reverse Primer Sequence (5’–3’) | Accession No. |
| IL-1β | CATGGGATAACGAGGCTTATG | ACAAAGGACATGGAGAACAC | NM_000576.3 |
| IL-6 | GACAGCCACTCACCTCTT | GGCAAGTCTCCTCATTGAATC | NM_000600.5 |
| IL-8 | CTGTGTGAAGGTGCAGTT | ACTTCTCCACAACCCTCT | NM_000584.4 |
| TNFα | AGCACTGAAAGCATGATCC | GCCAGAGGGCTGATTAGA | NM_000594.4 |
| GAPDH | TGGTATCGTGGAAGGACTCA | CAGTGAGCTTCCCGTTCAG | NM_002046.7 |
| RPL5 | TGGGCCAGAATGTTGCAGAT | AGGGACATTTTGGGACGGTT | NM_000969.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heckmann, M.; Sadova, N.; Drotarova, I.; Atzmüller, S.; Schwarzinger, B.; Guedes, R.M.C.; Correia, P.A.; Hirtenlehner, S.; Potthast, C.; Klanert, G.; et al. Extracts Prepared from Feed Supplements Containing Wood Lignans Improve Intestinal Health by Strengthening Barrier Integrity and Reducing Inflammation. Molecules 2022, 27, 6327. https://doi.org/10.3390/molecules27196327
Heckmann M, Sadova N, Drotarova I, Atzmüller S, Schwarzinger B, Guedes RMC, Correia PA, Hirtenlehner S, Potthast C, Klanert G, et al. Extracts Prepared from Feed Supplements Containing Wood Lignans Improve Intestinal Health by Strengthening Barrier Integrity and Reducing Inflammation. Molecules. 2022; 27(19):6327. https://doi.org/10.3390/molecules27196327
Chicago/Turabian StyleHeckmann, Mara, Nadiia Sadova, Ivana Drotarova, Stefanie Atzmüller, Bettina Schwarzinger, Roberto Mauricio Carvalho Guedes, Paula Angelica Correia, Stefan Hirtenlehner, Christine Potthast, Gerald Klanert, and et al. 2022. "Extracts Prepared from Feed Supplements Containing Wood Lignans Improve Intestinal Health by Strengthening Barrier Integrity and Reducing Inflammation" Molecules 27, no. 19: 6327. https://doi.org/10.3390/molecules27196327
APA StyleHeckmann, M., Sadova, N., Drotarova, I., Atzmüller, S., Schwarzinger, B., Guedes, R. M. C., Correia, P. A., Hirtenlehner, S., Potthast, C., Klanert, G., & Weghuber, J. (2022). Extracts Prepared from Feed Supplements Containing Wood Lignans Improve Intestinal Health by Strengthening Barrier Integrity and Reducing Inflammation. Molecules, 27(19), 6327. https://doi.org/10.3390/molecules27196327






