Essential Oils as a Source of Ecofriendly Insecticides for Drosophila suzukii (Diptera: Drosophilidae) and Their Potential Non-Target Effects
Abstract
1. Introduction
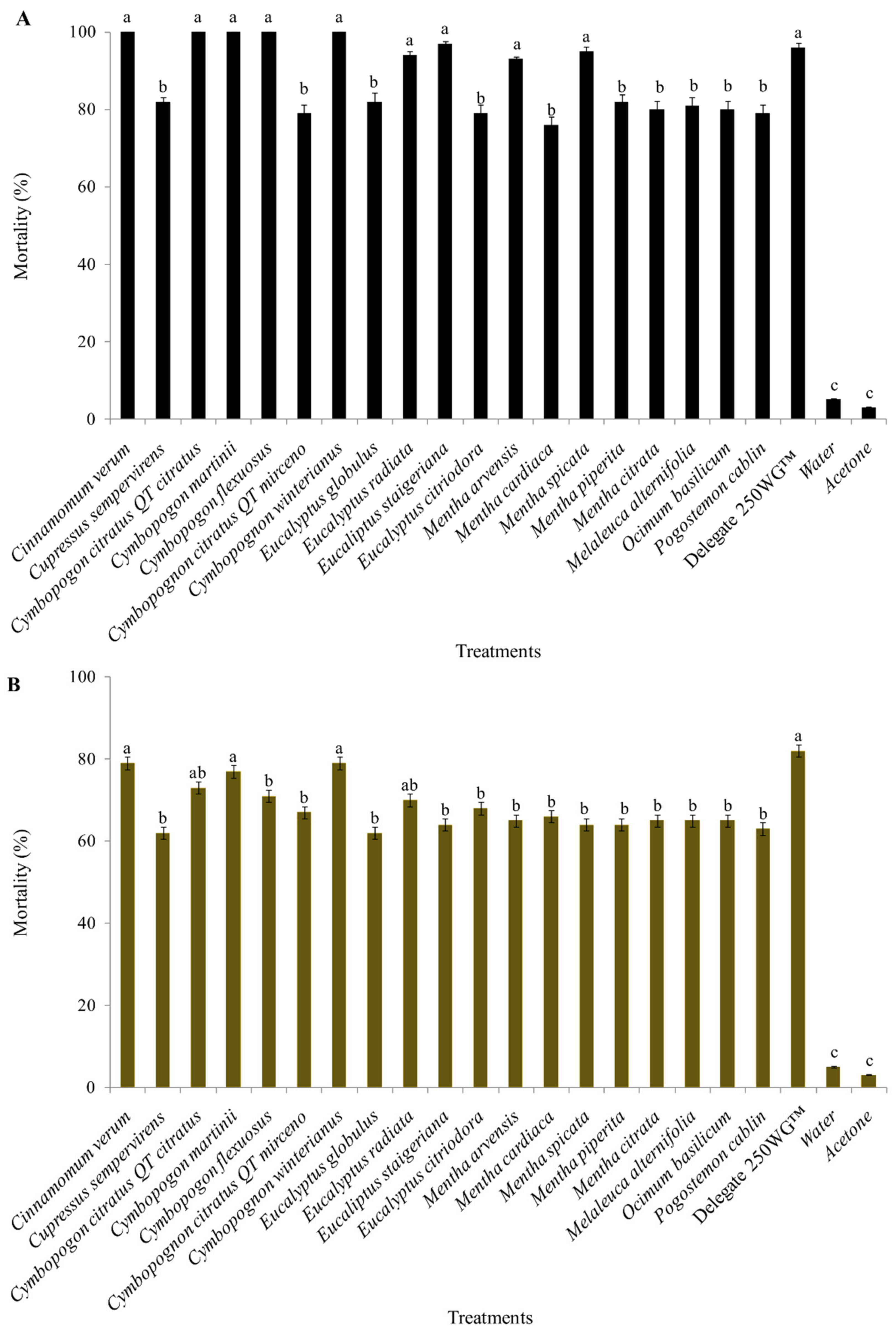
2. Results
3. Discussion
4. Materials and Methods
4.1. Insect Breeding
4.2. Essential Oils: Source and GC-MS Analysis
4.3. Bioassays
4.3.1. Essential Oil Toxicity on D. suzukii
4.3.2. Concentration–Response Curves
4.3.3. Oviposition Bioassay
4.3.4. Repellence Bioassay
4.3.5. Essential Oil Toxicity in T. anastrephae
4.4. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Little, C.M.; Chapman, T.W.; Hillier, N.K. Plasticity is key to success of Drosophila suzukii (Diptera: Drosophilidae) invasion. J. Insect Sci. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Lira-Noriega, A. Current and future global potential distribution of the fruit fly Drosophila suzukii (Diptera: Drosophilidae). Can. Entomol. 2020, 152, 587–599. [Google Scholar] [CrossRef]
- Wilson, J.K.; Gut, L.J.; Powers, K.; Huang, J.; Rothwell, N. Predicting the risk of tart cherry (Prunus cerasus) infestation by Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2020, 24, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Urbaneja-Bernat, P.; Waller, T.; Rodriguez-Saona, C. Repellent, oviposition-deterrent, and insecticidal activity of the fungal pathogen Colletotrichum fioriniae on Drosophila suzukii (Diptera: Drosophilidae) in highbush blueberries. Sci. Rep. 2020, 10, 14467. [Google Scholar] [CrossRef]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the chemical ecology of the spotted wing drosophila (Drosophila suzukii) and its applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef]
- Souza, M.T.; Souza, M.T.; Bernardi, D.; Melo, D.J.; Zarbin, P.H.G.; Zawadneak, M.A.C. Insecticidal and oviposition deterrent effects of essential oils of Baccharis spp. and histological assessment against Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2021, 11, 3944. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; Toni, D.C.; Valente, V.L.S. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Kenis, M.; Tonina, L.; Eschen, R.; Sulis, B.; Sancassani, M.; Mori, N.; Haye, T.; Helsen, H. Non-crop plants used as hosts by Drosophila suzukii in Europe. J. Pest Sci. 2016, 89, 735–748. [Google Scholar] [CrossRef]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef]
- DiGiacomo, G.; Hadrich, J.; Hutchison, W.D.; Peterson, H.; Rogers, M. Economic impact of Spotted Wing Drosophila (Diptera: Drosophilidae) yield loss on Minnesota raspberry farms: A grower survey. J. Integr. Pest Manag. 2019, 10, 11. [Google Scholar] [CrossRef]
- Benito, N.P.; Lopes-da-Silva, M.; Santos, R.S.S. Potential spread and economic impact of invasive Drosophila suzukii in Brazil. Pesq. Agropec. Bras. 2016, 51, 571–578. [Google Scholar] [CrossRef]
- Shaw, B.; Brain, P.; Wijnen, H.; Fountain, M.T. Implications of sub-lethal rates of insecticides and daily time of application on Drosophila suzukii lifecycle. Crop Prot. 2019, 121, 182–194. [Google Scholar] [CrossRef]
- Krutzler, M.; Brader, G.; Madercic, M.; Riedle-Bauer, M. Efficacy evaluation of alternative pest control products against Drosophila suzukii in Austrian elderberry orchards. J. Plant Dis. Prot. 2022, 1, 1–16. [Google Scholar] [CrossRef]
- Disi, J.O.; Sial, A.A. Laboratory selection and assessment of resistance risk in Drosophila suzukii (Diptera: Drosophilidae) to spinosad and malathion. Insects 2021, 4, 794. [Google Scholar] [CrossRef] [PubMed]
- Ganjisaffar, F.; Gress, B.E.; Demkovich, M.R.; Nicola, N.L.; Chiu, J.C.; Zalom, F.G. Spatio-temporal variation of spinosad susceptibility in Drosophila suzukii (Diptera: Drosophilidae), a three-year study in California’s Monterey Bay Region. J. Econ. Entomol. 2022, 115, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Schlesener, D.C.H.; Wollmann, J.; Pazini, J.B.; Padilha, A.C.; Grützmacher, A.D.; Garcia, F.R.M. Insecticide toxicity to Drosophila suzukii (Diptera: Drosophilidae) parasitoids: Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). J. Econ. Entomol. 2019, 22, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Gowton, C.M.; Reut, M.; Carrillo, J. Peppermint essential oil inhibits Drosophila suzukii emergence but reduces Pachycrepoideus vindemmiae parasitism rates. Sci. Rep. 2020, 10, 9090. [Google Scholar] [CrossRef]
- Morais, M.C.; Rakes, M.; Pasini, R.A.; Grützmacher, A.D.; Nava, D.E.; Bernardi, D. Toxicity and transgenerational effects of insecticides on Trichopria anastrephae (Hymenoptera: Diapriidae). Neotrop. Entomol. 2022, 51, 143–150. [Google Scholar] [CrossRef]
- VanWoerkom, A.H.; Whalon, M.E.; Gut, L.J.; Kunkel, D.L.; Wise, J.C. Impact of multiple applications of insecticides and post-harvest washing on residues at harvest and associated risk for cherry export. Int. J. Fruit Sci. 2022, 22, 346–357. [Google Scholar] [CrossRef]
- Van Timmeren, S.; Sial, A.A.; Lanka, S.K.; Spaulding, N.R.; Isaacs, R. Development of a rapid assessment method for detecting insecticide resistance in Spotted Wing Drosophila (Drosophila suzukii Matsumura). Pest Manage. Sci. 2019, 75, 1782–1793. [Google Scholar] [CrossRef]
- Gress, B.E.; Zalom, F.G. Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest Manage. Sci. 2019, 75, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.; Ribeiro, L.; Andreazza, F.; Neitzke, C.; Oliveira, E.E.; Botton, M.; Nava, D.E.; Vendramim, J.D. Potential use of Annona by products to control Drosophila suzukii and toxicity to its parasitoid Trichopria anastrephae. Ind. Crop Prod. 2017, 110, 30–35. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Gullickson, M.; Flavin, H.C.; Hegeman, A.; Rogers, M. Deterrent effects of essential oils on Spotted-Wing Drosophila (Drosophila suzukii): Implications for organic management in berry crops. Insects 2020, 11, e536. [Google Scholar] [CrossRef]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crop Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Synergistic interactions between major constituents of lemongrass essential oil against larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni. J. Pest Sci. 2017, 90, 735–744. [Google Scholar] [CrossRef]
- Gaire, S.; Scharf, M.E.; Gondhalekar, A.D. Synergistic toxicity interactions between plant essential oil components against the common bed bug (Cimex lectularius L.). Insects 2020, 11, 133. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Eben, A.; Sporer, F.; Vogt, H.; Wetterauer, P.; Wink, M. Search for alternative control strategies of Drosophila suzukii (Diptera: Drosophilidae): Laboratory assays using volatile natural plant compounds. Insects 2020, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Renkema, J.M.; Wright, D.; Buitenhuis, R.; Hallett, R.H. Plant essential oils and potassium metabisulfite as repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2016, 6, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, A.K.; Dong, H.C.; Gregory, M.L. Evaluating a push–pull strategy for management of Drosophila suzukii Matsumura in red raspberry. Pest Manag. Sci. 2018, 74, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Erland, L.A.E.; Rheault, M.R.; Mahmoud, S.S. Insecticidal and oviposition deterrent effects of essential oils and their constituents against the invasive pest Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Crop Prot. 2015, 78, 20–26. [Google Scholar] [CrossRef]
- Wernicke, M.; Lethmayer, C.; Blümel, S. Laboratory trials to investigate potential repellent/oviposition detrent effects of selected substances on Drosophila suzukii adults. Bull. Insectol. 2020, 73, 249–255. [Google Scholar]
- Andreazza, F.; Bernardi, D.; Nava, D.E.; Botton, M.; Costa, V.A. Parasitic Enemy. Cultiv. HF 2017, 102, 20–23. [Google Scholar]
- Oliveira, D.C.; Stupp, P.; Martins, L.N.; Wollmann, J.; Geisler, F.C.S.; Cardoso, T.D.N.; Bernardi, D.; Garcia, F.R.M. Interspecific competition in Trichopria anastrephae parasitism (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae) parasitism on pupae of Drosophila suzukii (Diptera: Drosophilidae). Phytoparasitica 2020, 49, 207–215. [Google Scholar] [CrossRef]
- Morais, M.C.; Rakes, M.; Padilha, A.C.; Grützmacher, A.D.; Nava, D.E.; Bernardi, O.; Bernardi, D. Susceptibility of Brazilian populations of Anastrepha fraterculus, Ceratitis capitata (Diptera: Tephritidae), and Drosophila suzukii (Diptera: Drosophilidae) to selected insecticides. J. Econ. Entomol. 2021, 114, 1291–1297. [Google Scholar] [CrossRef]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Kim, J.; Jang, M.; Shin, E.; Kim, J.; Lee, S.H.; Park, C.G. Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Pestic. Biochem. Phys. 2016, 133, 35–43. [Google Scholar] [CrossRef]
- Peach, D.A.H.; Almond, M.; Gries, R.; Gries, G. Lemongrass and cinnamon bark: Plant essential oil blend as a spatial repellent for mosquitoes in a field setting. J. Med. Entomol. 2019, 3, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, P.; Sanchez, S.; Duran, P.; Andreazza, F.; Isaacs, R.; Dong, K. Behavioral and physiological responses of Drosophila melanogaster and D. suzukii to volatiles from plant essential oils. Pest Manag. Sci. 2021, 77, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.; Souza, M.T.; Bernardi, D.; Oliveira, D.D.C.; Morais, M.C.; Melo, D.J.; Richardi, V.S.; Zarbin, P.H.G.; Zawadneak, M.A.C. Essential oil of Rosmarinus officinalis ecotypes and their major compounds: Insecticidal and histological assessment against Drosophila suzukii and their impact on a non-target parasitoid. J. Econ. Entomol. 2022, 115, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.; Souza, M.T.; Bernardi, D.; Krinski, D.; Melo, D.J.; Oliveira, D.C.; Rakes, M.; Zarbin, P.H.G.; Maia, B.H.L.N.S.; Zawadneak, M.A.C. Chemical composition of essential oils of selected species of Piper and their insecticidal activity against Drosophila suzukii and Trichopria anastrephae. Environ. Sci. Pollut. Res. 2020, 27, 13056–13065. [Google Scholar] [CrossRef]
- Souza, M.T.; Souza, M.T.; Bernardi, D.; Rakes, M.; Vidal, H.R.; Zawadneak, M.A.C. Physicochemical characteristics and superficial damage modulate persimmon infestation by Drosophila suzukii (Diptera: Drosophilidae) and Zaprionus indianus. Environ. Entomol. 2020, 49, 1290–1299. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system-a review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef]
- Dimetry, N.Z.; Amin, A.H.; Bayoumi, A.E.; Abdel-Raheem, M.A.; Youssef, D.A. Comparative toxicity of neem and peppermint oils Nano formulations against Agrotis ipsilon (Hufn.) larvae (Lepidoptera: Noctuidae). J. Bot. Res. 2019, 1, 13–19. [Google Scholar]
- Pérez-Guerrero, S.; Mateus, C. Field evaluation of commercial plant extracts against Drosophila suzukii (Diptera: Drosophlidae) in raspberry. J. Pest Manag. 2018, 65, 53–58. [Google Scholar] [CrossRef]
- Schlesener, D.C.H.; Wollmann, J.; Krüger, A.P.; Nunes, A.D.; Bernardi, D.; Garcia, F.R.M. Biology and fertility life table of Drosophila suzukii on artificial diets. Entomol. Bone Appl. 2018, 166, 932–936. [Google Scholar]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef]
- Stockton, D.G.; Hesler, S.P.; Wallingford, A.K.; Leskey, T.C.; McDermott, L.; Elsensohn, J.E.; Riggs, D.I.L.; Prits, M.; Loeb, G.M. Factors affecting the implementation of exclusion netting to control Drosophila suzukii on primocane raspberry. Crop Prot. 2020, 15, 105191. [Google Scholar] [CrossRef]
- Libs, E.; Salim, E. Formulation of Essential Oil Pesticides Technology and their Application. Agric. Res. Technol. 2017, 9, 555–759. [Google Scholar]
- Pascual-Villalobos, M.J.; Guirao, P.; Díaz-Baños, F.G.; Cantó-Tejero, M.; Villora, G. Oil in water nanoemulsion formulations of botanical active substances. In Nano-Biopesticides Today and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–247. [Google Scholar]
- Vieira, J.G.A.; Krüger, A.P.; Scheunemann, T.; Morais, M.C.; Speriogin, H.J.; Garcia, F.R.M.; Nava, D.E.; Bernardi, D. Some aspects of the biology of Trichopria anastrephae (Hymenoptera: Diapriidae), a resident parasitoid attacking Drosophila suzukii (Diptera: Drosophilidae) in Brazil. J. Econ. Entomol. 2020, 113, 81–87. [Google Scholar] [CrossRef] [PubMed]
- McLafferty, F.W.; Stauffer, D.A.; Loh, S.Y.; Wesdemiotis, C. Unknown identification using reference mass spectra. Quality evaluation of databases. J. Am. Exclusion Mass Spectr. 1999, 10, 1229–1240. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D.J.A. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Contr. 1925, 3, 302–303. [Google Scholar] [CrossRef]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Exclusion B 1972, 135, 370–384. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 20 May 2022).
- SAS Institute. SAS System–SAS/stat. Computer Program, Version 9.2 84; SAS Institute: Cary, NC, USA, 2011.
- Morimoto, Y.; Kawada, H.; Kuramoto, K.; Mitsuhashi, T.; Saitoh, T.; Minakawa, N. New mosquito repellency bioassay for evaluation of repellents and pyrethroids using an attractive blood-feeding device. Parasit. Vectors 2021, 14, 151. [Google Scholar] [CrossRef]

| Constituents | RI * | % Peak Area | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ** | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | ||
| α-pinene | 942 | --- | 51.5 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| α-terpinene | 1016 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 18.4 | --- | --- |
| 3-carene | 1022 | --- | 28.1 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| γ-terpinene | 1054 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 23.3 | --- | --- |
| Hydrocarbon monoterpene | 0 | 79.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41.7 | 0 | 0 | |
| 1,8-cineole | 1030 | --- | --- | --- | --- | --- | --- | --- | --- | --- | 36.8 | --- | 8.4 | --- | 40.1 | --- | --- | --- | --- | --- |
| linalool | 1096 | --- | --- | 5.0 | 10.6 | 10.3 | 11.0 | 10.3 | --- | 6.9 | --- | --- | --- | --- | 10.3 | --- | --- | --- | 23.2 | --- |
| cis-2-p-menthen-1-ol | 1116 | --- | --- | --- | 11.0 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| trans-2-p-menthen-1-ol | 1136 | --- | --- | --- | 10.2 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| menthone | 1148 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 14.5 | 16.0 | --- | --- | --- | --- |
| citronellal | 1151 | --- | --- | --- | --- | --- | --- | 68.1 | --- | --- | --- | 40.2 | --- | --- | --- | --- | --- | --- | --- | --- |
| iso-menthone | 1158 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 18.8 | --- | --- | --- |
| menthol | 1177 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 96.8 | --- | 45.3 | 56.4 | --- | --- | --- |
| terpinen-4-ol | 1180 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 49.9 | --- | --- |
| α-terpineol | 1186 | --- | --- | --- | 13.8 | --- | --- | --- | 86.8 | --- | --- | --- | --- | --- | --- | --- | --- | 8.3 | --- | --- |
| citronellol | 1222 | --- | --- | --- | --- | --- | --- | 21.6 | --- | --- | --- | 16.7 | --- | --- | --- | --- | --- | --- | --- | --- |
| neral | 1233 | --- | --- | 17.6 | --- | 28.7 | 19.4 | --- | --- | 12.8 | 25.8 | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| carvone | 1239 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 20.0 | --- | --- | --- |
| geraniol | 1246 | --- | --- | 18.9 | --- | 8.9 | 5.5 | --- | 13.2 | 17.6 | --- | 28.6 | 91.6 | --- | --- | --- | --- | --- | --- | --- |
| piperitone | 1253 | --- | --- | --- | 54.3 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| geranial | 1261 | --- | --- | 39.5 | --- | 44.6 | 60.5 | --- | --- | 24.1 | 22.1 | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| Oxygenated monoterpene | 0 | 0 | 81.0 | 99.9 | 85.0 | 96.4 | 100 | 100 | 61.4 | 84.7 | 85.5 | 100 | 96.8 | 64.9 | 61.3 | 95.2 | 58.2 | 23.2 | 0 | |
| β-patchoulene | 1385 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 9.0 |
| α-guaiene | 1437 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 19.0 |
| seychellene | 1450 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 19.1 |
| α-patchoulene | 1460 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 16.2 |
| α-bulnesene | 1504 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 15.1 |
| Hydrocarbon sesquiterpene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 78.4 | |
| cedrol | 1608 | --- | 7.6 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| bulnesol | 1668 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 4.0 |
| Oxygenated sesquiterpene | 0 | 7.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.0 | |
| estragole | 1197 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 76.7 | --- |
| (E)-cynnamaldeyde | 1270 | 86.6 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| (E)-cinnamyl acetate | 1441 | 13.4 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| Arylpropanoid | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 76.7 | 0 | |
| methyl nerolate | 1290 | --- | --- | --- | --- | --- | --- | --- | --- | 14.2 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| nerol acetate | 1362 | --- | --- | 19.0 | --- | 7.5 | --- | --- | --- | 19.1 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| Ester | 0 | 0 | 19.0 | --- | 7.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total of identification (%) | 100 | 87.2 | 100 | 99.9 | 92.5 | 96.4 | 100 | 100 | 94.7 | 84.7 | 85.5 | 100 | 96.8 | 64.9 | 61.3 | 95.2 | 99.9 | 99.9 | 82.4 | |
| Treatments | Slope ± SE | LC50 (95% CI) a | LC90 (95% CI) b | χ2 c | df d |
|---|---|---|---|---|---|
| Cinnamomum verum, | 2.74 ± 0.21 | 11.02 (10.12–13.45) | 17.12 (16.10–18.11) | 5.44 | 6 |
| Cupressus sempervirens | 2.78 ± 0.17 | 14.45 (13.76–15.44) | 24.21 (22.13–25.11) | 7.11 | 6 |
| Cymbopogon citratus QT citratus | 3.11 ± 0.11 | 10.12 (8.11–12.78) | 16.18 (15.11–17.98) | 8.12 | 6 |
| Cymbopogon martinii | 2.98 ± 0.21 | 11.73 (10.44–13.79) | 17.10 (16.13–18.11) | 5.10 | 6 |
| Cymbopogon flexuosus | 3.07 ± 0.16 | 6.11 (5.75–7.43) | 17.68 (16.13–19.17) | 6.03 | 6 |
| Cymbopognon citratus QT myrcene | 2.75 ± 0.11 | 12.67 (11.74–14.90) | 26.12 (25.01–28.17) | 5.44 | 6 |
| Cymbopognon winterianus | 2.74 ± 0.22 | 11.54 (10.23–14.98) | 18.17 (16.08–19.10) | 9.78 | 6 |
| Eucalyptus globulus | 2.95 ± 0.11 | 14.10 (12.76–16.89) | 24.56 (22.13–27.89) | 8.12 | 6 |
| Eucalyptus radiata | 2.87 ± 0.14 | 10.43 (9.72–11.13) | 18.15 (16.19–20.11) | 6.13 | 6 |
| Eucalyptus staigeriana | 3.08 ± 0.12 | 11.23 (9.55–13.75) | 16.12 (15.97–17.01) | 7.11 | 6 |
| Eucalyptus citriodora | 2.97 ± 0.22 | 12.12 (11.75–14.20) | 23.44 (21.76–25.16) | 8.19. | 6 |
| Mentha arvensis | 3.10 ± 0.14 | 5.07 (3.11–6.10) | 15.62 (14.45–17.12) | 9.90 | 6 |
| Mentha cardiaca | 2.98 ± 0.16 | 7.10 (5.95–9.24) | 16.23 (14.54–18.23) | 8.16 | 6 |
| Mentha spicata | 3.08 ± 0.18 | 8.14 (6.04–9.74) | 17.34 (15.23–18.12) | 7.35 | 6 |
| Mentha piperita | 2.95 ± 0.13 | 7.98 (5.24–9.11) | 16.78 (15.89–19.11) | 7.14 | 6 |
| Mentha citrata | 2.67 ± 0.15 | 8.97 (6.07–11.24) | 17.89 (16.78–19.34) | 8.45 | 6 |
| Melaleuca alternifolia | 2.87 ± 0.10 | 17.13 (15-60–19.20) | 22.78 (21.15–24.97) | 8.07 | 6 |
| Ocimum basilicum | 3.07 ± 0.14 | 18.10 (17.74–20.05) | 23.44 (22.14–25.17) | 6.40 | 6 |
| Pogostemon cablin | 2.76 ± 0.16 | 19.28 (17.18–22.40) | 20.79 (19.11–23.44) | 5.53 | 6 |
| Delegate 250 WG™ | 2.78 ± 0.09 | 30.12 (28.75–32.44) | 26.23 (24.24–28.79 | 6.12 | 6 |
| Treatments | Slope ± SE | LC50 (95% CI) a | LC90 (95% CI) b | χ2 c | df d |
|---|---|---|---|---|---|
| Cinnamomum verum, | 2.74 ± 0.21 | 15.78 (12.10–17.14) | 25.67 (23.44–28.97) | 7.10 | 6 |
| Cupressus sempervirens | 2.78 ± 0.17 | 15.16 (13.14–18.10) | 32.16 (30.11–35.78) | 9.11 | 6 |
| Cymbopogon citratus QT citratus | 3.11 ± 0.11 | 14.11 (13.14–18.67) | 27.11 (25.16–29.98) | 8.13 | 6 |
| Cymbopogon martinii | 2.98 ± 0.21 | 16.18 (14.76–18.45) | 28.78 (26.17–29.40) | 5.44 | 6 |
| Cymbopogon flexuosus | 3.07 ± 0.16 | 16.54 (15.11–19.76) | 32.97 (30.11–35.67) | 6.78 | 6 |
| Cymbopognon citratus QT myrcene | 2.75 ± 0.11 | 17.86 (16.10–20.06) | 33.67 (31.98–35.40) | 6.56 | 6 |
| Cymbopognon winterianus | 2.74 ± 0.22 | 15.67 (14.15–19.73) | 25.44 (24.56–28.76) | 7.10 | 6 |
| Eucalyptus globulus | 2.95 ± 0.11 | 18.34 (17.54–20.13) | 31.45 (30.08–33.45) | 8.12 | 6 |
| Eucalyptus radiata | 2.87 ± 0.14 | 16.14 (13.74–18.89) | 27.65 (26.11–29.80) | 9.11 | 6 |
| Eucalyptus staigeriana | 3.08 ± 0.12 | 15.17 (15.10–18.56) | 30.24 (29.78–33.70) | 6.14 | 6 |
| Eucalyptus citriodora | 2.97 ± 0.22 | 19.16 (17.67–20.14) | 32.45 (31.90–35.76) | 8.14 | 6 |
| Mentha arvensis | 3.10 ± 0.14 | 17.98 (15.11–21.34) | 34.56 (33.78–37.89) | 7.70 | 6 |
| Mentha cardiaca | 2.98 ± 0.16 | 16.74 (14.72–19.24) | 33.67 (32.89–38.75) | 8.15 | 6 |
| Mentha spicata | 3.08 ± 0.18 | 15.13 (13.20–18.19) | 34.89 (33.45–37.90) | 8.19. | 6 |
| Mentha piperia | 2.95 ± 0.13 | 20.11 (17.18–22.36) | 35.67 (33.14–38.97) | 8.23 | 6 |
| Mentha citrata | 2.67 ± 0.15 | 18.10 (16.34–19.55) | 34.65 (33.98–37.80) | 8.01 | 6 |
| Melaleuca alternifolia | 2.87 ± 0.10 | 17.12 (15.78–21.54) | 35.67 (34.98–38.11) | 9.70 | 6 |
| Ocimum basilicum | 3.07 ± 0.14 | 17.54 (14.98–18.76) | 34.78 (32.45–38.12) | 9.08 | 6 |
| Pogostemon cablin | 2.76 ± 0.16 | 18.34 (16.76–22.17) | 36.78 (34.50–39.11) | 6.23 | 6 |
| Delegate 250 WG™ | 2.78 ± 0.09 | 25.67 (22.34–28.45) | 82.34 (80.11–85.67) | 7.79 | 6 |
| Treatments | Number of Eggs a | Reduction of Oviposition (%) |
|---|---|---|
| Cinnamomum verum | 2.9 ± 0.32 C | 84.5 |
| Cupressus sempervirens | 17.1 ± 0.43 A | 10.9 |
| Cymbopogon citratus QT citratus | 5.5 ± 0.39 BC | 71.3% |
| Cymbopogon martinii | 8.8 ± 0.59 B | 54.2 |
| Cymbopogon flexuosus | 7.9 ± 0.58 B | 58.8 |
| Cymbopognon citratus QT myrcene | 17.3 ± 0.97 A | 9.9 |
| Cymbopognon winterianus | 7.2 ± 0.32 B | 62.5 |
| Eucalyptus globulus | 18.1 ± 0.67 A | 5.7 |
| Eucalyptus radiata | 7.3 ± 0.38 B | 61.9 |
| Eucalyptus staigeriana | 7.9 ± 0.87 B | 58.8 |
| Eucalyptus citriodora | 16.9 ±0.98 A | 11.9 |
| Mentha arvensis | 10.0 ± 0.62 B | 47.9 |
| Mentha cardiaca | 7.8 ± 0.55 B | 59.3 |
| Mentha spicata | 10.6 ± 0.62 B | 44.7 |
| Mentha piperita | 7.9 ± 0.58 B | 58.8 |
| Mentha citrata | 7.6 ± 0.37 B | 60.4 |
| Melaleuca alternifolia | 17.6 ± 1.12 A | 8.3 |
| Ocimum basilicum | 18.1 ± 1.75 A | 5.7 |
| Pogostemon cablin | 18.4 ± 0.52 A | 4.1 |
| Delegate 250 WG™ | 3.3 ± 0.55 C | 82.8 |
| F | 64.12 | |
| df | 21, 639 | |
| p | > 0.0001 |
| Treatments | Mortality a | P (%) a | |
|---|---|---|---|
| Topical Application | Ingestion | ||
| Cinnamomum verum | 7.1 ± 0.23 A | 19.1 ± 1.15 A | 50.1 ± 2.75 A |
| Cupressus sempervirens | 8.5 ± 0.11 A | 10.2 ± 0.88 A | 48.9 ± 1.89 A |
| Cymbopogon citratus QT citratus | 4.2 ± 0.15 A | 14.3 ± 1.10 A | 44.3 ± 1.12 A |
| Cymbopogon martinii | 6.4 ± 0.11 A | 12.2 ± 1.78 A | 50.4 ± 2.10 A |
| Cymbopogon flexuosus | 5.5 ± 0.78 A | 12.3 ± 1.15 A | 49.2 ± 3.07 A |
| Cymbopognon citratus QT myrcene | 6.1 ± 0.45 A | 10.3 ± 0.77 A | 46.1 ± 2.15 A |
| Cymbopognon winterianus | 4.5 ± 0.21 A | 16.1 ± 1.11 A | 45.2 ± 2.10 A |
| Eucalyptus globulus | 5.2 ± 0.56 A | 15.4 ± 1.34 A | 47.3 ± 1.75 A |
| Eucalyptus radiata | 6.4 ± 0.67 A | 18.4 ± 1.02 A | 49.9 ± 2.01 A |
| Eucalyptus staigeriana | 7.3 ± 0.34 A | 16.7 ± 1.32 A | 49.3 ± 1.86 A |
| Eucalyptus citriodora | 6.8 ± 0.24 A | 15.6 ± 1.44 A | 50.2 ± 1.67 A |
| Mentha arvensis | 6.7 ± 0.33 A | 13.2 ± 1.09 A | 43.4 ± 1.65 A |
| Mentha cardiaca | 5.7 ± 0.44 A | 14.2 ± 1.10 A | 50.8 ± 2.10 A |
| Mentha spicata | 6.2 ± 0.53 A | 18.3 ± 0.89 A | 49.6 ± 1.87 A |
| Mentha piperita | 9.8 ± 0.23 A | 13.4 ± 1.12 A | 48.2 ± 2.05 A |
| Mentha citrata | 8.7 ± 0.14 A | 11.3 ± 0.98 A | 55.3 ± 1.64 A |
| Melaleuca alternifolia | 9.1 ± 0.23 A | 12.4 ± 1.14 A | 48.6 ± 0.85 A |
| Ocimum basilicum | 8.3 ± 0.44 A | 15.7 ± 2.02 A | 49.3 ± 2.74 A |
| Pogostemon cablin | 7.8 ± 0.46 A | 13.5 ± 1.14 A | 50.1 ± 1.76 A |
| Delegate 250 WG™ | 29.6 ± 0.34 B | 35.7 ± 2.68 B | 47.2 ± 2.30 A |
| F | 6.5 ± 0.11 | 10.1 ± 1.74 | 46.2 ± 1.78 |
| df | 7.1 ± 0.23 | 11.5 ± 0.98 | 45.3 ± 2.35 |
| p | 119.11 | 78.45 | 11.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, M.T.d.; Souza, M.T.d.; Morais, M.C.; Oliveira, D.d.C.; Melo, D.J.d.; Figueiredo, L.; Zarbin, P.H.G.; Zawadneak, M.A.C.; Bernardi, D. Essential Oils as a Source of Ecofriendly Insecticides for Drosophila suzukii (Diptera: Drosophilidae) and Their Potential Non-Target Effects. Molecules 2022, 27, 6215. https://doi.org/10.3390/molecules27196215
Souza MTd, Souza MTd, Morais MC, Oliveira DdC, Melo DJd, Figueiredo L, Zarbin PHG, Zawadneak MAC, Bernardi D. Essential Oils as a Source of Ecofriendly Insecticides for Drosophila suzukii (Diptera: Drosophilidae) and Their Potential Non-Target Effects. Molecules. 2022; 27(19):6215. https://doi.org/10.3390/molecules27196215
Chicago/Turabian StyleSouza, Michele Trombin de, Mireli Trombin de Souza, Maíra Chagas Morais, Daiana da Costa Oliveira, Douglas José de Melo, Leonardo Figueiredo, Paulo Henrique Gorgatti Zarbin, Maria Aparecida Cassilha Zawadneak, and Daniel Bernardi. 2022. "Essential Oils as a Source of Ecofriendly Insecticides for Drosophila suzukii (Diptera: Drosophilidae) and Their Potential Non-Target Effects" Molecules 27, no. 19: 6215. https://doi.org/10.3390/molecules27196215
APA StyleSouza, M. T. d., Souza, M. T. d., Morais, M. C., Oliveira, D. d. C., Melo, D. J. d., Figueiredo, L., Zarbin, P. H. G., Zawadneak, M. A. C., & Bernardi, D. (2022). Essential Oils as a Source of Ecofriendly Insecticides for Drosophila suzukii (Diptera: Drosophilidae) and Their Potential Non-Target Effects. Molecules, 27(19), 6215. https://doi.org/10.3390/molecules27196215







