Synthesis, Molecular Docking, and Preclinical Evaluation of a New Succinimide Derivative for Cardioprotective, Hepatoprotective and Lipid-Lowering Effects
Abstract
1. Introduction
2. Results
2.1. Design Strategy
2.2. Chemistry of the Synthesized Compound
2.3. Acute Toxicity
2.4. Cardiac Markers
2.5. Liver Enzymes Analysis
3. Lipid Profile
4. Histopathological Study
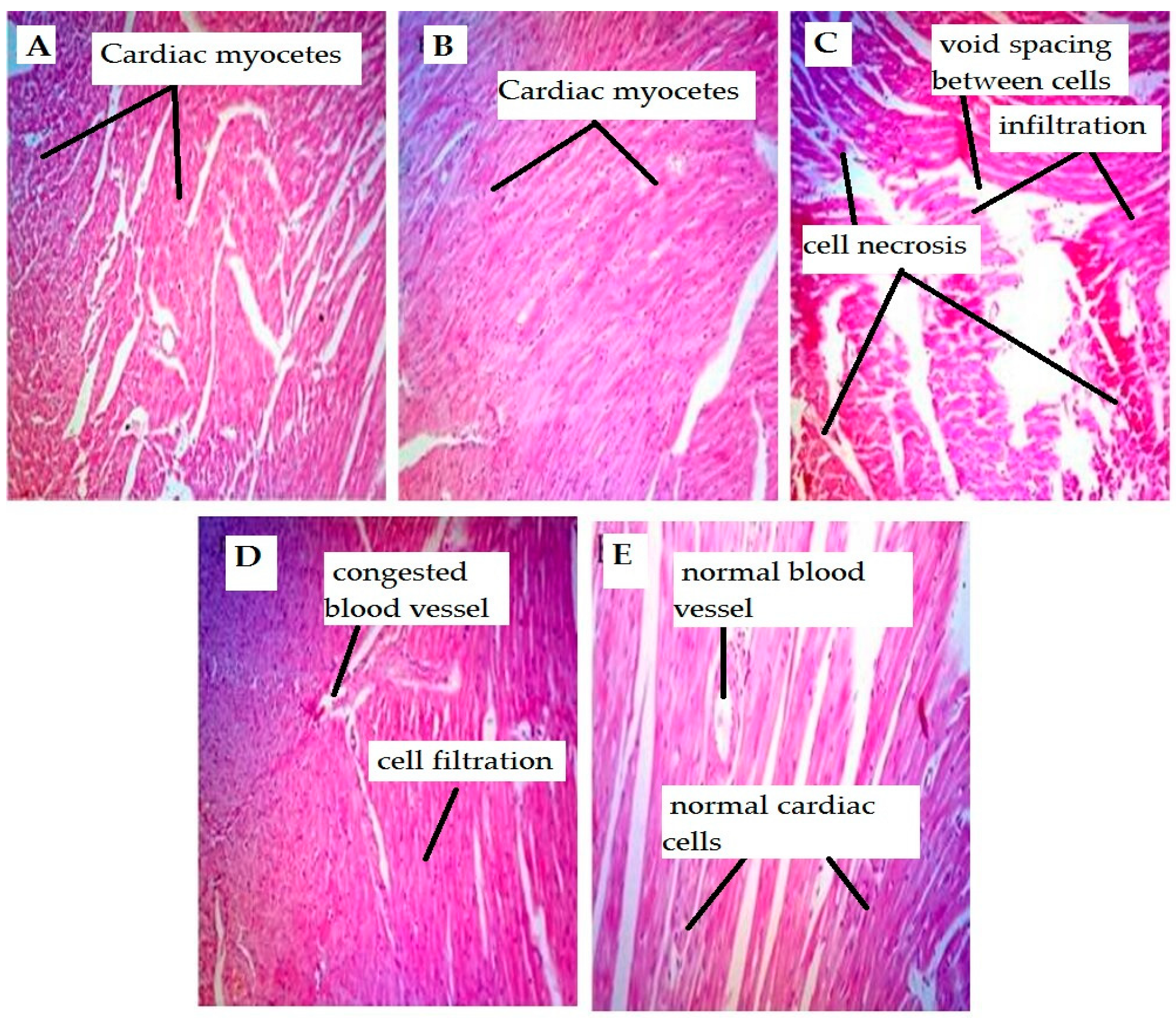
4.1. Effect on Cardiac Tissues
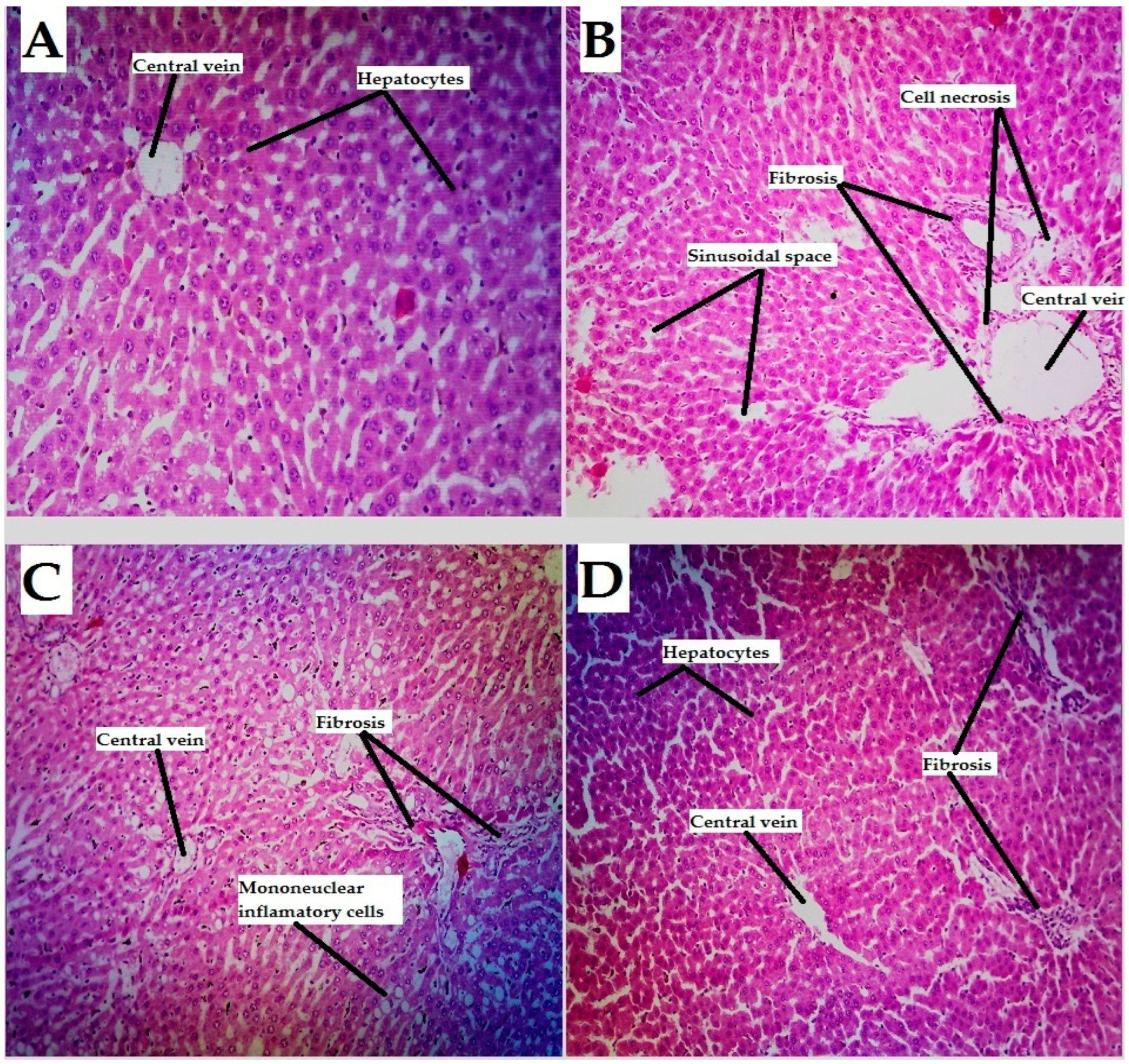
4.2. Liver Tissues
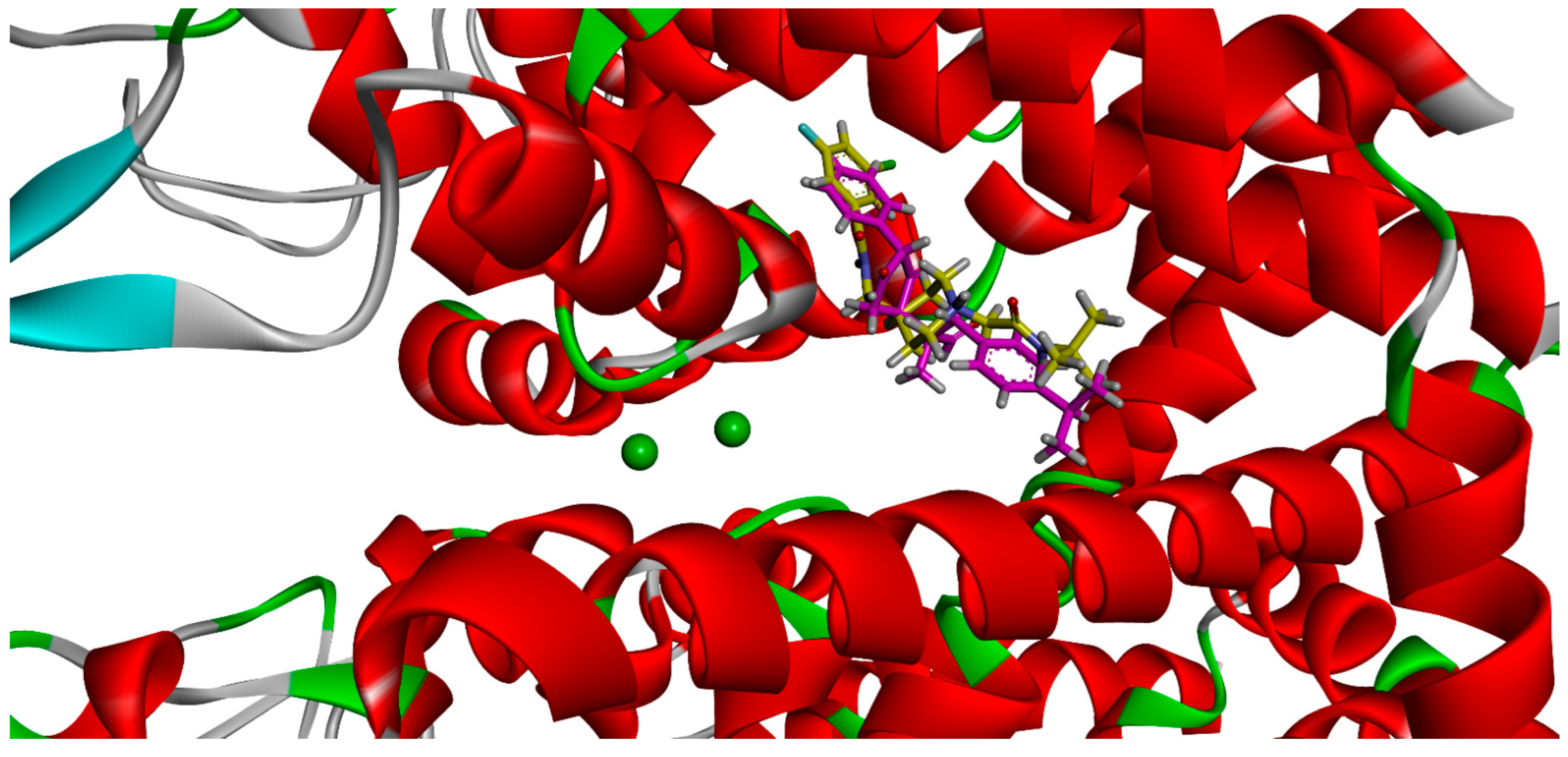
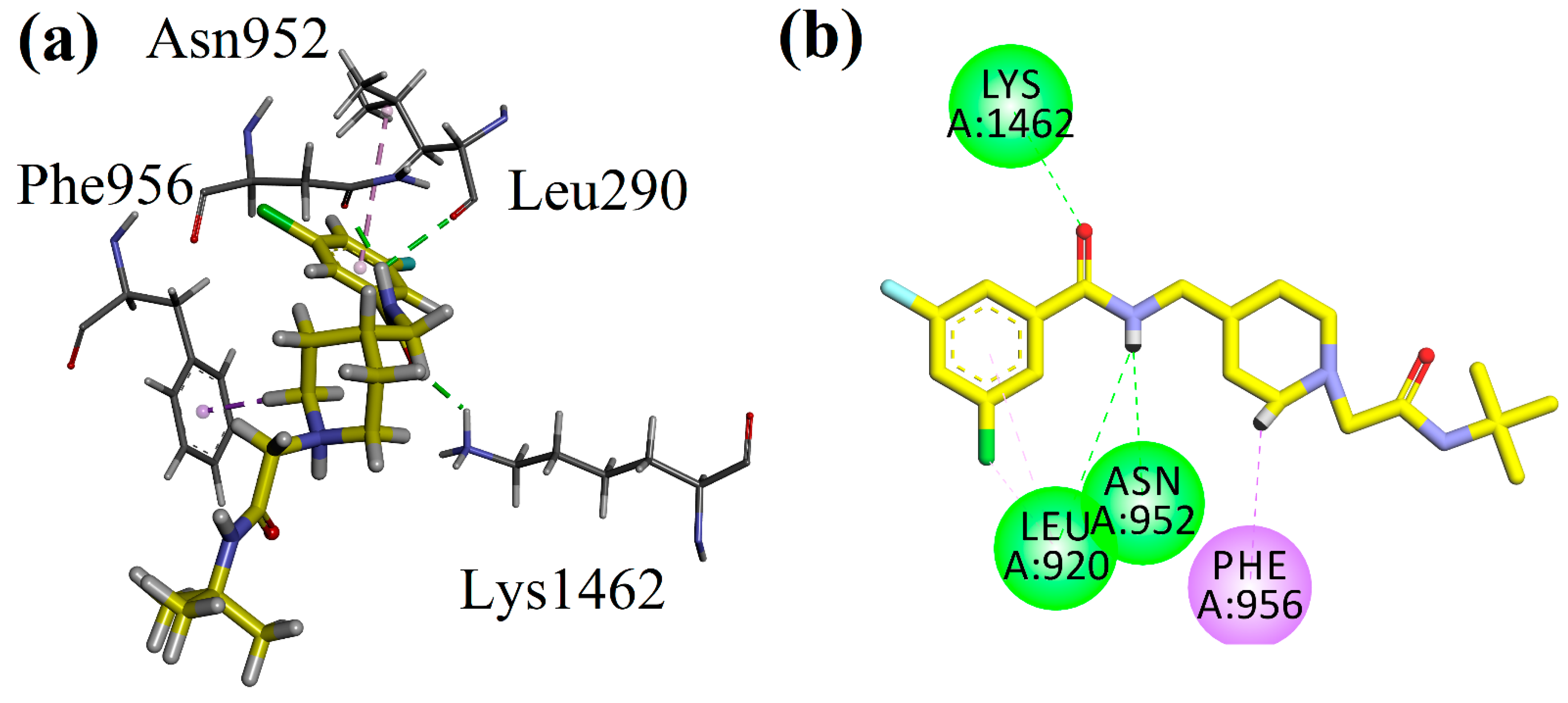
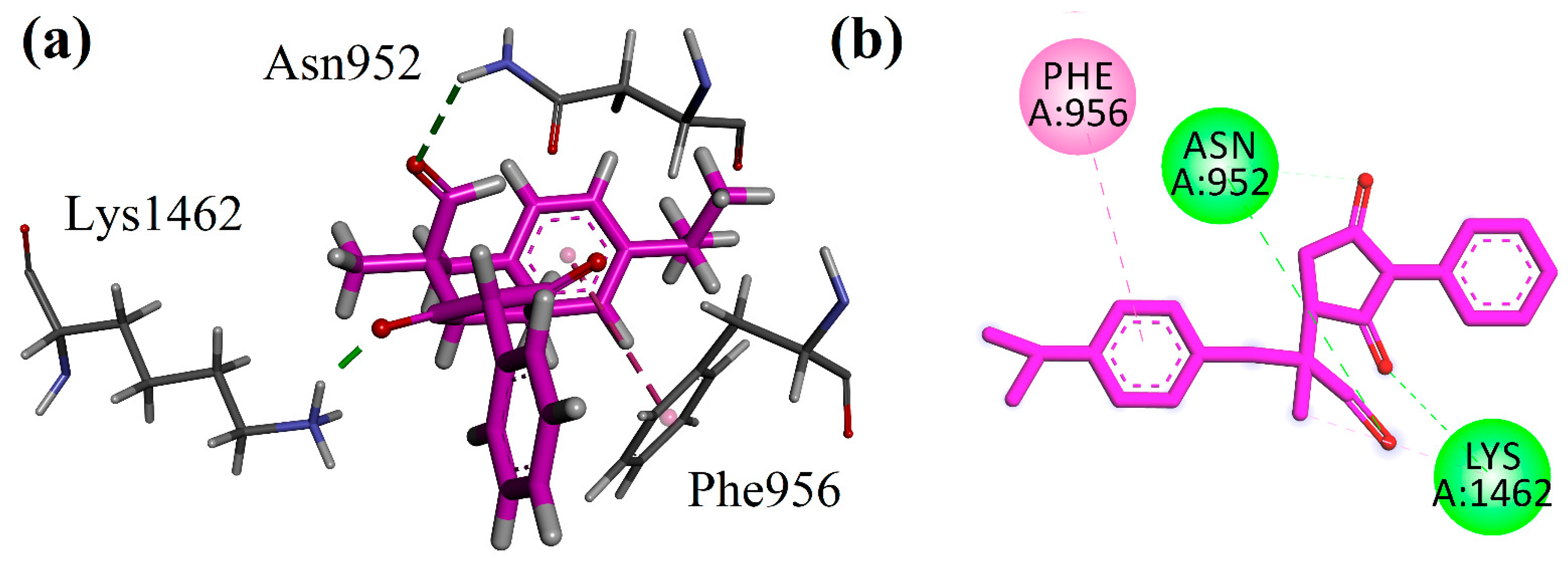
4.3. Docking Studies
5. Discussion
6. Materials and Methods
6.1. Chemicals and Reagents
6.2. Synthesis of 2-(2,5-Dioxo-1-phenylpyrrolidin-3-yl) -3-(4-isopropylphenyl)-2-methylpropanal
6.3. Animal Breeding and Ethical Approval
6.4. Experimental Procedures
6.4.1. Acute Toxicity Study
6.4.2. Preclinical Observations and Survival
6.4.3. Cardioprotective Study
6.4.4. Biochemical Assay
6.4.5. Histological Study
6.4.6. Docking Studies
6.4.7. Statistical Analysis
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jin, Y.; Yang, C.-J.; Xu, X.; Cao, J.-N.; Feng, Q.-T.; Yang, J. MiR-214 regulates the pathogenesis of patients with coronary artery disease by targeting VEGF. Mol. Cell. Biochem. 2015, 402, 111–122. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Zhu, J.-J.; Xu, Y.-Q.; He, J.-H.; Yu, H.-P.; Huang, C.-J.; Gao, J.-M.; Dong, Q.-X.; Xuan, Y.-X.; Li, C.-Q. Human cardiotoxic drugs delivered by soaking and microinjection induce cardiovascular toxicity in zebrafish. J. Appl. Toxicol. 2013, 34, 139–148. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P. (Ed.) Zebrafish: Methods for Assessing Drug Safety and Toxicity; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Amici, P. Intolerance of Uncertainty: From Transdiagnostic Model to Clinical Management. Psychiatr. Danub. 2021, 339, S22–S25. [Google Scholar]
- Redfern, W.S.; Bialecki, R.; Ewart, L.; Hammond, T.G.; Kinter, L.; Lindgren, S.; Pollard, C.E.; Rolf, M.; Valentin, J.-P. Impact and prevalence of safety pharmacology-related toxicities throughout the pharmaceutical life cycle. J. Pharmacol. Toxicol. Methods 2010, 62, e29. [Google Scholar] [CrossRef]
- An, J.; Shim, J.H.; Kim, S.O.; Lee, D.; Kim, K.M.; Lim, Y.S.; Lee, H.C.; Chung, Y.H.; Lee, Y.S. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: A registry-based matched case–control study. Circulation 2014, 130, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart failure and liver disease: Cardiohepatic interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Lei, J.; Dong, Y.; Hou, Q.; He, Y.; Lai, Y.; Liao, C.; Kawamura, Y.; Li, J.; Zhang, B. Intestinal Microbiota Regulate Certain Meat Quality Parameters in Chicken. Front. Nutr. 2022, 9, 747705. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, X.-M. Hepatocardiac or Cardiohepatic Interaction: From Traditional Chinese Medicine to Western Medicine. Evid.-Based Complement. Altern. Med. 2021, 2021, 6655335. [Google Scholar] [CrossRef]
- Møller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef]
- Samsky, M.D.; Patel, C.B.; DeWald, T.A.; Smith, A.D.; Felker, G.M.; Rogers, J.G.; Hernandez, A.F. Cardiohepatic interactions in heart failure: An overview and clinical implications. J. Am. Coll. Cardiol. 2013, 61, 2397–2405. [Google Scholar] [CrossRef]
- Henrion, J.; Descamps, O.; Luwaert, R.; Schapira, M.; Parfonry, A.; Heller, F. Hypoxic hepatitis in patients with cardiac failure: Incidence in a coronary care unit and measurement of hepatic blood flow. J. Hepatol. 1994, 21, 696–703. [Google Scholar] [CrossRef]
- Naschitz, J.E.; Yeshurun, D.; Shahar, J. Cardiogenic Hepatorenal Syndrome. Angiology 1990, 41, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Sachdeva, T.; Bafna, P. Drug-induced hepatotoxicity: A review. J. Appl. Pharm. Sci. 2012, 30, 233–243. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Y.; Chang, P.; Gong, S.; Shao, L.; Dong, L. Mesenchymal stem cell-based therapy for radiation-induced lung injury. Stem Cell Res. Ther. 2018, 9, 18. [Google Scholar] [CrossRef]
- Hunter, P.; Smaill, B. The analysis of cardiac function: A continuum approach. Prog. Biophys. Mol. Biol. 1988, 52, 101–164. [Google Scholar] [CrossRef]
- Schuster, D.; Laggner, C.; Langer, T. Why Drugs Fail—A Study on Side Effects in New Chemical Entities. Curr. Pharm. Des. 2005, 11, 3545–3559. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. -Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Last, A.R.; Ference, J.D.; Menzel, E.R. Hyperlipidemia: Drugs for Cardiovascular Risk Reduction in Adults. Am. Fam. Physician 2017, 95, 78–87. [Google Scholar]
- Schmitz, G.; Orsó, E. Lipoprotein(a) hyperlipidemia as cardiovascular risk factor: Pathophysiological aspects. Clin. Res. Cardiol. Suppl. 2015, 10, 21–25. [Google Scholar] [CrossRef]
- Payne, D.L.; Nohria, A. Prevention of Chemotherapy Induced Cardiomyopathy. Curr. Heart Fail. Rep. 2017, 14, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- More, L.A.; Lane, S.; Asnani, A. 5-FU Cardiotoxicity: Vasospasm, Myocarditis, and Sudden Death. Curr. Cardiol. Rep. 2021, 23, 17. [Google Scholar] [CrossRef]
- Chen, J.; Zou, Q.; Li, J. DeepM6ASeq-EL: Prediction of human N6-methyladenosine (m6A) sites with LSTM and ensemble learning. Front. Comput. Sci. 2021, 16, 162302. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Teschendorf, H.J.; Kretzschmar, R. Succinimides. In Antiepileptic Drugs; Springer: Berlin/Heidelberg, Germany, 1985; pp. 557–574. [Google Scholar] [CrossRef]
- Patil, M.M.; Rajput, S.S. Succinimides: Synthesis, reaction, and biological activity. Int. J. Pharm. Pharm. Sci. 2014, 6, 8–14. [Google Scholar]
- Aeberli, P.; Gogerty, J.H.; Houlihan, W.J.; Iorio, L.C. Synthesis and central nervous system depressant activity of some bicyclic amides. J. Med. Chem. 1976, 19, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.; Filho, V.C.; Rosa, P.W.; Pereira, C.I.; Schlemper, V.; Nunes, R.J. Synthesis of new succinimides and sulphonated derivatives with analgesic action in mice. Pharm. Pharmacol. Commun. 1997, 3, 67–71. [Google Scholar] [CrossRef]
- Crider, A.M.; Kolczynski, T.M.; Yates, K.M. Synthesis and anticancer activity of nitrosourea derivatives of phensuximide. J. Med. Chem. 1980, 23, 324–326. [Google Scholar] [CrossRef]
- Hall, I.H.; Wong, O.T.; Scovill, J.P. The cytotoxicity of N-pyridinyl and N-quinolinyl substituted derivatives of phthalimide and succinimide. Biomed. Pharmacother. 1995, 49, 251–258. [Google Scholar] [CrossRef]
- Rich, D.H.; Gardner, J.H. Synthesis of the cytostatic cyclic tetrapeptide, chlamydocin. Tetrahedron Lett. 1983, 24, 5305–5308. [Google Scholar] [CrossRef]
- Kaczorowski, G.J.; McManus, O.B.; Priest, B.T.; Garcia, M.L. Ion Channels as Drug Targets: The Next GPCRs. J. Gen. Physiol. 2008, 131, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Filho, V.C.; Nunes, R.J.; Calixto, J.B.; Yunes, R.A. Inhibition of guinea-pig ileum contraction by phyllanthimide analogues: Structure-activity relationships. Pharm. Pharmacol. Commun. 1995, 1, 399–401. [Google Scholar] [CrossRef]
- Musso, D.L.; Cochran, F.R.; Kelley, J.L.; McLean, E.W.; Selph, J.L.; Rigdon, G.C.; Orr, G.F.; Davis, R.G.; Cooper, B.R.; Styles, V.L.; et al. Indanylidenes. 1. Design and Synthesis of (E)-2-(4,6-Difluoro-1-indanylidene)acetamide, a Potent, Centrally Acting Muscle Relaxant with Antiinflammatory and Analgesic Activity. J. Med. Chem. 2002, 46, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Coram, W.M.; Brezenoff, H.E. The antihypertensive effect of a selective central muscarinic cholinergic antagonist: N-(4-diethylamino-2-butynyl)-succinimide. Drug Dev. Res. 1983, 3, 503–516. [Google Scholar] [CrossRef]
- Zentz, F.; Valla, A.; Le Guillou, R.; Labia, R.; Mathot, A.-G.; Sirot, D. Synthesis and antimicrobial activities of N-substituted imides. II Farm. 2002, 57, 421–426. [Google Scholar] [CrossRef]
- Hazra, B.; Pore, V.; Dey, S.; Datta, S.; Darokar, M.; Saikia, D.; Khanuja, S.P.; Thakur, A. Bile acid amides derived from chiral amino alcohols: Novel antimicrobials and antifungals. Bioorg. Med. Chem. Lett. 2004, 14, 773–777. [Google Scholar] [CrossRef]
- Kornet, M.J.; Crider, A.M.; Magarian, E.O. Potential long-acting anticonvulsants. 1. Synthesis and activity of succinimides containing an alkylating group at the 2 position. J. Med. Chem. 1977, 20, 405–409. [Google Scholar] [CrossRef]
- Isaka, M.; Prathumpai, W.; Wongsa, P.; Tanticharoen, M.; Tanticharoen, M. Hirsutellone F, a Dimer of Antitubercular Alkaloids from the Seed Fungus Trichoderma Species BCC 7579. ChemInform 2006, 37, 2815–2817. [Google Scholar] [CrossRef]
- Das, N. “Aberrant” neuronal stimulation and” cannabis psychosis”-hypothesis to a biological plausibility! Psychiatr. Danub. 2021, 33, 280–282. [Google Scholar] [CrossRef]
- Tang, C.-M.; Presser, F.; Morad, M. Amiloride Selectively Blocks the Low Threshold (T) Calcium Channel. Science 1988, 240, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Ullah, F.; Ayaz, M.; Tariq, M.; Sadiq, A.; Rashid, U. Synthesis of Michael Adducts as Key Building Blocks for Potential Analgesic Drugs: In vitro, in vivo and in silico Explorations. Drug Des. Dev. Ther. 2021, 15, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Nugent, T.C.; Bibi, A.; Sadiq, A.; Shoaib, M.; Umar, M.N.; Tehrani, F.N. Chiral picolylamines for Michael and aldol reactions: Probing substrate boundaries. Org. Biomol. Chem. 2012, 10, 9287–9294. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Nugent, T.C. Catalytic Access to Succinimide Products Containing Stereogenic Quaternary Carbons. ChemistrySelect 2020, 5, 11934–11938. [Google Scholar] [CrossRef]
- Nugent, T.C.; Sadiq, A.; Bibi, A.; Heine, T.; Zeonjuk, L.L.; Vankova, N.; Bassil, B.S. Noncovalent bifunctional organocatalysts: Powerful tools for contiguous quaternary-tertiary stereogenic carbon formation, scope, and origin of enantioselectivity. Chem. Eur. J. 2012, 18, 4088–4098. [Google Scholar] [CrossRef]
- Jan, M.S.; Ahmad, S.; Hussain, F.; Ahmad, A.; Mahmood, F.; Rashid, U.; Abid, O.-U.; Ullah, F.; Ayaz, M.; Sadiq, A. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2,5-dione derivatives as multitarget anti-inflammatory agents. Eur. J. Med. Chem. 2020, 186, 111863. [Google Scholar] [CrossRef]
- Nugent, T.C.; Negru, D.E.; El-Shazly, M.; Hu, D.; Sadiq, A.; Bibi, A.; Umar, M.N. Sequential Reductive Amination-Hydrogenolysis: A One-Pot Synthesis of Challenging Chiral Primary Amines. Adv. Synth. Catal. 2011, 353, 2085–2092. [Google Scholar] [CrossRef]
- Sadiq, A.; Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Alqarni, A.O.; Rashid, U. Tailoring the substitution pattern of Pyrrolidine-2,5-dione for discovery of new structural template for dual COX/LOX inhibition. Bioorg. Chem. 2021, 112, 104969. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Alqarni, A.O.; Jan, M.S.; Hussain, F.; Zafar, R.; Rashid, U.; Abbas, M.; Tariq, M.; et al. Antioxidant Molecules Isolated from Edible Prostrate Knotweed: Rational Derivatization to Produce More Potent Molecules. Oxid. Med. Cell. Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Huneif, M.A.; Alshehri, D.B.; Alshaibari, K.S.; Dammaj, M.Z.; Mahnashi, M.H.; Majid, S.U.; Javed, M.A.; Ahmad, S.; Rashid, U.; Sadiq, A. Design, synthesis and bioevaluation of new vanillin hybrid as multitarget inhibitor of α-glucosidase, α-amylase, PTP-1B and DPP4 for the treatment of type-II diabetes. Biomed. Pharm. 2022, 150, 113038. [Google Scholar] [CrossRef]
- Sadiq, A.; Mahmood, F.; Ullah, F.; Ayaz, M.; Ahmad, S.; Haq, F.U.; Khan, G.; Jan, M.S. Synthesis, anticholinesterase and antioxidant potentials of ketoesters derivatives of succinimides: A possible role in the management of Alzheimer’s. Chem. Central J. 2015, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Jan, M.S.; Ahmad, S.; Rashid, U.; Ayaz, M.; Ullah, F.; Hussain, F.; Ahmad, A.; Khan, A.U.; Aasim, M.; et al. Ethyl 3-oxo-2-(2, 5-dioxopyrrolidin-3-yl) butanoate derivatives: Anthelmintic and cytotoxic potentials, antimicrobial, and docking studies. Front. Chem 2017, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ullah, F.; Sadiq, A.; Ayaz, M.; Rahim, H.; Rashid, U.; Ahmad, S.; Jan, M.S.; Ullah, R.; Shahat, A.A.; et al. Pharmacological Evaluation of Aldehydic-Pyrrolidinedione Against HCT-116, MDA-MB231, NIH/3T3, MCF-7 Cancer Cell Lines, Antioxidant and Enzyme Inhibition Studies. Drug Des. Dev. Ther. 2019, 13, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Panteghini, M.; Pagani, F.; Cuccia, C. Activity of serum aspartate aminotransferase isoenzymes in patients with acute myocardial infarction. Clin. Chem. 1987, 33, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.H.S.; Anandan, R.; Kumar, S.H.S.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar] [CrossRef]
- Hammond, G.L.; Nadal-Ginard, B.; Talner, N.S.; Markert, C.L. Myocardial LDH isozyme distribution in the ischemic and hypoxic heart. Circulation 1976, 53, 637–643. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Mohamed, E.T.; Safwat, G.M. Evaluation of cardioprotective activity of Lepidium sativum seed powder in albino rats treated with 5-fluorouracil. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 208–215. [Google Scholar] [CrossRef]
- Cianci, G.; Morelli, M.F.; Cannita, K.; Morese, R.; Ricevuto, E.; Di Rocco, Z.C.; Porzio, G.; Baldi, P.L.; Ficorella, C. Prophylactic options in patients with 5-fluorouracil-associated cardiotoxicity. Br. J. Cancer 2003, 88, 1507–1509. [Google Scholar] [CrossRef]
- Ficorella, C.; Ricevuto, E.; Morelli, M.F.; Morese, R.; Cannita, K.; Cianci, G.; Porzio, G.; Di Rocco, Z.C.; De Galitiis, F.; De Tursi, M.; et al. Increased tolerability of bimonthly 12-hour timed flat infusion 5-fluorouracil/irinotecan regimen in advanced colorectal cancer: A dose-finding study. Oncol. Rep. 2006, 15, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.B.; Cheng, Y.C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990, 48, 381–395. [Google Scholar] [CrossRef]
- Polk, A.; Vistisen, K.; Vaage-Nilsen, M.; Nielsen, D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol. Toxicol. 2014, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Psaltopoulou, T.; Androulakis, E.; Papageorgiou, N.; Papaioannou, S.; Oikonomou, E.; Synetos, A.; Stefanadis, C. Oxidative Stress and Early Atherosclerosis: Novel Antioxidant Treatment. Cardiovasc. Drugs Ther. 2014, 29, 75–88. [Google Scholar] [CrossRef]
- Leitão, R.F.C.; Ribeiro, R.A.; Bellaguarda, E.A.L.; Macedo, F.D.B.; Silva, L.R.; Oriá, R.; Vale, M.; Cunha, F.Q.; Brito, G.A.C. Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster. Cancer Chemother. Pharmacol. 2006, 59, 603–612. [Google Scholar] [CrossRef]
- Hou, Q.; Huang, J.; Xiong, X.; Guo, Y.; Zhang, B. Role of Nutrient-sensing Receptor GPRC6A in Regulating Colonic Group 3 Innate Lymphoid Cells and Inflamed Mucosal Healing. J. Crohn’s Colitis 2022, 20, 1–13. [Google Scholar] [CrossRef]
- Bertolini, A.; Fiumanò, M.; Fusco, O.; Muffatti, A.; Scarinci, A.; Pontiggia, G.; Scopelliti, M. Acute cardiotoxicity during capecitabine treatment: A case report. Tumori J. 2001, 87, 200–206. [Google Scholar] [CrossRef]
- Hamilton, K.L. Antioxidants and cardioprotection. Med. Sci. Sports Exerc. 2007, 39, 1544–1553. [Google Scholar] [CrossRef]
- Cieślak, M.; Napiórkowska, M.; Kaźmierczak-Barańska, J.; Królewska-Golińska, K.; Hawrył, A.; Wybrańska, I.; Nawrot, B. New Succinimides with Potent Anticancer Activity: Synthesis, Activation of Stress Signaling Pathways and Characterization of Apoptosis in Leukemia and Cervical Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4318. [Google Scholar] [CrossRef]
- Shehab, N.G.; Abu-Gharbieh, E.; Bayoumi, F.A. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complement. Altern. Med. 2015, 15, 401. [Google Scholar] [CrossRef]
- Reid, A.B.; Kurten, R.C.; McCullough, S.S.; Brock, R.W.; Hinson, J.A. Mechanisms of Acetaminophen-Induced Hepatotoxicity: Role of Oxidative Stress and Mitochondrial Permeability Transition in Freshly Isolated Mouse Hepatocytes. J. Pharmacol. Exp. Ther. 2004, 312, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, N. Drug-induced liver disorders. Drug Saf. 2001, 24, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Wang, Y.; Chen, Y.; Li, D.; Li, G.; Xiao, H.; Hou, J.; Wang, Z.; Hu, L.; Wang, L.; et al. Angelica Polysaccharide Antagonizes 5-FU-Induced Oxidative Stress Injury to Reduce Apoptosis in the Liver Through Nrf2 Pathway. Front. Oncol. 2021, 11, 3216. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, G.; Cuomo, O.; Vasco, M.; Vennarecci, G.; Canonico, R.; Della Mura, N.; Alfano, R.; Napoli, C. Epigenetic-sensitive challenges of cardiohepatic interactions: Clinical and therapeutic implications in heart failure patients. Eur. J. Gastroenterol. Hepatol. 2020, 33, 1247–1253. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Serrano, J.; Knoben, J.E.; Navarro, V.J. LiverTox: A website on drug-induced liver injury. Hepatology 2012, 57, 873–874. [Google Scholar] [CrossRef]
- Abou-Zeid, N.R. Ameliorative effect of vitamin C against 5-fuorouracil-induced hepatotoxicity in mice: A light and electron microscope study. J. Basic Appl. Zool. 2014, 67, 109–118. [Google Scholar] [CrossRef]
- Krivoshein, A.V. Antiepileptic drugs based on the α-substituted amide group pharmacophore: From chemical crystallography to molecular pharmaceutics. Curr. Pharm. Des. 2016, 22, 5029–5040. [Google Scholar] [CrossRef]
- Saito, Y.; Takekuma, Y.; Komatsu, Y.; Sugawara, M. Hypertriglyceridemia induced by S-1: A novel case report and review of the literature. J. Oncol. Pharm. Pract. 2020, 27, 1020–1025. [Google Scholar] [CrossRef]
- Abdel-Hamid, H.F.; Soliman, A.; Helaly, F.M.; Ragab, S. Cytotoxic potency and induced biochemical parameters in mice serum of new furan derivatives against liver cancer cell line. Acta Pol. Pharm. -Drug Res. 2011, 68, 499–505. [Google Scholar]
- Javot, L.; Spaëth, D.; Scala-Bertola, J.; Gambier, N.; Petitpain, N.; Gillet, P. Severe hypertriglyceridaemia during treatment with capecitabine. Br. J. Cancer 2011, 104, 1238–1239. [Google Scholar] [CrossRef]
- Brockenbrough, J.S.; Morihara, J.K.; Hawes, S.E.; Stern, J.E.; Rasey, J.S.; Wiens, L.W.; Feng, Q.; Vesselle, H. Thymidine Kinase 1 and Thymidine Phosphorylase Expression in Non-Small-cell Lung Carcinoma in Relation to Angiogenesis and Proliferation. J. Histochem. Cytochem. 2009, 57, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Khan, J.A.; Mahnashi, M.H.; Jan, M.S.; Javed, M.A.; Rashid, U.; Sadiq, A.; Hassan, S.S.; Bungau, S. Anti-Inflammatory, Analgesic and Antioxidant Potential of New (2 S, 3 S)-2-(4-isopropylbenzyl)-2-methyl-4-nitro-3-phenylbutanals and Their Corresponding Carboxylic Acids through In Vitro, In Silico and In Vivo Studies. Molecules 2022, 27, 4068. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Mahnashi, M.H.; Rashid, U.; Jan, M.S.; Alshahrani, M.A.; Huneif, M.A. 3-(((1S,3S)-3-((R)-hydroxy(4-(trifluoromethyl)phenyl)methyl)-4-oxocyclohexyl)methyl)pentane-2,4-dione: Design and synthesis of new stereopure multi-target antidiabetic agent. Molecules 2022, 27, 3265. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 2248. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Al-Omar, M.A.; Khan, A.A.; Alanazi, A.M.; Naglah, A.M. Synthesis and antihepatotoxic activity of dihydropyrimidinone derivatives linked with 1,4-benzodioxane. Drug Des. Dev. Ther. 2019, 13, 2393–2404. [Google Scholar] [CrossRef]
- Kamil, M.; Fatima, A.; Ullah, S.; Ali, G.; Khan, R.; Ismail, N.; Qayum, M.; Irimie, M.; Dinu, C.; Ahmedah, H.; et al. Toxicological Evaluation of Novel Cyclohexenone Derivative in an Animal Model through Histopathological and Biochemical Techniques. Toxics 2021, 9, 119. [Google Scholar] [CrossRef]
- Malik, H.N.; Jabeen, A.; Ashraf, S.; Haq, Z.U.; Salar, U.; Arshia; Khan, K.M. Benzophenone and coumarin derivatives as 3-CLPro inhibitors: Targeting cytokine storm through in silico and in vitro approaches. J. Mol. Struct. 2022, 1265, 133478. [Google Scholar] [CrossRef]
- Tanoli, S.T.; Ramzan, M.; Hassan, A.; Sadiq, A.; Jan, M.S.; Khan, F.A.; Ullah, F.; Ahmad, H.; Bibi, M.; Mahmood, T.; et al. Design, synthesis and bioevaluation of tricyclic fused ring system as dual binding site acetylcholinesterase inhibitors. Bioorg. Chem. 2018, 83, 336–347. [Google Scholar] [CrossRef]
- Bibi, M.; Qureshi, N.A.; Sadiq, A.; Farooq, U.; Hassan, A.; Shaheen, N.; Asghar, I.; Umer, D.; Ullah, A.; Khan, F.A.; et al. Exploring the ability of dihydropyrimidine-5-carboxamide and 5-benzyl-2,4-diaminopyrimidine-based analogues for the selective inhibition of L. major dihydrofolate reductase. Eur. J. Med. Chem. 2020, 210, 112986. [Google Scholar] [CrossRef]
- Javed, M.A.; Ashraf, N.; Saeed, J.M.; Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Asiri, Y.I.; Ikram, M.; Sadiq, A.; et al. Structural Modification, In Vitro, In Vivo, Ex Vivo, and In Silico Exploration of Pyrimidine and Pyrrolidine Cores for Targeting Enzymes Associated with Neuroinflammation and Cholinergic Deficit in Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 4123–4143. [Google Scholar] [CrossRef] [PubMed]



| Groups | CK-MB(U/L) | CTnI (ng/mL) | LDH (U/L) |
|---|---|---|---|
| Normal control | 16.50 ± 0.619 *** | 0.029 ± 0.001 *** | 391.2 ± 9.495 *** |
| Standard drug control | 17.83 ± 0.654 *** | 0.085 ± 0.017 *** | 604.2 ± 19.58 *** |
| Toxic control | 35.50 ± 1.928 *** | 1.99 ± 0.045 *** | 1061 ± 22.20 *** |
| Comp-1 5 mg/kg (for 8 days) | 20.33 ± 0.421 *** | 1.529 ± 0.015 *** | 798.2 ± 22.01 *** |
| Comp-1 10 mg/kg (for 8 days) | 13.33 ± 0.614 *** | 1.017 ± 0.031 *** | 362.8 ± 13.53 *** |
| Groups | ALP(U/L) | ALT(U/L) | AST(U/L) | BT (mg/dL) | BD (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 35.51 ± 1.445 * | 33.13 ± 1.504 *** | 26.83 ± 0.872 *** | 7.142 ± 0.200 *** | 0.218 ± 0.015 *** |
| Standard drug Control | 35.00 ± 0.966 * | 38.68 ± 0.780 *** | 25.00 ± 1.155 *** | 8.012 ± 0.195 *** | 0.366 ± 0.022 *** |
| Toxic control | 40.83 ± 1.815 *** | 52.57 ± 1.235 *** | 37.56 ± 1.262 *** | 16.80 ± 0.173 *** | 1.907 ± 0.142 *** |
| Comp-1 5 mg/kg (for 8 days) | 25.68 ± 1.278 *** | 49.10 ± 1.865 ns | 26.00 ± 0.731 *** | 13.88 ± 0.634 *** | 2.047 ± 0.057 ns |
| Comp-1 10 mg/kg (for 8 days) | 20.49 ± 0.688 *** | 49.83 ± 1.217 ns | 26.17 ± 1.167 *** | 11.96 ± 0.963 *** | 1.680 ± 0.097 ns |
| Group | TC (mg/dL) | TG (mg/dL) | HDL-c (mg/dL) | LDL-c (mg/dL) | VLDL-c (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 27.95 ± 0.342 *** | 113.7 ± 1.404 *** | 63.12 ± 1.608 *** | 78.07 ± 0.947 *** | 24.27 ± 0.841 *** |
| Standard control | 37.89 ± 0.960 *** | 133.0 ± 6.005 *** | 68.66 ± 2.989 *** | 81.44 ± 0.989 *** | 23.23 ± 0.812 *** |
| Toxic control | 63.43 ± 0.945 *** | 209.6 ± 4.037 *** | 36.56 ± 1.626 *** | 150.0 ± 1.891 *** | 42.47 ± 0.662 *** |
| Comp-1 5 mg/kg (for 8 days) | 23.21 ± 1.710 *** | 193.1 ± 4.201 ns | 46.84 ± 0.497 ns | 121.1 ± 2.357 *** | 36.21 ± 1.356 *** |
| Comp-1 10 mg/kg (for 8 days) | 13.39 ± 1.325 *** | 148.0 ± 3.856 *** | 66.89 ± 2.429 *** | 92.18 ± 1.433 *** | 28.62 ± 1.363 *** |
| GROUPS | Normal Control (Normal Saline) | Standard Drug Control Atenolol + 5-FU-Treated Group | Toxic Control 5-FU-Treated Group | Comp-1 5 mg/kg Treated Group | Comp-1 10 mg/kg Treated Group |
|---|---|---|---|---|---|
| Cellular infiltration | 0 | 1 | 2 | 1 | 1 |
| Necrosis | 0 | 0 | 3 | 1 | 0 |
| Arterial congestion | 0 | 0 | 2 | 1 | 0 |
| Fibrosis | 0 | 0 | 3 | 1 | 0 |
| Groups | Normal Control (Normal Saline) | Toxic Control 5-FU-Treated Group | Comp-1 5 mg/kg Treated Group | Comp-1 10 mg/kg Treated Group |
|---|---|---|---|---|
| Sinusoidal spaces | 0 | 2 | 1 | 1 |
| Necrosis | 0 | 3 | 1 | 0 |
| hepatocyte degeneration | 0 | 2 | 1 | 0 |
| Fibrosis | 0 | 3 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qayyum, M.I.; Ullah, S.; Rashid, U.; Sadiq, A.; Obaidullah; Mahnashi, M.H.; Alshehri, O.M.; Jalal, M.M.; Alzahrani, K.J.; Halawani, I.F. Synthesis, Molecular Docking, and Preclinical Evaluation of a New Succinimide Derivative for Cardioprotective, Hepatoprotective and Lipid-Lowering Effects. Molecules 2022, 27, 6199. https://doi.org/10.3390/molecules27196199
Qayyum MI, Ullah S, Rashid U, Sadiq A, Obaidullah, Mahnashi MH, Alshehri OM, Jalal MM, Alzahrani KJ, Halawani IF. Synthesis, Molecular Docking, and Preclinical Evaluation of a New Succinimide Derivative for Cardioprotective, Hepatoprotective and Lipid-Lowering Effects. Molecules. 2022; 27(19):6199. https://doi.org/10.3390/molecules27196199
Chicago/Turabian StyleQayyum, Muhammad Imran, Sami Ullah, Umer Rashid, Abdul Sadiq, Obaidullah, Mater H. Mahnashi, Osama M. Alshehri, Mohammed M. Jalal, Khalid J. Alzahrani, and Ibrahim F. Halawani. 2022. "Synthesis, Molecular Docking, and Preclinical Evaluation of a New Succinimide Derivative for Cardioprotective, Hepatoprotective and Lipid-Lowering Effects" Molecules 27, no. 19: 6199. https://doi.org/10.3390/molecules27196199
APA StyleQayyum, M. I., Ullah, S., Rashid, U., Sadiq, A., Obaidullah, Mahnashi, M. H., Alshehri, O. M., Jalal, M. M., Alzahrani, K. J., & Halawani, I. F. (2022). Synthesis, Molecular Docking, and Preclinical Evaluation of a New Succinimide Derivative for Cardioprotective, Hepatoprotective and Lipid-Lowering Effects. Molecules, 27(19), 6199. https://doi.org/10.3390/molecules27196199







