Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects
Abstract
1. Introduction
2. Results
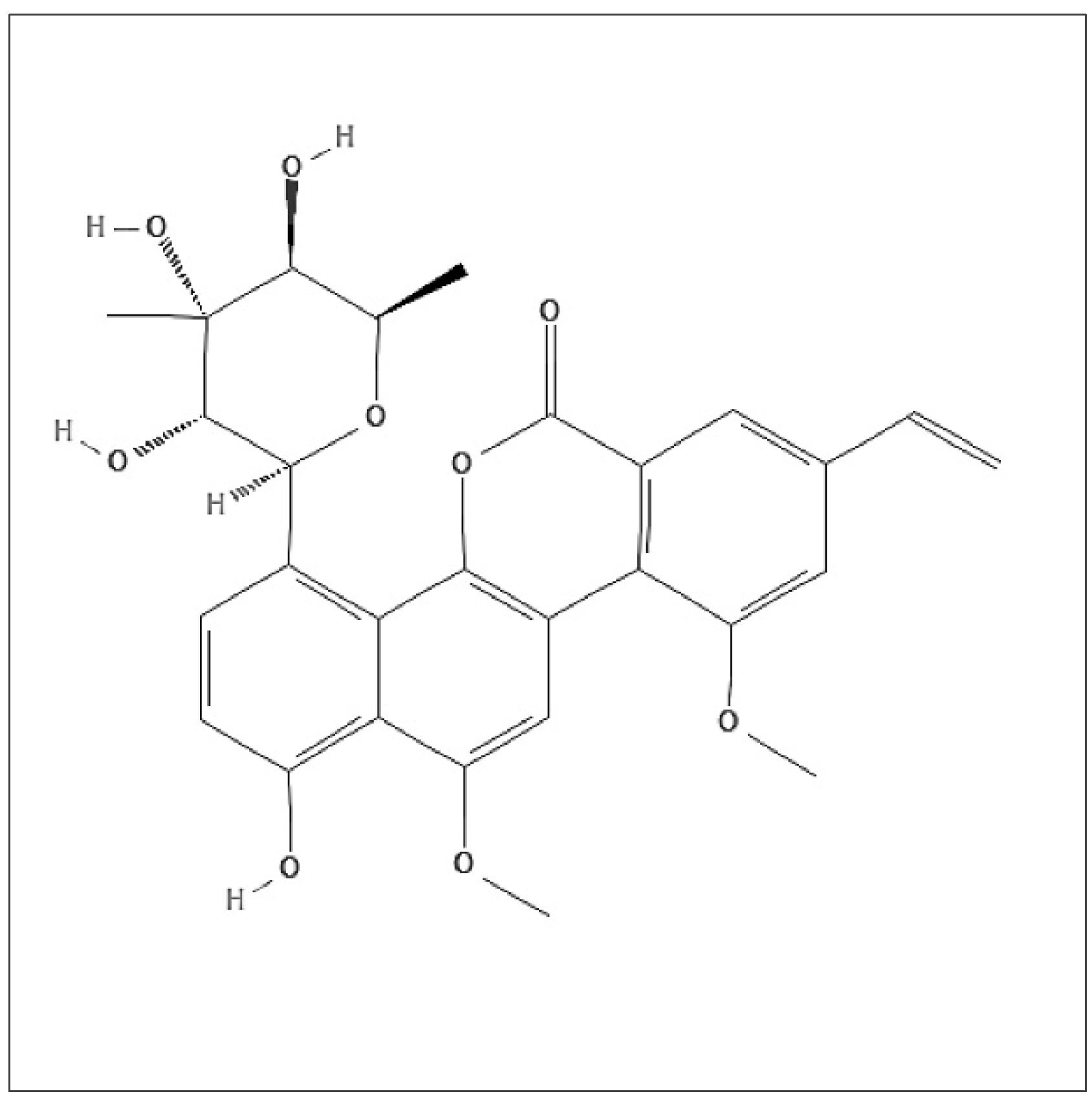
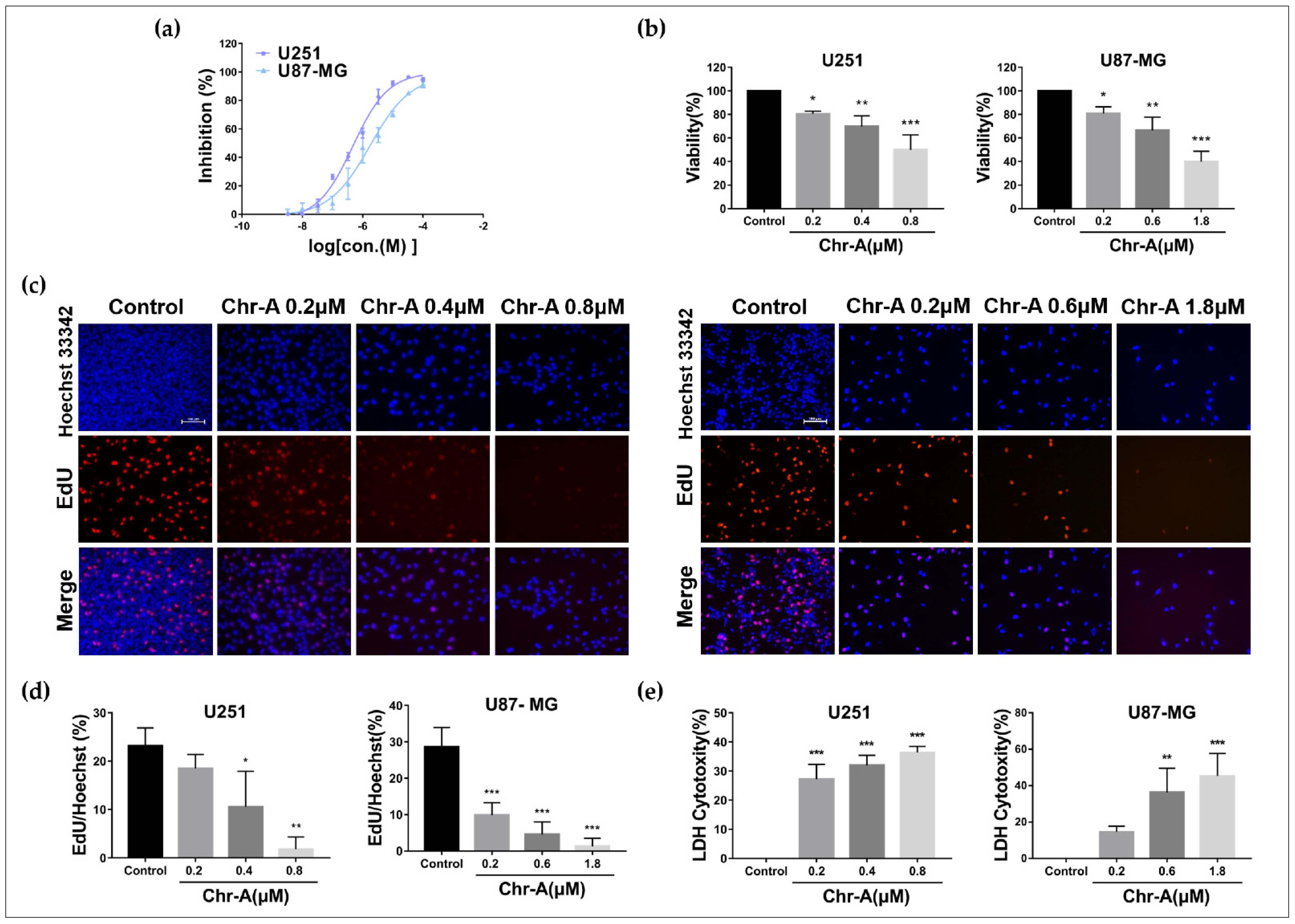
2.1. Chr-A Inhibits Viability and Proliferation of U251 and U87-MG Cells
2.2. Chr-A Attenuates Migration and Invasion of U251 and U87-MG Cells
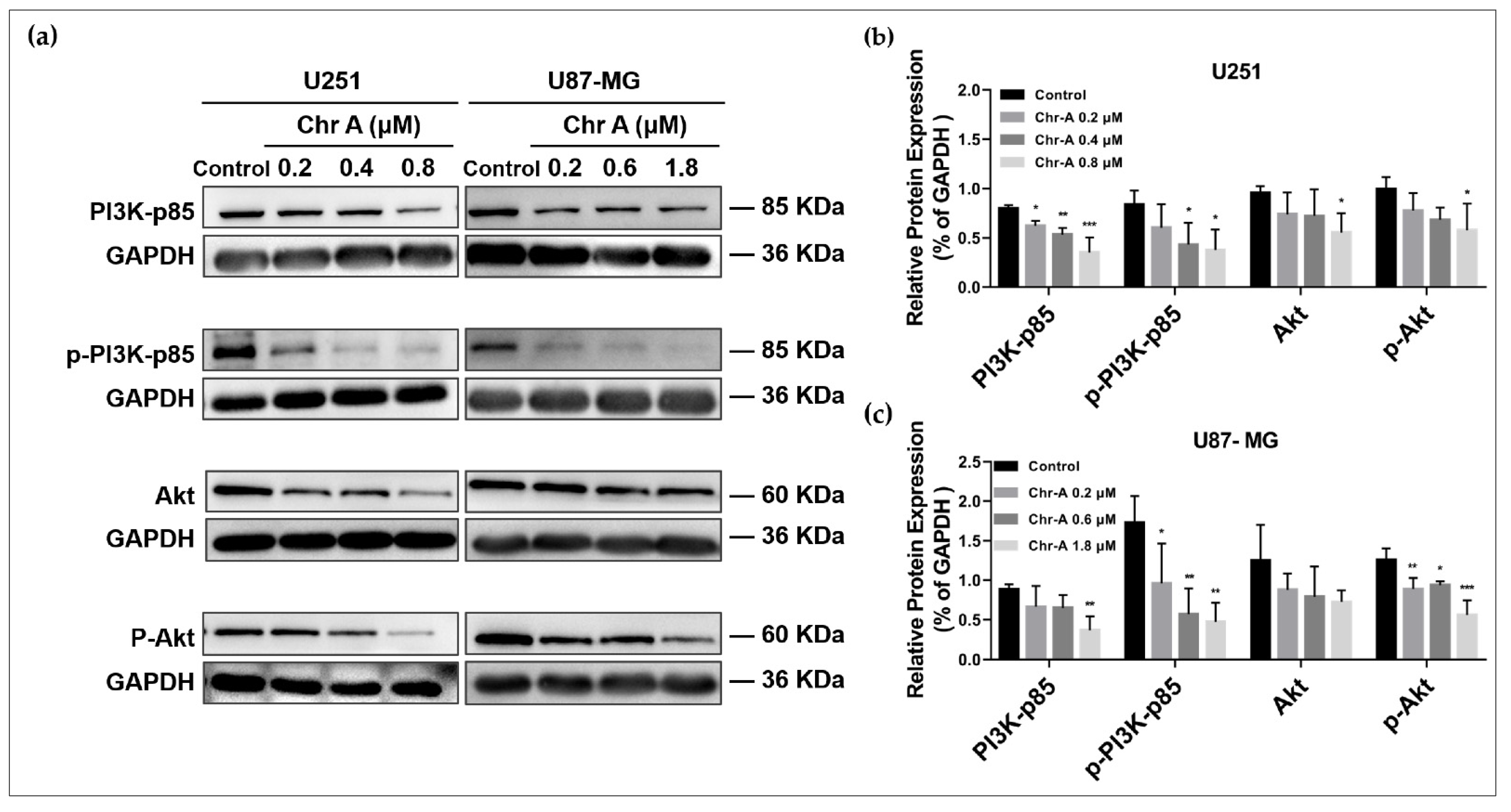
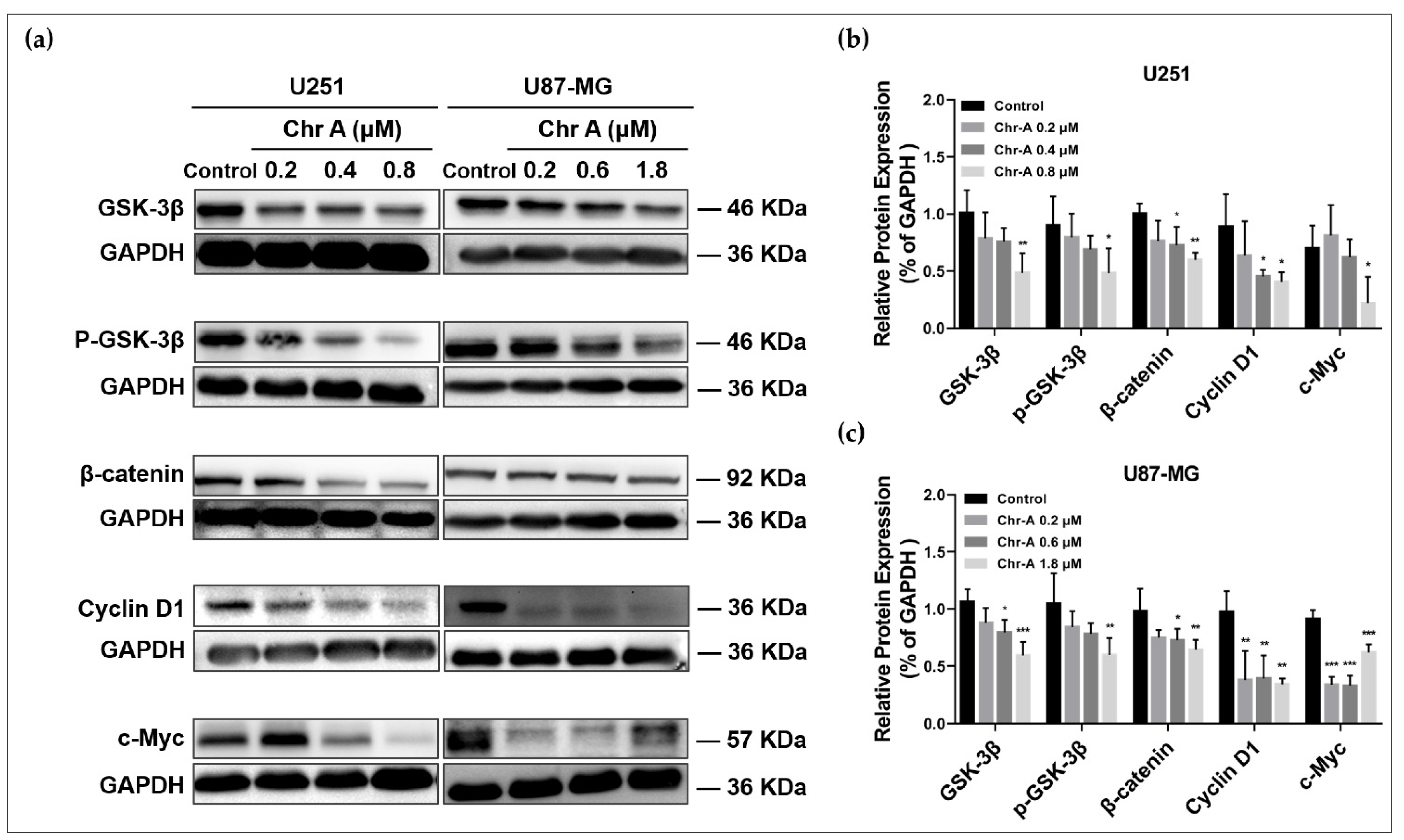
2.3. Chr-A Mediated Akt/GSK-3β/β-Catenin Signaling Pathway in U251 and U87-MG Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Cell Viability Assay
4.3. EdU-DNA Synthesis Assay
4.4. Lactate Dehydrogenase (LDH) Detection
4.5. Transwell Migration and Invasion Detection
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Y.; Lu, J.; Zhang, Z.; Zhu, L.; Dong, S.; Guo, G.; Li, R.; Nan, Y.; Yu, K.; Zhong, Y.; et al. Amlexanox, a selective inhibitor of IKBKE, generates anti-tumoral effects by disrupting the Hippo pathway in human glioblastoma cell lines. Cell Death Dis. 2017, 8, e3022. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; He, X.; Yu, W.; Chen, Y.; Long, Y.; Wang, K.; Zhu, S.; Liu, Q. p53-targeted lncRNA ST7-AS1 acts as a tumour suppressor by interacting with PTBP1 to suppress the Wnt/beta-catenin signalling pathway in glioma. Cancer Lett. 2021, 503, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Balca-Silva, J.; Matias, D.; do Carmo, A.; Girao, H.; Moura-Neto, V.; Sarmento-Ribeiro, A.B.; Lopes, M.C. Tamoxifen in combination with temozolomide induce a synergistic inhibition of PKC-pan in GBM cell lines. Biochim. Biophys. Acta 2015, 1850, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Strelitz, F.; Flon, H.; Asheshov, I.N. Chrysomycin: A new antibiotic substance for bacterial viruses. J. Bacteriol. 1955, 69, 280–283. [Google Scholar] [CrossRef]
- Li, Y.Q.; Huang, X.S.; Ishida, K.; Maier, A.; Kelter, G.; Jiang, Y.; Peschel, G.; Menzel, K.D.; Li, M.G.; Wen, M.L.; et al. Plasticity in gilvocarcin-type C-glycoside pathways: Discovery and antitumoral evaluation of polycarcin V from Streptomyces polyformus. Org. Biomol. Chem. 2008, 6, 3601–3605. [Google Scholar] [CrossRef]
- Wada, S.I.; Sawa, R.; Iwanami, F.; Nagayoshi, M.; Kubota, Y.; Iijima, K.; Hayashi, C.; Shibuya, Y.; Hatano, M.; Igarashi, M.; et al. Structures and biological activities of novel 4’-acetylated analogs of chrysomycins A and B. J. Antibiot. 2017, 70, 1078–1082. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, S.; Gao, X.; Hong, Y.; Cai, Y.; Zhang, Q.; Xiang, J.; Xie, D.; Song, F.; Zhang, H.; et al. Mechanochemical preparation of chrysomycin A self-micelle solid dispersion with improved solubility and enhanced oral bioavailability. J. Nanobiotechnol. 2021, 19, 164. [Google Scholar] [CrossRef]
- Ni, H.J.; Lv, S.Y.; Sheng, Y.T.; Wang, H.; Chu, X.H.; Zhang, H.W. Optimization of fermentation conditions and medium compositions for the production of chrysomycin a by a marine-derived strain Streptomyces sp. 891. Prep. Biochem. Biotechnol. 2021, 51, 998–1003. [Google Scholar] [CrossRef]
- Wei, T.T.; Byrne, K.M.; Warnick-Pickle, D.; Greenstein, M. Studies on the mechanism of actin of gilvocarcin V and chrysomycin A. J. Antibiot. 1982, 35, 545–548. [Google Scholar] [CrossRef][Green Version]
- Weiss, U.; Yoshihira, K.; Highet, R.J.; White, R.J.; Wei, T.T. The chemistry of the antibiotics chrysomycin A and B. Antitumor activity of chrysomycin A. J. Antibiot. 1982, 35, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, J.; Song, F.; Wang, S.; Guo, H.; Wei, Q.; Dai, H.; Chen, X.; Xia, X.; Liu, X.; et al. Chrysomycin A Derivatives for the Treatment of Multi-Drug-Resistant Tuberculosis. ACS Cent. Sci. 2020, 6, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, S.S.; Liu, D.N.; Yang, Y.L.; Wang, Y.H.; Du, G.H. Chrysomycin A Attenuates Neuroinflammation by Down-Regulating NLRP3/Cleaved Caspase-1 Signaling Pathway in LPS-Stimulated Mice and BV2 Cells. Int. J. Mol. Sci. 2021, 22, 6799. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Chen, L.; Qi, S.; Yu, S.; Weng, Z.; Hu, Z.; Zhou, Q.; Xin, Z.; Shi, L.; et al. HERC3-Mediated SMAD7 Ubiquitination Degradation Promotes Autophagy-Induced EMT and Chemoresistance in Glioblastoma. Clin. Cancer Res. 2019, 25, 3602–3616. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, J.; Dai, X.; Ye, L.; Wang, B.; Cheng, H.; Wang, W. Raddeanin A inhibited epithelial-mesenchymal transition (EMT) and angiogenesis in glioblastoma by downregulating beta-catenin expression. Int. J. Med. Sci. 2021, 18, 1609–1617. [Google Scholar] [CrossRef]
- Hwang, K.E.; Kim, H.J.; Song, I.S.; Park, C.; Jung, J.W.; Park, D.S.; Oh, S.H.; Kim, Y.S.; Kim, H.R. Salinomycin suppresses TGF-beta1-induced EMT by down-regulating MMP-2 and MMP-9 via the AMPK/SIRT1 pathway in non-small cell lung cancer. Int. J. Med. Sci. 2021, 18, 715–726. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Talaie, Z.; Jusheghani, F.; Los, M.J.; Klonisch, T.; Ghavami, S. Wnt and PI3K/Akt/mTOR Survival Pathways as Therapeutic Targets in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 1353. [Google Scholar] [CrossRef]
- Khabibov, M.; Garifullin, A.; Boumber, Y.; Khaddour, K.; Fernandez, M.; Khamitov, F.; Khalikova, L.; Kuznetsova, N.; Kit, O.; Kharin, L. Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review). Int. J. Oncol. 2022, 60, 69. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Jin, R.; Xu, H.; Li, Y.; Chen, Y.; Zhao, G. Pyrvinium pamoate regulates MGMT expression through suppressing the Wnt/beta-catenin signaling pathway to enhance the glioblastoma sensitivity to temozolomide. Cell Death Discov. 2021, 7, 288. [Google Scholar] [CrossRef]
- Walker, B.C.; Mittal, S. Antitumor Activity of Curcumin in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 9435. [Google Scholar] [CrossRef]
- Nager, M.; Bhardwaj, D.; Canti, C.; Medina, L.; Nogues, P.; Herreros, J. Beta-Catenin Signalling in Glioblastoma Multiforme and Glioma-Initiating Cells. Chemother. Res. Pract. 2012, 2012, 192362. [Google Scholar] [PubMed]
- Guan, R.; Zhang, X.; Guo, M. Glioblastoma stem cells and Wnt signaling pathway: Molecular mechanisms and therapeutic targets. Chin. Neurosurg. J. 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, Y.; Wu, J.; Kong, S. Effects of IGFBP-3 and GalNAc-T14 on proliferation and cell cycle of glioblastoma cells and its mechanism. J. Pharm. Pharmacol. 2020, 72, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, R.; Oliva, M.A.; Staffieri, S.; Castaldo, S.; Giangaspero, F.; Arcella, A. Implication of Lactucopicrin in Autophagy, Cell Cycle Arrest and Oxidative Stress to Inhibit U87Mg Glioblastoma Cell Growth. Molecules 2020, 25, 5843. [Google Scholar] [CrossRef]
- Feng, S.; Cai, X.; Li, Y.; Jian, X.; Zhang, L.; Li, B. Tripartite motif-containing 14 (TRIM14) promotes epithelial-mesenchymal transition via ZEB2 in glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 57. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Yan, X.; Wang, P. The role of miR-382-5p in glioma cell proliferation, migration and invasion. Onco Targets Ther. 2019, 12, 4993–5002. [Google Scholar] [CrossRef]
- Huang, Q.; Fu, Y.; Zhang, S.; Zhang, Y.; Chen, S.; Zhang, Z. Ethyl pyruvate inhibits glioblastoma cells migration and invasion through modulation of NF-kappaB and ERK-mediated EMT. PeerJ 2020, 8, e9559. [Google Scholar] [CrossRef]
- Cheng, B.; Morales, L.D.; Zhang, Y.; Mito, S.; Tsin, A. Niclosamide induces protein ubiquitination and inhibits multiple pro-survival signaling pathways in the human glioblastoma U-87 MG cell line. PLoS ONE 2017, 12, e0184324. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; Xu, H.; Liu, J.; Ren, L.; Yang, Y.; Li, S.; Wang, J.; Ji, T.; Du, G. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm. Sin. B 2021, 11, 3465–3480. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Zhao, P.; Zhao, H.; Gao, W.; Wang, L. Celastrol Suppresses Glioma Vasculogenic Mimicry Formation and Angiogenesis by Blocking the PI3K/Akt/mTOR Signaling Pathway. Front. Pharmacol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Tchokonte-Nana, V.; Lotfi, M.; Lotfi, M.; Ghorbani, A.; Sadeghnia, H.R. Wnt/beta-catenin and PI3K/Akt/mTOR Signaling Pathways in Glioblastoma: Two Main Targets for Drug Design: A Review. Curr. Pharm. Des. 2020, 26, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Q.; Zhao, N.; Zhang, X.; Cui, W.; Sun, J.; Fu, J.; Hao, J. Sohlh1 suppresses glioblastoma cell proliferation, migration, and invasion by inhibition of Wnt/beta-catenin signaling. Mol. Carcinog. 2018, 57, 494–502. [Google Scholar] [CrossRef]
- Chen, X.J.; Shen, Y.S.; He, M.C.; Yang, F.; Yang, P.; Pang, F.X.; He, W.; Cao, Y.M.; Wei, Q.S. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/beta-catenin signaling pathway. Biomed. Pharmacother. 2019, 112, 108746. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/beta-catenin signaling via transactivation of GSK-3beta and Axin2 expression. Cell Death Dis. 2019, 10, 26. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Li, M.Y.; Ng, C.S.H.; Yang, S.L.; Wang, S.; Zou, C.; Dong, Y.; Du, J.; Long, X.; et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/beta-catenin signaling pathway and EMT in non-small cell lung cancer. Mol. Cancer 2017, 16, 124. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-N.; Liu, M.; Zhang, S.-S.; Shang, Y.-F.; Song, F.-H.; Zhang, H.-W.; Du, G.-H.; Wang, Y.-H. Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects. Molecules 2022, 27, 6148. https://doi.org/10.3390/molecules27196148
Liu D-N, Liu M, Zhang S-S, Shang Y-F, Song F-H, Zhang H-W, Du G-H, Wang Y-H. Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects. Molecules. 2022; 27(19):6148. https://doi.org/10.3390/molecules27196148
Chicago/Turabian StyleLiu, Dong-Ni, Man Liu, Shan-Shan Zhang, Yu-Fu Shang, Fu-Hang Song, Hua-Wei Zhang, Guan-Hua Du, and Yue-Hua Wang. 2022. "Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects" Molecules 27, no. 19: 6148. https://doi.org/10.3390/molecules27196148
APA StyleLiu, D.-N., Liu, M., Zhang, S.-S., Shang, Y.-F., Song, F.-H., Zhang, H.-W., Du, G.-H., & Wang, Y.-H. (2022). Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects. Molecules, 27(19), 6148. https://doi.org/10.3390/molecules27196148








