Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells
Abstract
1. Introduction
2. Results
2.1. GC-MS Analysis and Antioxidant Potential
2.2. Antimicrobial Activity
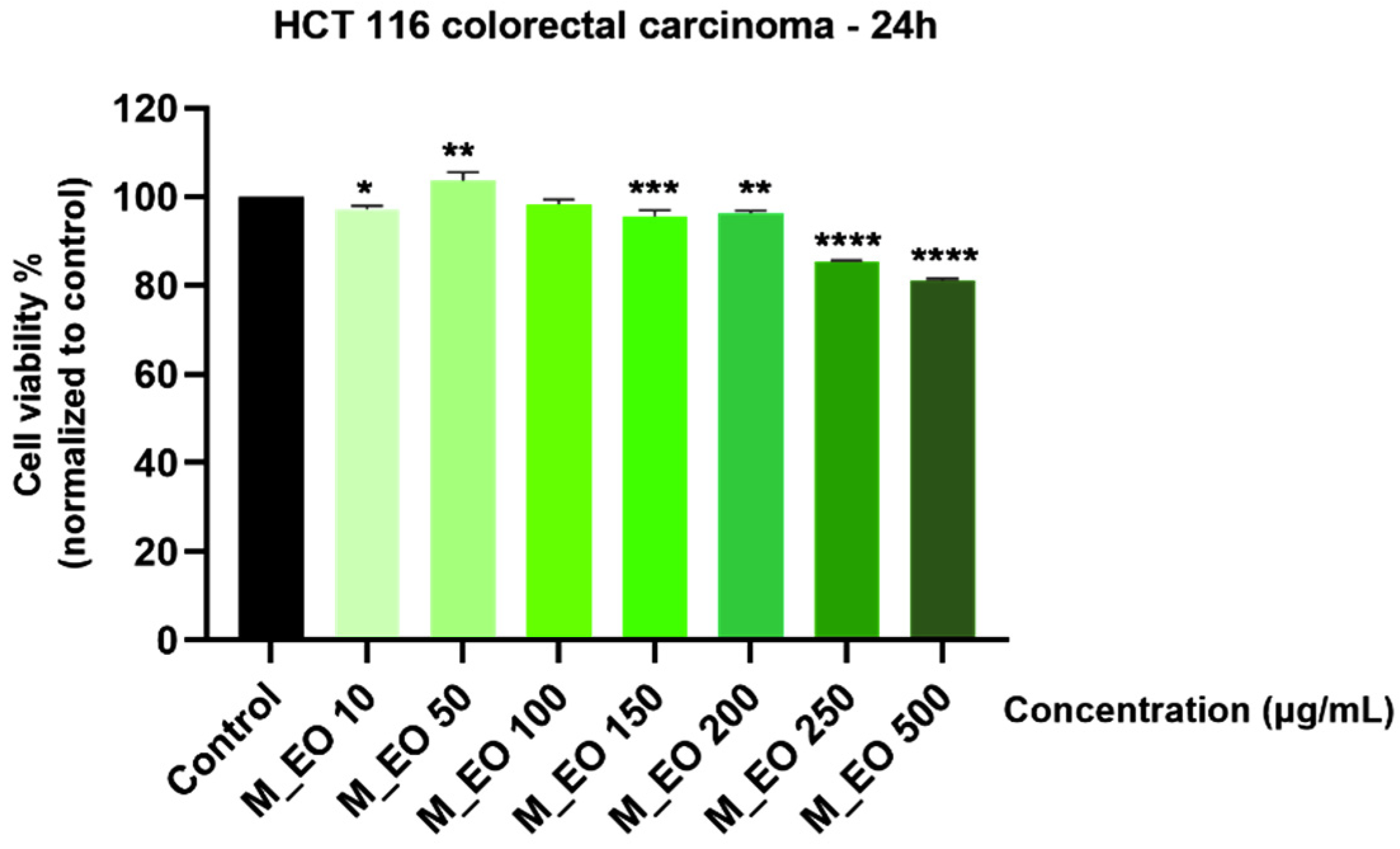
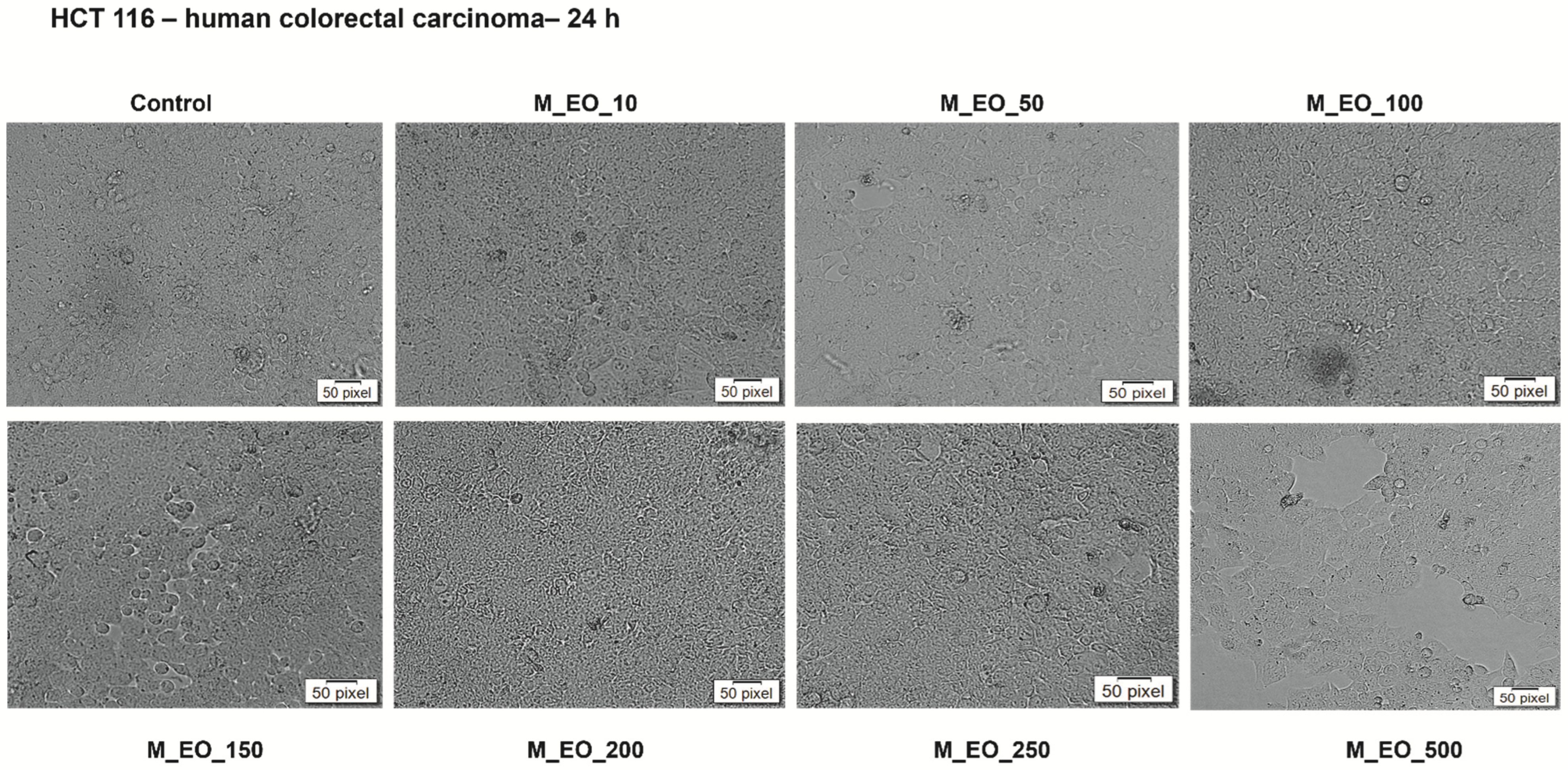
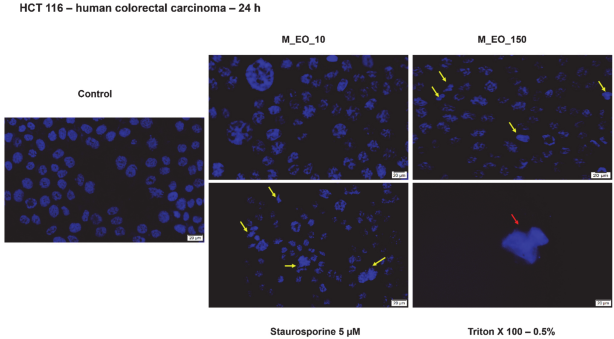
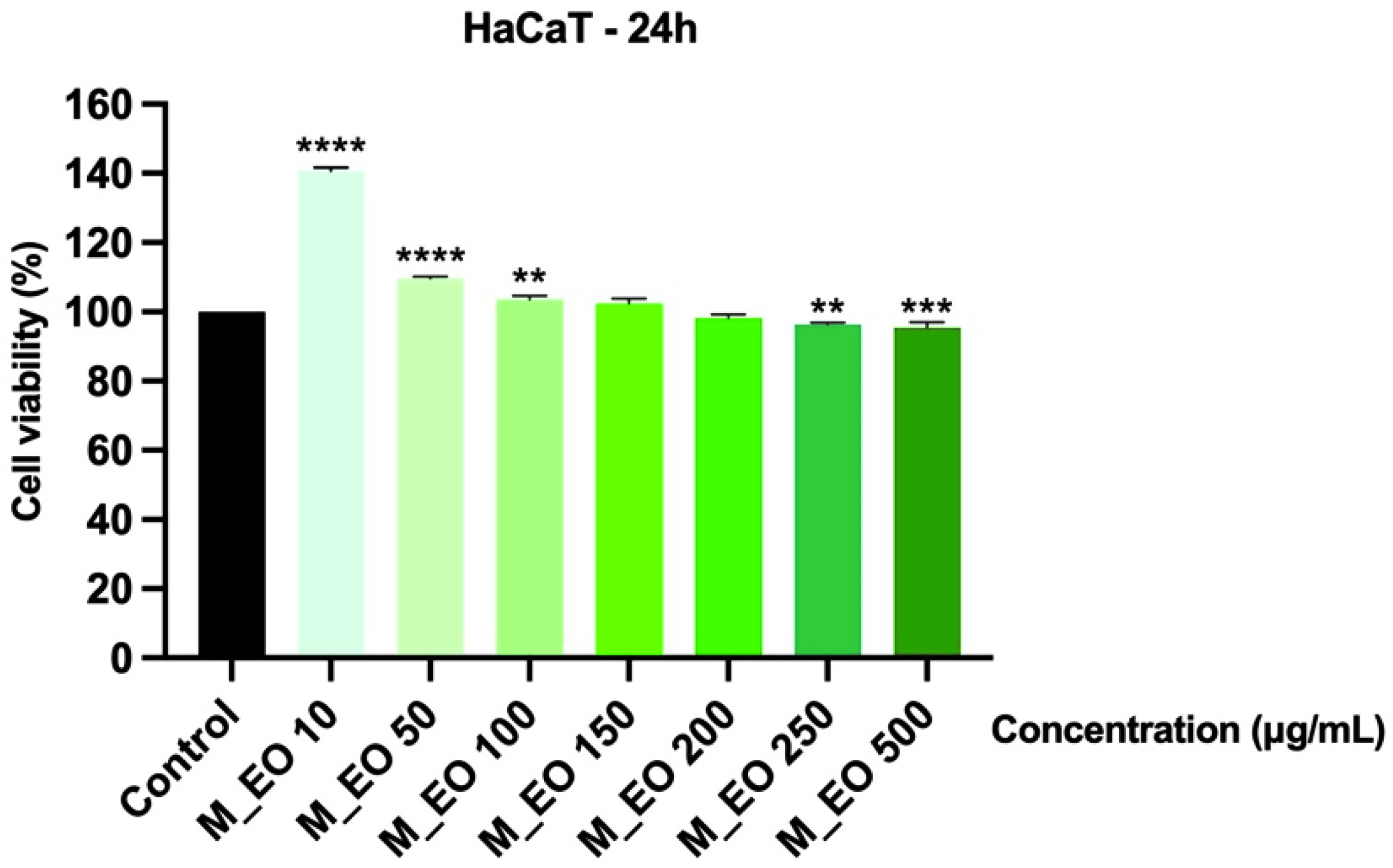
2.3. The Cytotoxic Effect of Mentha piperita L. Essential Oil
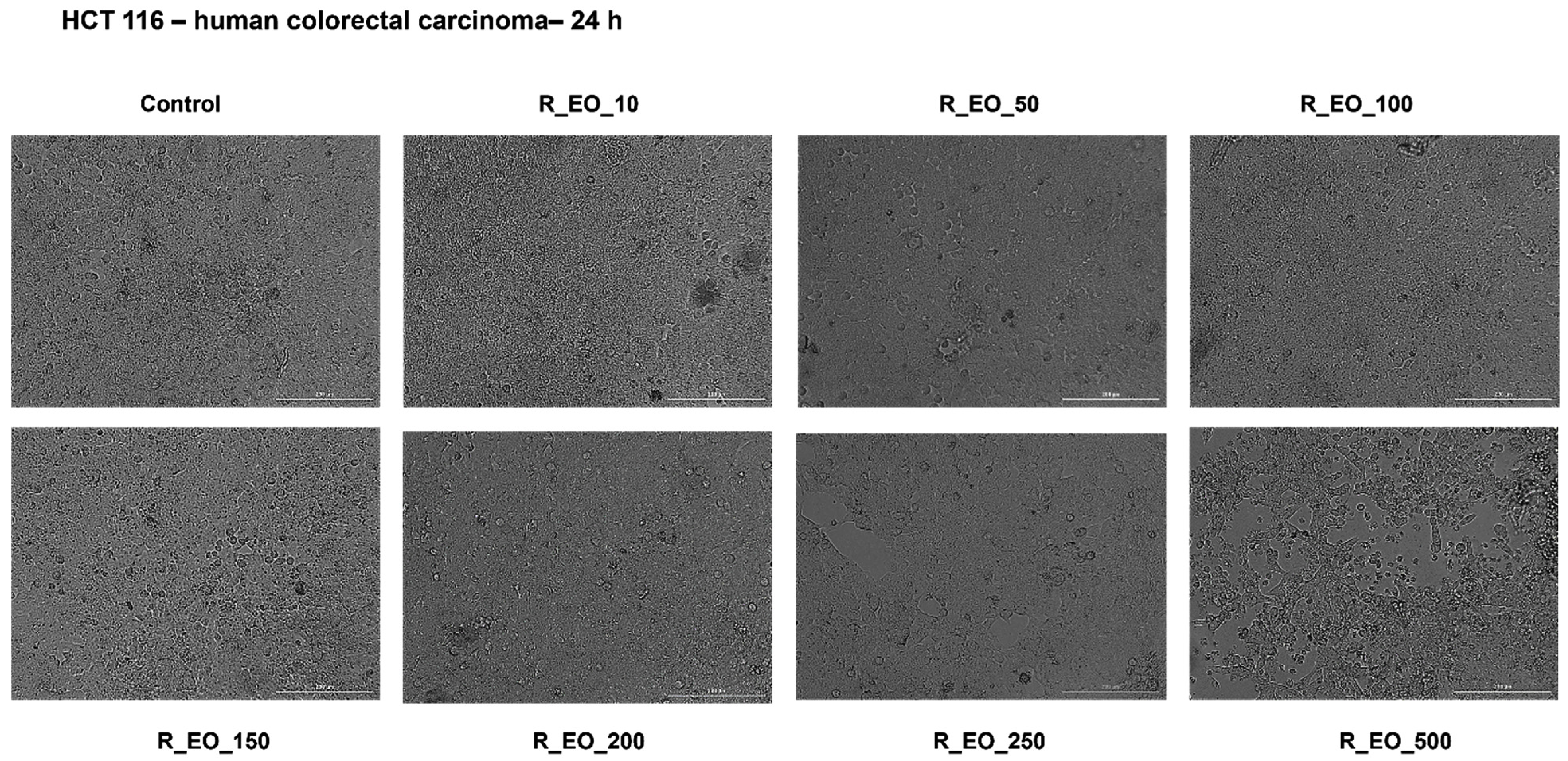
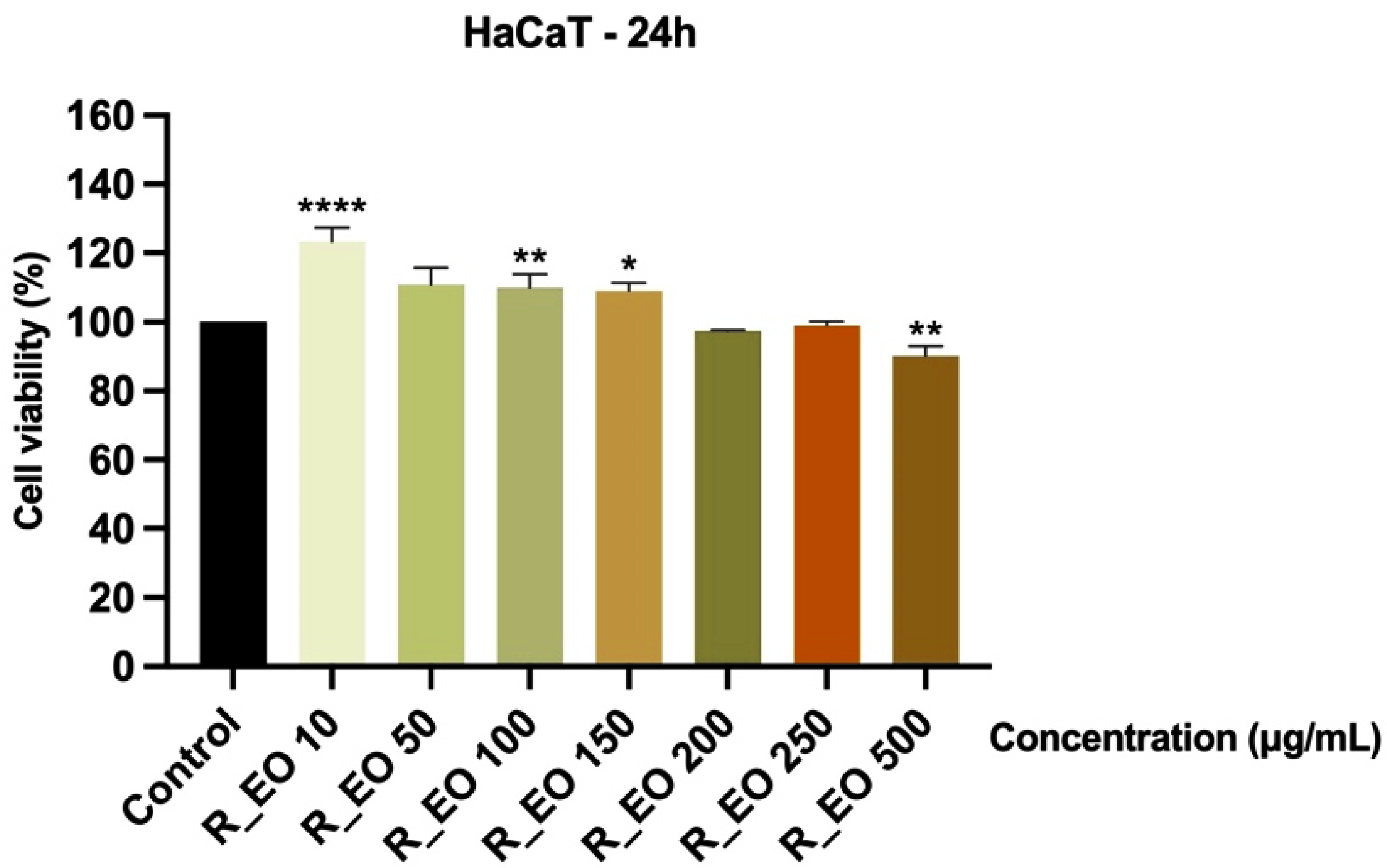
2.4. The Cytotoxic Effects of Rosmarinus officinalis L. Essential Oil
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.3. Assessment of the Antioxidant Capacity
4.4. Antimicrobial Activity
4.5. Cell Culture
4.6. Cell Viability Evaluation
4.7. Assessment of Cellular Morphology
4.8. Nuclear Staining Using Hoechst 33342 Dye
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Colorectal Cancers: An Update on Their Molecular Pathology. Cancers 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, An-ti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Grady, W.M.; Markowitz, S.D. The molecular pathogenesis of colorectal cancer and its po-tential application to colorectal cancer screening. Dig. Dis. Sci. 2015, 60, 762–772. [Google Scholar] [CrossRef]
- Guesmi, F.; Tyagi, A.K.; Prasad, S.; Landoulsi, A. Terpenes from essential oils and hydrolate of Teucrium alopecurus triggered apoptotic events dependent on caspases activation and PARP cleavage in human colon cancer cells through decreased protein expressions. Oncotarget 2018, 9, 32305–32320. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Feng, Y.; Deng, L.; Guo, H.; Zhao, Y.; Peng, F.; Wang, G.; Yu, C. The Anti-Colon Cancer Effects of Essential Oil of Curcuma phaeocaulis Through Tumour Vessel Normalisation. Front. Oncol. 2021, 11, 728464. [Google Scholar] [CrossRef]
- Athamneh, K.; Alneyadi, A.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.H.; Al Dhaheri, Y.; Iratni, R. Origanum majorana Es-sential Oil Triggers p38 MAPK-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef]
- Chandan, G.; Kumar, C.; Verma, M.K.; Satti, N.K.; Saini, A.K.; Saini, R.V. Datura stramonium essential oil composition and it’s immunostimulatory potential against colon cancer cells. 3 Biotech 2020, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. Biomed. Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef]
- Kadereit, J.; Kubitzki, K.; Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; de Kok, R.; et al. The Families and Genera of Vascular Plants; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 7, p. 190. [Google Scholar]
- İşcan, G.; Kïrïmer, N.; Kürkcüoǧlu, M.; Başer, H.C.; Demirci, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phyther. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Hirvonen, T.; Mennen, L.I.; de Bree, A.; Castetbon, K.; Galan, P.; Bertrais, S.; Arnault, N.; Hercberg, S. Consumption of Anti-oxidant-Rich Beverages and Risk for Breast Cancer in French Women. Ann. Epidemiol. 2006, 16, 503–508. [Google Scholar] [CrossRef]
- Kasem, R.F.; Hegazy, R.H.; Arafa, M.A.A.; Abdelmohsen, M.M. Chemopreventive effect of Mentha piperita on dime-thylbenz[a]anthracene and formaldehyde-induced tongue carcinogenesis in mice (histological and immunohistochemical study). J. Oral Pathol. Med. 2014, 43, 484–491. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Palni, U.T.; Tripathi, N.N. Use of essential oils of aromatic plants for the management of pigeon pea infestation by pulse bruchids during storage. Int. J. Agric. Technol. 2011, 7, 1615–1624. [Google Scholar]
- Efe Ertürk, N.; Taşcı, S. The Effects of Peppermint Oil on Nausea, Vomiting and Retching in Cancer Patients Undergoing Chemotherapy: An Open Label Quasi-Randomized Controlled Pilot Study. Complement. Ther. Med. 2021, 56, 102587. [Google Scholar] [CrossRef]
- Sharafi, S.M.; Rasooli, I.; Owlia, P.; Taghizadeh, M.; Astaneh, S.D. Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn. Mag. 2010, 6, 147–153. [Google Scholar] [PubMed]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Abrams, T.R.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; Kirkwood, C.D.; Giese, N.; Hoehn, K.; Iovin, R.; et al. An evidence-based systematic review of rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2010, 7, 351–413. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosma-rinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, K.; Assiri, A.M.A.; Tadros, M.; Khider, M.; Assaeedi, A.; Mohdaly, A.A.A.; Ramadan, M.F. Rosemary (Rosmarinus officinalis) oil: Composition and functionality of the cold-pressed extract. J. Food Meas. Charact. 2018, 12, 1601–1609. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 23 September 2021).
- Coricovac, D.E.; Moacă, E.A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef]
- Nikolic, M.; Jovanovic, K.K.; Markovic, T.; Markovic, D.; Gligorijevic, N.; Radulovic, S.; Sokovic, M. Chemical composition, antimicrobial, and cytotoxicproperties of five Lamiaceae essential oils. Ind. Crop. Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef]
- Gezici, S.; Sekeroglu, N.; Kijjoa, A. In vitro anticancer activity and antioxidant properties of essential oils from Populus alba L. and Rosmarinus officinalis L. fromSouth Eastern Anatolia of Turkey. Indian J. Pharm. Educ. Res. 2017, 51, S498–S503. [Google Scholar] [CrossRef]
- Santos, P.A.; Avanço, G.B.; Nerilo, S.B.; Marcelino, R.I.; Janeiro, V.; Valadares, M.C.; Machinski, M. Assessment of Cytotoxic Activity of Rosemary (Rosmarinus officinalis L.), Turmeric (Curcuma longa L.), and Ginger (Zingiber officinale R.) Essential Oils in Cervical Cancer Cells (HeLa). Sci. World J. 2016, 2016, 9273078. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, M.; Milosevic, M.; Petrovic, N.; Petrovic, S.; Damante, G.; Milasin, J.; Milovanovic, B. Cytotoxic Effects of Different Aromatic Plants Essential Oils on Oral Squamous Cell Carcinoma- an in vitro Study. Balk. J. Dent. Med. 2019, 23, 73–79. [Google Scholar] [CrossRef]
- Melusova, M.; Slamenova, D.; Kozics, K.; Jantova, S.; Horvathova, E. Carvacrol and rosemary essential oil manifest cytotoxic, DNA-protective and pro-apoptotic effect having no effect on DNA repair. Neoplasma 2014, 61, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Contini, A.; Di Bello, D.; Azzarà, A.; Giovanelli, S.; D’Urso, G.; Piaggi, S.; Pinto, B.; Pistelli, L.; Scarpato, R.; Testi, S. Assessing the cytotoxic/genotoxic activity and estrogen-ic/antiestrogenic potential of essential oils from seven aromatic plants. Food Chem. Toxicol. 2020, 138, 111205. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef]
- Moghaddam, M.; Pourbaige, M.; Tabar, H.K.; Farhadi, N.; Hosseini, S.M.A. Composition and antifungal activity of peppermint (Mentha piperita) essential oil from Iran. J. Essent. Oil Bear. Plants 2012, 16, 506–512. [Google Scholar] [CrossRef]
- Sadowska, U.; Żabiński, A.; Szumny, A.; Dziadek, K. An effect of peppermint herb (Mentha piperita L.) pressing on physico-chemical parameters of the resulting product. Ind. Crop. Prod. 2016, 94, 909–919. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ben Hsouna, A.; Athmouni, K.; van Pelt, J.; Ayadi, F.M.; Rebai, T.; Elfeki, A. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats. Lipids Health Dis. 2018, 17, 9. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Riabov, P.A.; Micić, D.; Božović, R.B.; Jovanović, D.V.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Ostojić, S.; Blagojević, S.; et al. The chemical, biological and thermal characteristics and gastronomical perspectives of Laurus nobilis essential oil from different geographical origin. Ind. Crop. Prod. 2020, 151, 112498. [Google Scholar] [CrossRef]
- Park, Y.J.; Baskar, T.B.; Yeo, S.K.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, S.S.; Park, S.U. Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. Springerplus 2016, 5, 1628. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Assessment Report on Mentha × Piperita L., Folium and Aetheroleum Final–Revision 1, 15 Jan 2020. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-mentha-x-piperita-l-folium-aetheroleum-revision-1_en.pdf (accessed on 20 May 2022).
- Tayarani-Najaran, Z.; Talasaz-Firoozi, E.; Nasiri, R.; Jalali, N.; Hassanzadeh, M. Antiemetic activity of volatile oil from Mentha spicata and Mentha × piperita in chemotherapy-induced nausea and vomiting. Ecancermedicalscience 2013, 7, 290. [Google Scholar] [CrossRef] [PubMed]
- Ruzauskas, M.; Bartkiene, E.; Stankevicius, A.; Bernatoniene, J.; Zadeike, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Grigas, J.; Zokaityte, E.; et al. The Influence of Essential Oils on Gut Microbial Profiles in Pigs. Animals 2020, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Shipradeep; Karmakar, S.; Khare, R.S.; Ojha, S.; Kundu, K.; Kundu, S. Development of Probiotic Candidate in Combination with Essential Oils from Medicinal Plant and Their Effect on Enteric Pathogens: A Review. Gastroenterol. Res. Pract. 2012, 2012, 457150. [Google Scholar] [CrossRef]
- Silva, W.M.F.; Bona, N.P.; Pedra, N.S.; Cunha, K.F.D.; Fiorentini, A.M.; Stefanello, F.M.; Zavareze, E.R.; Dias, A.R.G. Risk assessment of in vitro cytotoxicity, antioxidant and antimicrobial activities of Mentha piperita L. essential oil. J. Toxicol. Environ. Health A 2022, 85, 230–242. [Google Scholar] [CrossRef]
- Nikolić, B.; Vasilijević, B.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knežević-Vukćević, J. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem. Biol. Interact. 2015, 242, 263–271. [Google Scholar] [CrossRef]
- Takaki, I.; Bersani-Amado, L.E.; Vendruscolo, A.; Sartoretto, S.M.; Diniz, S.P.; Bersani-Amado, C.A.; Cuman, R.K. Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experimental animal models. J. Med. Food. 2008, 11, 741–746. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Hamza, B.; Boumediene, M.; Aicha, T.T.; Sekeroglu, N. Chemical composition and DNA damage protective effect of essential oil of Rosmarinus officinalis and Populus alba. Int. J. Phytopharm. 2016, 7, 196–201. [Google Scholar]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.G.; Andrade, L.N.; Azevedo Doria, G.A.; Barbosa-Filho, J.M.; Pergentino de Sousa, D.; Carvalho, A.A.; Thomazzi, S.M. Antitumor effects of the essential oil from Mentha x villosa combined with 5-fluorouracil in mice. Flavour Flagrance J. 2016, 31, 250–254. [Google Scholar] [CrossRef]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; de la Cueva, A.; Vargas, T.; Santoyo, S.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; de Molina, A.R. Antitumor effect of 5-fluorouracil is enhanced by rosemary extract in both drug sensitive and resistant colon cancer cells. Pharmacol. Res. 2013, 172, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration en-hancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2014, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Kijpornyongpan, T.; Sereemaspun, A.; Chanchao, C. Dose-Dependent Cytotoxic Effects of Menthol on Human Malignant Melanoma A-375 Cells: Correlation with TRPM8 Tran-script Expression. Asian Pac. J. Cancer Prev. 2014, 15, 1551–1556. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Z.; Yang, S.; Guo, S.; Dai, X.; Qiao, Y.; Shi, X. A Molecular Interpretation on the Different Penetration Enhancement Effect of Borneol and Menthol towards 5-Fluorouracil. Int. J. Mol. Sci. 2017, 18, 2747. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Langley, K.; Exinger, F.; Gosse, F.; Raul, F. Geraniol, a component of plant essential oils, sensitizes human colonic cancer cells to 5-Fluorouracil treatment. J. Pharmacol. Exp. Ther. 2002, 301, 625–630. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Bras-Gonçalves, R.; Bradaia, A.; Zeisel, M.; Gossé, F.; Poupon, M.F.; Raul, F. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004, 215, 53–59. [Google Scholar] [CrossRef]
- Popovici, R.A.; Vaduva, D.; Pinzaru, I.; Dehelean, C.A.; Farcas, C.G.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V.; et al. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef]
- EUCAST-AFST; Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07Ed11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Muntean, D.; Licker, M.; Alexa, E.; Popescu, I.; Jianu, C.; Buda, V.; Dehelean, C.A.; Ghiulai, R.; Horhat, F.; Horhat, D.; et al. Evaluation of essential oil obtained from Mentha×piperita L. against multidrug-resistant strains. Infect. Drug Resist. 2019, 12, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and Biological Screening of Oenothera biennis L. Hydroalcoholic Extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab-Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef]




| Compounds Name | Retention Time | LRI Rep [28] | LRI Exp | Conc. (%) |
|---|---|---|---|---|
| α-pinene (MH) | 6.375 | 1015–1030 | 1017 | 1.974 |
| β-pinene (MH) | 8.652 | 1105–1108 | 1106 | 2.204 |
| Thujene (MH) | 9.006 | 1022–1027 | 1023 | 0.585 |
| Limonene (MH) | 11.286 | 1196–1199 | 1196 | 3.816 |
| Eucalyptol (MH) | 11.504 | 1200–1211 | 1201 | 12.556 |
| γ-terpinene (MH) | 12.634 | 1243 | 1247 | 0.128 |
| Benzene, tert-butyl- | 13.253 | 1215–1249 | 1232 | 0.122 |
| Cyclohexanone, 3-methyl-, (R)- | 14.779 | 1376–1381 | 1372 | 0.233 |
| Menthone (MO) | 18.745 | 1438–1448 | 1446 | 28.970 |
| Menthofuran | 19.171 | 1477–1503 | 1491 | 1.681 |
| Mentha-3 ona, cis- | 19.440 | - | 1502 | 7.044 |
| α-bourbonene (SH) | 20.510 | 1514–1515 | 1512 | 0.339 |
| Linalool (MO) | 20.819 | 1550–1552 | 1553 | 0.144 |
| Mentholacetate | 21.235 | - | 1633 | 8.436 |
| Citronellol acetate | 21.721 | 1645–1662 | 1655 | 0.035 |
| Germacrene (SH) | 21.895 | 1684–1702 | 1694 | 0.327 |
| Caryophyllene (SH) | 22.484 | 1576–1597 | 1580 | 3.760 |
| Pulegone (MO) | 23.312 | 1631 | 1640 | 0.869 |
| Estragole | 23.674 | 1624–1685 | 1662 | 0.129 |
| β-citral | 23.992 | 1663–1696 | 1681 | 0.775 |
| α-caryophyllene (SH) | 24.220 | 1636–1670 | 1648 | 0.156 |
| α-terpineol | 24.882 | 1674–1692 | 1681 | 0.288 |
| Piperitone | 25.204 | 1715–1743 | 1723 | 2.686 |
| β-farnesene | 25.711 | 1660–1662 | 1658 | 0.196 |
| Cyclopropane, pentyl- | 26.803 | - | 1649 | 0.087 |
| Menthol | 27.736 | 1618–1637 | 1620 | 22.396 |
| Butanoic acid, 3,7-dimethyl-6-octenyl ester | 31.129 | 1765 | 1749 | 0.063 |
| Total | 99.999 | |||
| monoterpenes | 70.36 | |||
| sesquiterpenes | 11.11 | |||
| others | 18.52 | |||
| Total | 99.99 |
| Compounds Name | Retention Time | LRI Rep [28] | LRI Exp | Conc. (%) |
|---|---|---|---|---|
| α-Pinene (MH) | 6.382 | 1015–1030 | 1022 | 12.239 |
| α-Fenchene (MH) | 7.270 | 1048–1060 | 1051 | 0.032 |
| Camphene (MH) | 7.512 | 1060–180 | 1077 | 5.723 |
| β-Pinene (MH) | 8.657 | 1105–1108 | 1100 | 9.435 |
| β-Thujene (MH) | 8.657 | 1100–1133 | 1108 | 9.709 |
| Carane MH | - | - | - | N.D. |
| β-trans Ocimene (MH) | 9.838 | 1232–1250 | 1240 | 0.109 |
| β -Myrcene (MH) | 10.180 | 1155–1164 | 1154 | 1.546 |
| α-Phellandrene (MH) | 10.180 | 1153–1168 | 1158 | 1.546 |
| 1,4-Cineole | - | - | - | N.D. |
| Limonene (MH) | 11.307 | 1189–1205 | 1192 | 1.474 |
| Eucalyptol (MH) | 11.542 | 1198–1211 | 1201 | 33.592 |
| γ-Terpinene (MH) | 12.616 | 1243 | 1252 | 0.891 |
| p-Cymol (MH) | 13.253 | 1261–1283 | 1268 | 1.743 |
| Terpinolene (MH) | 13.685 | 1266–1274 | 1271 | 0.209 |
| D-Fenchone | - | - | - | N.D. |
| 2-Camphanone | - | - | - | N.D. |
| L-Camphor (MO) | 20.042 | 1532 | 1544 | 12.222 |
| β-Linalool (MO) | 20.796 | 1531–1552 | 1538 | 0.552 |
| Bornyl acetate (MO) | 21.676 | 1545–1567 | 1554 | 1.186 |
| Caryophyllene SH | 22.479 | 1576–1597 | 1582 | 2.859 |
| Estragole | 23.673 | 1624–1685 | 1658 | 0.151 |
| α-Caryophyllene (SH) | 24.231 | 1636–1670 | 1642 | 0.335 |
| α-Terpinyl acetate (MO) | 24.231 | 1676–1692 | 1680 | 0.339 |
| p-menth-1-en-8-ol (MO) | 24.944 | 1692–1704 | 1701 | 2.074 |
| Vinyl crotonate | - | - | - | N.D. |
| Menthol | - | - | - | N.D. |
| Borneol (MO) | 30.458 | 1692–1706 | 1698 | 1.999 |
| Nerolidol (SO) | 30.458 | 2006 | 1998 | 0.034 |
| Cinnamaldehyde | - | - | - | N.D. |
| Total | 99.999 | |||
| monoterpenes | 82.61 | |||
| sesquiterpenes | 13.04 | |||
| others | 4.34 | |||
| Total | 99.99 |
| Conc. µg/mL | IP (%) M_EO | IP (%) R_EO |
|---|---|---|
| 5 | 36.52 ± 0.04 **** | 34.25 ± 0.02 **** |
| 10 | 37.51 ± 0.04 **** | 34.55 ± 0.03 **** |
| 25 | 45.00 ± 0.07 **** | 35.14 ± 0.04 **** |
| 50 | 52.44 ± 0.1 **** | 35.66 ± 0.04 **** |
| 75 | 57.38 ± 0.2 **** | 35.83 ± 0.04 **** |
| 100 | 58.28 ± 0.2 **** | 37.21 ± 0.04 **** |
| 200 | 65.89 ± 0.2 **** | 38.56 ± 0.02 **** |
| Microbial Strain | Test Compound | IZ (mm) | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|---|
| Streptococcus mutans (+) | M_EO_1 | 31.67 | 1.25 | 1.25 |
| M_EO_2 | 24.33 | 0.625 | 0.625 | |
| M_EO_3 | 21.67 | 0.625 | 0.625 | |
| R_EO_1 | 13.00 | - | - | |
| R_EO_2 | 12.33 | - | - | |
| R_EO_3 | 8.67 | - | - | |
| Positive control (GM) | 19.67 | - | - | |
| Negative control (EtOH) | 7.67 | - | - | |
| Streptococcus pyogenes (+) | M_EO_1 | 33.33 | 1.25 | 1.25 |
| M_EO_2 | 31.33 | 0.625 | 0.625 | |
| M_EO_3 | 24.33 | 0.625 | 0.625 | |
| R_EO_1 | 12.67 | - | 10 | |
| R_EO_2 | 12.00 | - | - | |
| R_EO_3 | 9.00 | - | - | |
| Positive control (GM) | 20.67 | - | - | |
| Negative control (EtOH) | 8.00 | - | - | |
| Staphylococcus aureus (+) | M_EO_1 | 18.00 | 2.5 | 2.5 |
| M_EO_2 | 18.00 | 1.25 | 1.25 | |
| M_EO_3 | 12.00 | 1.25 | 1.25 | |
| R_EO_1 | 20.00 | 2.5 | 2.5 | |
| R_EO_2 | 12.00 | - | - | |
| R_EO_3 | 11.67 | - | - | |
| Positive control (GM) | 20 | - | - | |
| Negative control (EtOH) | 7 | - | - | |
| Escherichia coli (−) | M_EO_1 | 17.67 | 2.5 | 2.5 |
| M_EO_2 | 17.33 | 1.25 | 1.25 | |
| M_EO_3 | 15.33 | 1.25 | 1.25 | |
| R_EO_1 | 18.33 | 2.5 | 2.5 | |
| R_EO_2 | 11.67 | - | - | |
| R_EO_3 | 11.00 | - | - | |
| Positive control (GM) | 20.33 | - | - | |
| Negative control (EtOH) | 7 | - | - | |
| Pseudomonas aeruginosa (−) | M_EO_1 | 11.33 | - | - |
| M_EO_2 | 11.00 | - | - | |
| M_EO_3 | 8.67 | - | - | |
| R_EO_1 | 20.33 | 5 | 5 | |
| R_EO_2 | 11.33 | - | - | |
| R_EO_3 | 11.00 | - | - | |
| Positive control (GM) | 17.67 | - | - | |
| Negative control (EtOH) | 7 | - | - |
| Fungi | Test Compound | IZ (mm) | MIC (µg/mL) | MFC (µg/mL) |
|---|---|---|---|---|
| Candida albicans | M_EO_1 | 32.33 | 1.25 | 1.25 |
| M_EO_2 | 30.67 | 0.625 | 0.625 | |
| M_EO_3 | 23.67 | 0.625 | 1.25 | |
| R_EO_1 | 12.67 | - | - | |
| R_EO_2 | 12.00 | - | - | |
| R_EO_3 | 8.33 | - | - | |
| Positive control (FCZ) | 17.33 | - | - | |
| Negative control (EtOH) | 7 | - | - | |
| Candida parapsilosis | M_EO_1 | 31.33 | 1.25 | 1.25 |
| M_EO_2 | 30.33 | 0.625 | 0.625 | |
| M_EO_3 | 23.00 | 0.625 | 0.625 | |
| R_EO_1 | 12.33 | - | - | |
| R_EO_2 | 12.33 | - | - | |
| R_EO_3 | 8.00 | - | - | |
| Positive control (FCZ) | 18.33 | - | - | |
| Negative control (EtOH) | 7 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells. Molecules 2022, 27, 6106. https://doi.org/10.3390/molecules27186106
Dolghi A, Coricovac D, Dinu S, Pinzaru I, Dehelean CA, Grosu C, Chioran D, Merghes PE, Sarau CA. Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells. Molecules. 2022; 27(18):6106. https://doi.org/10.3390/molecules27186106
Chicago/Turabian StyleDolghi, Alina, Dorina Coricovac, Stefania Dinu, Iulia Pinzaru, Cristina Adriana Dehelean, Cristina Grosu, Doina Chioran, Petru Eugen Merghes, and Cristian Andrei Sarau. 2022. "Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells" Molecules 27, no. 18: 6106. https://doi.org/10.3390/molecules27186106
APA StyleDolghi, A., Coricovac, D., Dinu, S., Pinzaru, I., Dehelean, C. A., Grosu, C., Chioran, D., Merghes, P. E., & Sarau, C. A. (2022). Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells. Molecules, 27(18), 6106. https://doi.org/10.3390/molecules27186106








