Differential Expression and Localization of ADAMTS Proteinases in Proliferative Diabetic Retinopathy
Abstract
:1. Introduction
2. Results
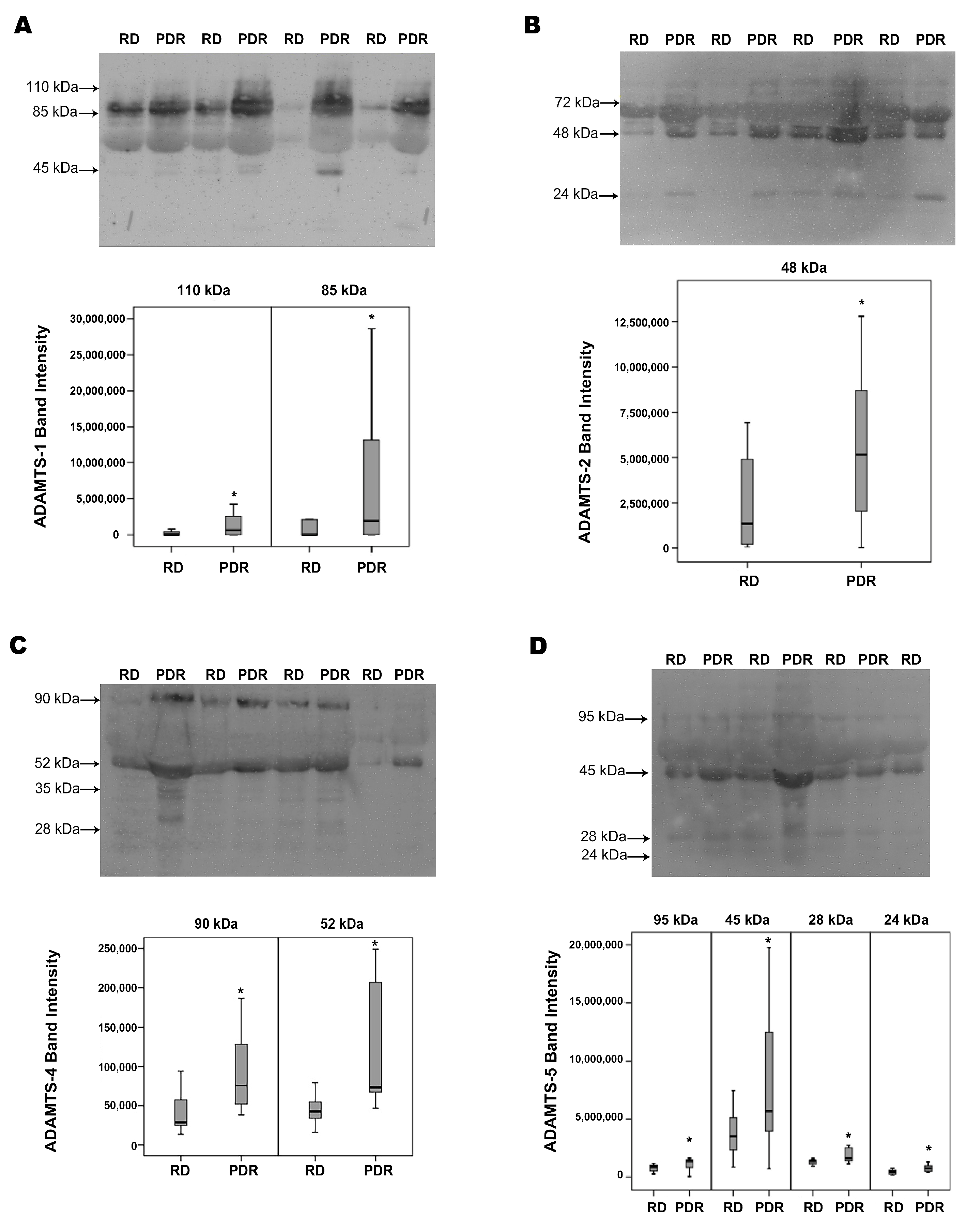
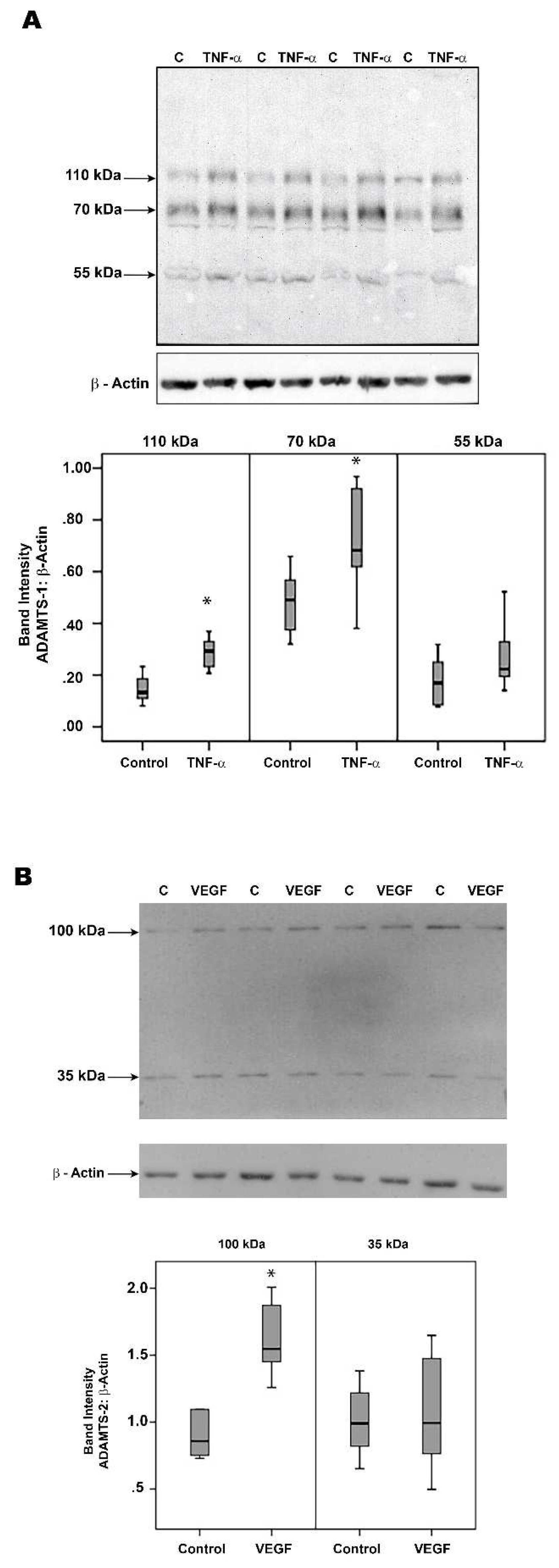
2.1. Western Blot Analysis of Vitreous Samples from Patients with PDR and Nondiabetic Control Patients
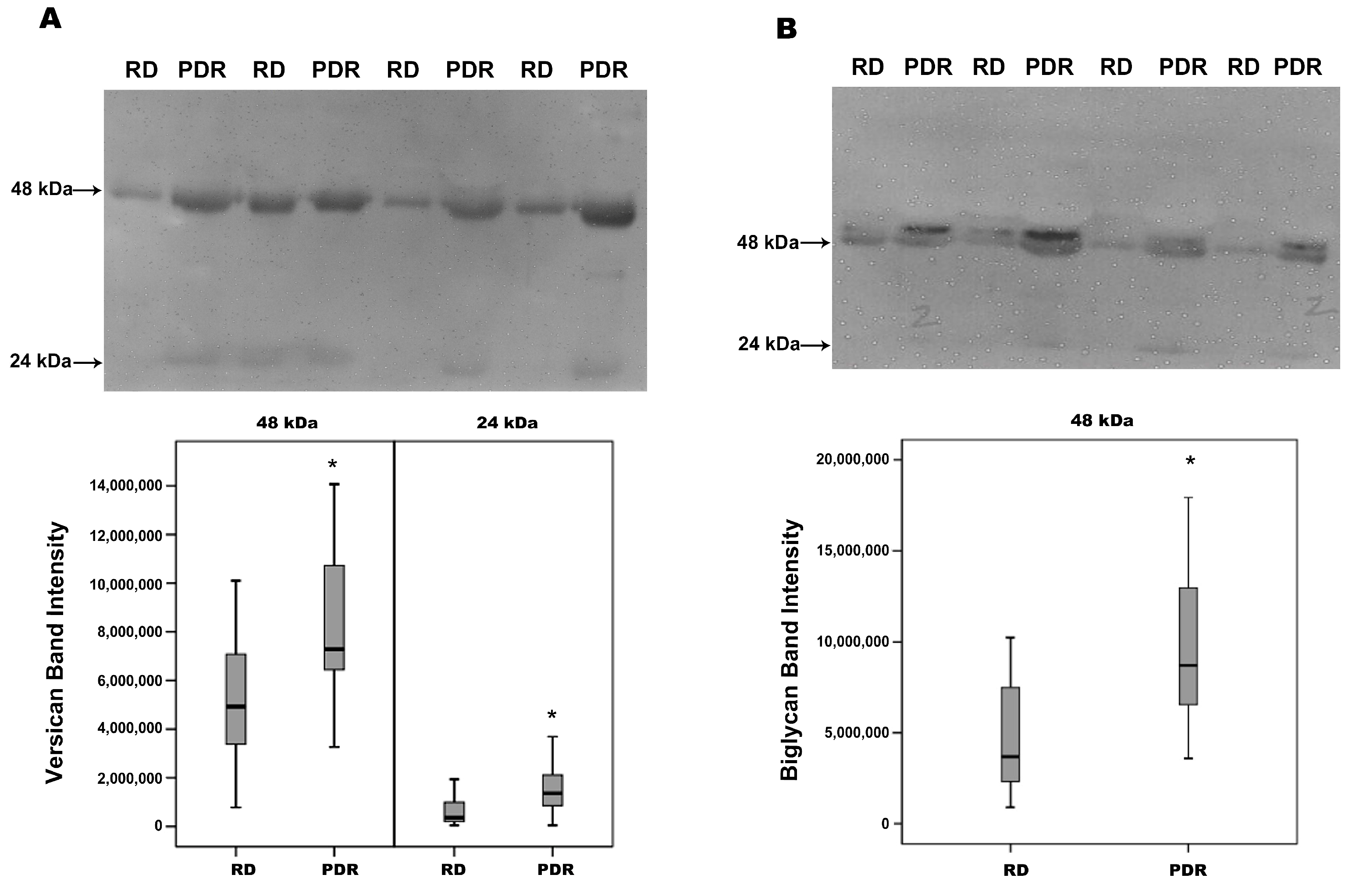
2.2. Increased Degradation Products of Versican and Biglycan in Vitreous Samples from Patients with PDR
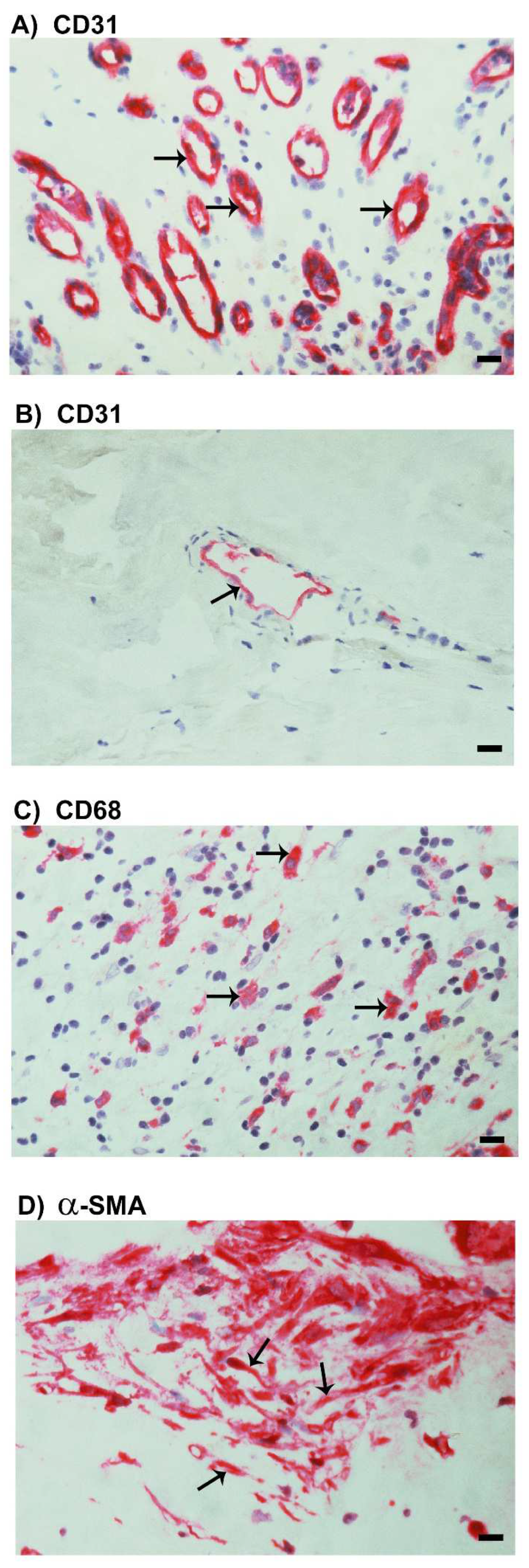
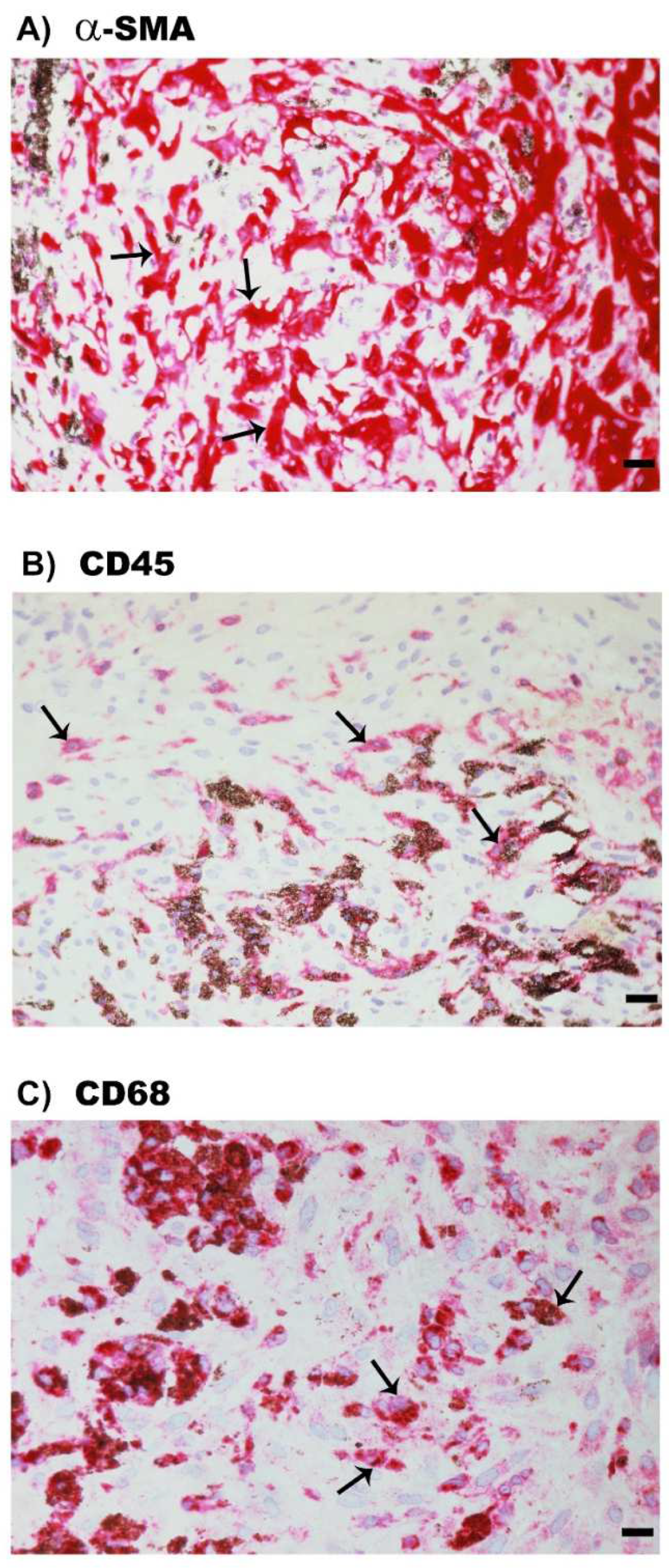
2.3. Neovascularization and Expression of the Monocyte/Macrophage Marker CD68 and the Myofibroblast Marker α-SMA in Epiretinal Fibrovascular Membranes from Patients with PDR
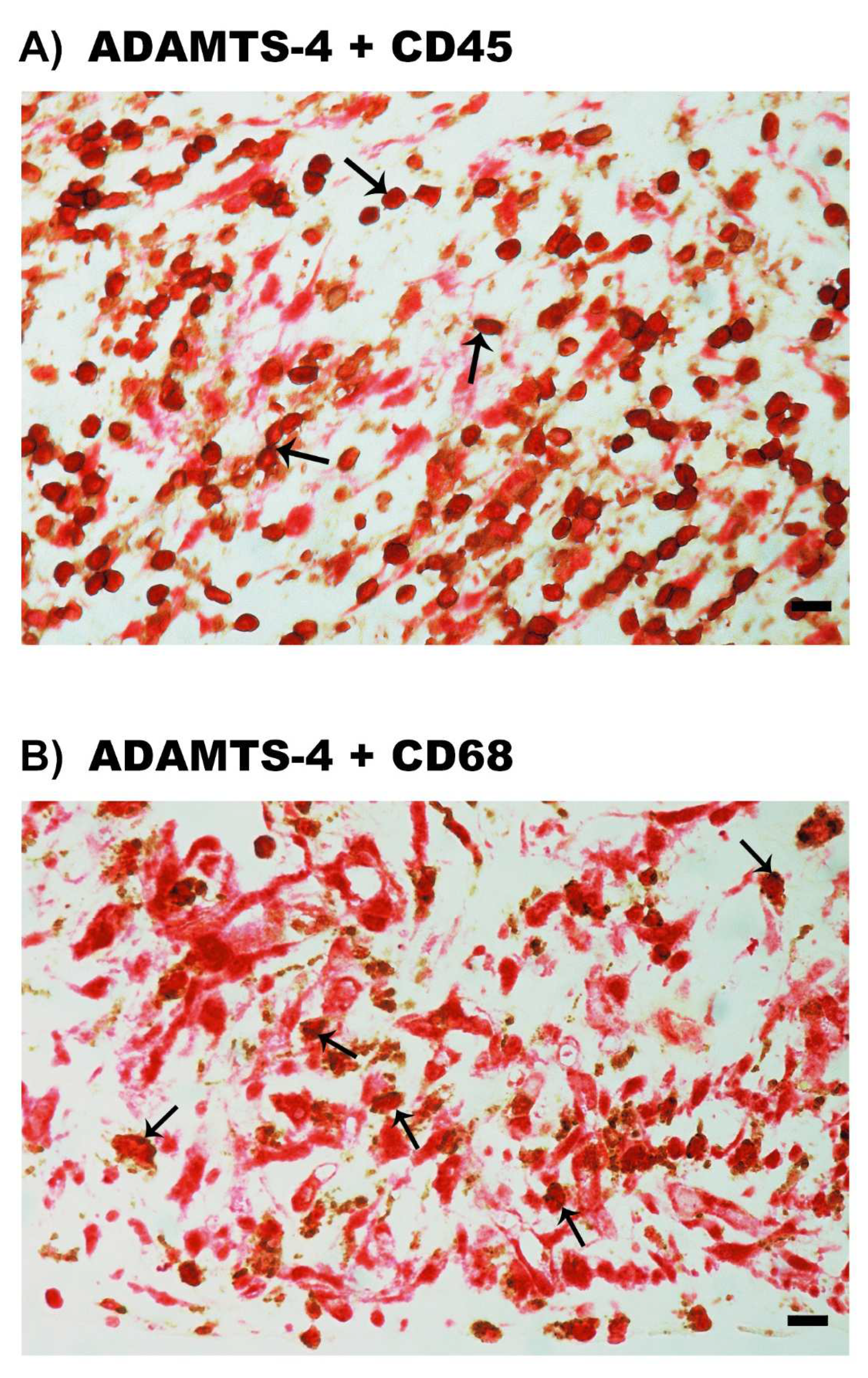
2.4. Expression of ADAMTS Proteinases in Epiretinal Fibrovascular Membranes from Patients with PDR
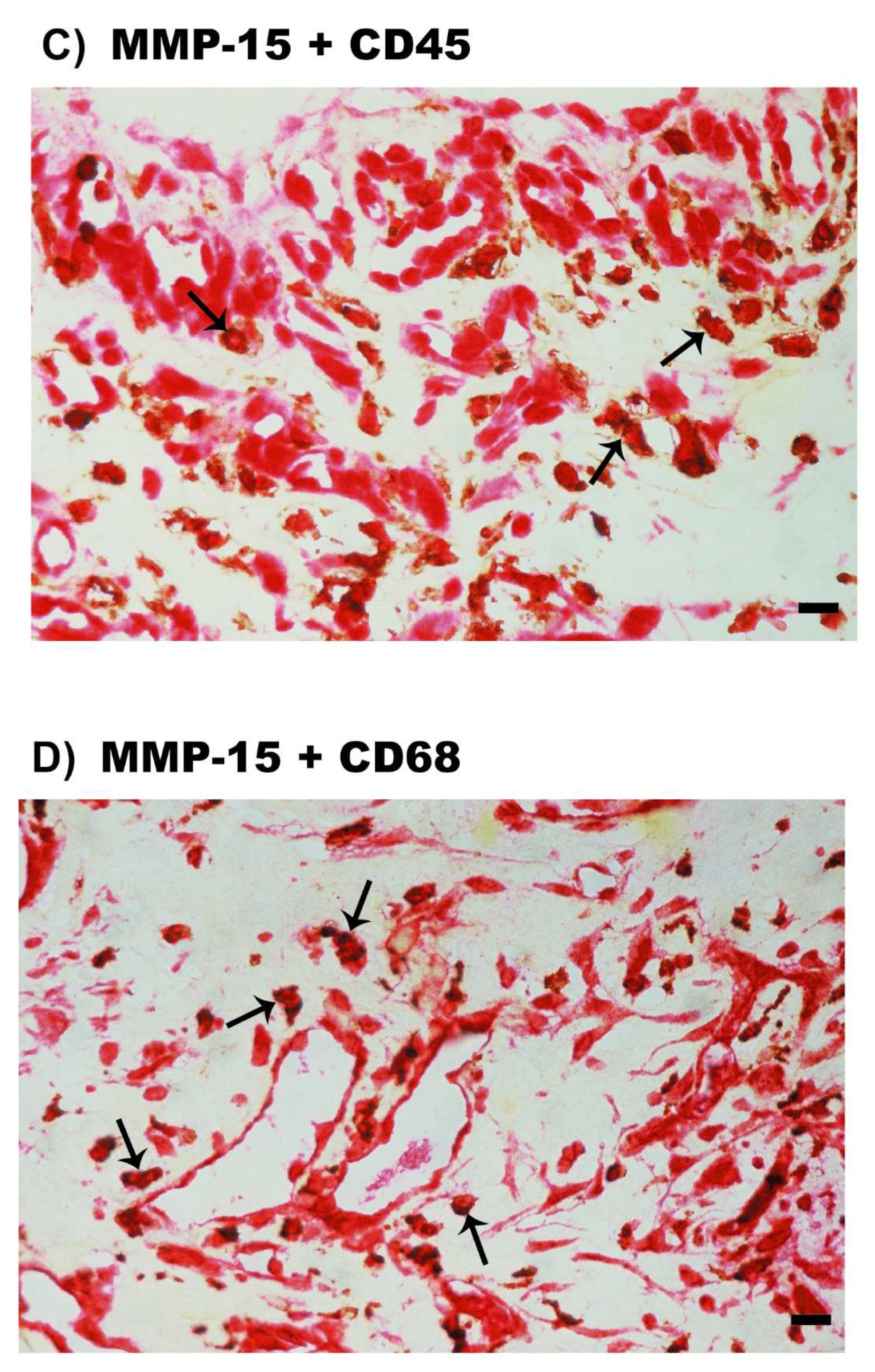
2.5. Expression of MMP-15 in Epiretinal Fibrovascular Membranes from Patients with PDR
2.6. Expression of α-SMA, the Leukocyte Common Antigen CD45 and the Monocyte/Macrophage Marker CD68 in Epiretinal Fibrocellular Membranes from Patients with PVR
2.7. Expression of ADAMTS Proteinases in Epiretinal Fibrocellular Membranes from Patients with PVR
2.8. Correlations between ADAMTS Proteinases Expression and Angiogenic Activity in Epiretinal Fibrovascular Membranes from Patients with PDR
2.9. Effect of Diabetes on the Retinal Expression of ADAMTS Proteinases and MMP-15 in Experimental Rats
2.10. Human Retinal Müller Glial Cells and Human Retinal Microvascular Endothelial Cells Constitutively Express ADAMTS-1, -2, -4, -5 and -13 but Not MMP-15
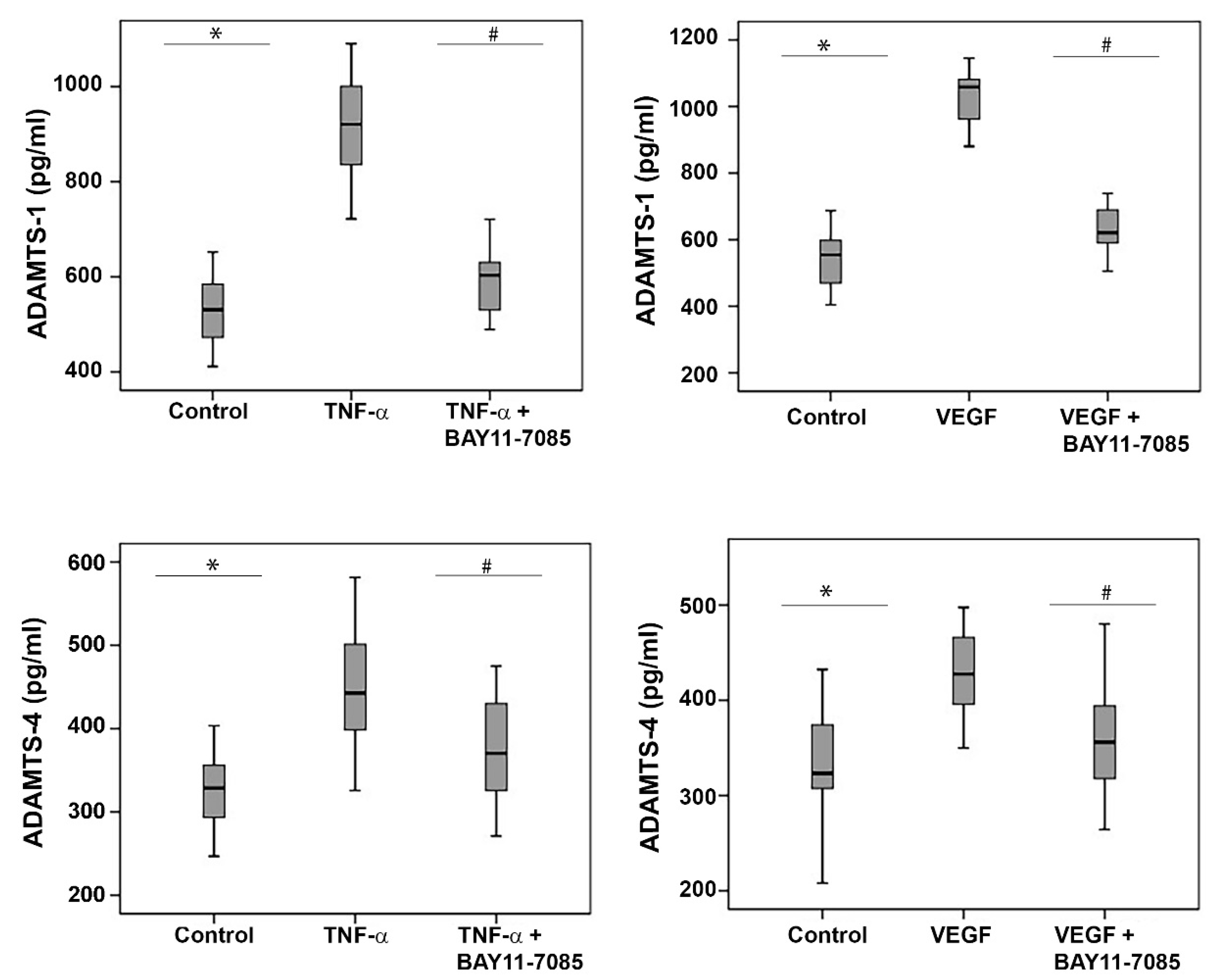
2.11. The NF-κB Inhibitor BAY11-7085 Attenuates the TNF-α-and-VEGF-Induced Upregulation of ADAMTS-1 and -4 in Human Retinal Microvascular Endothelial Cells and Human Retinal Müller Glial Cells
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Immunohistochemical Staining of Human Epiretinal Membranes and Quantifications
4.3. Induction of Streptozotocin-Induced Diabetes in Rats
4.4. Human Retinal Müller Glial Cell and Human Retinal Microvascular Endothelial Cell Cultures
4.5. Enzyme-Linked Immunosorbent Assays
4.6. Western Blot Analysis of Human Vitreous Fluid, Human Retinal Müller Glial Cell and Human Retinal Microvascular Endothelial Cell Lysates and Rat Retinas
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu El-Asrar, A.M.; Mohammad, G.; Nawaz, M.I.; Siddiquei, M.M.; Van den Eynde, K.; Mousa, A.; De Hertogh, G.; Opdenakker, G. Relationship between vitreous levels of matrix metalloproteinases and vascular endothelial growth factor in proliferative diabetic retinopathy. PLoS ONE 2013, 8, e85857. [Google Scholar]
- Abu El-Asrar, A.M.; Ahmad, A.; Alam, K.; Siddiquei, M.M.; Mohammad, G.; Hertogh, G.; Mousa, A.; Opdenakker, G. Extracellular matrix metalloproteinase inducer (EMMPRIN) is a potential biomarker of angiogenesis in proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Mohammad, G.; Allegaert, E.; Ahmad, A.; Siddiquei, M.M.; Alam, K.; Gikandi, P.W.; De Hertogh, G.; Opdenakker, G. Matrix metalloproteinase-14 is a biomarker of angiogenic activity in proliferative diabetic retinopathy. Mol. Vis. 2018, 24, 394–406. [Google Scholar] [PubMed]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Ahmad, A.; De Zutter, A.; Siddiquei, M.M.; Blanter, M.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Van Damme, J.; et al. Evaluation of Proteoforms of the Transmembrane Chemokines CXCL16 and CX3CL1, Their Receptors, and Their Processing Metalloproteinases ADAM10 and ADAM17 in Proliferative Diabetic Retinopathy. Front. Immunol. 2020, 11, 601639. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Ahmad, A.; Siddiquei, M.M.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Opdenakker, G. CD146/Soluble CD146 Pathway Is a Novel Biomarker of Angiogenesis and Inflammation in Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 32. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Ahmad, A.; Siddiquei, M.M.; De Zutter, A.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Van Damme, J.; Opdenakker, G.; Struyf, S. The Proinflammatory and Proangiogenic Macrophage Migration Inhibitory Factor Is a Potential Regulator in Proliferative Diabetic Retinopathy. Front. Immunol. 2019, 10, 2752. [Google Scholar] [CrossRef]
- Rezzola, S.; Loda, A.; Corsini, M.; Semeraro, F.; Annese, T.; Presta, M.; Ribatti, D. Angiogenesis-Inflammation Cross Talk in Diabetic Retinopathy: Novel Insights From the Chick Embryo Chorioallantoic Membrane/Human Vitreous Platform. Front. Immunol. 2020, 11, 581288. [Google Scholar] [CrossRef]
- Wu, G.; Liu, B.; Wu, Q.; Tang, C.; Du, Z.; Fang, Y.; Hu, Y.; Yu, H. Correlations Between Different Angiogenic and Inflammatory Factors in Vitreous Fluid of Eyes With Proliferative Diabetic Retinopathy. Front. Med. 2021, 8, 727407. [Google Scholar] [CrossRef]
- Handsley, M.M.; Edwards, D.R. Metalloproteinases and their inhibitors in tumor angiogenesis. Int. J. Cancer 2005, 115, 849–860. [Google Scholar] [CrossRef]
- Apte, S.S. ADAMTS Proteins: Concepts, Challenges, and Prospects. Methods Mol. Biol. 2020, 2043, 1–12. [Google Scholar]
- Mead, T.J.; Apte, S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71–72, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rao, N.; Ge, R. Emerging Roles of ADAMTSs in Angiogenesis and Cancer. Cancers 2012, 4, 1252–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Nagy, J.A.; Brown, L.F.; Shih, S.C.; Johnson, P.Y.; Chan, C.K.; Dvorak, H.F.; Wight, T.N. Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J. Histochem. Cytochem. 2011, 59, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Obika, M.; Vernon, R.B.; Gooden, M.D.; Braun, K.R.; Chan, C.K.; Wight, T.N. ADAMTS-4 and biglycan are expressed at high levels and co-localize to podosomes during endothelial cell tubulogenesis in vitro. J. Histochem. Cytochem. 2014, 62, 34–49. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, L.; Xu, G.; Palmero, L.C.; Albini, P.T.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2013, 95, 570–577. [Google Scholar] [CrossRef]
- Longpre, J.M.; Leduc, R. Identification of prodomain determinants involved in ADAMTS-1 biosynthesis. J. Biol. Chem. 2004, 279, 33237–33245. [Google Scholar] [CrossRef]
- Rao, N.; Ke, Z.; Liu, H.; Ho, C.J.; Kumar, S.; Xiang, W.; Zhu, Y.; Ge, R. ADAMTS4 and its proteolytic fragments differentially affect melanoma growth and angiogenesis in mice. Int. J. Cancer 2013, 133, 294–306. [Google Scholar] [CrossRef]
- Wang, P.; Tortorella, M.; England, K.; Malfait, A.M.; Thomas, G.; Arner, E.C.; Pei, D. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network. J. Biol. Chem. 2004, 279, 15434–15440. [Google Scholar] [CrossRef]
- Gao, G.; Westling, J.; Thompson, V.P.; Howell, T.D.; Gottschall, P.E.; Sandy, J.D. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J. Biol. Chem. 2002, 277, 11034–11041. [Google Scholar] [CrossRef]
- Tortorella, M.D.; Arner, E.C.; Hills, R.; Gormley, J.; Fok, K.; Pegg, L.; Munie, G.; Malfait, A.M. ADAMTS-4 (aggrecanase-1): N-terminal activation mechanisms. Arch. Biochem. Biophys. 2005, 444, 34–44. [Google Scholar] [CrossRef]
- Kumar, S.; Sharghi-Namini, S.; Rao, N.; Ge, R. ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. Am. J. Pathol. 2012, 181, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Deforche, L.; Roose, E.; Vandenbulcke, A.; Vandeputte, N.; Feys, H.B.; Springer, T.A.; Mi, L.Z.; Muia, J.; Sadler, J.E.; Soejima, K.; et al. Linker regions and flexibility around the metalloprotease domain account for conformational activation of ADAMTS-13. J. Thromb. Haemost. 2015, 13, 2063–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Manzaneque, J.C.; Milchanowski, A.B.; Dufour, E.K.; Leduc, R.; Iruela-Arispe, M.L. Characterization of METH-1/ADAMTS1 processing reveals two distinct active forms. J. Biol. Chem. 2000, 275, 33471–33479. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Duh, E.J. Vascular endothelial growth factor upregulates expression of ADAMTS1 in endothelial cells through protein kinase C signaling. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4059–4066. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Ahmad, A.; Siddiquei, M.M.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Opdenakker, G. Proprotein convertase furin is a driver and potential therapeutic target in proliferative diabetic retinopathy. Clin. Exp. Ophthalmol. 2022, 50, 632–652. [Google Scholar] [CrossRef] [PubMed]
- Kenagy, R.D.; Plaas, A.H.; Wight, T.N. Versican degradation and vascular disease. Trends Cardiovasc. Med. 2006, 16, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Hughes, M.; Krishnamoorthy, S.; Zou, S.; Zhang, L.; Wu, D.; Zhang, C.; Curci, J.A.; Coselli, J.S.; Milewicz, D.M.; et al. Critical Role of ADAMTS-4 in the Development of Sporadic Aortic Aneurysm and Dissection in Mice. Sci. Rep. 2017, 7, 12351. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xu, Y.; Yu, Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene 2006, 25, 2452–2467. [Google Scholar] [CrossRef]
- Kuno, K.; Bannai, K.; Hakozaki, M.; Matsushima, K.; Hirose, K. The carboxyl-terminal half region of ADAMTS-1 suppresses both tumorigenicity and experimental tumor metastatic potential. Biochem. Biophys. Res. Commun. 2004, 319, 1327–1333. [Google Scholar]
- Krampert, M.; Kuenzle, S.; Thai, S.N.; Lee, N.; Iruela-Arispe, M.L.; Werner, S. ADAMTS1 proteinase is up-regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J. Biol. Chem. 2005, 280, 23844–23852. [Google Scholar] [CrossRef]
- Lee, M.; Rodansky, E.S.; Smith, J.K.; Rodgers, G.M. ADAMTS13 promotes angiogenesis and modulates VEGF-induced angiogenesis. Microvasc. Res. 2012, 84, 109–115. [Google Scholar] [CrossRef]
- Wagsater, D.; Bjork, H.; Zhu, C.; Bjorkegren, J.; Valen, G.; Hamsten, A.; Eriksson, P. ADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis 2008, 196, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Ashlin, T.G.; Kwan, A.P.; Ramji, D.P. Regulation of ADAMTS-1, -4 and -5 expression in human macrophages: Differential regulation by key cytokines implicated in atherosclerosis and novel synergism between TL1A and IL-17. Cytokine 2013, 64, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashlin, T.G.; Buckley, M.L.; Salter, R.C.; Johnson, J.L.; Kwan, A.P.; Ramji, D.P. The anti-atherogenic cytokine interleukin-33 inhibits the expression of a disintegrin and metalloproteinase with thrombospondin motifs-1, -4 and -5 in human macrophages: Requirement of extracellular signal-regulated kinase, c-Jun N-terminal kinase and phosphoinositide 3-kinase signaling pathways. Int. J. Biochem. Cell Biol. 2014, 46, 113–123. [Google Scholar] [PubMed]
- Norata, G.D.; Bjork, H.; Hamsten, A.; Catapano, A.L.; Eriksson, P. High-density lipoprotein subfraction 3 decreases ADAMTS-1 expression induced by lipopolysaccharide and tumor necrosis factor-alpha in human endothelial cells. Matrix Biol. 2004, 22, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, S.; Gutierrez-Canas, I.; Seoane, I.V.; Fernandez, J.; Mellado, M.; Leceta, J.; Tio, L.; Villanueva-Romero, R.; Juarranz, Y.; Gomariz, R.P. Healthy and Osteoarthritic Synovial Fibroblasts Produce a Disintegrin and Metalloproteinase with Thrombospondin Motifs 4, 5, 7, and 12: Induction by IL-1beta and Fibronectin and Contribution to Cartilage Damage. Am. J. Pathol. 2016, 186, 2449–2461. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Miller, J.W.; Le Couter, J.; Strauss, E.C.; Ferrara, N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 2013, 120, 106–114. [Google Scholar] [CrossRef]
- Spranger, J.; Pfeiffer, A.F. New concepts in pathogenesis and treatment of diabetic retinopathy. Exp. Clin. Endocrinol. Diabetes 2001, 109, S438–S450. [Google Scholar] [CrossRef]
- Hsu, Y.P.; Staton, C.A.; Cross, N.; Buttle, D.J. Anti-angiogenic properties of ADAMTS-4 in vitro. Int. J. Exp. Pathol. 2012, 93, 70–77. [Google Scholar] [CrossRef]
- Turner, S.J.; Dharmasena, A.; Deane, J. Bilateral rubeosis iridis and rubeotic glaucoma due to peripheral occlusive vasculitis associated with multiple sclerosis. Ocul. Immunol. Inflamm. 2011, 19, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yuan, W.; Fujita, N.; Wang, J.; Wang, H.; Shapiro, I.M.; Risbud, M.V. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am. J. Pathol. 2013, 182, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Cilek, M.Z.; de Vega, S.; Shiozawa, J.; Yoshinaga, C.; Miyamae, Y.; Chijiiwa, M.; Mochizuki, S.; Ito, M.; Kaneko, H.; Kaneko, K.; et al. Synergistic upregulation of ADAMTS4 (aggrecanase-1) by cytokines and its suppression in knee osteoarthritic synovial fibroblasts. Lab. Investig. 2022, 102, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.A.; Liu, C.J. The role of ADAMTSs in arthritis. Protein Cell 2010, 1, 33–47. [Google Scholar] [CrossRef]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Ahmed, E.; Carrasquillo, K.G.; Amano, S.; Hida, T.; Oguchi, Y.; Adamis, A.P. VEGF164 is proinflammatory in the diabetic retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2155–2162. [Google Scholar] [CrossRef]
- Navaratna, D.; Menicucci, G.; Maestas, J.; Srinivasan, R.; McGuire, P.; Das, A. A peptide inhibitor of the urokinase/urokinase receptor system inhibits alteration of the blood-retinal barrier in diabetes. FASEB J. 2008, 22, 3310–3317. [Google Scholar] [CrossRef]
- Navaratna, D.; McGuire, P.G.; Menicucci, G.; Das, A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 2007, 56, 2380–2387. [Google Scholar] [CrossRef]
- Poulaki, V.; Joussen, A.M.; Mitsiades, N.; Mitsiades, C.S.; Iliaki, E.F.; Adamis, A.P. Insulin-like growth factor-I plays a pathogenetic role in diabetic retinopathy. Am. J. Pathol. 2004, 165, 457–469. [Google Scholar] [CrossRef] [Green Version]






| Immunoreactive Blood Vessels Mean ± SD (Range) | Immunoreactive Stromal Cells Mean ± SD (Range) | |
|---|---|---|
| ADAMTS-1 | 47.3 ± 20.6 (19–80) | 78.6 ± 29.4 (27–145) |
| ADAMTS-2 | 61.6 ± 29.4 (21–115) | 114.9 ± 55.1 (20–230) |
| ADAMTS-4 | 62.3 ± 25.5 (25–120) | 146.5 ± 49.8 (62–270) |
| ADAMTS-5 | 38.3 ± 16.8 (11–70) | 86.1 ± 34.9 (33–145) |
| ADAMTS-13 | 2.6 ± 9.4 (0–34) | 28.5 ± 21 (10–65) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu El-Asrar, A.M.; Nawaz, M.I.; Allegaert, E.; Siddiquei, M.M.; Ahmad, A.; Gikandi, P.; De Hertogh, G.; Opdenakker, G. Differential Expression and Localization of ADAMTS Proteinases in Proliferative Diabetic Retinopathy. Molecules 2022, 27, 5977. https://doi.org/10.3390/molecules27185977
Abu El-Asrar AM, Nawaz MI, Allegaert E, Siddiquei MM, Ahmad A, Gikandi P, De Hertogh G, Opdenakker G. Differential Expression and Localization of ADAMTS Proteinases in Proliferative Diabetic Retinopathy. Molecules. 2022; 27(18):5977. https://doi.org/10.3390/molecules27185977
Chicago/Turabian StyleAbu El-Asrar, Ahmed M., Mohd Imtiaz Nawaz, Eef Allegaert, Mohammad Mairaj Siddiquei, Ajmal Ahmad, Priscilla Gikandi, Gert De Hertogh, and Ghislain Opdenakker. 2022. "Differential Expression and Localization of ADAMTS Proteinases in Proliferative Diabetic Retinopathy" Molecules 27, no. 18: 5977. https://doi.org/10.3390/molecules27185977
APA StyleAbu El-Asrar, A. M., Nawaz, M. I., Allegaert, E., Siddiquei, M. M., Ahmad, A., Gikandi, P., De Hertogh, G., & Opdenakker, G. (2022). Differential Expression and Localization of ADAMTS Proteinases in Proliferative Diabetic Retinopathy. Molecules, 27(18), 5977. https://doi.org/10.3390/molecules27185977







