Antioxidant Activity of New Copolymer Conjugates of Methoxyoligo(Ethylene Glycol)Methacrylate and Betulin Methacrylate with Cerium Oxide Nanoparticles In Vitro
Abstract
1. Introduction
2. Results
2.1. Polymer Properties
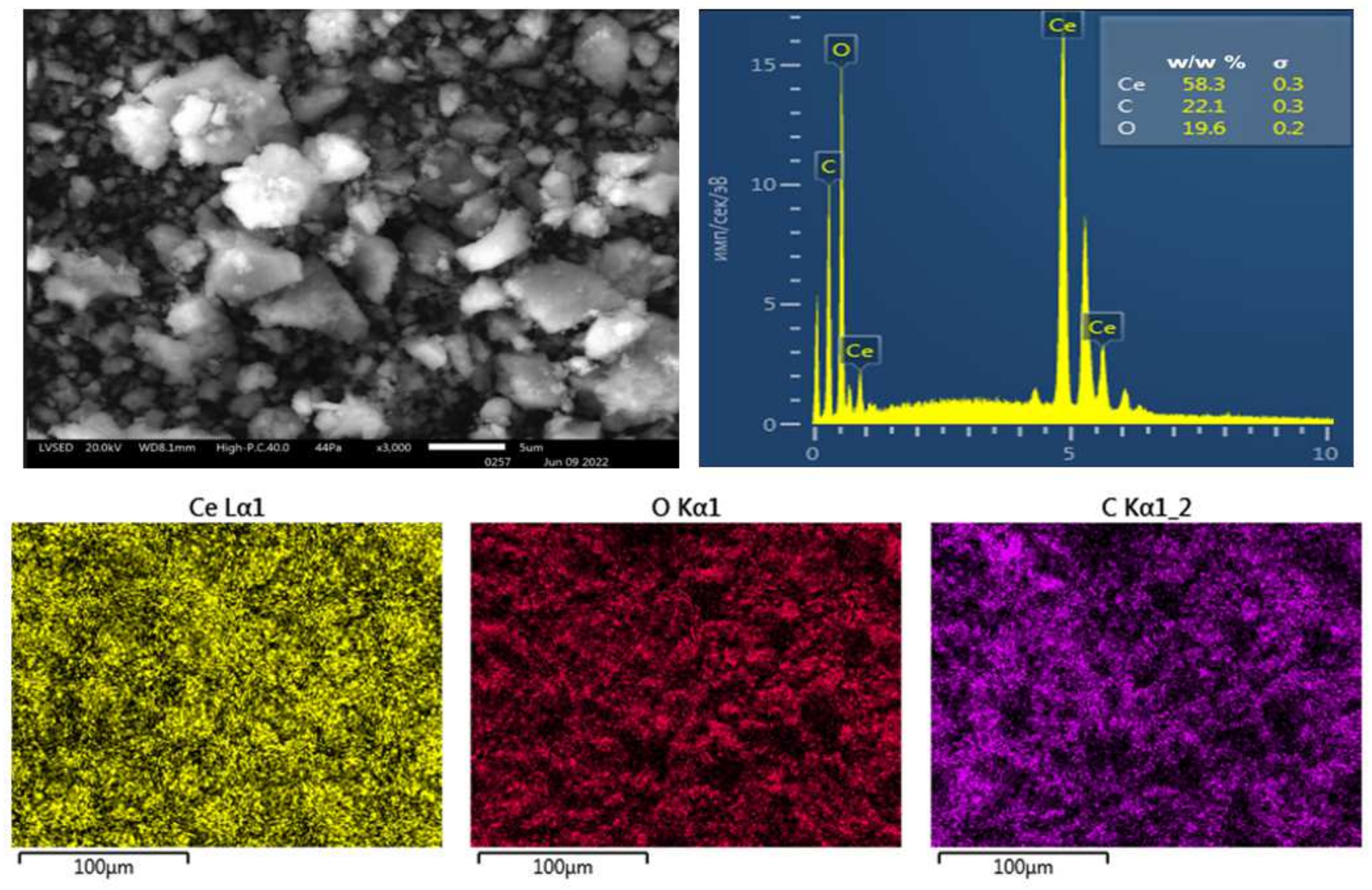
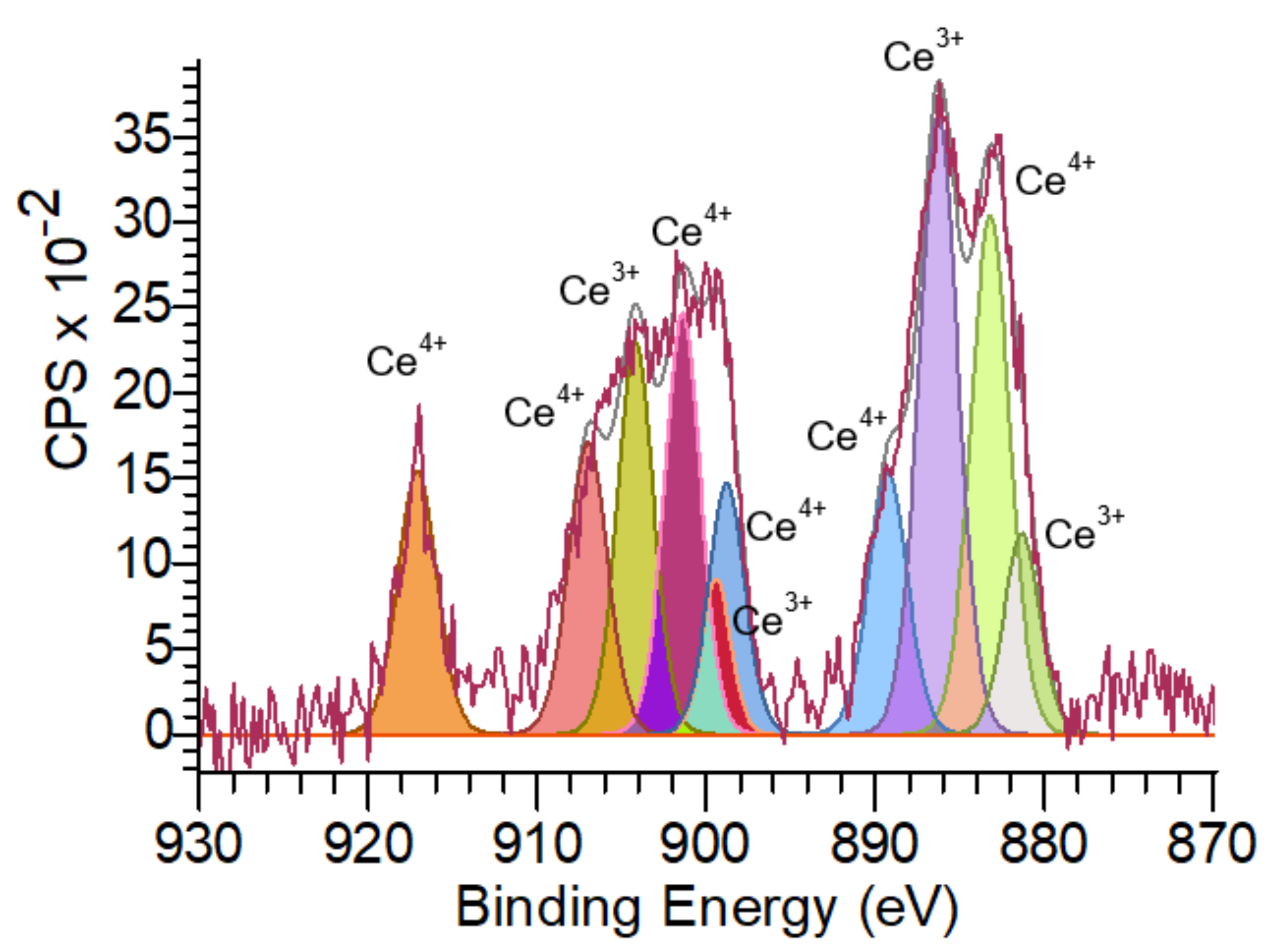
2.2. Properties of Cerium Oxide Nanoparticles
2.2.1. Physicochemical Properties
2.2.2. The Activity of Ceria Nanoparticles Assayed Using Cytochrome C
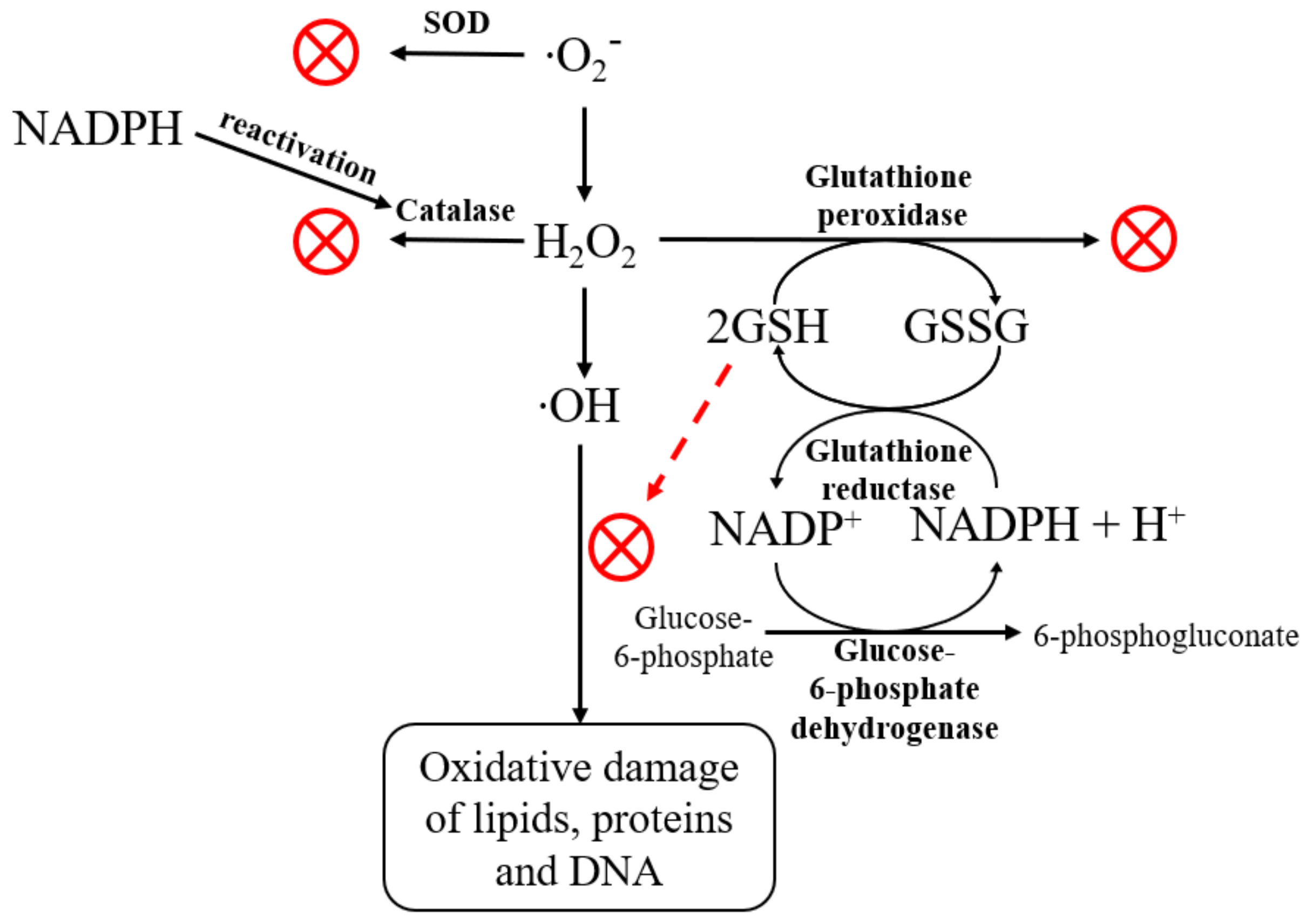
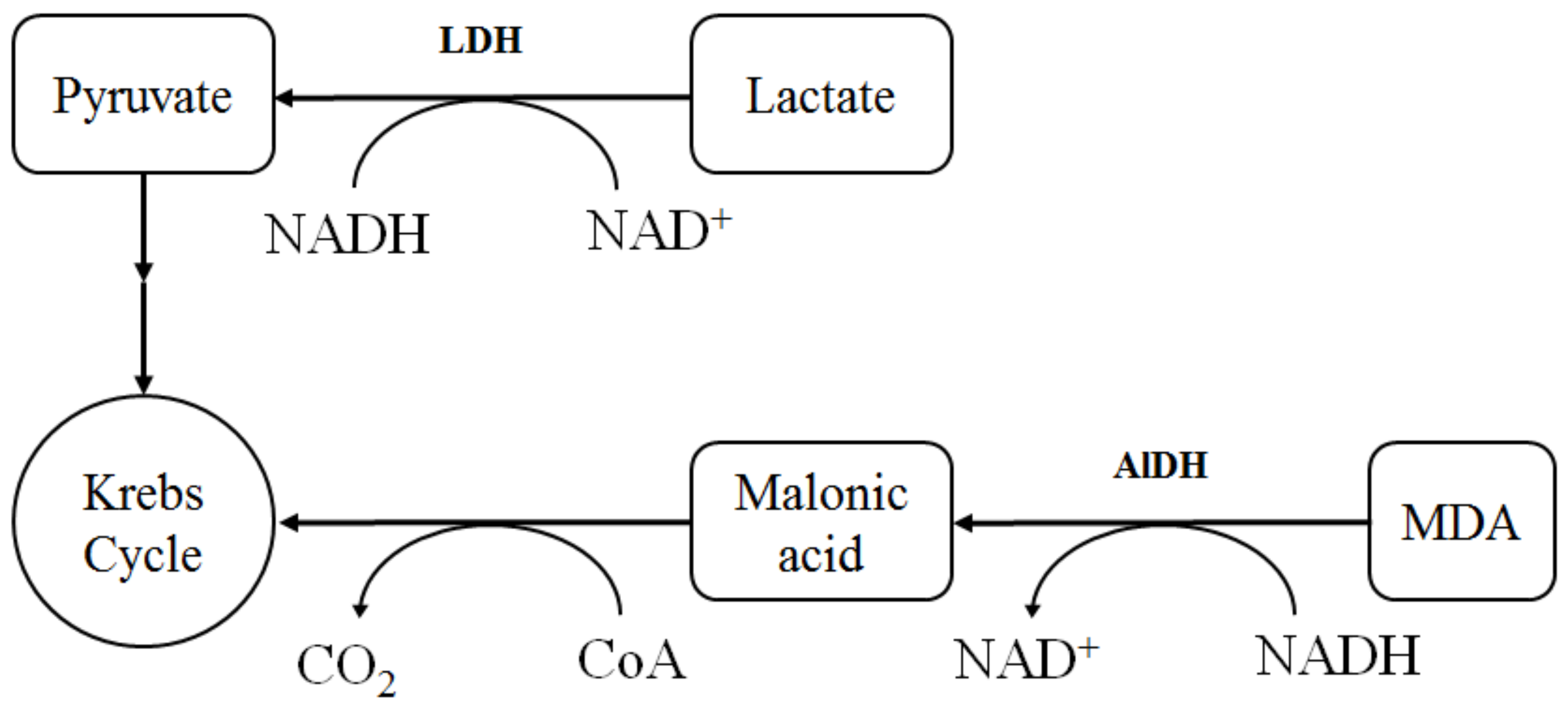
2.3. The Assessment of Biological Activity In Vitro
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Copolymer Conjugates of Methoxyoligo(Ethylene Glycol)Methacrylate and Betulin Methacrylate
4.2.1. Synthesis of Methoxyoligo(Ethylene Glycol)Methacrylate
4.2.2. Synthesis of Betulin Methacrylate
4.2.3. Synthesis of Copolymers
4.3. Synthesis of Cerium Oxide Nanoparticles Stabilized Using Maltodextrin
4.4. Analysis Methods
4.4.1. Determination of the Critical Micelle Concentration (CMC)
4.4.2. SEM and EDXMA Studies
4.4.3. Specific Area Estimation
4.4.4. Surface Charge and Dynamic Light Scattering Measurements
4.4.5. NMR
4.4.6. Specific Area Estimation
4.4.7. UV Spectrophotometry
4.4.8. Powder X-ray Diffractometry
4.4.9. HPLC
4.4.10. XPS Analysis
4.5. Biological Experiments
4.5.1. Biological Activity
4.5.2. Samples for Biological Experiments
4.5.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, Q.; Zhou, X.; Liu, Le.; Fu, S.B. Research Progress in the Promissing Natural Product-Betulin. Biomed. Sci. Tech. Res. 2018, 8, 6378–6384. [Google Scholar] [CrossRef]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. Biomed. Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef] [PubMed]
- Boparai, A.; Niazi, J.; Bajwa, N.; Singh, P.A. Betulin a pentacyclic tri–terpenoid: An hour to rethink the compound. J. Trans. Med. Res. 2017, 1, 53–59. [Google Scholar] [CrossRef]
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 11. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Jiang, D.; Lin, Y.; Wang, Y.; Li, Q.; Liu, L.; Jin, Y.H. Betulin induces reactive oxygen species-dependent apoptosis in human gastric cancer SGC7901 cells. Arch. Pharm. Res. 2016, 39, 1257–1265. [Google Scholar] [CrossRef]
- Yamashita, K.; Lu, H.; Lu, J.; Chen, G.; Yokoyama, T.; Sagara, Y.; Manabe, M.; Kodama, H. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta. 2002, 325, 91–96. [Google Scholar] [CrossRef]
- Yang, M.S.; Chan, H.W.; Yu, L.C. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicology 2006, 226, 126–130. [Google Scholar] [CrossRef]
- Chunhua, M.; Long, H.; Zhu, W.; Liu, Z.; Jie, R.; Zhang, Y.; Wang, Y. Betulin inhibited cigarette smoke-induced COPD in mice. Biomed. Pharmacother. 2017, 85, 679–686. [Google Scholar] [CrossRef]
- Dai, L.; Li, D.; Cheng, J.; Liu, J.; Deng, L.-H.; Wang, L.-Y.; Lei, J.-D.; He, J. Water soluble multiarm-polyethylene glycol–betulinic acid prodrugs: Design, synthesis, and in vivo effectiveness. Polym. Chem. 2014, 5, 5775–5783. [Google Scholar] [CrossRef]
- Saneja, A.; Sharma, L.; Dubey, R.D.; Mintoo, M.J.; Singh, A.; Kumar, A.; Sangwan, P.L.; Tasaduq, S.A.; Singh, G.; Mondhe, D.M.; et al. Synthesis, characterization and augmented anticancer potential of PEG-betulinic acid conjugate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Lomkova, E.A.; Chytil, P.; Janoušková, O.; Mueller, T.; Lucas, H.; Filippov, S.K.; Trhlíková, O.; Aleshunin, P.A.; Skorik, Y.A.; Ulbrich, K.; et al. Biodegradable Micellar HPMA-Based Polymer-Drug Conjugates with Betulinic Acid for Passive Tumor Targeting. Biomacromolecules 2016, 17, 3493–3507. [Google Scholar] [CrossRef] [PubMed]
- Mustafaev, M.; Mustafaeva, Z.; Ergen, E.; Uraki, Y.; Sano, Y. Novel Betulin-Containing Polyelectrolyte Conjugates. J. Bioact. Compat. Polym. 2002, 17, 251–269. [Google Scholar] [CrossRef]
- Auclair, N.; Kaboorani, A.; Riedl, B.; Landry, V. Acrylated betulin as a comonomer for bio-based coatings. Part I: Characterization, photo-polymerization behavior and thermal stability. Ind. Crops. Prod. 2015, 76, 530–537. [Google Scholar] [CrossRef]
- Niewolik, D.; Krukiewicz, K.; Bednarczyk-Cwynar, B.; Ruszkowski, P.; Jaszcz, K. Novel polymeric derivatives of betulin with anticancer activity. RSC Adv. 2019, 9, 20892–20900. [Google Scholar] [CrossRef]
- Gorbunova, M.N.; Kraynova, G.F.; Voronina, A.O. Synthesis of Triterpene Polymer Constructions. Khimiya Rastitel’nogo Syr’ya 2020, 1, 49–56. (In Russian) [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Wang, R.; Zhu, M. Synthesis and Characterization of Methacrylate-Functionalized Betulin Derivatives as Antibacterial Comonomer for Dental Restorative Resins. ACS Biomater. Sci. Eng. 2021, 7, 3132–3140. [Google Scholar] [CrossRef]
- Ma, Z.; Jia, Y.-G.; Zhu, X.X. Glycopolymers Bearing Galactose and Betulin: Synthesis, Encapsulation, and Lectin Recognition. Biomacromolecules 2017, 18, 3812–3818. [Google Scholar] [CrossRef]
- Zamani, K.; Allah-Bakhshi, N.; Akhavan, F.; Yousefi, M.; Golmoradi, R.; Ramezani, M.; Bach, H.; Razavi, S.; Irajian, G.R.; Gerami, M.; et al. Antibacterial effect of cerium oxide nanoparticle against Pseudomonas aeruginosa. BMC Biotechnol. 2021, 21, 68. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Shydlovska, O.; Kharchenko, E.; Osenniy, I.; Spivak, M.; Shcherbakov, A.; Zholobak, N. Nanoparticles of cerium dioxide – an effective antiviral agent and adjuvant of biologically active molecules. Sci. Biol. Sci. 2018, 1, 26–30. [Google Scholar] [CrossRef][Green Version]
- Neal, C.J.; Fox, C.R.; Sakthivel, T.S.; Kumar, U.; Fu, Y.; Drake, C.; Parks, G.D.; Seal, S. Metal-Mediated Nanoscale Cerium Oxide Inactivates Human Coronavirus and Rhinovirus by Surface Disruption. ACS Nano 2021, 15, 14544–14556. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Ali, M.; Zohra, T.; Akhtar, R.; Ikram, A.; Shinwari, Z.K.; Maaza, M. Promising antiviral, antimicrobial and therapeutic properties of green nanoceria. Nanomedicine 2020, 15, 467–488. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K. Biological, biomedical and pharmaceutical applications of cerium oxide. In Cerium Oxide (CeO2): Synthesis, Properties and Applications, 1st ed.; Scire, S., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–358. [Google Scholar]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 Nanoparticle-Containing Polymers for Biomedical Applications: A Review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Krysanov, E.; Demidova, T.; Ivanova, O.; Ordzhonikidze, K.; Shcherbakov, A.; Ivanov, V. Synergetic action of ceria nanoparticles and doxorubicin on the early development of two fish species, Danio rerio and Puntius tetrazona. Nanosyst. Phys. Chem. Math. 2019, 10, 289–302. [Google Scholar] [CrossRef]
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of Conventional Chemotherapeutics with Redox-Active Cerium Oxide Nanoparticles – A Novel Aspect in Cancer Therapy. Mol. Cancer Ther. 2014, 13, 1740–1749. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Lord, M.S.; Berret, J.F.; Singh, S.; Vinu, A.; Karakoti, A.S. Redox Active Cerium Oxide Nanoparticles: Current Status and Burning Issues. Small 2021, 17, e2102342. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.K. Betulin Project NMR Spectra—2014. Available online: https://hdl.handle.net/2144/12964 (accessed on 19 August 2022).
- Skrabania, K.; Miasnikova, A.; Bivigou-Koumba, A.M.; Zehm, D.; Laschewsky, A. Examining the UV-vis absorption of RAFT chain transfer agents and their use for polymer analysis. Polym. Chem. 2011, 2, 2074–2083. [Google Scholar] [CrossRef]
- Smith, D.W.; Williams, R.J.P. The spectra of ferric haems and haemoprotein. In Structure and Bonding, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1970; Volume 7, pp. 1–45. [Google Scholar]
- Chottard, G.; Michelon, M.; Herve, M.; Herve, G. Modification of the Structural and Redox Properties of Cytochrome c by Heteropolytungstate Binding. Biochim. Biophys. Acta 1987, 916, 402–410. [Google Scholar] [CrossRef]
- Ni, P.; Wei, X.; Guo, J.; Ye, X.; Yang, S. On the origin of the oxidizing ability of ceria nanoparticles. RSC Adv. 2015, 5, 97512–97519. [Google Scholar] [CrossRef]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 10, 1056–1058. [Google Scholar] [CrossRef]
- Selektor, S.L.; Shokurov, A.V.; Raitman, O.A.; Sheinina, L.S.; Arslanov, V.V.; Birin, K.P.; Tsivadze, A.Y. Orientation-induced redox transformations in Langmuir monolayers of double-decker cerium bis[tetra-(15-crown-5)-phthalocyaninate] and multistability of its Langmuir-Blodgett films. Colloid J. 2012, 74, 334–345. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef]
- Orekhov, D.; Kamorin, D.; Simagin, A.; Arifullin, I.; Kazantsev, O.; Sivokhin, A.; Savinova, M. Molecular brushes based on copolymers of alkoxy oligo(ethylene glycol)methacrylates and dodecyl(meth)acrylate: Features of synthesis by conventional free radical polymerization. Polym. Bull. 2021, 78, 5833–5850. [Google Scholar] [CrossRef]
- Matsumoto, M.; Takenaka, M.; Sawamoto, M.; Terashima, T. Self-assembly of amphiphilic block pendant polymers as microphase separation materials and folded flower micelles. Polym. Chem. 2019, 10, 4954–4961. [Google Scholar] [CrossRef]
- Rubio-Cervilla, J.; González, E.; Pomposo, J. Advances in Single-Chain Nanoparticles for Catalysis Applications. Nanomaterials 2017, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Rothfuss, H.; Knöfel, N.D.; Roesky, P.W.; Barner-Kowollik, C. Single-Chain Nanoparticles as Catalytic Nanoreactors. J. Am. Chem. Soc. 2018, 140, 5875–5881. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Arbe, A.; Kohlbrecher, J.; Colmenero, J.; Pomposo, J.A. Efficient Synthesis of Single-Chain Globules Mimicking the Morphology and Polymerase Activity of Metalloenzymes. Macromol. Rapid Commun. 2015, 36, 1592–1597. [Google Scholar] [CrossRef]
- Guzik, T.J.; Griendling, K.K. NADPH oxidases: Molecular understanding finally reaching the clinical level? Antioxid. Redox. Signal. 2009, 11, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, H.N.; Rolfo, M.; Ferraris, A.M.; Gaetani, G.F. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J. Biol. Chem. 1999, 274, 13908–13914. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sivilotti, M.L. Oxidant stress and haemolysis of the human erythrocyte. Toxicol. Rev. 2004, 23, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Jose, C.; Bellance, N.; Rossignol, R. Choosing between glycolysis and oxidative phosphorylation: A tumor’s dilemma? Biochim. Biophys. Acta 2011, 1807, 552–561. [Google Scholar] [CrossRef]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Sivokhin, A.; Orekhov, D.; Kazantsev, O.; Sivokhina, O.; Orekhov, S.; Kamorin, D.; Otopkova, K.; Smirnov, M.; Karpov, R. Random and Diblock Thermoresponsive Oligo(ethylene glycol)-Based Copolymers Synthesized via Photo-Induced RAFT Polymerization. Polymers 2022, 14, 137. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K.; Ivanova, O.S.; Marchevsky, A.V.; Baranchikov, A.E.; Spivak, N.Ya.; Tretyakov, Yu.D. Synthesis and antioxidant activity of biocompatible maltodextrin-stabilized aqueous sols of nanocrystalline ceria. Russ. J. Inorg. Chem. 2012, 57, 1411–1418. [Google Scholar] [CrossRef]
- Sivokhin, A.P.; Orekhov, D.V.; Kazantsev, O.A.; Gubanova, O.V.; Kamorin, D.M.; Zarubina, I.S.; Bolshakova, E.A.; Zaitsev, S.D. Amphiphilic thermoresponsive copolymer bottlebrushes: Synthesis, characterization, and study of their self-assembly into flower-like micelles. Polym. J. 2021, 53, 655–665. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Khyshiktuev, B.S.; Khyshiktueva, N.A.; Ivanov, V.N. Metody opredeleniia produktov perekisnogo okisleniia lipidov v kondensate vydykhaemogo vozdukha i ikh klinicheskoe znachenie [Methods of measuring lipid peroxidation products in exhaled air condensate and their clinical significance]. Klin. Lab. Diagn. 1996, 3, 13–15. (In Russian) [Google Scholar]
- Sirota, T.V. A new approach to studying the autoxidation of adrenaline: Possibility of the determination of superoxide dismutase activity and the antioxidant properties of various preparations by polarography. Biomed. Khi. 2012, 58, 77–87. [Google Scholar] [CrossRef][Green Version]
- Packer, L. Catalase in vitro. In Methods in Enzymology, 1st ed.; Kaplan, N.P., Colowick, N.P., Sies, H., Eds.; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Sibgatullina, G.V.; Khartendinova, L.R.; Gumerova, E.A.; Akulov, A.N.; Kostyukova, Y.A.; Nikonorova, N.A.; Rumyantseva, N.I. Methods for Determining the Redox Status of Cultured Plant Cells; Kazan (Privolzhsky) Federal University: Kazan, Russia, 2011; pp. 18–20. [Google Scholar]
- Kochetov, G.A. Practical Guide to Enzymology, 2nd ed.; Severin, S.E., Ed.; High School: Moscow, Russia, 1980; p. 272. [Google Scholar]
- Solov’eva, A.G.; Zimin, Y.V. A new way to assess the dynamics of blood metabolism in patients with thermal trauma. Mod. Technol. Med. 2012, 2, 116–117. [Google Scholar]
- Guru, S.C.; Shetty, K.T. Methodological aspects of aldehyde dehydrogenase assay by spectrophotometric technique. Alcohol 1990, 7, 397–401. [Google Scholar] [CrossRef]
- Dawson, J.M.; Heatlic, P.L. Lowry method of protein quantification evidence for photosensitivity. Anal. Biochem. 1984, 140, 391–393. [Google Scholar] [CrossRef]
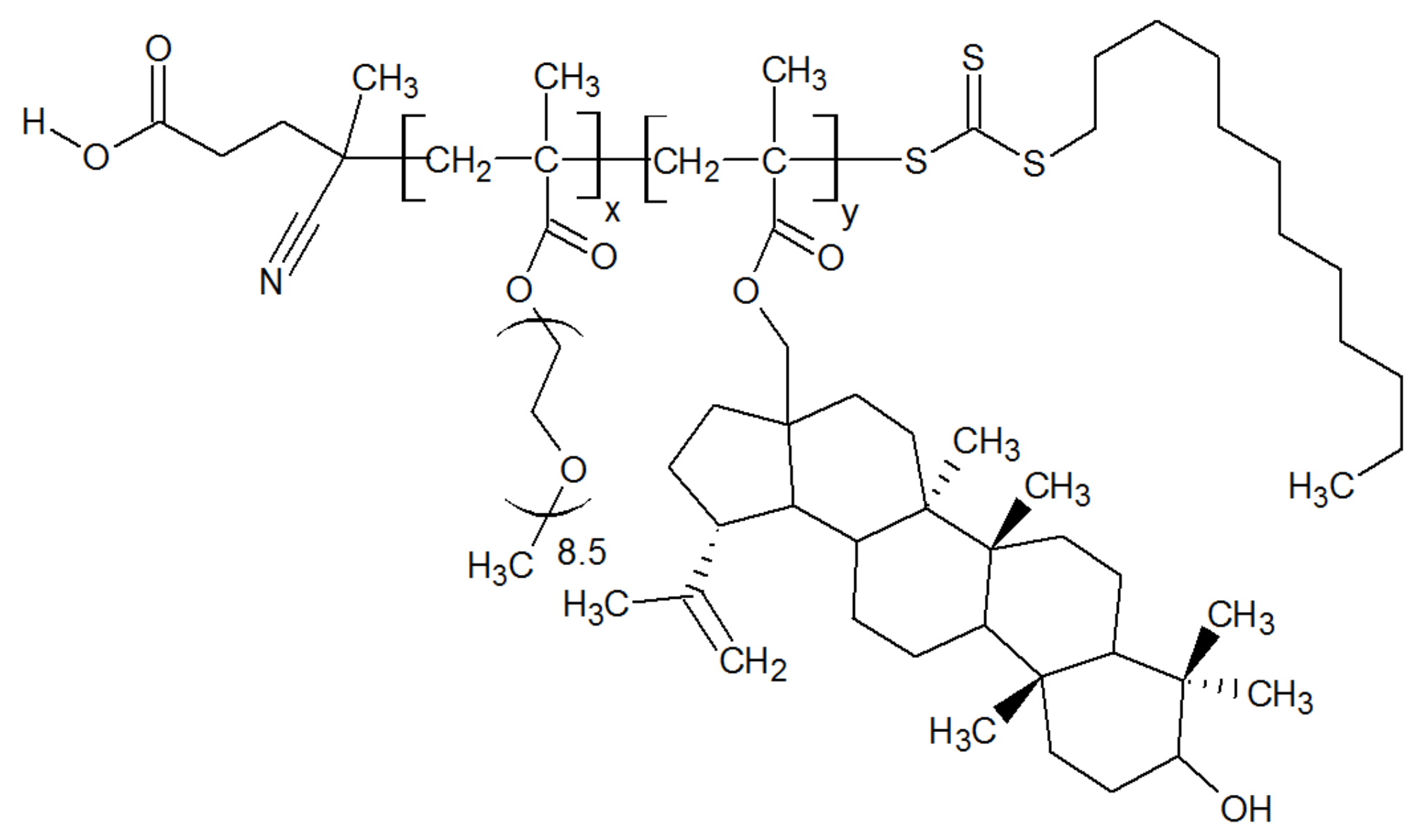
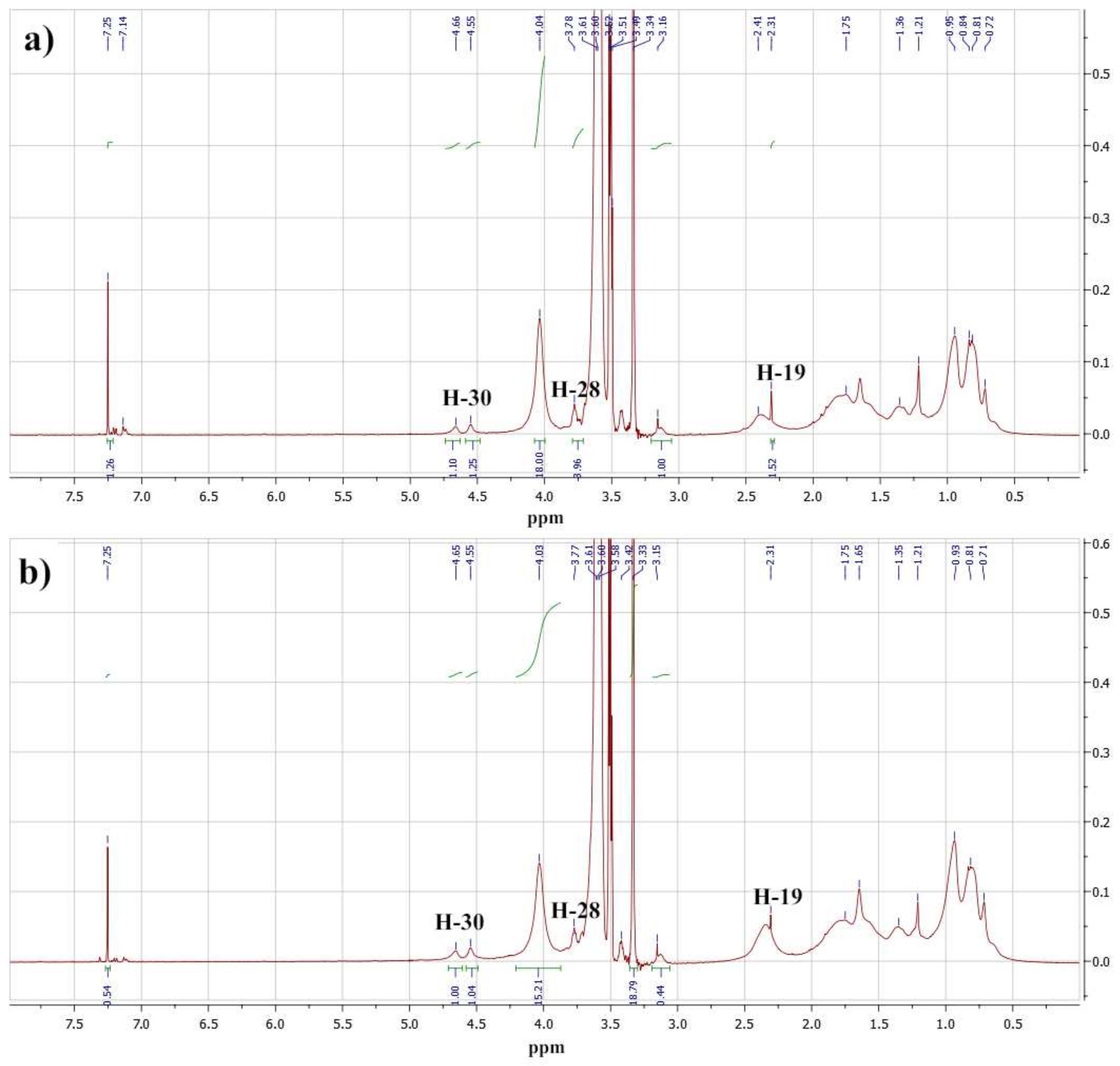
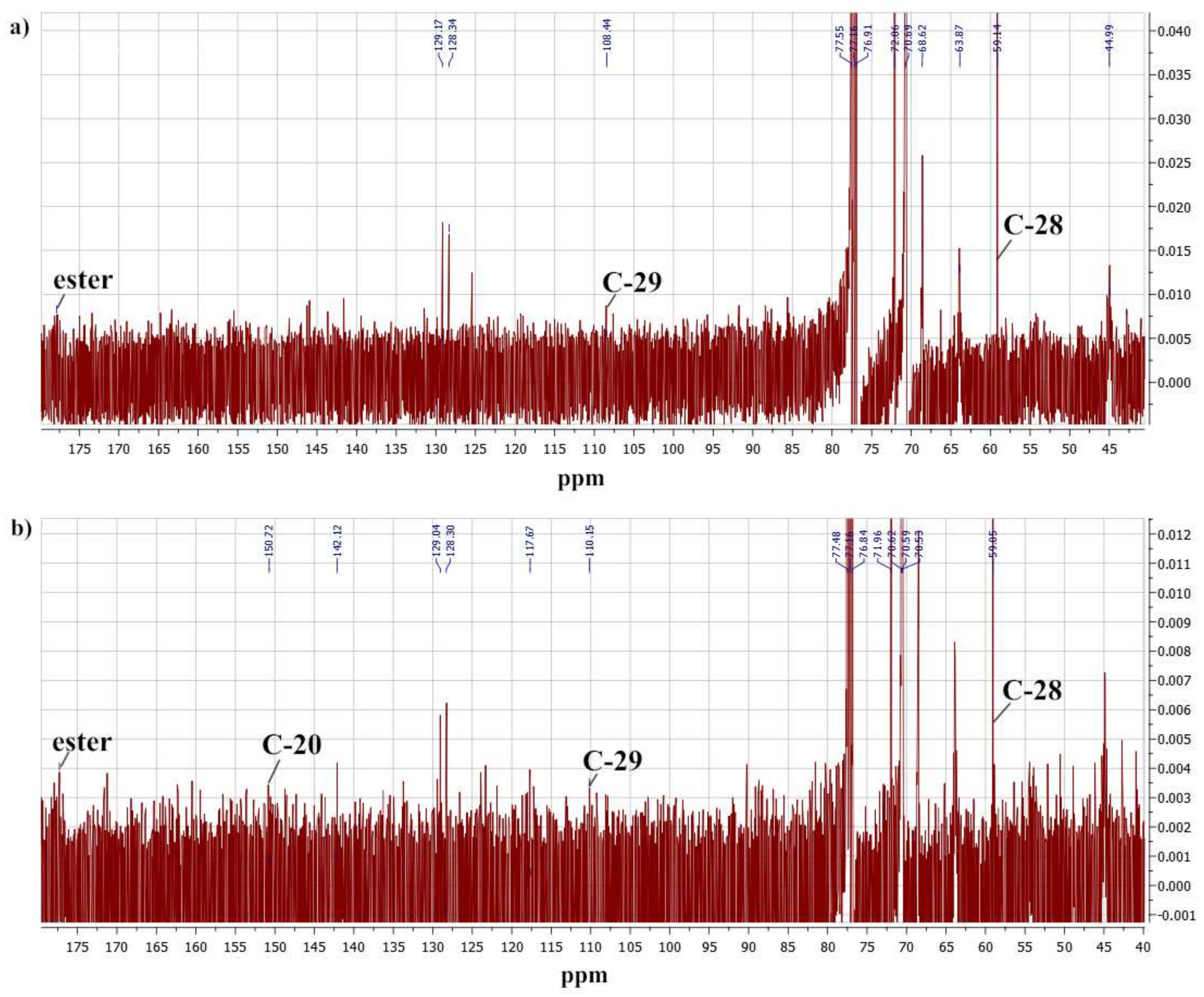
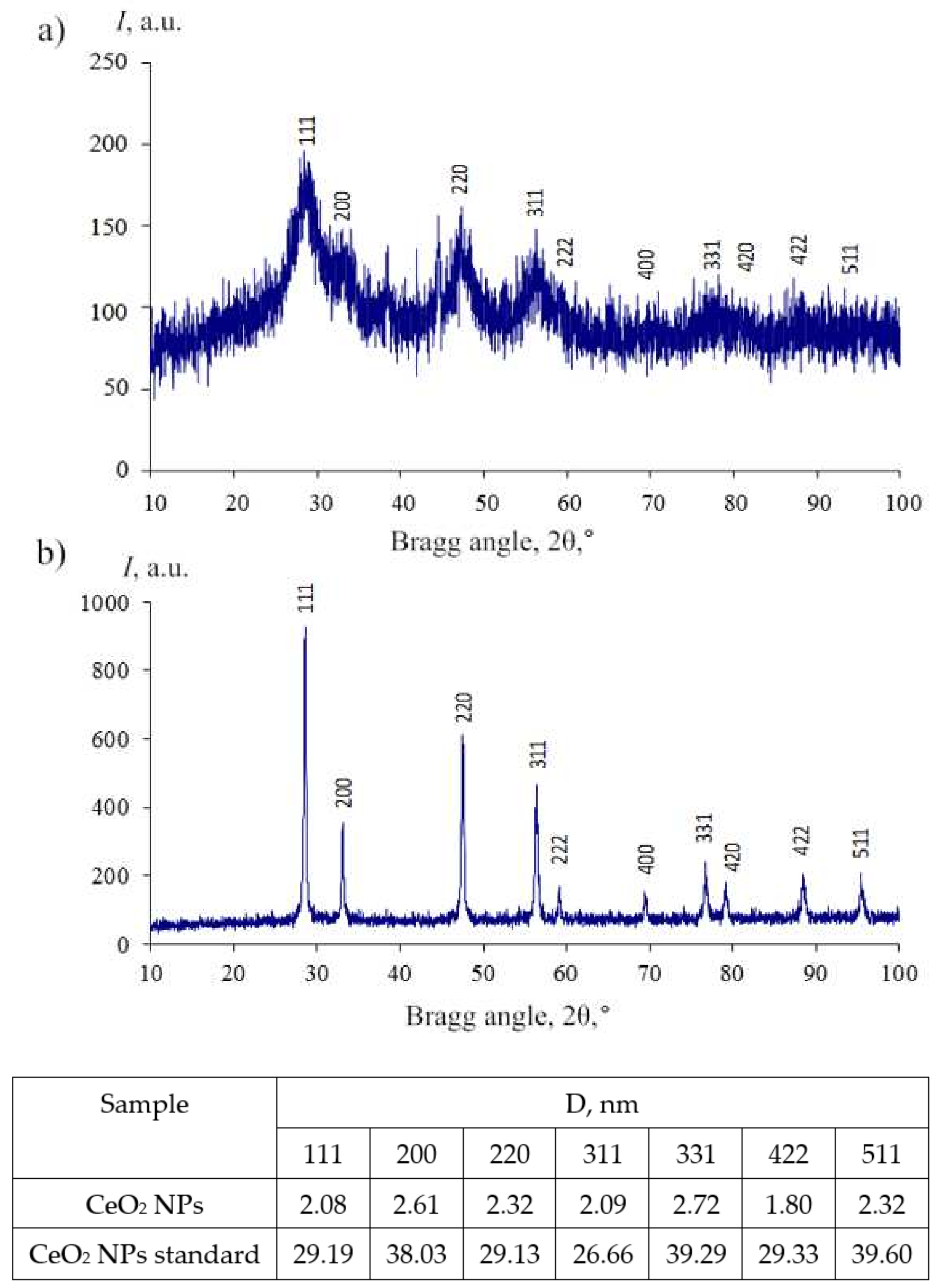


| Sample | Copolymer Composition | Average Units | Molar Weight (UV Spectral Assay) | CMC, wt% | ||
|---|---|---|---|---|---|---|
| Betulin Methacrylate Units, mol% | MOEGM Units, mol% | x | y | |||
| Bet-1 1 | 10.4 | 89.7 | 219.1 | 25.3 | 115 000 | 0.00010 |
| Bet-1 2 | 9.3 | 90.8 | 222.1 | 22.6 | ||
| Bet-2 1 | 15.8 | 84.2 | 132.4 | 24.8 | 75 000 | 0.00007 |
| Bet-2 2 | 13.3 | 86.7 | 136.7 | 20.9 | ||
| No. of Curves | CeO2 NPs Concentration, mg/mL | Time, min | λ, nm (A) | ||
|---|---|---|---|---|---|
| Cyt c | |||||
| γ-Band | β-Band | α-Band | |||
| 1 | 0 | 0 | 415.0 (0.384) | 520.5 (0.043) | 549.5 (0.066) |
| 2 | 0.25 | 0 | 414.0 (0.479) | 520.5 (0.124) | 549.5 (0.155) |
| 3 | 0.50 | 0 | 413.5 (0.651) | 520.0 (0.279) | 549.5 (0.298) |
| 4 | 0.75 | 0 | 412.0 (0.835) | 520.0 (0.457) | 549.5 (0.459) |
| 5 | 1.00 | 0 | 409.00 (1.055) | 520.0 (0.633) | 549.5 (0.626) |
| 7 | 5 | 407.0 (0.964) | 520.0 diff. 1 (0.583) | - | |
| 8 | 10 | 408.0 (0.891) | 520.0 diff. (0.539) | - | |
| 9 | 15 | 407.5 (0.806) | 520.0 diff. (0.486) | - | |
| 10 | 20 | 407.5 (0.734) | 520.0 diff. (0.439) | - | |
| 11 | 25 | 407.5 (0.694) | 520.0 diff. (0.397) | - | |
| 12 | 30 | 408.0 (0.638) | 520.0 diff. (0.363) | - | |
| 13 | 35 | 408.0 (0.573) | 520.0 diff. (0.322) | - | |
| 14 | 40 | 408.0 (0.520) | 518.5 diff. (0.285) | - | |
| 15 | 45 | 408.0 (0.427) | 518.5 diff. (0.226) | - | |
| Sample in PBS, pH 7.4 (mg/mL) | Dose, µL/mL | Index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GR | G6PDH | SOD | Catalase | ||||||
| NADPH/min·mg Protein | % of Control | NADPH/min·mg Protein | % of Control | a.u./min·mg Protein | % of Control | μmol H2O2/ min·mg Protein | % of Control | ||
| Control (100%) | - | 90.21 ± 1.49 | 100.0 | 40.86 ± 1.17 | 100.0 | 959.23 ± 11.28 | 100.0 | 35.76 ± 1.26 | 100.0 |
| Bet-1 | 20 | 89.03 ± 4.05 | 98.7 | 49.39 ± 1.68 | 120.9 | 1052.01 ± 9.61 | 109.7 | 35.33 ± 0.92 | 98.8 |
| 50 | 89.43 ± 2.42 | 99.1 | 58.73 ± 1.55 | 143.7 | 1111.16 ± 12.97 | 115.8 | 35.49 ± 1.25 | 99.3 | |
| 100 | 93.73 ± 4.54 | 103.9 | 61.60 ± 2.35 | 150.8 | 1246.47 ± 8.904 | 130.0 | 38.90 ± 2.12 | 108.8 | |
| Bet-2 | 20 | 98.12 ± 0.91 | 108.8 | 58.13 ± 1.41 | 142.3 | 1086.69 ± 10.57 | 113.3 | 32.40 ± 1.11 | 90.6 |
| 50 | 114.05 ± 3.74 | 126.4 | 80.48 ± 1.04 | 197.0 | 1161.87 ± 20.93 | 121.1 | 33.80 ± 0.40 | 94.5 | |
| 100 | 208.91 ± 4.64 | 231.6 | 90.03 ± 1.81 | 220.4 | 1281.13 ± 10.89 | 133.6 | 36.43 ± 0.85 | 101.9 | |
| CeO2 NPs | 20 | 88.00 ± 0.85 | 97.6 | 58.92 ± 0.82 | 144.2 | 1215.40 ± 15.83 | 126.7 | 37.16 ± 1.50 | 103.9 |
| 50 | 100.45 ± 2.20 | 111.4 | 99.15 ± 1.16 | 242.7 | 1273.05 ± 26.20 | 132.7 | 46.60 ± 3.10 | 130.3 | |
| 100 | 105.99 ± 1.43 | 117.5 | 112.58 ± 0.98 | 275.6 | 1272.14 ± 34.47 | 132.6 | 48.11 ± 3.90 | 134.6 | |
| Combination of Bet-2 and CeO2 NPs | 20 | 83.09 ± 0.92 | 92.1 | 62.76 ± 1.70 | 153.6 | 1268.97 ± 6.66 | 132.3 | 40.40 ± 3.25 | 113.0 |
| 50 | 102.60 ± 3.22 | 113.7 | 102.54 ± 2.82 | 251.0 | 1301.67 ± 12.54 | 135.7 | 51.72 ± 0.39 | 144.6 | |
| 100 | 134.19 ± 6.04 | 148.8 | 95.87 ± 1.41 | 234.7 | 1318.35 ± 25.60 | 137.4 | 46.92 ± 4.29 | 131.2 | |
| Kruskal–Wallis test | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p = 0.002 | |||||
| Sample in PBS, pH 7.4 (mg/mL) | Dose, µL/mL | Index (% of Control) | LDHrev/ LDHdir, ([NADH]/ [NAD+]) | |||||
|---|---|---|---|---|---|---|---|---|
| AlDH | LDHdir | LDHrev | ||||||
| nmol NADH/ min·mg Protein | % of Control | nmol NAD+/ min·mg Protein | % of Control | nmol NADH/ min·mg Protein | % of Control | |||
| Control (100%) | - | 40.07 ± 1.76 | 100.0 | 42.48 ± 1.15 | 100.0 | 176.47 ± 3.66 | 100.0 | 4.2 |
| Bet-1 | 20 | 36.79 ± 2.45 | 91.8 | 36.78 ± 0.66 | 86.6 | 198.37 ± 0.87 | 112.4 | 5.4 |
| 50 | 39.81 ± 0.95 | 99.4 | 40.00 ± 1.64 | 94.1 | 156.23 ± 3.09 | 88.5 | 3.9 | |
| 100 | 49.92 ± 1.48 | 124.6 | 41.95 ± 1.58 | 98.7 | 149.09 ± 3.62 | 84.5 | 3.6 | |
| Bet-2 | 20 | 35.69 ± 1.21 | 89.1 | 37.94 ± 0.98 | 89.3 | 197.38 ± 2.01 | 111.8 | 5.2 |
| 50 | 40.19 ± 2.01 | 100.3 | 39.73 ± 0.99 | 93.5 | 212.54 ± 5.22 | 120.4 | 5.3 | |
| 100 | 38.60 ± 2.50 | 96.3 | 46.23 ± 1.00 | 108.8 | 204.18 ± 6.30 | 115.7 | 4.4 | |
| CeO2 NPs | 20 | 37.52 ± 1.52 | 93.6 | 39.19 ± 0.63 | 92.2 | 221.28 ± 1.19 | 125.4 | 5.6 |
| 50 | 44.01 ± 1.82 | 109.8 | 54.30 ± 0.78 | 127.8 | 237.24 ± 1.70 | 134.4 | 4.4 | |
| 100 | 46.19 ± 1.50 | 115.3 | 42.99 ± 0.97 | 101.2 | 229.69 ± 3.63 | 130.2 | 5.3 | |
| Combination of Bet-2 and CeO2 NPs | 20 | 40.75 ± 2.30 | 101.7 | 35.61 ± 0.68 | 83.8 | 205.94 ± 2.95 | 116.7 | 5.8 |
| 50 | 39.36 ± 0.60 | 98.2 | 39.61 ± 1.19 | 93.2 | 217.31 ± 3.84 | 123.1 | 5.5 | |
| 100 | 34.18 ± 0.73 | 85.3 | 40.69 ± 0.85 | 95.8 | 217.98 ± 3.71 | 123.5 | 5.4 | |
| Kruskal–Wallis test | p = 0.003 | p ≤ 0.0001 | p ≤ 0.0001 | |||||
| Sample in PBS, pH 7.4 (mg/mL) | Dose, µL/mL | Concentration, % of Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MDAer | DC | TC | SB | ||||||
| μmol/L | % of Control | a.u. | % of Control | a.u. | % of Control | a.u. | % of Control | ||
| Control (100%) | - | 7.28 ± 0.25 | 100.0 | 0.67 ± 0.02 | 100.0 | 0.27 ± 0.01 | 100.0 | 0.14 ± 0.01 | 100.0 |
| Bet-1 | 20 | 7.95 ± 0.10 | 109.2 | 0.68 ± 0.01 | 102.5 | 0.28 ± 0.01 | 103.0 | 0.15 ± 0.01 | 104.3 |
| 50 | 8.29 ± 0.03 | 113.8 | 0.72 ± 0.01 | 108.5 | 0.27 ± 0.01 | 101.1 | 0.17 ± 0.01 | 120.7 | |
| 100 | 8.48 ± 0.17 | 116.5 | 0.77 ± 0.01 | 116.0 | 0.26 ± 0.02 | 96.6 | 0.17 ± 0.01 | 122.9 | |
| Bet-2 | 20 | 7.55 ± 0.03 | 103.7 | 0.63 ± 0.01 | 94.6 | 0.26 ± 0.01 | 98.1 | 0.15 ± 0.01 | 104.3 |
| 50 | 7.76 ± 0.03 | 106.5 | 0.65 ± 0.01 | 96.7 | 0.27 ± 0.01 | 100.7 | 0.15 ± 0.01 | 110.0 | |
| 100 | 7.66 ± 0.03 | 105.1 | 0.67 ± 0.01 | 100.6 | 0.28 ± 0.01 | 103.4 | 0.15 ± 0.01 | 110.0 | |
| CeO2 NPs | 20 | 7.23 ± 0.24 | 99.2 | 0.71 ± 0.01 | 106.6 | 0.27 ± 0.01 | 99.6 | 0.14 ± 0.01 | 99.6 |
| 50 | 6.38 ± 0.10 | 87.6 | 0.70 ± 0.01 | 105.1 | 0.26 ± 0.01 | 96.3 | 0.13 ± 0.01 | 95.7 | |
| 100 | 6.15 ± 0.06 | 84.5 | 0.67 ± 0.10 | 101.0 | 0.25 ± 0.01 | 92.5 | 0.13 ± 0.01 | 92.1 | |
| Combination of Bet-2 and CeO2 NPs | 20 | 7.18 ± 0.09 | 98.6 | 0.67 ± 0.01 | 99.9 | 0.28 ± 0.01 | 103.0 | 0.14 ± 0.01 | 102.1 |
| 50 | 6.70 ± 0.27 | 92.0 | 0.69 ± 0.04 | 104.0 | 0.28 ± 0.01 | 103.7 | 0.17 ± 0.01 | 120.0 | |
| 100 | 6.78 ± 0.09 | 93.1 | 0.72 ± 0.01 | 108.1 | 0.26 ± 0.01 | 98.5 | 0.17 ± 0.01 | 122.1 | |
| Kruskal–Wallis test | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | |||||
| Sample | Preparation |
|---|---|
| Bet-1 | 1.41 mg of Bet-1 was dissolved in 1 mL of PBS |
| Bet-2 | 1.41 mg of Bet-2 was dissolved in 1 mL of PBS |
| CeO2-MD 1 | 0.8 mg of CeO2-MD was dispersed in 1 mL of PBS |
| Mixture of Bet-2 and CeO2-MD | 0.71 mg of Bet-2 and 0.4 mg of CeO2-MD were dispersed in 1 mL of PBS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, N.; Orekhov, D.; Simagin, A.; Malygina, D.; Korokin, V.; Kosmachova, K.; Al-Azzavi, H.; Solovyeva, A.; Kazantsev, O. Antioxidant Activity of New Copolymer Conjugates of Methoxyoligo(Ethylene Glycol)Methacrylate and Betulin Methacrylate with Cerium Oxide Nanoparticles In Vitro. Molecules 2022, 27, 5894. https://doi.org/10.3390/molecules27185894
Melnikova N, Orekhov D, Simagin A, Malygina D, Korokin V, Kosmachova K, Al-Azzavi H, Solovyeva A, Kazantsev O. Antioxidant Activity of New Copolymer Conjugates of Methoxyoligo(Ethylene Glycol)Methacrylate and Betulin Methacrylate with Cerium Oxide Nanoparticles In Vitro. Molecules. 2022; 27(18):5894. https://doi.org/10.3390/molecules27185894
Chicago/Turabian StyleMelnikova, Nina, Dmitry Orekhov, Alexander Simagin, Darina Malygina, Vitaly Korokin, Karina Kosmachova, Haider Al-Azzavi, Anna Solovyeva, and Oleg Kazantsev. 2022. "Antioxidant Activity of New Copolymer Conjugates of Methoxyoligo(Ethylene Glycol)Methacrylate and Betulin Methacrylate with Cerium Oxide Nanoparticles In Vitro" Molecules 27, no. 18: 5894. https://doi.org/10.3390/molecules27185894
APA StyleMelnikova, N., Orekhov, D., Simagin, A., Malygina, D., Korokin, V., Kosmachova, K., Al-Azzavi, H., Solovyeva, A., & Kazantsev, O. (2022). Antioxidant Activity of New Copolymer Conjugates of Methoxyoligo(Ethylene Glycol)Methacrylate and Betulin Methacrylate with Cerium Oxide Nanoparticles In Vitro. Molecules, 27(18), 5894. https://doi.org/10.3390/molecules27185894






