Abstract
To discover novel herbicidal compounds with favorable activity, a range of phenylpyridine-moiety-containing α-trifluorothioanisole derivatives were designed, synthesized, and identified via NMR and HRMS. Preliminary screening of greenhouse-based herbicidal activity revealed that compound 5a exhibited >85% inhibitory activity against broadleaf weeds Amaranthus retroflexus, Abutilon theophrasti, and Eclipta prostrate at 37.5 g a.i./hm2, which was slightly superior to that of fomesafen. The current study suggests that compound 5a could be further optimized as an herbicide candidate to control various broadleaf weeds.
1. Introduction
Organosulfur compounds are widely present in animals and plants due to their physiological activities, such as diallyl disulfide compounds in garlic, isothiocyanate compounds in cruciferous vegetables and some fruits, and sulfur-containing amino acids and vitamins. These molecules play a vital role in maintaining the metabolism of organisms and various life activities. Therefore, many researchers have conducted in-depth research on these compounds and found that some molecules containing thioheterocycles, thioureas, sulfonamides, thioethers, sulfoxides, sulfones, and other structures show biological activity [1,2,3,4,5,6,7,8]. For instance, Wang et al. [9] reported a class of sulfonylurea compounds with high herbicidal activities against Echinochloa crusgalli and Digitaria sanguinalis for pre- and post-emergence treatment and noted that they were safe for peanut as post-emergence treatment. A range of 5-substituted sulfonylurea derivatives discovered by Li et al. [10] showed good herbicidal activities against Amaranthus retroflexus and Brassica campestris for pre-emergence treatment. It is worth noting that compounds containing sulfide, sulfoxide, and sulfone structures have been widely used in materials [11,12], medicine [13,14,15], and pesticides [16,17,18,19,20,21,22] in the past few decades (Figure 1). The pre-emergence herbicide pyroxasulfone, discovered by Todoroki et al. [23], exhibited excellent herbicidal activity and crop safety, and it is currently used in the field.
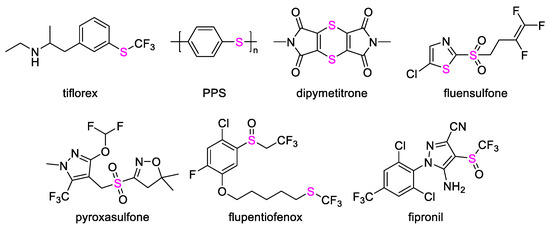
Figure 1.
Structures of reported sulfur-containing compounds.
Substituted 2-phenylpyridines discovered by Schaefer et al. exhibited good inhibition activity against weeds [24,25]. Substituted 3-(pyridin-2-yl)benzenesulfonamide derivatives disclosed by Liu et al. showed excellent inhibitory activity against a variety of weeds [26,27,28]. Du et al. also reported that a range of kresoxim-methyl derivatives containing phenylpyridine moieties exhibited higher inhibitory activities against broadleaf weeds than mesotrione [29,30].
Herein, 18 novel α-trifluorothioanisole derivatives were synthesized through introducing phenylpyridine moieties into α-trifluorothioanisole. The inhibitory activities of the resultant compounds against broadleaf and grass weeds were determined.
2. Results and Discussions
2.1. Chemistry
The synthesis procedures for the target compounds used in this work are outlined in Scheme 1. Intermediates 3a–3f were obtained according to the literature [31]. The target compounds 5a–5f were prepared via nucleophilic substitution reaction from intermediates 3a–3f and compound 4. In addition, compounds 5a–5f were oxidized to yield compounds 6a–6f and 7a–7f using 3-chloroperbenzoic acid or hydrogen peroxide as oxidants according to previously disclosed methods [32,33]. After synthesis, all target compounds were characterized via HRMS and NMR. X-ray diffraction crystallography was further used to confirm the structure of compound 5a (Figure 2). The crystal data of compound 5a and the NMR spectrum of all target compounds are shown in the Supplementary Materials.
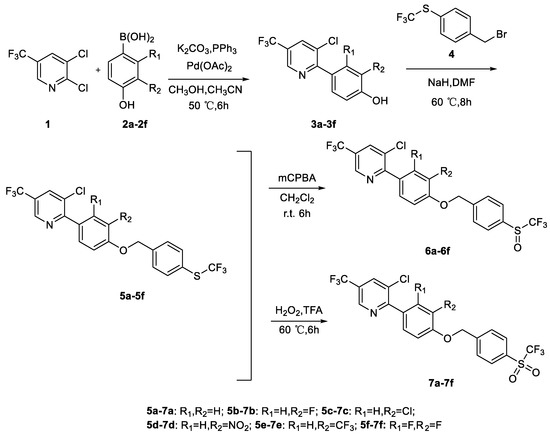
Scheme 1.
Overview synthesis methods of α-trifluorothioanisole derivatives.
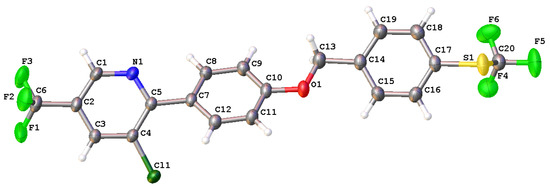
Figure 2.
The X-ray crystal structure of compound 5a.
2.2. Greenhouse Herbicidal Activity Assays
As can be seen from Table 1, some target compounds exhibited excellent inhibitory activity against the tested dicotyledonous weeds but proved ineffective against monocotyledonous weeds. Of these, at 150 g a.i./hm2, compounds 5a, 5f, 6b, and 7a exhibited >80% inhibitory activity when used for the post-emergence treatment of the broadleaf weeds AT, AR, and EP, while compounds 6f and 7f exhibited >80% activity against AT and AR. Furthermore, compound 5a also effectively suppressed the growth of DS and SV, which was slightly better than the positive control fomesafen. Other compounds exhibited varying levels of general herbicidal activity. Further analysis revealed that compound 5a exhibited >85% inhibition against AT, AR, and EP for post-emergence treatment at 37.5 g a.i./hm2, which was slightly superior to the inhibition ability of fomesafen, whereas compound 5f also exhibited >70% inhibition against these three weeds.

Table 1.
The structures and herbicidal activities of α-trifluoroanisole derivatives for post-emergence treatment in a greenhouse assay setting.
From Table 1, we can see that the herbicidal activities of compound 5 were slightly better than those of compounds 6 and 7. According to the SAR of compound 5 in the field of herbicidal activity, when R1 and R2 were both substituted by hydrogen atoms (5a), the activities of compound 5 against three broadleaf weeds were better than those of other compounds. For compound 6, when R1 was substituted by a hydrogen atom and R2 was substituted by a fluorine atom (6b), the herbicidal activities of compound 6 were optimal. For compound 7, the optimal herbicidal activities were observed when R1 and R2 were substituted by either hydrogen atoms or fluorine atoms (7a, 7f).
3. Materials and Methods
3.1. Instrumentation
All reagents and other materials were purchased from commercial sources and used without additional purification unless otherwise noted. A B-545 melting point instrument was used to determine melting point without calibration. A Bruker AV-400 or AV-500 MHz spectrometer was used to generate NMR spectra with DMSO-d6 or CDCl3 serving as solvents. An Agilent 6545 Q-TOF LCMS spectrometer was used for mass spectrometry. A Bruker D8 Venture diffractometer was utilized to collect crystallographic data.
3.2. Synthesis
The synthesis methods of the title compounds are outlined in Scheme 1.
3.2.1. General Approach to the Synthesis of Compounds 3a–3f
2,3-Dichloro-5-(trifluoromethyl)pyridine 1 (5 mmol), triphenylphosphorus (0.5 mmol), K2CO3 (10 mmol), substituted p-hydroxybenzeneboronic acid 2a–2f (5.5 mmol), and palladium(II) acetate (0.25 mmol) were mixed and stirred for 6 h with CH3CN (10 mL) and CH3OH (5 mL) at 50 °C under N2. Thereafter, the mixture was extracted using ethyl acetate (30 mL × 3), rinsed using brine, and concentrated. The remaining residue was then recrystallized using ethanol and water as solvents at 70 °C to obtain compounds 3a–3f [31].
3.2.2. General Approach to the Synthesis of Compounds 5a–5f
Substituted phenylpyridines 3a–3f (2 mmol), N,N-dimethylformamide (10 mL), and NaH (3 mmol, 0.12 g) were mixed and stirred at 20 °C for 30 min under N2. Next, 4-trifluoromethylthiobenzyl bromide (2.4 mmol) was added and stirred for 8 h at 60 °C. The mixture was extracted thrice using ethyl acetate (30 mL × 3), rinsed using brine, and concentrated. Residues were then purified via silica gel column chromatography using ethyl acetate (EA) and petroleum ether (PE) (VEA:VPE=1:15) to obtain compounds 5a–5f [31].
3.2.3. General Approach to the Synthesis of Compounds 6a–6f
Compounds 5a–5f (0.5 mmol), 85% 3-chloroperbenzoic acid (0.5 mmol), and dichloromethane (10 mL) were mixed and stirred at 20 °C for 6 h. The mixture was evaporated to remove the solvent. Residues were then purified via silica gel column chromatography using ethyl acetate (EA) and petroleum ether (PE) (VEA:VPE=1:7) to obtain compounds 6a–6f [32].
3.2.4. General Approach to the Synthesis of Compounds 7a–7f
Compounds 5a–5f (0.4 mmol), 30% hydrogen peroxide (1.6 mmol), and trifluoroacetic acid (2 mL) were mixed and stirred at 60 °C for 6 h. Thereafter, the mixture was made alkaline using a sodium hydroxide solution, extracted thrice using ethyl acetate (30 mL × 3), rinsed using Na2SO3, and concentrated. Residues were then purified via silica gel column chromatography using ethyl acetate (EA) and petroleum ether (PE) (VEA:VPE=1:10) to obtain compounds 7a–7f [33].
3-chloro-5-(trifluoromethyl)-2-(4-((4-((trifluoromethyl)thio)benzyl)oxy)phenyl)pyridine (5a): White solid, yield 78.8%, m.p. 93.2–95.4 °C. 1H NMR (500 MHz, DMSO-d6) δ: 8.99 (s, 1H), 8.51 (s, 1H), 7.76 (dd, J = 8.4, 3.4 Hz, 4H), 7.64 (d, J = 8.1 Hz, 2H), 7.17 (d, J = 8.8 Hz, 2H), 5.29 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 159.17, 158.79, 144.27 (q, J = 4.0 Hz), 140.63, 136.27, 135.71 (q, J = 3.1 Hz), 131.02, 129.57 (q, J = 308.4 Hz), 129.37, 129.17, 128.82, 124.39 (q, J = 33.0 Hz), 122.86 (q, J = 273.4 Hz), 122.29 (q, J = 2.0 Hz), 114.37, 68.43. HRMS (ESI): calculated for C20H13ClF6NOS [M+H]+ 464.0305, 466.0276, found 464.0308, 466.0277.
3-chloro-2-(3-fluoro-4-((4-((trifluoromethyl)thio)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (5b): White solid, yield 85.9%, m.p. 63.5–65.0 °C. 1H NMR (500 MHz, DMSO-d6) δ: 9.01 (d, J = 0.9 Hz, 1H), 8.55 (s, 1H), 7.79 (d, J = 8.1 Hz, 2H), 7.70–7.64 (m, 3H), 7.61 (d, J = 8.6 Hz, 1H), 7.41 (t, J = 8.7 Hz, 1H), 5.38 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 157.57, 150.96 (d, J = 244.7 Hz), 147.15 (d, J = 10.6 Hz), 144.33 (q, J = 3.9 Hz), 140.06, 136.34, 135.89 (q, J = 3.3 Hz), 129.79 (d, J = 6.7 Hz), 129.58 (q, J = 308.3 Hz), 129.42, 128.92, 126.27 (d, J = 3.2 Hz), 124.85 (q, J = 33.1 Hz), 122.80 (q, J = 273.4 Hz), 122.59 (q, J = 1.9 Hz), 117.22 (d, J = 20.0 Hz), 114.71, 69.36. HRMS (ESI): calculated for C20H10ClF7NOS [M−H]− 480.0065, 482.0036, found 480.0064, 482.0043.
3-chloro-2-(3-chloro-4-((4-((trifluoromethyl)thio)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (5c): White solid, yield 94.0%, m.p. 82.0–83.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.02 (s, 1H), 8.57 (s, 1H), 7.88 (s, 1H), 7.78 (t, J = 10.5 Hz, 3H), 7.67 (d, J = 7.2 Hz, 2H), 7.40 (d, J = 8.3 Hz, 1H), 5.41 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 157.49, 154.37, 144.38 (q, J = 3.9 Hz), 140.14, 136.38, 135.89 (q, J = 3.1 Hz), 130.94, 130.25, 129.68, 129.60 (q, J = 309.1 Hz), 129.45, 128.62, 124.87 (q, J = 33.4 Hz), 122.81 (q, J = 274.1 Hz), 122.48, 121.21, 113.62, 69.28. HRMS (ESI): calculated for C20H10Cl2F6NOS [M−H]− 495.9770, 497.9740, found 495.9767, 497.9740.
3-chloro-2-(3-nitro-4-((4-((trifluoromethyl)thio)benzyl)oxy)phenyl)-5 (trifluoromethyl)pyridine (5d): White solid, yield 86.5%, m.p. 114.5–116.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.06 (s, 1H), 8.63 (s, 1H), 8.35 (d, J = 2.2 Hz, 1H), 8.11 (dd, J = 8.8, 2.2 Hz, 1H), 7.80 (d, J = 8.1 Hz, 2H), 7.65 (d, J = 8.1 Hz, 2H), 7.61 (d, J = 8.9 Hz, 1H), 5.51 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 156.76, 151.71, 144.63 (q, J = 3.6 Hz), 139.64, 138.99, 136.51, 136.12 (q, J = 3.5 Hz), 135.49, 129.74, 129.66 (q, J = 308.8 Hz), 129.27, 128.63, 126.42, 125.33 (q, J = 33.3 Hz), 122.83 (q, J = 274.1 Hz), 122.66 (q, J = 1.9 Hz), 115.28, 69.85. HRMS (ESI): calculated for C20H10ClF6N2O3S [M−H]− 507.0010, 508.9981, found 507.0010, 508.9983.
3-chloro-5-(trifluoromethyl)-2-(3-(trifluoromethyl)-4-((4 ((trifluoromethyl)thio)benzyl)oxy)phenyl)pyridine (5e): White solid, yield 93.4%, m.p. 111.6–113.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.07 (s, 1H), 8.62 (s, 1H), 8.13 (d, J = 8.8 Hz, 1H), 8.10 (s, 1H), 7.83 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 8.7 Hz, 1H), 5.52 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 157.42, 156.64, 144.47 (q, J = 3.6 Hz), 139.94, 136.39, 135.93 (q, J = 3.5 Hz), 135.35, 129.60 (q, J = 308.7 Hz), 129.51, 129.06, 128.33, 128.15 (q, J = 5.1 Hz), 125.02 (q, J = 33.3 Hz), 123.45 (q, J = 273.6 Hz), 122.80 (q, J = 274.1 Hz), 122.48 (q, J = 1.8 Hz), 117.04 (q, J = 30.7 Hz), 113.76, 69.21. HRMS (ESI): calculated for C21H10ClF9NOS [M−H]− 530.0033, 532.0004, found 530.0031, 532.0010.
3-chloro-2-(2,3-difluoro-4-((4-((trifluoromethyl)thio)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (5f): White solid, yield 80.2%, m.p. 79.5–82.0 °C. 1H NMR (500 MHz, CDCl3) δ: 8.84 (d, J = 1.1 Hz, 1H), 8.06 (d, J = 1.5 Hz, 1H), 7.71 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 8.3 Hz, 2H), 7.19–7.15 (m, 1H), 6.92–6.89 (m, 1H), 5.26 (s, 2H). 13C NMR (126 MHz, CDCl3) δ: 155.42, 149.29 (dd, J = 252.8, 11.0 Hz), 149.28 (dd, J = 8.2, 2.8 Hz), 144.45 (q, J = 3.6 Hz), 141.76 (dd, J = 249.8, 14.4 Hz), 139.03, 136.78, 134.93 (q, J = 3.2 Hz), 132.36, 129.68 (q, J = 308.6 Hz), 128.26, 127.20 (q, J = 33.9 Hz), 124.84 (t, J = 3.6 Hz), 124.65, 122.74 (q, J = 273.6 Hz), 120.22 (d, J = 12.2 Hz), 110.36 (q, J = 2.6 Hz), 70.97. HRMS (ESI): calculated for C20H9ClF8NOS [M−H]− 497.9971, 499.9942, found 497.9969, 499.9949.
3-chloro-5-(trifluoromethyl)-2-(4-((4-((trifluoromethyl)sulfinyl)benzyl)oxy)phenyl)pyridin (6a): White solid, yield 41.7%, m.p. 123.3–124.7 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.00 (s, 1H), 8.53 (s, 1H), 7.94 (d, J = 8.1 Hz, 2H), 7.82 (d, J = 8.3 Hz, 2H), 7.76 (d, J = 8.7 Hz, 2H), 7.19 (d, J = 8.8 Hz, 2H), 5.35 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 159.17, 158.87, 144.40 (q, J = 3.7 Hz), 143.20, 135.85 (q, J = 3.4 Hz), 134.70 (q, J = 1.4 Hz), 131.14, 129.50, 129.28, 128.67, 126.26, 124.83 (q, J = 337.5 Hz), 124.47 (q, J = 32.9 Hz), 122.96 (q, J = 273.7 Hz), 114.45, 68.47. HRMS (ESI): calculated for C20H13ClF6NO2S [M+H]+ 480.0254, 482.0225, found 480.0254, 482.0227.
3-chloro-2-(3-fluoro-4-((4-((trifluoromethyl)sulfinyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (6b): White solid, yield 80.3%, m.p. 113.0–115.0 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.02 (s, 1H), 8.57 (s, 1H), 7.97 (d, J = 7.8 Hz, 2H), 7.84 (d, J = 8.0 Hz, 2H), 7.69 (dd, J = 12.2, 1.5 Hz, 1H), 7.62 (d, J = 8.2 Hz, 1H), 7.43 (t, J = 8.6 Hz, 1H), 5.45 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 157.59, 150.96 (d, J = 245.2 Hz), 147.10 (d, J = 10.4 Hz), 144.36 (q, J = 3.7 Hz), 142.56, 135.91 (q, J = 3.2 Hz), 134.94 (q, J = 1.0 Hz), 129.88 (d, J = 6.6 Hz), 129.44, 128.68, 126.31, 126.25, 124.86 (q, J = 33.1 Hz), 124.77 (q, J = 337.7 Hz), 122.81 (q, J = 273.9 Hz), 117.26 (d, J = 19.9 Hz), 114.75, 69.38. HRMS (ESI): calculated for C20H10ClF7NO2S [M−H]− 496.0014, 497.9985, found 496.0015, 497.9993.
3-chloro-2-(3-chloro-4-((4-((trifluoromethyl)sulfinyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (6c): White solid, yield 50.1%, m.p. 119.3–120.3 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.01 (s, 1H), 8.58 (s, 1H), 7.96 (d, J = 8.1 Hz, 2H), 7.87–7.83 (m, 3H), 7.76 (dd, J = 8.6, 1.9 Hz, 1H), 7.40 (d, J = 8.7 Hz, 1H), 5.46 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 157.55, 154.35, 144.47 (q, J = 3.9 Hz), 142.68, 135.98 (q, J = 3.3 Hz), 134.86 (q, J = 1.0 Hz), 131.02, 130.37, 129.78, 129.53, 128.44, 126.33, 124.93 (q, J = 33.2 Hz), 124.83 (q, J = 337.6 Hz), 122.88 (q, J = 274.1 Hz), 121.23, 113.67, 69.30. HRMS (ESI): calculated for C20H10Cl2F6NO2S [M−H]− 511.9719, 513.9689, found 511.9718, 513.9692.
3-chloro-2-(3-nitro-4-((4-((trifluoromethyl)sulfinyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (6d): Yellow solid, yield 96.2%, m.p. 116.0–118.0 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.09 (s, 1H), 8.66 (s, 1H), 8.41 (d, J = 2.1 Hz, 1H), 8.17 (dd, J = 8.8, 2.1 Hz, 1H), 8.01 (d, J = 8.1 Hz, 2H), 7.87 (d, J = 8.2 Hz, 2H), 7.67 (d, J = 8.9 Hz, 1H), 5.61 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 156.67, 151.67, 144.54 (q, J = 3.6 Hz), 142.05, 138.94, 136.03 (q, J = 3.5 Hz), 135.44, 134.97, 129.67, 129.31, 128.30, 126.38, 126.28, 125.30 (q, J = 33.3 Hz), 124.77 (q, J = 337.8 Hz), 122.76 (q, J = 274.1 Hz), 115.28, 69.86. HRMS (ESI): calculated for C20H10ClF6N2O4S [M−H]− 522.9959, 524.9930, found 522.9961, 524.9921.
3-chloro-5-(trifluoromethyl)-2-(3-(trifluoromethyl)-4-((4-((trifluoromethyl)sulfinyl)benzyl)oxy)phenyl)pyridine (6e): White solid, yield 51.1%, m.p. 117.9–120.1 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.05 (s, 1H), 8.60 (s, 1H), 8.09 (d, J = 9.8 Hz, 2H), 7.98 (s, 2H), 7.82 (d, J = 5.1 Hz, 2H), 7.54 (d, J = 6.4 Hz, 1H), 5.55 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 157.40, 156.58, 144.48 (q, J = 2.7 Hz), 142.44, 135.95, 135.37, 134.86 (q, J = 1.5 Hz), 129.53, 129.15, 128.20, 128.09, 126.29, 125.04 (q, J = 33.2 Hz), 124.77 (q, J = 339.3 Hz), 123.44 (q, J = 273.4 Hz), 122.80 (q, J = 274.3 Hz), 117.06 (q, J = 30.8 Hz), 113.78, 69.23. HRMS (ESI): calculated for C21H10ClF9NO2S [M−H]− 545.9983, 547.9953, found 545.9985, 547.9960.
3-chloro-2-(2,3-difluoro-4-((4-((trifluoromethyl)sulfinyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (6f): White solid, yield 48.4%, m.p. 122.4–24.4 °C. 1H NMR (500 MHz, CDCl3) δ: 8.79-8.75 (m, 1H), 7.99 (d, J = 1.5 Hz, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.66 (d, J = 8.5 Hz, 2H), 7.13–7.10 (m, 1H), 6.86–6.83 (m, 1H), 5.25 (s, 2H). 13C NMR (126 MHz, CDCl3) δ: 154.15, 148.13 (dd, J = 253.0, 11.6 Hz), 147.93 (dd, J = 8.1, 3.1 Hz), 143.30 (q, J = 3.9 Hz), 140.90, 140.59 (dd, J = 250.4, 14.4 Hz), 134.73 (q, J = 1.5 Hz), 133.79 (q, J = 3.5 Hz), 131.19, 127.08, 126.08 (q, J = 33.9 Hz), 125.42, 123.76 (t, J = 4.0 Hz), 123.66 (q, J = 335.8 Hz), 121.56 (q, J = 273.6 Hz), 119.28 (d, J = 12.4 Hz), 109.16 (d, J = 3.1 Hz), 69.60. HRMS (ESI): calculated for C20H9ClF8NO2S [M−H]− 513.9920, 515.9891, found 513.9916, 515.9887.
3-chloro-5-(trifluoromethyl)-2-(4-((4-((trifluoromethyl)sulfonyl)benzyl)oxy)phenyl)pyridine (7a): White solid, yield 40.1%, m.p. 132.7–134.8 °C. 1H NMR (500 MHz, DMSO-d6) δ: 9.02–8.97 (m, 1H), 8.53 (d, J = 1.5 Hz, 1H), 8.19 (d, J = 8.4 Hz, 2H), 7.94 (d, J = 8.5 Hz, 2H), 7.78–7.75 (m, 2H), 7.21–7.18 (m, 2H), 5.45 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 158.92, 158.78, 147.74, 144.32 (q, J = 3.8 Hz), 135.76 (q, J = 3.5 Hz), 131.12, 131.03, 129.65, 129.23, 128.92, 128.58, 124.46 (q, J = 32.9 Hz), 122.88 (q, J = 273.3 Hz), 119.41 (q, J = 326.7 Hz), 114.42, 68.10. HRMS (ESI): calculated for C20H11ClF6NO3S [M−H]− 494.0058, 496.0028, found 494.0061, 496.0035.
3-chloro-2-(3-fluoro-4-((4-((trifluoromethyl)sulfonyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (7b): White solid, yield 48.4%, m.p. 111.8–114.0 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.02 (s, 1H), 8.58 (s, 1H), 8.23 (d, J = 8.2 Hz, 2H), 7.95 (d, J = 8.4 Hz, 2H), 7.71 (dd, J = 12.3, 1.9 Hz, 1H), 7.62 (d, J = 8.5 Hz, 1H), 7.41 (t, J = 8.7 Hz, 1H), 5.54 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 157.57, 150.93 (d, J = 245.4 Hz), 147.12, 146.91 (d, J = 10.5 Hz), 144.37 (q, J = 3.9 Hz), 135.92 (q, J = 3.4 Hz), 131.11, 130.09 (d, J = 6.8 Hz), 129.47, 128.96, 128.79, 126.34 (d, J = 3.2 Hz), 124.90 (q, J = 33.3 Hz), 122.81 (q, J = 274.0 Hz), 119.41 (q, J = 327.6 Hz), 117.33 (d, J = 19.8 Hz), 114.76, 68.99. HRMS (ESI): calculated for C20H10ClF7NO3S [M−H]− 511.9964, 513.9934, found 511.9968, 513.9937.
3-chloro-2-(3-chloro-4-((4-((trifluoromethyl)sulfonyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (7c): White solid, yield 68.9%, m.p. 142.6–144.8 °C. 1H NMR (500 MHz, DMSO-d6) δ: 9.04–9.00 (m, 1H), 8.57 (d, J = 1.5 Hz, 1H), 8.24 (d, J = 8.4 Hz, 2H), 7.97 (d, J = 8.5 Hz, 2H), 7.90 (d, J = 2.2 Hz, 1H), 7.78 (dd, J = 8.6, 2.2 Hz, 1H), 7.40 (d, J = 8.7 Hz, 1H), 5.57 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 157.43, 154.12, 147.14, 144.36 (d, J = 3.7 Hz), 135.86 (d, J = 3.3 Hz), 131.07, 130.97, 130.50, 129.71, 129.45, 128.74, 128.66, 124.91 (q, J = 33.3 Hz), 122.78 (q, J = 273.4 Hz), 121.23, 119.39 (q, J = 326.9 Hz), 113.64, 68.94. HRMS (ESI): calculated for C20H10Cl2F6NO3S [M−H]− 527.9668, 529.9639, found 527.9664, 529.9640.
3-chloro-2-(3-nitro-4-((4-((trifluoromethyl)sulfonyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (7d): Yellow solid, yield 24.8%, m.p. 129.1–131.6 °C. 1H NMR (500 MHz, DMSO-d6) δ: 9.06 (d, J = 0.9 Hz, 1H), 8.62 (d, J = 1.4 Hz, 1H), 8.40 (d, J = 2.3 Hz, 1H), 8.25 (d, J = 8.4 Hz, 2H), 8.15 (dd, J = 8.8, 2.3 Hz, 1H), 7.95 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.9 Hz, 1H), 5.67 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ: 156.61, 151.50, 146.52, 144.51 (q, J = 3.8 Hz), 138.85, 136.00 (q, J = 3.6 Hz), 135.50, 131.09, 129.65, 129.47, 128.86, 128.61, 126.43, 125.30 (q, J = 33.3 Hz), 122.72 (q, J = 273.5 Hz), 119.38 (q, J = 326.8 Hz), 115.26, 69.51. HRMS (ESI): calculated for C20H10ClF6N2O5S [M−H]− 538.9909, 540.9879, found 538.9905, 540.9882.
3-chloro-5-(trifluoromethyl)-2-(3-(trifluoromethyl)-4-((4-((trifluoromethyl)sulfonyl)benzyl)oxy)phenyl)pyridine (7e): White solid, yield 35.2%, m.p. 111.2–113.4 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.05 (s, 1H), 8.61 (s, 1H), 8.26 (d, J = 8.1 Hz, 2H), 8.13 (d, J = 9.0 Hz, 1H), 8.09 (s, 1H), 7.93 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 8.7 Hz, 1H), 5.65 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 157.39, 156.37, 146.94, 144.49 (q, J = 3.6 Hz), 135.95 (q, J = 3.4 Hz), 135.44, 131.17, 129.55, 129.34, 128.77, 128.46, 128.22 (q, J = 5.3 Hz), 125.07 (q, J = 33.0 Hz), 123.44 (q, J = 273.5 Hz), 122.80 (q, J = 274.1 Hz), 119.41 (q, J = 327.3 Hz), 117.04 (q, J = 30.7 Hz), 113.77, 68.90. HRMS (ESI): calculated for C21H10ClF9NO3S [M−H]− 561.9932, 563.9902, found 561.9935, 563.9913.
3-chloro-2-(2,3-difluoro-4-((4-((trifluoromethyl)sulfonyl)benzyl)oxy)phenyl)-5-(trifluoromethyl)pyridine (7f): White solid, yield 37.7%, m.p. 102.8–104.8 °C. 1H NMR (500 MHz, CDCl3) δ: 8.77 (d, J = 1.1 Hz, 1H), 8.02 (d, J = 8.3 Hz, 2H), 7.99 (d, J = 1.6 Hz, 1H), 7.72 (d, J = 8.4 Hz, 2H), 7.15–7.11 (m, 1H), 6.87–6.82 (m, 1H), 5.29 (s, 2H). 13C NMR (126 MHz, CDCl3) δ: 155.22, 149.30 (dd, J = 253.0, 11.3 Hz), 148.83 (dd, J = 8.2, 3.1 Hz), 145.53, 144.47 (q, J = 3.8 Hz), 141.75 (dd, J = 250.2, 14.3 Hz), 134.96 (q, J = 3.5 Hz), 132.35, 131.42, 131.27, 128.14, 127.29 (q, J = 33.9 Hz), 124.99 (t, J = 3.9 Hz), 122.70 (q, J = 273.6 Hz), 119.92 (q, J = 326.4 Hz), 120.71 (d, J = 12.5 Hz), 110.29 (d, J = 3.1 Hz), 70.38. HRMS (ESI): calculated for C20H9ClF8NO3S [M−H]− 529.9869, 531.9840, found 529.9869, 531.9824.
3.3. Herbicidal Activity Test
Levels of herbicidal activity for compounds 5a–5f, 6a–6f, and 7a–7f against the monocotyledonous weeds Digitaria sanguinalis (DS), Echinochloa crusgalli (EC), and Setaria viridis (SV), and the dicotyledonous weeds Abutilon theophrasti (AT), Amaranthus retroflexus (AR), and Eclipta prostrate (EP) were determined using previously disclosed methods [29,30,31,34].
All target compounds were dissolved in N,N-dimethylformamide, diluted to an appropriate dose with distilled water containing 0.1% Tween-80 emulsifier, and used for seedlings at the three-leaf stage with three replicates. Seedlings treated with N,N-dimethylformamide and distilled water containing 0.1% Tween-80 emulsifier served as blank controls, with fomesafen-treated seedlings serving as positive controls. The application rates were 150, 75, and 37.5 g a.i./ha. Herbicidal activity was assessed visually after a 20-day period, with the results being listed in Table 1.
4. Conclusions
In conclusion, 18 novel α-trifluorothioanisole derivatives containing phenylpyridine moieties were prepared as candidate herbicides. The preliminary herbicidal activity assay results show that some target compounds exhibited good herbicidal activities against broadleaf weeds at 150 g a.i./hm2. Among these, compound 5a possessed excellent activity (>85%) against AT, AR, and EP at 37.5 g a.i./hm2, which was slightly superior to fomesafen. Furthermore, compound 5f possessed good activity (>70%) against AT, AR, and EP at 37.5 g a.i./hm2, which was equivalent to that of fomesafen. Thus, compound 5a may be a lead compound for further structural optimization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27185879/s1, Table S1: Crystal data and structure refinement parameters of compound 5a; Table S2: Bond angles of compound 5a; Table S3: Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 × 103) of the nonhydrogen atoms; Table S4: Hydrogen coordinates (× 104) and isotropic displacement parameters (Å2 × 103); Table S5: Bond lengths of compound 5a; Figure S1–S36:1H NMR and 13C NMR spectra of target compounds 5a-5f, 6a-6f, and 7a-7f.
Author Contributions
Z.C. and W.Z. carried out experimental work; Z.C. prepared the manuscript; X.D. designed the material and supervised the project; and Z.Y. and X.D. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Samples of the compounds are not available from the authors.
Acknowledgments
We are grateful to Yong-Hua Li (Zhejiang Research Institute of Chemical Industry) for assistance with the bioactivity assay.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-M.; Tan, X.-Y.; Feng, J.; Ding, N.; Li, Y.-P.; Jin, Z.; Meng, Q.-G.; Liu, X.-P.; Hu, C. Design, synthesis and biological evaluation of a new series of 1-aryl-3-{4-[(pyridin-2-ylmethyl)thio]phenylurea derivatives as antiproliferative agents. Molecules 2019, 24, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, V.; Cichero, E.; Schenone, S.; Naesens, L.; Tonelli, M. Synthesis and biological evaluation of novel (thio)semicarbazone-based benzimidazoles as antiviral agents against human respiratory viruses. Molecules 2020, 25, 1487–1507. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Al-Hussain, S.A.; Zaki, M.E.A. Design, synthesis and anticancer activity of new polycyclic: Imidazole, thiazine, oxathiine, pyrrolo-quinoxaline and thienotriazolopyrimidine derivatives. Molecules 2021, 26, 2031–2050. [Google Scholar] [CrossRef]
- Li, Y.-T.; Yao, W.-Q.; Lin, J.; Li, F.-L.; Wu, Y.; Xu, J.-X. Design, synthesis, and biological activity of novel triazole sulfonamide derivatives containing a benzylamine moiety. J. Heterocycl. Chem. 2019, 56, 2170–2178. [Google Scholar] [CrossRef]
- Yu, X.-L.; Liu, Y.-X.; Li, Y.-Q.; Wang, Q.-M. Design, synthesis, acaricidal/insecticidal activity, and structure-activity relationship studies of novel oxazolines containing sulfone/sulfoxide groups based on the sulfonylurea receptor protein-binding site. J. Agric. Food Chem. 2016, 64, 3034–3040. [Google Scholar] [CrossRef]
- Fu, Q.; Cai, P.-P.; Cheng, L.; Zhong, L.-K.; Tan, C.-X.; Shen, Z.-H.; Han, L.; Xu, T.-M.; Liu, X.-H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag. Sci. 2020, 76, 679–868. [Google Scholar] [CrossRef]
- Hao, S.-L.; Cai, Z.-F.; Cao, Y.-Y.; Du, X.-H. Design, synthesis, and acaricidal activity of phenyl methoxyacrylates containing 2-alkenylthiopyrimidine. Molecules 2020, 25, 3379–3390. [Google Scholar] [CrossRef]
- Wang, H.-L.; Li, H.-R.; Zhang, Y.-C.; Yang, W.-T.; Yao, Z.; Wu, R.-J.; Niu, C.-W.; Li, Y.-H.; Wang, J.-G. Discovery of ortho-alkoxy substituted novel sulfonylurea compounds that display strong herbicidal activity against monocotyledon grasses. J. Agric. Food Chem. 2021, 69, 8415–8427. [Google Scholar] [CrossRef]
- Zhang, D.K.; Hua, X.W.; Liu, M.; Wu, C.C.; Wei, W.; Liu, Y.; Chen, M.G.; Zhou, S.; Li, Y.H.; Li, Z.M. Design, synthesis and herbicidal activity of novel sulfonylureas containing triazole and oxadiazole moieties. Chem. Res. Chin. U. 2016, 32, 607–614. [Google Scholar] [CrossRef]
- Wang, S.-H.; Li, M.; Su, B.; Lu, Q.-H. Preparation and properties of superhydrophobic polyphenylene sulfide composite coatings. Acta Polym. Sin. 2010, 10, 449–455. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Gao, Y.; Zhang, Y.-X.; Zhang, M.-G.; Gao, Y.; Cheng, B.-W.; Li, Z.-H. Preparation and properties of polyphenylene sulfide/oxidized-polyphenylene sulfide composite membranes. React. Funct. Polym. 2021, 160, 104842. [Google Scholar] [CrossRef]
- Zheng, H.-D.; Huang, Y.-J.; Weng, Z.-Q. Recent advances in trifluoromethylthiolation using nucleophilic trifluoromethylthiolating reagents. Tetrahedron Lett. 2016, 57, 1397–1409. [Google Scholar] [CrossRef]
- Nohara, T.; Fujiwara, Y.; El-Aasr, M.; Ikeda, T.; Ono, M.; Nakano, D.; Kinjoet, J. Antitumor allium sulfides. Chem. Pharm. Bull. 2017, 65, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Crozet, M.D.; Rathelot, P.; Azas, N.; Vanelle, P. Synthesis and promising in vitro antiproliferative activity of sulfones of a 5-nitrothiazole series. Molecules 2012, 18, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Liu, X.-H.; Zhao, W.; Shen, Z.-H.; Xing, J.-H.; Xu, T.-M.; Peng, W.-L. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 2017, 125, 881–889. [Google Scholar] [CrossRef]
- Khalil, Y.; Flower, K.; Siddique, K.H.M.; Ward, P. Pyroxasulfone efficacy for annual ryegrass control is affected by wheat residue height, amount, and orientation. Pest Manag. Sci. 2019, 76, 861–867. [Google Scholar] [CrossRef]
- Li, P.; Wang, L.; Wang, X. Recent advances on the pesticidal activity evaluations of sulfone derivatives: A 2010 to 2020 decade in mini-review. J. Heterocycl. Chem. 2020, 58, 28–39. [Google Scholar] [CrossRef]
- Su, S.-H.; Zhou, X.; Liao, G.-P.; Qi, P.-Y.; Jin, L.-H. Synthesis and antibacterial evaluation of new sulfone derivatives containing 2-aroxymethyl-1,3,4-oxadiazole/thiadiazole moiety. Molecules 2016, 22, 64–80. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.-M.; He, J.; He, M.; Han, F.-F.; Chen, X.-H.; Pan, Z.-X.; Wang, J.; Tong, M. Synthesis and antifungal activity of novel sulfone derivatives containing 1,3,4-oxadiazole moieties. Molecules 2011, 16, 9129–9141. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, Y.-H.; Zhou, J.-L.; Luo, H.; Yan, J.-W.; Mao, Y.-Y.; Wang, Z. The efficacy and underlying mechanism of sulfone derivatives containing 1,3,4-oxadiazole on citrus canker. Molecules 2015, 20, 14103–14117. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, M.; Ito, M.; Yoshimura, T.; Miyazaki, M.; Ueno, R.; Kawasaki, H.; Takahashi, S.; Todoroki, Y. Synthesis and herbicidal activity of 3-{[(hetero)aryl]methanesulfonyl}-4,5-dihydro-1,2-oxazole derivative; Discovery of the novel pre-emergence herbicide pyroxasulfone. J. Pestic. Sci. 2016, 41, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, P.; Hampreche, G.; Puhl, M.; Westphalen, K.O.; Zagaret, C. Synthesis and herbicidal activity of phenylpyridines—A new lead. Chim. Int. J. Chem. 2003, 57, 715–719. [Google Scholar] [CrossRef]
- Schaefer, P.; Hampreche, G.; Heistracher, E.; Koenig, H.; Klintz, R.; Muenster, P.; Rang, H.; Westphalen, K.O.; Gerber, M.; Walter, H. Preparation of Substituted 2-Phenylpyriden Herbicides. DE Patent DE4323916A1, 19 January 1995. [Google Scholar]
- Xie, Y.; Chi, H.W.; Guan, A.Y.; Liu, C.L.; Ma, H.J.; Cui, D.L. Design, synthesis, and herbicidal activity of novel substituted 3-(pyridin-2-yl)benzenesulfonamide derivatives. J. Agric. Food Chem. 2014, 62, 12491–12496. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, W.; Ding, F.; Liu, S.J.; Ma, H.J.; Liu, C.L. Quantitative structure-activity relationship (QSAR) directed the discovery of 3-(pyridin-2-yl)benzenesulfonamide derivatives as novel herbicidal agents. Pest Manag. Sci. 2017, 74, 189–199. [Google Scholar] [CrossRef]
- Xie, Y.; Chi, H.W.; Guan, A.Y.; Liu, C.L.; Ma, H.J.; Cui, D.L. Synthesis and evaluation of substituted 3-(pyridin-2-yl)-benzenesulfonamide derivatives as potent herbicidal agents. Bioorg. Med. Chem. 2016, 24, 428–434. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Mao, D.J.; Wang, W.W.; Du, X.H. Kresoxim-methyl derivatives: Synthesis and herbicidal activities of (pyridinylphenoxymethylene)phenyl methoxyiminoacetates. J. Agric. Food Chem. 2017, 65, 6114–6121. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Wang, W.W.; Du, X.H. Synthesis, crystal structure and herbicidal activity of methyl (E)-α-(methoxyimino)-2-((4-(3-chloro-5-(trifluoromethyl)-pyridine-2-yl)phenoxy)methyl)benzeneacetate. Chin. J. Struct. Chem. 2019, 38, 1123–1128. [Google Scholar]
- Cao, Y.-Y.; Cai, Z.-F.; Zhang, W.-L.; Du, X.-H. Synthesis and herbicidal activity of novel β-methoxyacrylate derivatives containing a substituted phenylpyridine moiety. Chem. Res. Chin. U. 2019, 35, 1008–1011. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Li, Y.; Xiao, Y.; Zhu, L.; Cheng, C.-M.; Liu, Y. Advances in synthesis and application of sulfoxide compounds. Chin. J. Org. Chem. 2011, 31, 925–931. [Google Scholar]
- Horvat, M.; Kodri, G.; Jereb, M.; Iskra, J. One pot synthesis of trifluoromethyl aryl sulfoxides by trifluoromethylthiolation of arenes and subsequent oxidation with hydrogen peroxide. RSC Adv. 2020, 10, 34534–34540. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-F.; Zhang, W.-L.; Cao, Y.-Y.; Du, X.-H. Synthesis and herbicidal activities of 2-phenylpyridine compounds containing alkenyl moieties. J. Heterocycl. Chem. 2022, 59, 1247–1252. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).