Tocotrienol as a Protecting Agent against Glucocorticoid-Induced Osteoporosis: A Mini Review of Potential Mechanisms
Abstract
1. Introduction
2. Glucocorticoids and Bone
2.1. Direct Action of Glucocorticoid on Osteoblasts
2.2. Action of Glucocorticoid on Oxidative Stress in Osteoblasts
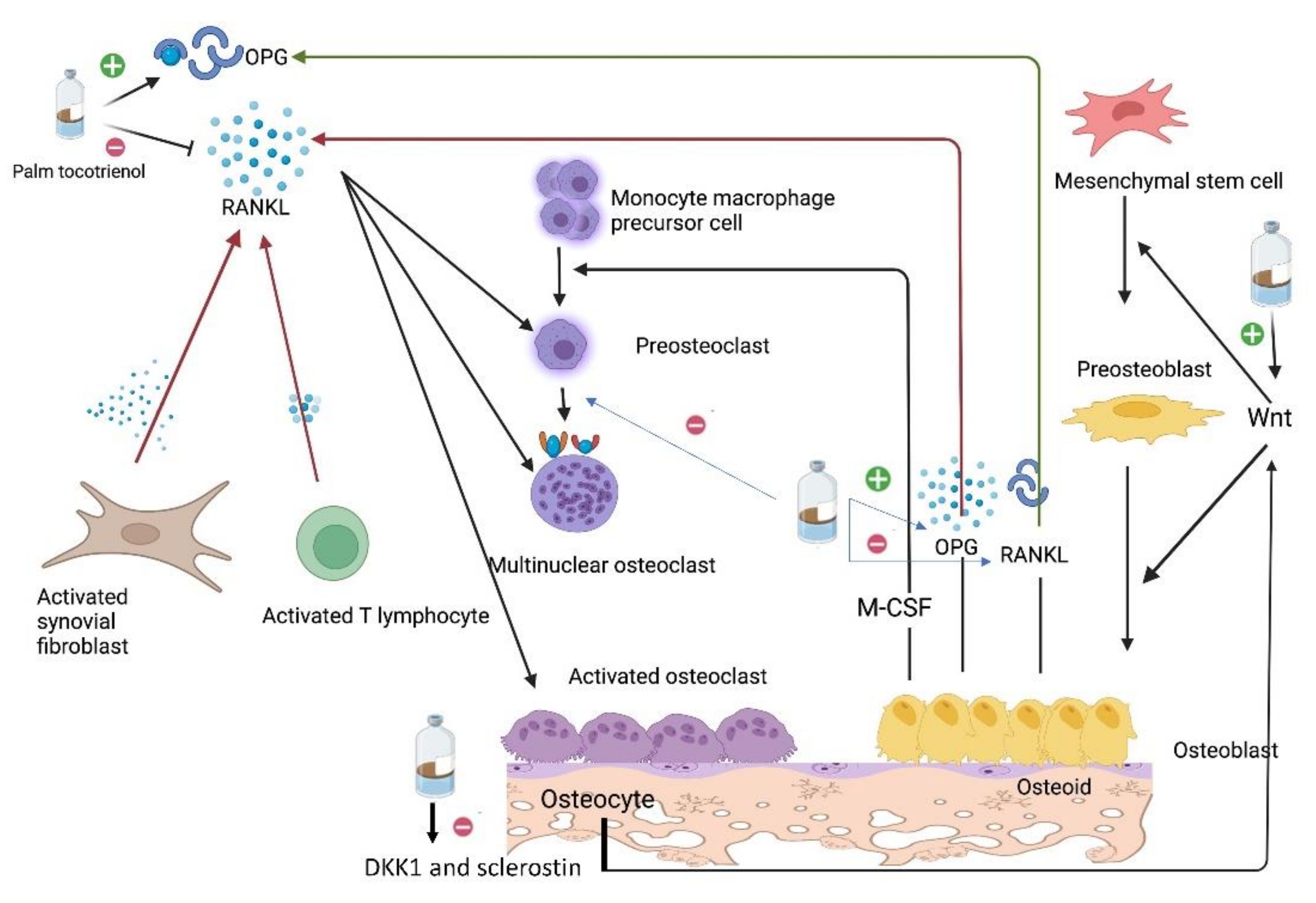
2.3. Action of Glucocorticoid on Osteoclasts
3. Tocotrienol and Bone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galindo, M.; Pratap, J.; Young, D.W.; Hovhannisyan, H.; Im, H.J.; Choi, J.Y.; Lian, J.B.; Stein, J.L.; Stein, G.S.; van Wijnen, A.J. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J. Biol. Chem. 2005, 280, 20274–20285. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H.; Ohlsson, C. The WNT system: Background and its role in bone. J. Intern. Med. 2015, 277, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ekeuku, S.O.; Pang, K.-L. Sclerostin in the development of osteoarthritis: A mini review. Malays. J. Pathol. 2022, 44, 1–18. [Google Scholar]
- Wong, S.K.; Mohamad, N.-V.; Ibrahim, N.I.; Chin, K.-Y.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef]
- Iba, K.; Chiba, H.; Sawada, N.; Hirota, S.; Ishii, S.; Mori, M. Glucocorticoids induce mineralization coupled with bone protein expression without influence on growth of a human osteoblastic cell line. Cell Struct. Funct. 1995, 20, 319–330. [Google Scholar] [CrossRef][Green Version]
- Herbertson, A.; Aubin, J.E. Dexamethasone alters the subpopulation make-up of rat bone marrow stromal cell cultures. J. Bone Min. Res. 1995, 10, 285–294. [Google Scholar] [CrossRef]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem. Cell Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Cui, X.; Li, W.; Xue, Y.; Shang, P.; Zhang, H. Blocking glucocorticoid signaling in osteoblasts and osteocytes prevents mechanical unloading-induced cortical bone loss. Bone 2020, 130, 115108. [Google Scholar] [CrossRef]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Mandic, V.; Takacz, A.; Schmidt-Ullrich, R.; et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef]
- Chen, T.L.; Cone, C.M.; Feldman, D. Glucocorticoid modulation of cell proliferation in cultured osteoblast-like bone cells: Differences between rat and mouse. Endocrinology 1983, 112, 1739–1745. [Google Scholar] [CrossRef]
- Ohnaka, K.; Tanabe, M.; Kawate, H.; Nawata, H.; Takayanagi, R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem. Biophys. Res. Commun. 2005, 329, 177–181. [Google Scholar] [CrossRef]
- Raterman, H.G.; Bultink, I.E.M.; Lems, W.F. Current Treatments and New Developments in the Management of Glucocorticoid-induced Osteoporosis. Drugs 2019, 79, 1065–1087. [Google Scholar] [CrossRef]
- Allen, C.S.; Yeung, J.H.; Vandermeer, B.; Homik, J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst. Rev. 2016, 10, CD001347. [Google Scholar] [CrossRef]
- Saag, K.G.; Zanchetta, J.R.; Devogelaer, J.P.; Adler, R.A.; Eastell, R.; See, K.; Krege, J.H.; Krohn, K.; Warner, M.R. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: Thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009, 60, 3346–3355. [Google Scholar] [CrossRef]
- Dore, R.K.; Cohen, S.B.; Lane, N.E.; Palmer, W.; Shergy, W.; Zhou, L.; Wang, H.; Tsuji, W.; Newmark, R. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann. Rheum. Dis. 2010, 69, 872–875. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Devel. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef]
- Chin, K.Y.; Mo, H.; Soelaiman, I.N. A review of the possible mechanisms of action of tocotrienol-a potential antiosteoporotic agent. Curr. Drug Targets 2013, 14, 1533–1541. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L.; Soelaiman, I.N. Tocotrienol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 97–130. [Google Scholar]
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, N.I.; Shuid, N.A.; Saad, Q.M.; Abdullah, A.; et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, E259. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Vitamin E as an Antiosteoporotic Agent via Receptor Activator of Nuclear Factor Kappa-B Ligand Signaling Disruption: Current Evidence and Other Potential Research Areas. Evid. Based Complement. Alternat. Med. 2012, 2012, 747020. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Yang, L.; Mu, S.; Fang, T.; Fu, Q. Gastrodin alleviates glucocorticoid induced osteoporosis in rats via activating the Nrf2 signaling pathways. Oncotarget 2018, 9, 11528–11540. [Google Scholar] [CrossRef]
- Frenkel, B.; White, W.; Tuckermann, J. Glucocorticoid-Induced Osteoporosis. Adv. Exp. Med. Biol. 2015, 872, 179–215. [Google Scholar] [PubMed]
- Tang, Y.H.; Yue, Z.S.; Li, G.S.; Zeng, L.R.; Xin, D.W.; Hu, Z.Q.; Xu, C. Effect of β-ecdysterone on glucocorticoid-induced apoptosis and autophagy in osteoblasts. Mol. Med. Rep. 2018, 17, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Eijken, M.; Hewison, M.; Cooper, M.S.; de Jong, F.H.; Chiba, H.; Stewart, P.M.; Uitterlinden, G.; Pols, H.A.P.; van Leeuwen, J.P.T.M. 11beta-Hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol. Endocrinol. 2005, 19, 621–631. [Google Scholar] [CrossRef]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell Biochem. 1997, 64, 295–312. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Shi, C.; Huang, P.; Kang, H.; Hu, B.; Qi, J.; Jiang, M.; Zhou, H.; Guo, L.; Deng, L. Glucocorticoid inhibits cell proliferation in differentiating osteoblasts by microRNA-199a targeting of WNT signaling. J. Mol. Endocrinol. 2015, 54, 325–337. [Google Scholar] [CrossRef]
- Mak, W.; Shao, X.; Dunstan, C.R.; Seibel, M.J.; Zhou, H. Biphasic glucocorticoid-dependent regulation of Wnt expression and its inhibitors in mature osteoblastic cells. Calcif. Tissue Int. 2009, 85, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.; Cooper, M.S. Glucocorticoid-induced osteoporosis-a disorder of mesenchymal stromal cells? Front. Endocrinol. 2011, 2, 24. [Google Scholar] [CrossRef]
- Sui, B.; Hu, C.; Liao, L.; Chen, Y.; Zhang, X.; Fu, X.; Zheng, C.; Li, M.; Wu, L.; Zhao, X.; et al. Mesenchymal progenitors in osteopenias of diverse pathologies: Differential characteristics in the common shift from osteoblastogenesis to adipogenesis. Sci. Rep. 2016, 6, 30186. [Google Scholar] [CrossRef]
- Vande Berg, B.C.; Malghem, J.; Lecouvet, F.E.; Devogelaer, J.P.; Maldague, B.; Houssiau, F.A. Fat conversion of femoral marrow in glucocorticoid-treated patients: A cross-sectional and longitudinal study with magnetic resonance imaging. Arthritis Rheum. 1999, 42, 1405–1411. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; Parlee, S.D.; Pham, H.A.; Learman, B.S.; Redshaw, C.M.; Sulston, R.J.; Burr, A.A.; Das, A.K.; Simon, B.R.; et al. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia. Endocrinology 2016, 157, 508–521. [Google Scholar] [CrossRef]
- Justesen, J.; Mosekilde, L.; Holmes, M.; Stenderup, K.; Gasser, J.; Mullins, J.J.; Seckl, J.R.; Kassem, M. Mice deficient in 11beta-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology 2004, 145, 1916–1925. [Google Scholar] [CrossRef][Green Version]
- Ohnaka, K.; Taniguchi, H.; Kawate, H.; Nawata, H.; Takayanagi, R. Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: Novel mechanism of glucocorticoid-induced osteoporosis. Biochem. Biophys. Res. Commun. 2004, 318, 259–264. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Ambrogini, E.; Weinstein, R.S.; Manolagas, S.C. Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J. Biol. Chem. 2011, 286, 44326–44335. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Manolagas, S.C.; Bellido, T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival. Evidence for inside-out signaling leading to anoikis. J. Biol. Chem. 2007, 282, 24120–24130. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Weinstein, R.S.; Parfitt, A.M.; Roberson, P.K.; Manolagas, S.C.; Bellido, T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Investig. 1999, 104, 1363–1374. [Google Scholar] [CrossRef]
- Espina, B.; Liang, M.; Russell, R.G.G.; Hulley, P.A. Regulation of bim in glucocorticoid-mediated osteoblast apoptosis. J. Cell Physiol. 2008, 215, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.K.; Li, C.J.; Liao, H.J.; Wang, C.K.; Wang, G.J.; Ho, M.L. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology 2009, 258, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qian, W.; Weng, X.; Wu, Z.; Li, H.; Zhuang, Q.; Feng, B.; Bian, Y. Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS ONE 2012, 7, e37030. [Google Scholar] [CrossRef] [PubMed]
- Centrella, M.; McCarthy, T.L.; Canalis, E. Glucocorticoid regulation of transforming growth factor beta 1 activity and binding in osteoblast-enriched cultures from fetal rat bone. Mol. Cell Biol. 1991, 11, 4490–4496. [Google Scholar]
- Bennett, A.; Chen, T.; Feldman, D.; Hintz, R.L.; Rosenfeld, R.G. Characterization of insulin-like growth factor I receptors on cultured rat bone cells: Regulation of receptor concentration by glucocorticoids. Endocrinology 1984, 115, 1577–1583. [Google Scholar] [CrossRef]
- McCarthy, T.L.; Centrella, M.; Canalis, E. Cortisol inhibits the synthesis of insulin-like growth factor-I in skeletal cells. Endocrinology 1990, 126, 1569–1575. [Google Scholar] [CrossRef]
- Sato, A.Y.; Richardson, D.; Cregor, M.; Davis, H.M.; Au, E.D.; McAndrews, K.; Zimmers, T.A.; Organ, J.M.; Peacock, M.; Plotkin, L.I.; et al. Glucocorticoids Induce Bone and Muscle Atrophy by Tissue-Specific Mechanisms Upstream of E3 Ubiquitin Ligases. Endocrinology 2017, 158, 664–677. [Google Scholar]
- Chrousos, G.P.; Torpy, D.J.; Gold, P.W. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: Clinical implications. Ann. Intern. Med. 1998, 129, 229–240. [Google Scholar] [CrossRef]
- Straub, R.H.; Cutolo, M.; Pacifici, R. Evolutionary medicine and bone loss in chronic inflammatory diseases--A theory of inflammation-related osteopenia. Semin. Arthritis Rheum. 2015, 45, 220–228. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. The impact of diabetes and diabetes medications on bone health. Endocr. Rev. 2015, 36, 194–213. [Google Scholar] [CrossRef]
- Shanbhogue, V.V.; Mitchell, D.M.; Rosen, C.J.; Bouxsein, M.L. Type 2 diabetes and the skeleton: New insights into sweet bones. Lancet Diabetes Endocrinol. 2016, 4, 159–173. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Park, B.G.; Yoo, C.I.; Kim, H.T.; Kwon, C.H.; Kim, Y.K. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology 2005, 215, 115–125. [Google Scholar] [CrossRef]
- Zhang, S.; Li, D.; Yang, J.Y.; Yan, T.B. Plumbagin protects against glucocorticoid-induced osteoporosis through Nrf-2 pathway. Cell Stress Chaperones 2015, 20, 621–629. [Google Scholar] [CrossRef]
- Feng, Y.L.; Tang, X.L. Effect of glucocorticoid-induced oxidative stress on the expression of Cbfa1. Chem. Biol. Interact. 2014, 207, 26–31. [Google Scholar] [CrossRef]
- Yuzefovych, L.V.; LeDoux, S.P.; Wilson, G.L.; Rachek, L.I. Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: Crosstalk, links and signaling. PLoS ONE 2013, 8, e83349. [Google Scholar] [CrossRef]
- Liu, S.; Fang, T.; Yang, L.; Chen, Z.; Mu, S.; Fu, Q. Gastrodin protects MC3T3-E1 osteoblasts from dexamethasone-induced cellular dysfunction and promotes bone formation via induction of the NRF2 signaling pathway. Int. J. Mol. Med. 2018, 41, 2059–2069. [Google Scholar] [CrossRef]
- Elvy Suhana, M.; Fairus, A.; Norazlina, M.; Mohamad Fairuz, Y.; Ima Nirwana, S. Protective Effects of Palm Tocotrienol Against Glucocorticoid Induced Osteoporosis via Regulation of Gene Expressions. Med. Health 2018, 13, 175–197. [Google Scholar]
- Suhana, M.R.; Fairus, A.; Nur Izrin, S.; Ahmad, A.I.; Chen Hee, C.; Sanghari, N.K.; Izam, A.M.; Fairuz, Y.; Nirwana, S.I. Beneficial Effects of Annatto (Bixa Orellana) Tocotrienol on Bone Histomorphometry and Expres-sion of Genes Related to Bone Formation and Resorption in Osteoporosis Induced by Dexamethasone. Int. J. Med. Res. Health Sci. 2018, 103, 85–100. [Google Scholar]
- Rushworth, S.A.; Macewan, D.J. The role of nrf2 and cytoprotection in regulating chemotherapy resistance of human leukemia cells. Cancers 2011, 3, 1605–1621. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Gori, F.; Riggs, B.L.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: Potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 1999, 140, 4382–4389. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.O.; Brändström, H.; Jonsson, K.B.; Ohlsson, C. Osteoprotegerin mRNA is expressed in primary human osteoblast-like cells: Down-regulation by glucocorticoids. J. Endocrinol. 1998, 159, 191–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brändström, H.; Björkman, T.; Ljunggren, O. Regulation of osteoprotegerin secretion from primary cultures of human bone marrow stromal cells. Biochem. Biophys. Res. Commun. 2001, 280, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Kobza, A.O.; Herman, D.; Papaioannou, A.; Lau, A.N.; Adachi, J.D. Understanding and Managing Corticosteroid-Induced Osteoporosis. Open Access Rheumatol. 2021, 13, 177–190. [Google Scholar] [CrossRef]
- Shi, C.; Qi, J.; Huang, P.; Jiang, M.; Zhou, Q.; Zhou, H.; Kang, H.; Qian, N.; Yang, Q.; Guo, L.; et al. MicroRNA-17/20a inhibits glucocorticoid-induced osteoclast differentiation and function through targeting RANKL expression in osteoblast cells. Bone 2014, 68, 67–75. [Google Scholar] [CrossRef]
- Zhao, B.; Luo, M.; Wang, A.; Zhang, W. MiR-182 antagonist alleviates glucocorticoid-induced secondary bone degeneration and osteoclast differentiation. Cell Mol. Biol. 2022, 67, 123–130. [Google Scholar] [CrossRef]
- Conaway, H.H.; Henning, P.; Lie, A.; Tuckermann, J.; Lerner, U.H. Activation of dimeric glucocorticoid receptors in osteoclast progenitors potentiates RANKL induced mature osteoclast bone resorbing activity. Bone 2016, 93, 43–54. [Google Scholar] [CrossRef]
- Fu, L.; Wu, W.; Sun, X.; Zhang, P. Glucocorticoids Enhanced Osteoclast Autophagy Through the PI3K/Akt/mTOR Signaling Pathway. Calcif. Tissue Int. 2020, 107, 60–71. [Google Scholar] [CrossRef]
- Vanderoost, J.; Søe, K.; Merrild, D.M.; Delaissé, J.M.; van Lenthe, G.H. Glucocorticoid-induced changes in the geometry of osteoclast resorption cavities affect trabecular bone stiffness. Calcif. Tissue Int. 2013, 92, 240–250. [Google Scholar] [CrossRef]
- Chen, Y.H.; Peng, S.Y.; Cheng, M.T.; Hsu, Y.P.; Huang, Z.X.; Cheng, W.T.-K.; Wu, S.-C. Different susceptibilities of osteoclasts and osteoblasts to glucocorticoid-induced oxidative stress and mitochondrial alterations. Chin. J. Physiol. 2019, 62, 70–79. [Google Scholar]
- Teitelbaum, S.L. Glucocorticoids and the osteoclast. Clin. Exp. Rheumatol. 2015, 33, S37–S39. [Google Scholar]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Effect of tocotrienol from Bixa orellana (annatto) on bone microstructure, calcium content, and biomechanical strength in a model of male osteoporosis induced by buserelin. Drug Des. Devel. Ther. 2018, 12, 555–564. [Google Scholar] [CrossRef]
- Xu, W.; He, P.; He, S.; Cui, P.; Mi, Y.; Yang, Y.; Li, Y.; Zhou, S. Gamma-Tocotrienol Stimulates the Proliferation, Differentiation, and Mineralization in Osteoblastic MC3T3-E1 Cells. J. Chem. 2018, 2018, 3805932. [Google Scholar] [CrossRef]
- Bruzzaniti, A.; Baron, R. Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 2006, 7, 123–139. [Google Scholar] [CrossRef]
- Ottewell, P.D. The role of osteoblasts in bone metastasis. J. Bone Oncol. 2016, 5, 124–127. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef]
- Yahaya, M.F.; Zainodin, A.; Pupathy, R.; Edwin Ong, H.M.; Abu Bakar, N.H.; Zamri, N.; Ismail, H.; Ramli, E.S.M. The Effect of Palm Tocotrienol on Surface Osteoblast and Osteoclast in Excess Glucocorticoid Osteoporotic Rat Model. Sains Malays. 2018, 47, 2731–2739. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. The Role of Tocotrienol in Protecting Against Metabolic Diseases. Molecules 2019, 24, 923. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Self-emulsified annatto tocotrienol improves bone histomorphometric parameters in a rat model of oestrogen deficiency through suppression of skeletal sclerostin level and RANKL/OPG ratio. Int. J. Med. Sci. 2021, 18, 3665–3673. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin. Interv. Aging. 2014, 9, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. Int. J. Env. Res. Public Health 2019, 16, 3313. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.; Ahmad, F.; Ima-Nirwana, S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients 2016, 8, 347. [Google Scholar] [CrossRef]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef]
- Challa, T.D.; Rais, Y.; Ornan, E.M. Effect of adiponectin on ATDC5 proliferation, differentiation and signaling pathways. Mol. Cell. Endocrinol. 2010, 323, 282–291. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lee, C.Y.; Chen, M.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. Adiponectin increases BMP-2 expression in osteoblasts via AdipoR receptor signaling pathway. J. Cell Physiol. 2010, 224, 475–483. [Google Scholar] [CrossRef]
- Ha, H.; Lee, J.H.; Kim, H.N.; Lee, Z.H. α-Tocotrienol inhibits osteoclastic bone resorption by suppressing RANKL expression and signaling and bone resorbing activity. Biochem. Biophys. Res. Commun. 2011, 406, 546–551. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Brooks, R.; Kalia, P.; Ireland, D.C.; Beeton, C.; Rushton, N. Direct inhibition of osteoclast formation and activity by the vitamin E isomer gamma-tocotrienol. Int. J. Vitam. Nutr. Res. 2011, 81, 358–367. [Google Scholar] [CrossRef]
- Shen, C.L.; Yang, S.; Tomison, M.D.; Romero, A.W.; Felton, C.K.; Mo, H. Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: A 12-week randomized double-blinded placebo-controlled trial. Osteoporos. Int. 2018, 29, 881–891. [Google Scholar] [CrossRef]
- Shen, C.L.; Mo, H.; Dunn, D.M.; Watkins, B.A. Tocotrienol Supplementation Led to Higher Serum Levels of Lysophospholipids but Lower Acylcarnitines in Postmenopausal Women: A Randomized Double-Blinded Placebo-Controlled Clinical Trial. Front. Nutr. 2021, 8, 766711. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, S.; Yang, S.; Tomison, M.D.; Abbasi, M.; Hao, L.; Scott, S.; Khan, M.S.; Romero, A.W.; Felton, C.K.; et al. A 12-week evaluation of annatto tocotrienol supplementation for postmenopausal women: Safety, quality of life, body composition, physical activity, and nutrient intake. BMC Complement. Altern. Med. 2018, 18, 198. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekeuku, S.O.; Mohd Ramli, E.S.; Abdullah Sani, N.; Abd Ghafar, N.; Soelaiman, I.N.; Chin, K.-Y. Tocotrienol as a Protecting Agent against Glucocorticoid-Induced Osteoporosis: A Mini Review of Potential Mechanisms. Molecules 2022, 27, 5862. https://doi.org/10.3390/molecules27185862
Ekeuku SO, Mohd Ramli ES, Abdullah Sani N, Abd Ghafar N, Soelaiman IN, Chin K-Y. Tocotrienol as a Protecting Agent against Glucocorticoid-Induced Osteoporosis: A Mini Review of Potential Mechanisms. Molecules. 2022; 27(18):5862. https://doi.org/10.3390/molecules27185862
Chicago/Turabian StyleEkeuku, Sophia Ogechi, Elvy Suhana Mohd Ramli, Norfarahin Abdullah Sani, Norzana Abd Ghafar, Ima Nirwana Soelaiman, and Kok-Yong Chin. 2022. "Tocotrienol as a Protecting Agent against Glucocorticoid-Induced Osteoporosis: A Mini Review of Potential Mechanisms" Molecules 27, no. 18: 5862. https://doi.org/10.3390/molecules27185862
APA StyleEkeuku, S. O., Mohd Ramli, E. S., Abdullah Sani, N., Abd Ghafar, N., Soelaiman, I. N., & Chin, K.-Y. (2022). Tocotrienol as a Protecting Agent against Glucocorticoid-Induced Osteoporosis: A Mini Review of Potential Mechanisms. Molecules, 27(18), 5862. https://doi.org/10.3390/molecules27185862






