Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites
Abstract
:1. Introduction
2. Results and Discussions
2.1. Morphological and Structural Characterization of the Composites
2.2. Optical Properties
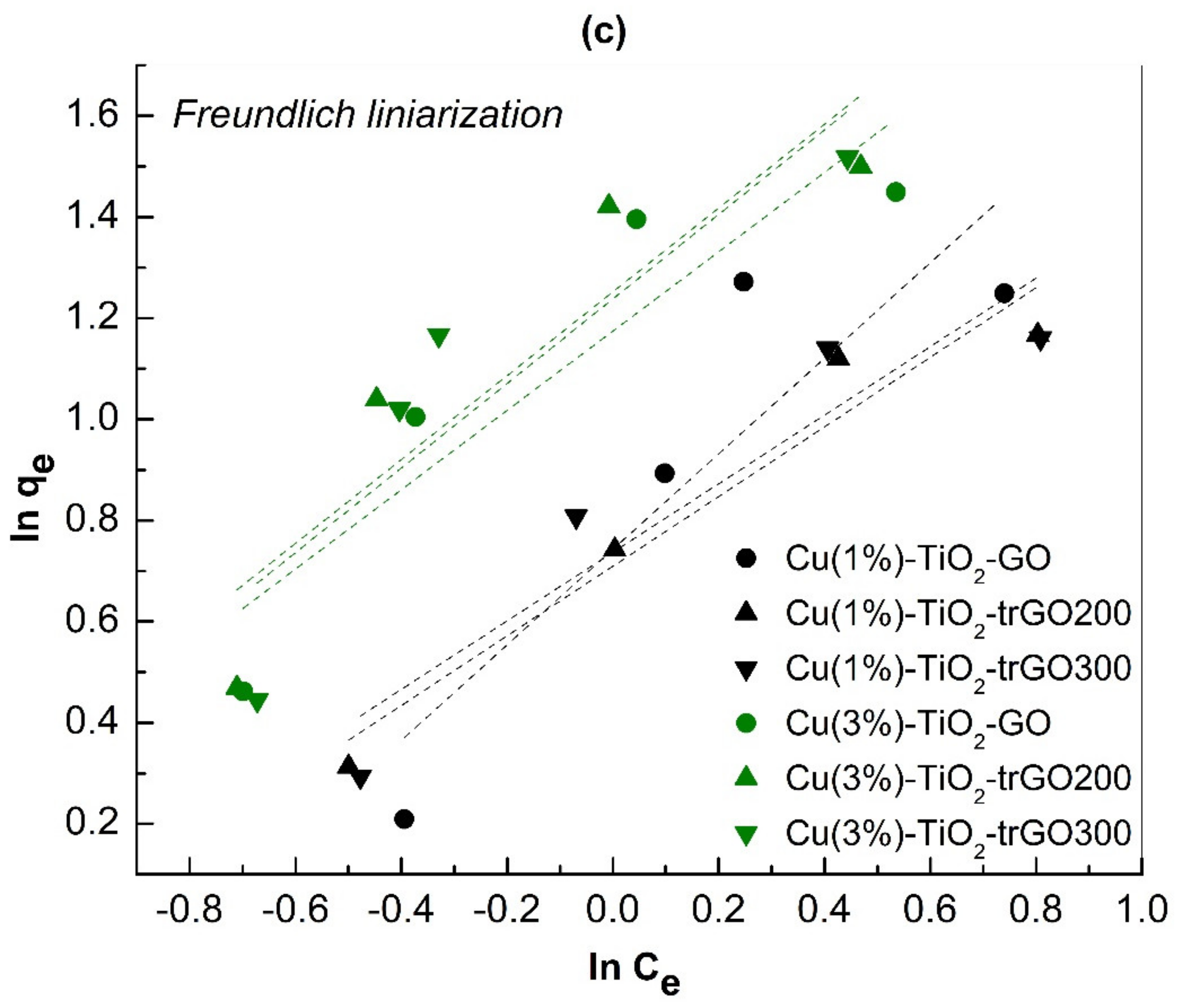
2.3. Adsorption of Methylene Blue
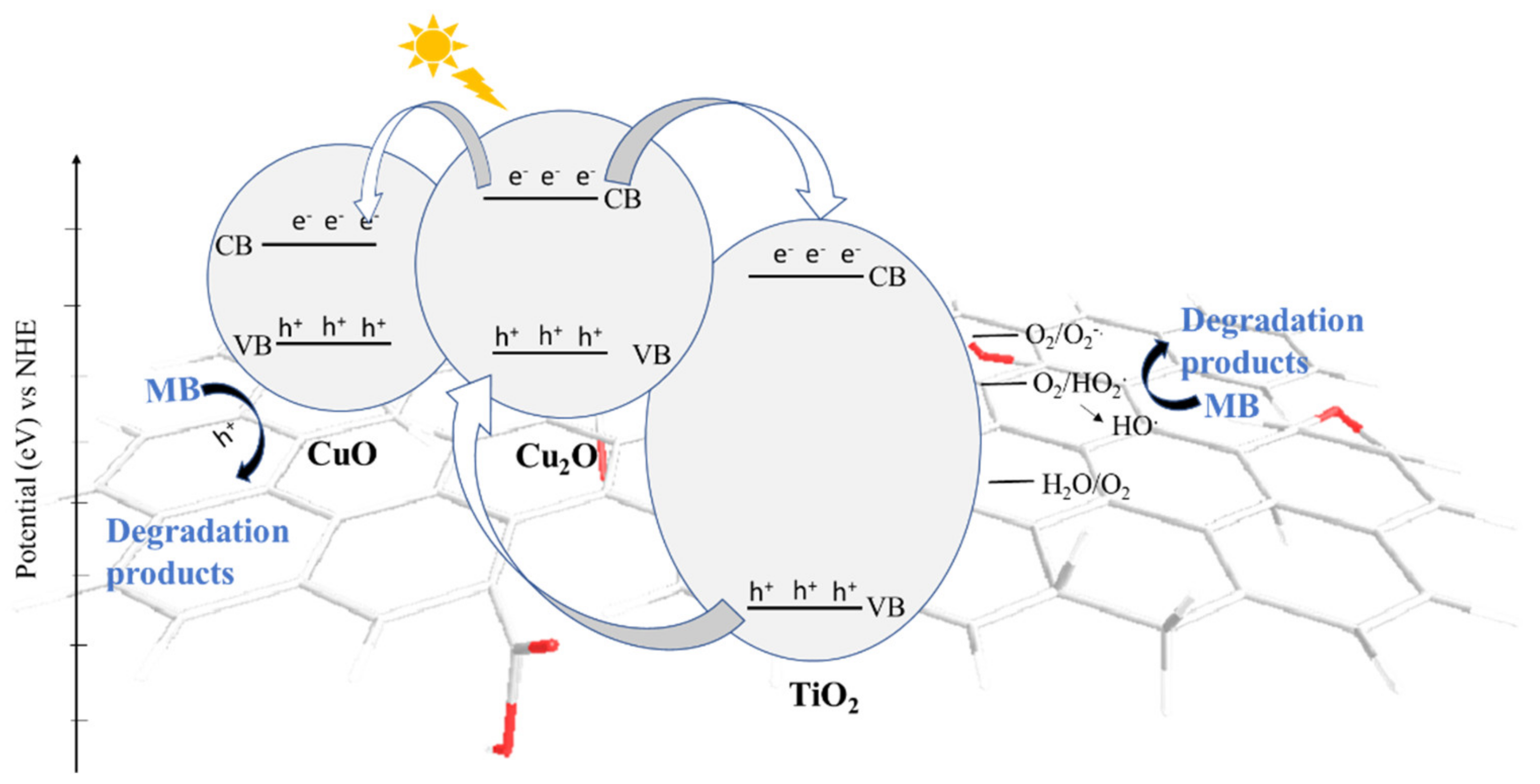
2.4. Photodegradation Experiments
3. Materials and Methods
3.1. Preparation of Starting Cu(1,2,3%)-TiO2
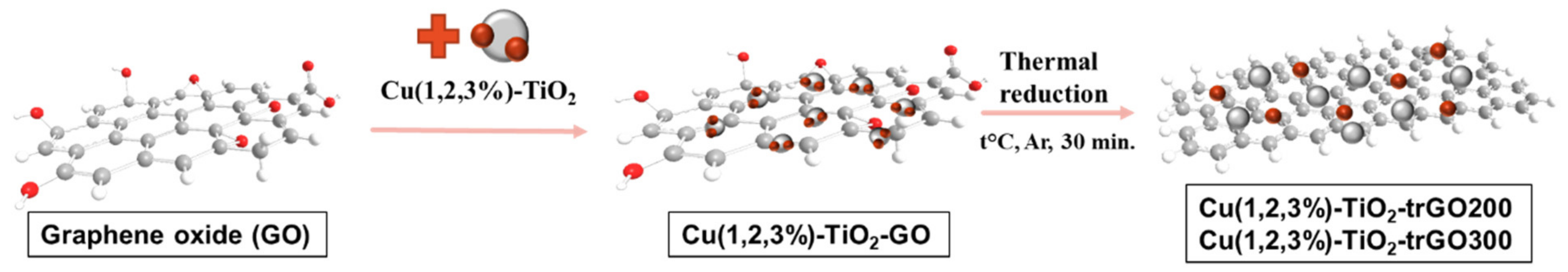
3.2. Preparation of Cu-TiO2-Graphene Composites
3.3. Adsorption Isotherms/Photocatalytic Degradation of Methylene Blue (MB)
3.4. Instrumental Part
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Santoyo, V.; Andrade-Espinoza, B.A.; Romero-Toledo, R.; Anaya-Esparza, L.M.; Villagrán, Z.; Guerra-Contreras, A. Use of Nanostructured Photocatalysts for Dye Degradation: A Reviewtle. Period. Polytech. Chem. Eng. 2022, 66, 367–393. [Google Scholar] [CrossRef]
- Prakruthi, K.; Ujwal, M.P.; Yashas, S.R.; Mahesh, B.; Kumara Swamy, N.; Shivaraju, H.P. Recent advances in photocatalytic remediation of emerging organic pollutants using semiconducting metal oxides: An overview. Environ. Sci. Pollut. Res. 2022, 29, 4930–4957. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Zhou, X.; Zhang, S.; Qian, S.; Li, D.S.; Liu, X.; Zhang, S. Sustainable engineering of TiO2-based advanced oxidation technologies: From photocatalyst to application devices. J. Mater. Sci. Technol. 2021, 78, 202–222. [Google Scholar] [CrossRef]
- Parangi, T.; Mishra, M.K. Titania Nanoparticles as Modified Photocatalysts: A Review on Design and Development. Comments Inorg. Chem. 2019, 39, 90–126. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Karim, A.V.; Selvaraj, A. Graphene composites in photocatalytic oxidation of aqueous organic contaminants–A state of art. Process Saf. Environ. Prot. 2021, 146, 136–160. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wei, Y.-S.; Lu, J.-S. Visible-light-driven photodegradation of sulfamethoxazole and methylene blue by Cu2O/rGO photocatalysts. Chemosphere 2016, 154, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, L.; Tao, T.; Ma, F.; Xu, M.; Zhang, Z. Contrastive study of structure and photocatalytic performance with three-dimensionally ordered macroporous CuO–TiO2 and CuO/TiO2. Appl. Surf. Sci. 2014, 288, 363–368. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Jiang, X. Room-temperature synthesis of cuprous oxide and its heterogeneous nanostructures for photocatalytic applications. J. Alloys Compd. 2019, 772, 447–459. [Google Scholar] [CrossRef]
- Polat, K. Cuprous oxide film sputtered on monolayer graphene for visible light sensitive heterogeneous photocatalysis. Thin Solid Films. 2020, 709, 138254. [Google Scholar] [CrossRef]
- Sahu, K.; Dhonde, M.; Murty, V.V.S. Preparation of copper/TiO2/graphene oxide ternary nanocomposites and their structural, surface morphology, and optical properties. J. Mater. Sci. Mater. Electron. 2021, 32, 15971–15980. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Basnet, P.; Zhao, Y. Tuning the CuxO nanorod composition for efficient visible light induced photocatalysis. Catal. Sci. Technol. 2016, 6, 2228–2238. [Google Scholar] [CrossRef]
- Bayat, F.; Sheibani, S. Enhancement of photocatalytic activity of CuO-Cu2O heterostructures through the controlled content of Cu2O. Mater. Res. Bull. 2022, 145, 111561. [Google Scholar] [CrossRef]
- Sabzehei, K.; Hadavi, S.H.; Bajestani, M.G.; Sheibani, S. Comparative evaluation of copper oxide nano-photocatalyst characteristics by formation of composite with TiO2 and ZnO. Solid State Sci. 2020, 107, 106362. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K.; Bhorolua, D.; Das, J.; Kumar, A.; Hazarika, A. Facile synthesis of CuO nanowires and Cu2O nanospheres grown on rGO surface and exploiting its photocatalytic, antibacterial and supercapacitive properties. Phys. B Condens. Matter. 2019, 558, 74–81. [Google Scholar] [CrossRef]
- Kottappara, R.; Palantavida, S.; Pillai, S.C.; Vijayan, B.K. Composition tuning in copper - oxide decorated reduced graphene oxide yields efficient photo- and reduction catalysts. Surf. Interfaces 2021, 22, 100792. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Pham, T.-T.; Nguyen-Huy, C.; Lee, H.-J.; Nguyen-Phan, T.-D.; Son, T.H.; Kim, C.-K.; Shin, E.W. Cu-doped TiO2/reduced graphene oxide thin-film photocatalysts: Effect of Cu content upon methylene blue removal in water. Ceram. Int. 2015, 41, 11184–11193. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Han, C.; Ma, X.; Tong, Z.; Tan, B. Cu2O/CuO heterojunction formed by thermal oxidation and decorated with Pt co-catalyst as an efficient photocathode for photoelectrochemical water splitting. J. Nanopart. Res. 2021, 23, 268. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, L.; Wang, K.; Miao, L.; Lan, Q.; Jiang, K.; Lu, H.; Li, M.; Li, Y.; Shen, B.; et al. Analysis of oxidation degree of graphite oxide and chemical structure of corresponding reduced graphite oxide by selecting different-sized original graphite. RSC Adv. 2018, 8, 17209–17217. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Sawant, S.S.; Bhagwat, A.D.; Mahajan, C.M. Synthesis of Cuprous Oxide (Cu2O) Nanoparticles—A Review. J. Nano-Electron. Phys. 2016, 8, 1035. [Google Scholar] [CrossRef]
- Sáenz-Trevizo, A.; Pizá-Ruiz, P.; Chávez-Flores, D.; Ogaz-Parada, J.; Amézaga-Madrid, P.; Vega-Ríos, A.; Miki-Yoshida, M. On the Discoloration of Methylene Blue by Visible Light. J. Fluoresc. 2019, 29, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, H.E.; Knauber, T.; Lévesque, F.; Moschetta, E.G.; Susanne, F.; Edwards, L.J. Photons as a 21st century reagent. Nat. Commun. 2020, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Esparza, M.A.; Rivera, L.P.; Pérez-Centeno, A.; Zamudio-Ojeda, A.; González, D.R.; Chávez-Chávez, A.; Santana-Aranda, M.A.; Santos-Cruz, J.; Quiñones-Galván, J.G. UV and Visible light photodegradation of methylene blue with graphene decorated titanium dioxide. Mater. Res. Express. 2020, 7, 35504. [Google Scholar] [CrossRef]
- Zhou, F.; Fu, Y.Q.; Wan, X. Photocatalytic Enhancement in Methylene Blue Degradation of TiO2 Photocatalysts via Graphene Hybridization. Key Eng. Mater. 2012, 512–515, 1677–1681. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2020, 357, 630–637. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Liu, S.; Wang, S. Graphene facilitated visible light photodegradation of methylene blue over titanium dioxide photocatalysts. Chem. Eng. J. 2013, 214, 298–303. [Google Scholar] [CrossRef]
- Alamelu, K.; Raja, V.; Shiamala, L.; Jaffar Ali, B.M. Biphasic TiO2 nanoparticles decorated graphene nanosheets for visible light driven photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2018, 430, 145–154. [Google Scholar] [CrossRef]
- Wanag, A.; Kusiak-Nejman, E.; Czyżewski, A.; Moszyński, D.; Morawski, A.W. Influence of rGO and Preparation Method on the Physicochemical and Photocatalytic Properties of TiO2/Reduced Graphene Oxide Photocatalysts. Catalysts 2021, 11, 1333. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.L.; Van Tran, T.T.; Juang, R.-S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, R.; Jiang, G.; Jin, H.E.; Wang, Y.I.N.; Sun, X.; Wang, S.; Wang, T.A.O. CuO/TiO2 nanocrystals grown on graphene as visible-light responsive photocatalytic hybrid materials. Bull. Mater. Sci. 2012, 35, 495–499. [Google Scholar] [CrossRef]
- Ding, R.C.; Fan, Y.Z.; Wang, G.S. High Efficient Cu2O/TiO2 Nanocomposite Photocatalyst to Degrade Organic Polluant under Visible Light Irradiation. ChemistrySelect 2018, 3, 1682–1687. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B Environ. 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Lopes, D.; Daniel-da-Silva, A.L.; Sarabando, A.R.; Arias-Serrano, B.I.; Rodríguez-Aguado, E.; Rodríguez-Castellón, E.; Trindade, T.; Frade, J.R.; Kovalevsky, A.V. Design of Multifunctional Titania-Based Photocatalysts by Controlled Redox Reactions. Materials 2020, 13, 758. [Google Scholar] [CrossRef]
- Luna, A.L.; Valenzuela, M.A.; Colbeau-Justin, C.; Vázquez, P.; Rodriguez, J.L.; Avendaño, J.R.; Alfaro, S.; Tirado, S.; Garduño, A.; De la Rosa, J.M. Photocatalytic degradation of gallic acid over CuO–TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A Gen. 2016, 521, 140–148. [Google Scholar] [CrossRef]
- Vu Nu, T.T.; Thi Tran, N.H.; Truong, P.L.; Phan, B.T.; Nguyen Dinh, M.T.; Dinh, V.-P.; Phan, T.S.; Go, S.; Chang, M.; Loan Trinh, K.T.; et al. Green synthesis of microalgae-based carbon dots for decoration of TiO2 nanoparticles in enhancement of organic dye photodegradation. Environ. Res. 2022, 206, 112631. [Google Scholar] [CrossRef] [PubMed]
- Pogacean, F.; Socaci, C.; Pruneanu, S.; Biris, A.R.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide—A comparison study of their intrinsic peroxidase catalytic behavior. Sens. Actuators B Chem. 2015, 213, 474–483. [Google Scholar] [CrossRef]

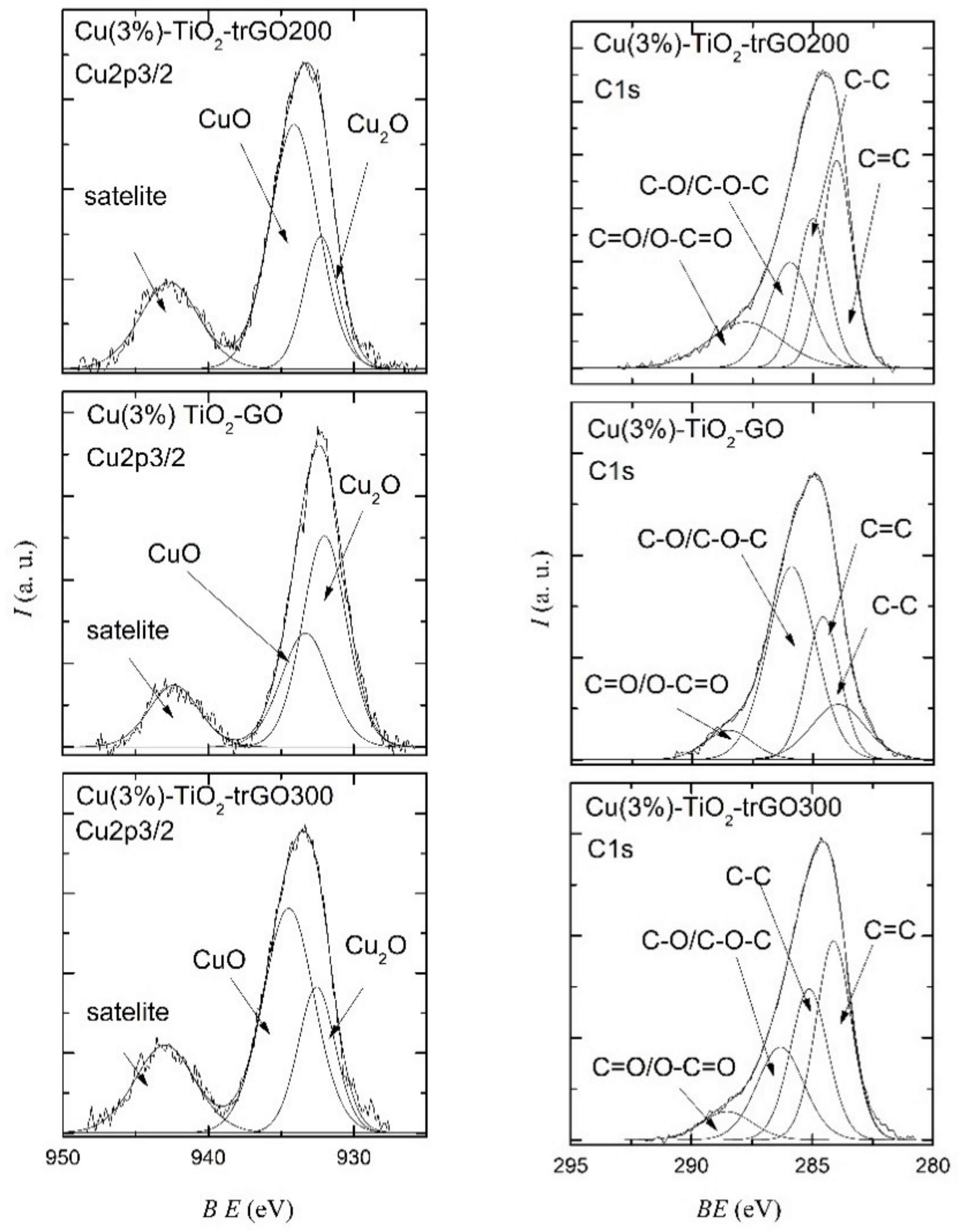




| Copper Content (x%) | Cu(x%)-TiO2 | Cu(x%)-TiO2-GO | Cu(x%)-TiO2-trGO200 | Cu(x%)-TiO2-trGO300 |
|---|---|---|---|---|
| 1% | 2.9 eV | 2.75 eV | 2.58 eV | 2.5 eV |
| 2% | 2.94 eV | 2.56 eV | 2.34 eV | 2.16 eV |
| 3% | 2.86 eV | 2.76 eV | 2.66 eV | 2.56 eV |
| Light Source—Initial MB Concentration | Composite | MB Photodegradation | Ref |
|---|---|---|---|
| UV source—America brand lamp model F17T8/BLB 17W; (λmax = 360 nm)—2.3 × 10−4 M | TiO2 | 59% | [27] |
| TiO2/graphene | 87% | ||
| Visible source—LED Philips 3PH5 with 14W-2.3 × 10−4 M | TiO2 | - | |
| TiO2/graphene | 40% | ||
| UV with λmax = 254 nm—1 × 10−5 M | TiO2/graphene (3wt%) | 90% | [28] |
| UV–Vis light with high UV intensity—6 lamps with the power of 20W each, 110 W/m2 UV and 5 W/m2 Visible—10 mg/L | TiO2 | 29.42% (60 min) | [29] |
| TiO2 500 | 55% (60 min) | ||
| TiO2/rGO (1wt%) | 63% (60 min) | ||
| TiO2/rGO (8wt%) | 91.5% (60 min) | ||
| UV–Vis—light source consisting of 2.31 µW/cm2 (220–280 nm), 6.94 mW/cm2 (315–400 nm), 129.3 mW/cm2 (400–1050 nm)—10 mg/L | MB alone | 30% (50 min) | [30] |
| TiO2 P25 | 100% (50 min) | ||
| Graphene-P25 3% | 100% (80 min) | ||
| Graphene-TiO2-5% | 100% (90 min) | ||
| Visible light—84 mW/cm2 (400–1050 nm)—10 mg/L | Graphene-TiO2-3% | 95% (150 min) | |
| Visible light—natural sunlight with a UV filter (11.45 a.m. to 17.45 p.m., 11.93oN; 79.13oE)—2 × 10−5 M | TiO2 | 40% (60 min) | [31] |
| TiO2-graphene | 80% (60 min) | ||
| UV light—6 lamps of 20W each (310–430 nm), 110 W/cm2—10 mg/L | TiO2 | 38.78% (90 min) | [32] |
| TiO2-700 | 86.48% (90 min) | ||
| TiO2/rGO-700 | 100% (90 min) | ||
| Visible light—artificial solar light, halogen lamp, 60W—10 mg/L | TiO2 | 1.02% (300 min) | |
| TiO2-700 | 7.11% (300 min) | ||
| TiO2/rGO-700 | 32.13% (300 min) | ||
| UV light—H2100CH—5 lamps (λmax = 254 nm)—20 mg/L | TiO2/ZnO/rGO | 99.6% (120 min) | [33] |
| Visible light—simulated solar light, Xenon lamp, 300W—20 mg/L | TiO2/ZnO/rGO | 80% (180 min) | |
| Visible light—Xenon lamp, 500W—30 mg/L | TiO2-P25 | 2% (480 min) | [34] |
| CuO/TiO2-GR | 80% (480 min) | ||
| Visible light—a Xenon lamp | Cu2O/TiO2 | 93.63% (45 min) | [35] |
| Visible light—150W lamp (OSRAM) with a 420 nm cutoff filter—5 mg/L | CuO-Cu2O/TiO2 | 90% (180 min) | [15] |
| Solar light—1 × 10−5 M | CuO | 35% (60 min) | [16] |
| CuO-rGO | 50% (60 min) | ||
| Cu2O | 45% (60 min) | ||
| Cu2O-rGO | 52% (60 min) | ||
| UV source—300W light source mainly UVA (315–400 nm) with some UVB (280–315 nm)—10 mg/L | Cu-doped TiO2/RGO (5 wt%) | 64% | [19] |
| Visible light—Xenon lamp 300 W with a 420 nm cutoff filter—5 mg/L | Cu2O/rGO | 100% | [7] |
| Cu(3%)-TiO2 | Cu(3%)-TiO2-GO | Cu(3%)-TiO2-trGO200 | Cu(3%)-TiO2-trGO300 | Cu(1%)-TiO2-GO | Cu(2%)-TiO2-GO | |
|---|---|---|---|---|---|---|
| kapp×103 (min−1) | 10.10 | 11.40 | 5.85 | 10.10 | 4.91 | 6.15 |
| t1/2 (min) | 68.6 | 60.8 | 118.4 | 68.6 | 141.1 | 112.7 |
| R2 | 0.9826 | 0.9885 | 0.9942 | 0.9990 | 0.9916 | 0.9914 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosma, D.; Urda, A.; Radu, T.; Rosu, M.C.; Mihet, M.; Socaci, C. Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules 2022, 27, 5803. https://doi.org/10.3390/molecules27185803
Cosma D, Urda A, Radu T, Rosu MC, Mihet M, Socaci C. Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules. 2022; 27(18):5803. https://doi.org/10.3390/molecules27185803
Chicago/Turabian StyleCosma, Dragos, Alexandra Urda, Teodora Radu, Marcela C. Rosu, Maria Mihet, and Crina Socaci. 2022. "Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites" Molecules 27, no. 18: 5803. https://doi.org/10.3390/molecules27185803
APA StyleCosma, D., Urda, A., Radu, T., Rosu, M. C., Mihet, M., & Socaci, C. (2022). Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules, 27(18), 5803. https://doi.org/10.3390/molecules27185803







