Isatin Bis-Indole and Bis-Imidazothiazole Hybrids: Synthesis and Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
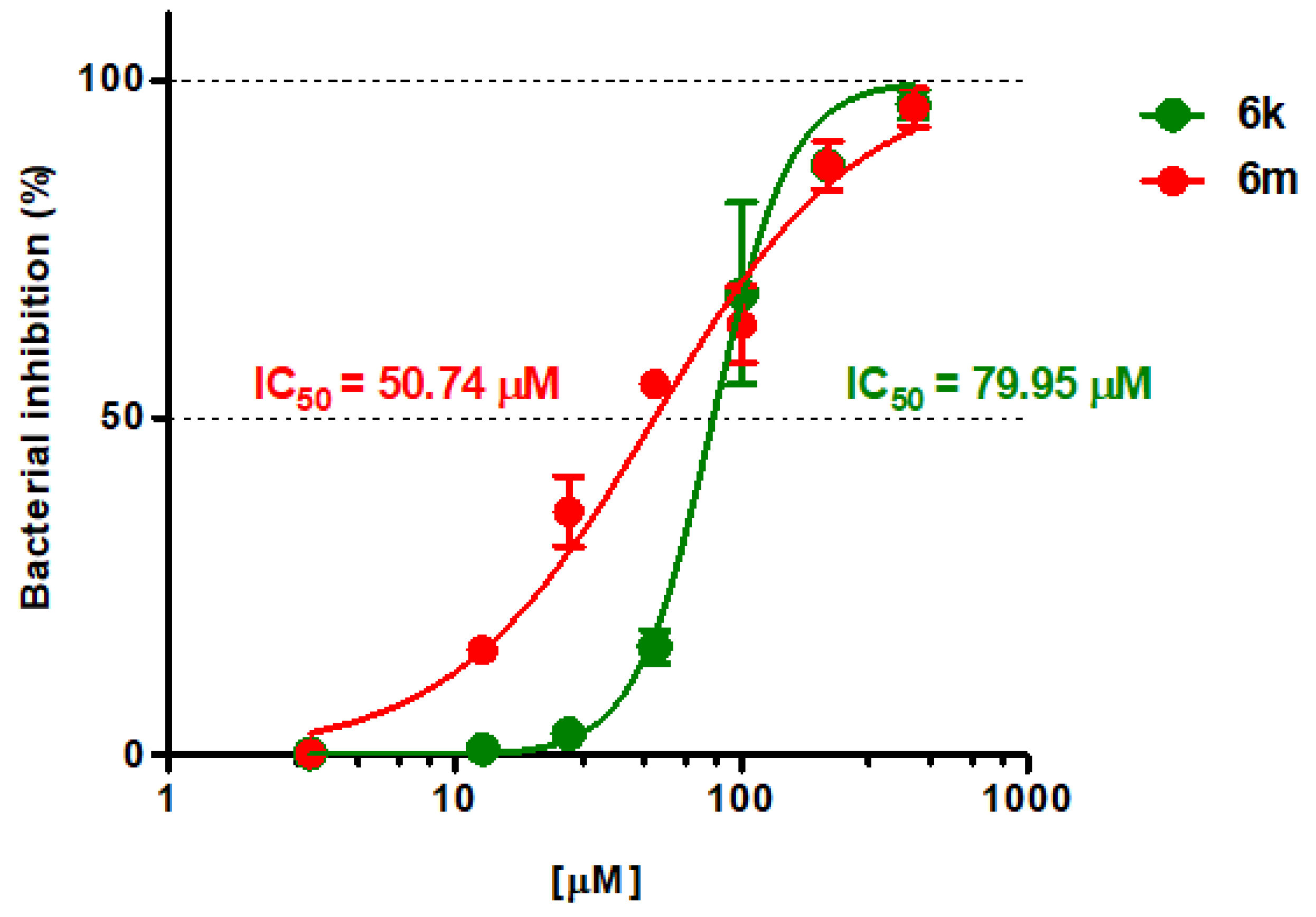
2.2. Biological Evaluation
3. Materials and Methods
3.1. Chemistry
Synthesis of New Compounds, 4d-i, 4l-m, 5d, 5m, 6c, 6g, 6k, and 6m
3.2. In Vitro Susceptibility Testing
3.3. Cytotoxicity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, M.I.; Adnan, M.; et al. Therapeutic Outcomes of Isatin and Its Derivatives against Multiple Diseases: Recent Developments in Drug Discovery. Pharmaceuticals 2022, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Guo, H. Isatin Derivatives and Their Anti-Bacterial Activities. Eur. J. Med. Chem. 2019, 164, 678–688. [Google Scholar] [CrossRef]
- Andreani, A.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. Synthesis and Antitubercular Activity of Imidazo[2,1-b]Thiazoles. Eur. J. Med. Chem. 2001, 36, 743–746. [Google Scholar] [CrossRef]
- Fascio, M.L.; Errea, M.I.; D’Accorso, N.B. Imidazothiazole and Related Heterocyclic Systems. Synthesis, Chemical and Biological Properties. Eur. J. Med. Chem. 2015, 90, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Güzeldemirci, N.U.; Küçükbasmacı, Ö. Synthesis and Antimicrobial Activity Evaluation of New 1,2,4-Triazoles and 1,3,4-Thiadiazoles Bearing Imidazo[2,1-b]Thiazole Moiety. Eur. J. Med. Chem. 2010, 45, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rajurkar, V.G.; Patil, R.B.; Miniyar, P.B. Synthesis and antimicrobial activity of novel 3, 6-disubstituted imidazo [2, 1-B] [1, 3] thiazoles. Int. J. Pharm. Sci. Res. 2011, 2, 1537–1542. [Google Scholar]
- Malik, J.K.; Soni, H.; Singhai, A.K. Synthesis, Characterization and Evaluation for Antifungal Activity of Substituted Diaryl Imidazo [2, 1, b]-Benzothiazole. J. Pharm. Res. 2013, 7, 39–46. [Google Scholar] [CrossRef]
- Andreani, A.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Lannigan, D.; Smith, J.; Scudiero, D.; et al. Imidazo[2,1-b]Thiazole Guanylhydrazones as RSK2 Inhibitors. Eur. J. Med. Chem. 2011, 46, 4311–4323. [Google Scholar] [CrossRef]
- Andreani, A.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Cervellati, R.; Greco, E.; Kondratyuk, T.P.; Park, E.-J.; Huang, K.; et al. Chemopreventive and Antioxidant Activity of 6-Substituted Imidazo[2,1-b]Thiazoles. Eur. J. Med. Chem. 2013, 68, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Prencipe, F.; Balzarini, J.; Liekens, S.; Estévez, F. Design, Synthesis and Antiproliferative Activity of Novel Heterobivalent Hybrids Based on Imidazo[2,1-b][1,3,4]Thiadiazole and Imidazo[2,1-b][1,3]Thiazole Scaffolds. Eur. J. Med. Chem. 2015, 101, 205–217. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.S.; Kim, M.-R.; El-Gamal, M.I.; Gamal El-Din, M.M.; Tae, J.; Choi, H.S.; Lee, K.-T.; Yoo, K.H.; Oh, C.-H. Design, Synthesis, in Vitro Antiproliferative Evaluation, and Kinase Inhibitory Effects of a New Series of Imidazo[2,1-b]Thiazole Derivatives. Eur. J. Med. Chem. 2015, 95, 453–463. [Google Scholar] [CrossRef]
- Kamal, A.; Reddy, M.K.; Viswanath, A. The design and development of imidazothiazole-chalcone derivatives as potential anticancer drugs. Expert Opin. Drug Discov. 2013, 8, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, H.; Aberi, M.; Shiri, P. Supported Benzimidazole—Salen Cu(II) Complex: An Efficient, Versatile and Highly Reusable Nanocatalyst for One-pot Synthesis of Hybrid Molecules. Appl. Organomet. Chem. 2018, 32, e4446. [Google Scholar] [CrossRef]
- Shiri, P. Novel Hybrid Molecules Based on Triazole-β-Lactam as Potential Biological Agents. Mini-Rev. Med. Chem. 2021, 21, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh, K.; Shiri, P. An Overview of Recent Advances in the Applications of Click Chemistry in the Synthesis of Bioconjugates with Anticancer Activities. Chem. Sel. 2019, 4, 13459–13478. [Google Scholar] [CrossRef]
- Pinto, H.; Simões, M.; Borges, A. Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 825. [Google Scholar] [CrossRef]
- Murphy, S.E.; Bicanic, T. Drug Resistance and Novel Therapeutic Approaches in Invasive Candidiasis. Front. Cell. Infect. Microbiol. 2021, 11, 759408. [Google Scholar] [CrossRef] [PubMed]
- Paira, P.; Hazra, A.; Kumar, S.; Paira, R.; Sahu, K.B.; Naskar, S.; Saha, P.; Mondal, S.; Maity, A.; Banerjee, S.; et al. Efficient Synthesis of 3,3-Diheteroaromatic Oxindole Analogues and Their in Vitro Evaluation for Spermicidal Potential. Bioorg. Med. Chem. Lett. 2009, 19, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shen, T.; Lin, Y.; Zhou, Y.; Song, Q. Rapid and Efficient Synthesis of 3,3-Di(1 H -Indol-3-Yl)Indolin-2-Ones and 2,2-Di(1H-Indol-3-Yl)-2H-Acenaphthen-1-Ones Catalyzed by p-TSA. Synth. Commun. 2014, 44, 2029–2036. [Google Scholar] [CrossRef]
- Paolini, J.P.; Lendvay, L.J. Heterocyclic Systems with a Bridgehead Nitrogen. II. 6-Chloroimidazo[2,1-b]Thiazole and Some of Its 5-Substituted Derivatives. J. Med. Chem. 1969, 12, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Aimi, N.; Kubo, A.; Kitagawa, M.; Hanasawa, M.; Katano, K.; Yamaguchi, K.; Haginiwa, J. Structure of Gardneramine and 18-Demethylgardneramine. Chem. Pharm. Bull. (Tokyo) 1975, 23, 2805–2817. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z. Sandmeyer Isatin Synthesis: (Sandmeyer Synthesis). In Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, X.; Zhao, N.; Xu, S.; An, Z.; Zhuang, X.; Lan, Z.; Wen, L.; Wan, X. Reductive Ring Closure Methodology toward Heteroacenes Bearing a Dihydropyrrolo[3,2-b]Pyrrole Core: Scope and Limitation. J. Org. Chem. 2014, 79, 11339–11348. [Google Scholar] [CrossRef] [PubMed]
- Shintani, R.; Inoue, M.; Hayashi, T. Rhodium-Catalyzed Asymmetric Addition of Aryl- and Alkenylboronic Acids to Isatins. Angew. Chem. Int. Ed. 2006, 45, 3353–3356. [Google Scholar] [CrossRef]
- Samineni, R.; Madapa, J.; Srihari, P.; Mehta, G. Spiroannulation of Oxindoles via Aryne and Alkyne Incorporation: Substituent-Diverted, Transition-Metal-Free, One-Pot Access to Spirooxindoles. Org. Lett. 2017, 19, 3119–3122. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Raghuwanshi, K.; Patel, V.K.; Jain, D.K.; Veerasamy, R.; Dixit, A.; Rajak, H. Assessment of 5-Substituted Isatin as Surface Recognition Group: Design, Synthesis, and Antiproliferative Evaluation of Hydroxamates as Novel Histone Deacetylase Inhibitors. Pharm. Chem. J. 2017, 51, 366–374. [Google Scholar] [CrossRef]
- Lackey, K.; Besterman, J.M.; Fletcher, W.; Leitner, P.; Morton, B.; Sternbach, D.D. Rigid Analogs of Camptothecin as DNA Topoisomerase I Inhibitors. J. Med. Chem. 1995, 38, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabli, R.; Zakaria, A.; Attia, M. Synthesis, Spectroscopic Characterization and Antimicrobial Potential of Certain New Isatin-Indole Molecular Hybrids. Molecules 2017, 22, 1958. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.A.; Cottrell, D.M.; Paquette, L.A. Demonstration and Analysis of Bridging Regioselectivity Operative during Di-.Pi.-Methane Photorearrangement of Ortho-Substituted Benzonorbornadienes and Anti-7,8-Benzotricyclo[4.2.2.02,5]Deca-3,7,9-Trienes. J. Am. Chem. Soc. 1977, 99, 3734–3744. [Google Scholar] [CrossRef]
- Ebejer, J.-P.; Charlton, M.H.; Finn, P.W. Are the Physicochemical Properties of Antibacterial Compounds Really Different from Other Drugs? J. Cheminform. 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Manet, I.; Belluti, F.; Gobbi, S.; Rampa, A.; Gentilomi, G.A.; Bisi, A. Targeting the Bacterial Membrane with a New Polycyclic Privileged Structure: A Powerful Tool To Face Staphylococcus Aureus Infections. ACS Infect. Dis. 2019, 5, 1524–1534. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Wright, G.D. Intrinsic Antibiotic Resistance: Mechanisms, Origins, Challenges and Solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Pervez, H.; Iqbal, M.S.; Tahir, M.Y.; Nasim, F.-H.; Choudhary, M.I.; Khan, K.M. In Vitro Cytotoxic, Antibacterial, Antifungal and Urease Inhibitory Activities of Some N4—Substituted Isatin-3-Thiosemicarbazones. J. Enzym. Inhib. Med. Chem. 2008, 23, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-Infective Potential of Natural Products: How to Develop a Stronger in Vitro ‘Proof-of-Concept. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Potente, G.; Bonvicini, F.; Gentilomi, G.A.; Antognoni, F. Anti-Candida Activity of Essential Oils from Lamiaceae Plants from the Mediterranean Area and the Middle East. Antibiotics 2020, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Özil, M.; Menteşe, E.; Yilmaz, F.; İslamoğlu, F.; Kahveci, B. Synthesis of Novel Triazol Compounds Containing Isatin as Potential Antibacterial and Antifungal Agents by Microwave and Conventional Methods. J. Chem. Res. 2011, 35, 268–271. [Google Scholar] [CrossRef]
- Lian, Z.-M.; Sun, J.; Zhu, H.-L. Design, Synthesis and Antibacterial Activity of Isatin Derivatives as FtsZ Inhibitors. J. Mol. Struct. 2016, 1117, 8–16. [Google Scholar] [CrossRef]
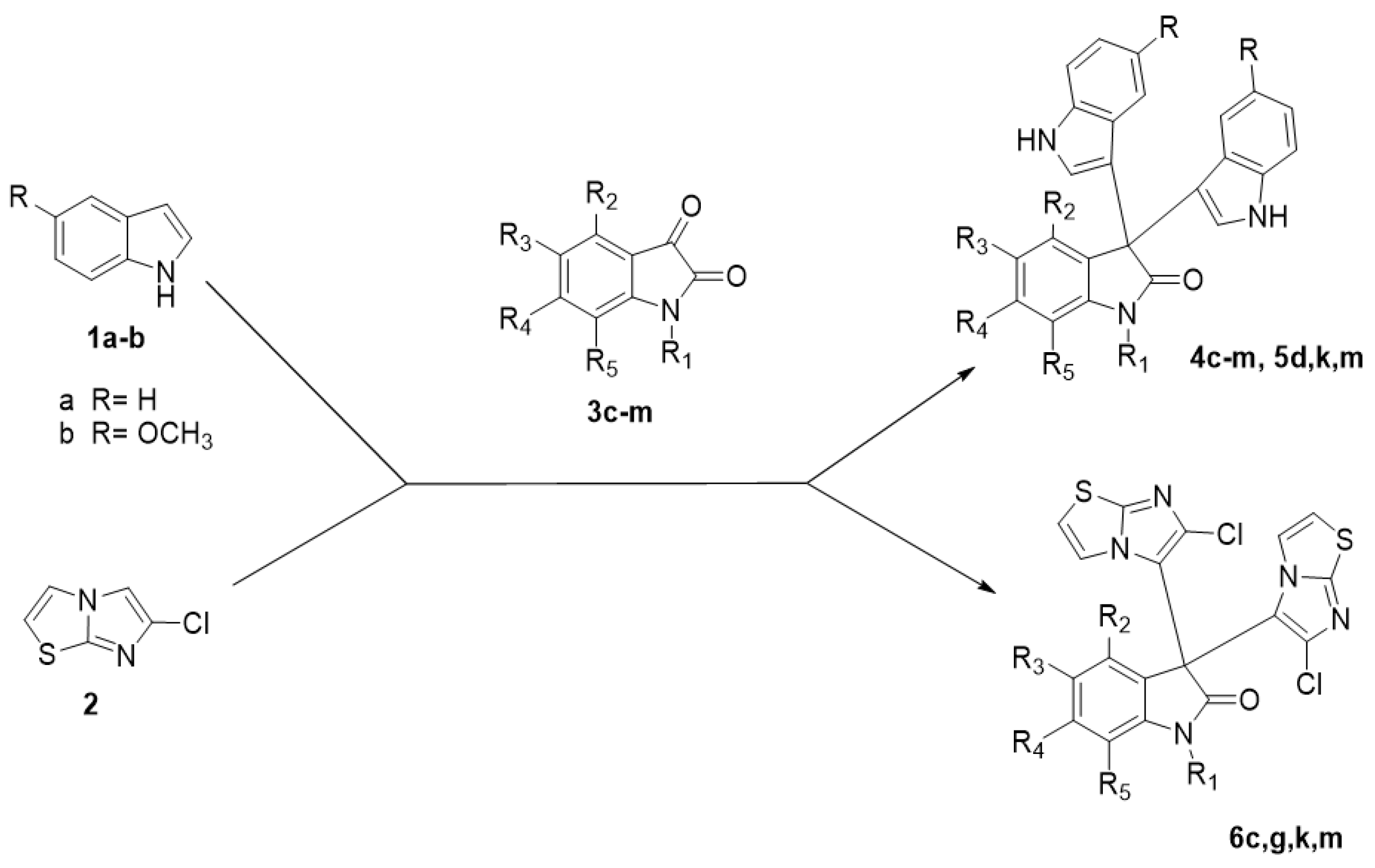
| Comp. | R | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|
| 4c [18] | H | H | H | H | H | H |
| 4d | H | H | OCH3 | OCH3 | OCH3 | H |
| 4e | H | H | CH3 | H | CH3 | H |
| 4f | H | H | Cl | H | H | Cl |
| 4g | H | 4-MBn | H | OCH3 | H | H |
| 4h | H | H | I | H | H | H |
| 4i | H | CH3 | H | Cl | H | H |
| 4j [19] | H | H | F | H | H | H |
| 4k [19] | H | H | OCH3 | H | H | H |
| 4l | H | H | H | H | OCF3 | H |
| 4m | H | 4-ClBn | H | H | H | H |
| 5d | OCH3 | H | OCH3 | OCH3 | OCH3 | H |
| 5k [19] | OCH3 | H | H | OCH3 | H | H |
| 5m | OCH3 | 4-ClBn | H | H | H | H |
| 6c | H | H | H | H | H | |
| 6g | 4-MBn | H | OCH3 | H | H | |
| 6k | H | H | OCH3 | H | H | |
| 6m | 4-ClBn | H | H | H | H |
| Comp. | S. aureus | E. coli | C. albicans | Vero |
|---|---|---|---|---|
| 4c | 1.3 ± 1.5 | 111.2 ± 1.5 | 106.8 ± 5.0 | 9.9 ± 1.3 |
| 4d | 84.3 ± 5.8 | 116.4 ± 0.6 | 120.8 ± 7.6 | 41.2 ± 0.1 |
| 4e | 71.2 ± 8.7 | 103.3 ± 1.4 | 68.8 ± 2.7 | 8.5 ± 0.3 |
| 4f | 75.8 ± 1.8 | 96.9 ± 1.7 | 106.7 ± 4.2 | 9.8 ± 0.5 |
| 4g | 86.2 ± 5.7 | 100.3 ± 0.7 | 50.6 ± 6.6 | 107.9 ± 8.9 |
| 4h | 72.8 ± 6.1 | 82.0 ± 1.5 | 23.2 ±2.1 | 10.6 ± 0.3 |
| 4i | 64.2 ± 4.3 | 103.6 ± 1.2 | 51.7 ± 3.9 | 69.7 ± 8.6 |
| 4j | 66.4 ± 9.9 | 98.1 ± 3.0 | 77.8 ± 10.5 | 9.2 ± 0.3 |
| 4k | 62.2 ± 4.1 | 110.5 ± 1.1 | 67.8 ± 3.7 | 9.4 ± 0.9 |
| 4l | 72.0 ± 2.2 | 103.2 ± 1.6 | 93.3 ± 2.1 | 8.9 ±0.4 |
| 4m | 77.9 ± 6.7 | 107.2 ± 1.2 | 106.1 ± 2.7 | 58.2 ± 5.9 |
| 5d | 89.1 ± 7.0 | 117.0 ± 1.1 | 120.0 ± 2.0 | 54.3 ± 0.9 |
| 5k | 76.0 ± 5.0 | 113.3 ± 1.1 | 66.0 ± 5.2 | 99.6 ± 6.8 |
| 5m | 95.1 ± 3.9 | 113.0 ± 0.6 | 88.2 ± 5.2 | 87.0 ± 5.7 |
| 6c | 95.4 ± 2.4 | 117.3 ± 3.1 | 126.3 ± 2.1 | 92.8 ± 4.8 |
| 6g | 67.4 ± 8.4 | 73.6 ± 9.1 | 92.4 ± 3.6 | 84.3 ± 0.9 |
| 6k | 43.4 ± 5.6 | 102.7 ± 2.4 | 104.1 ± 1.9 | 70.9 ± 5.6 |
| 6m | 48.1 ± 11.4 | 88.6 ± 6.2 | 81.5 ± 2.0 | 90.7 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonvicini, F.; Locatelli, A.; Morigi, R.; Leoni, A.; Gentilomi, G.A. Isatin Bis-Indole and Bis-Imidazothiazole Hybrids: Synthesis and Antimicrobial Activity. Molecules 2022, 27, 5781. https://doi.org/10.3390/molecules27185781
Bonvicini F, Locatelli A, Morigi R, Leoni A, Gentilomi GA. Isatin Bis-Indole and Bis-Imidazothiazole Hybrids: Synthesis and Antimicrobial Activity. Molecules. 2022; 27(18):5781. https://doi.org/10.3390/molecules27185781
Chicago/Turabian StyleBonvicini, Francesca, Alessandra Locatelli, Rita Morigi, Alberto Leoni, and Giovanna Angela Gentilomi. 2022. "Isatin Bis-Indole and Bis-Imidazothiazole Hybrids: Synthesis and Antimicrobial Activity" Molecules 27, no. 18: 5781. https://doi.org/10.3390/molecules27185781
APA StyleBonvicini, F., Locatelli, A., Morigi, R., Leoni, A., & Gentilomi, G. A. (2022). Isatin Bis-Indole and Bis-Imidazothiazole Hybrids: Synthesis and Antimicrobial Activity. Molecules, 27(18), 5781. https://doi.org/10.3390/molecules27185781






