Investigation of Photosystem II Functional Size in Higher Plants under Physiological and Stress Conditions Using Radiation Target Analysis and Sucrose Gradient Ultracentrifugation
Abstract
1. Introduction
2. Results
2.1. PSII Core Oligomeric Forms from Solubilised PSII Particles of Various Higher Plants
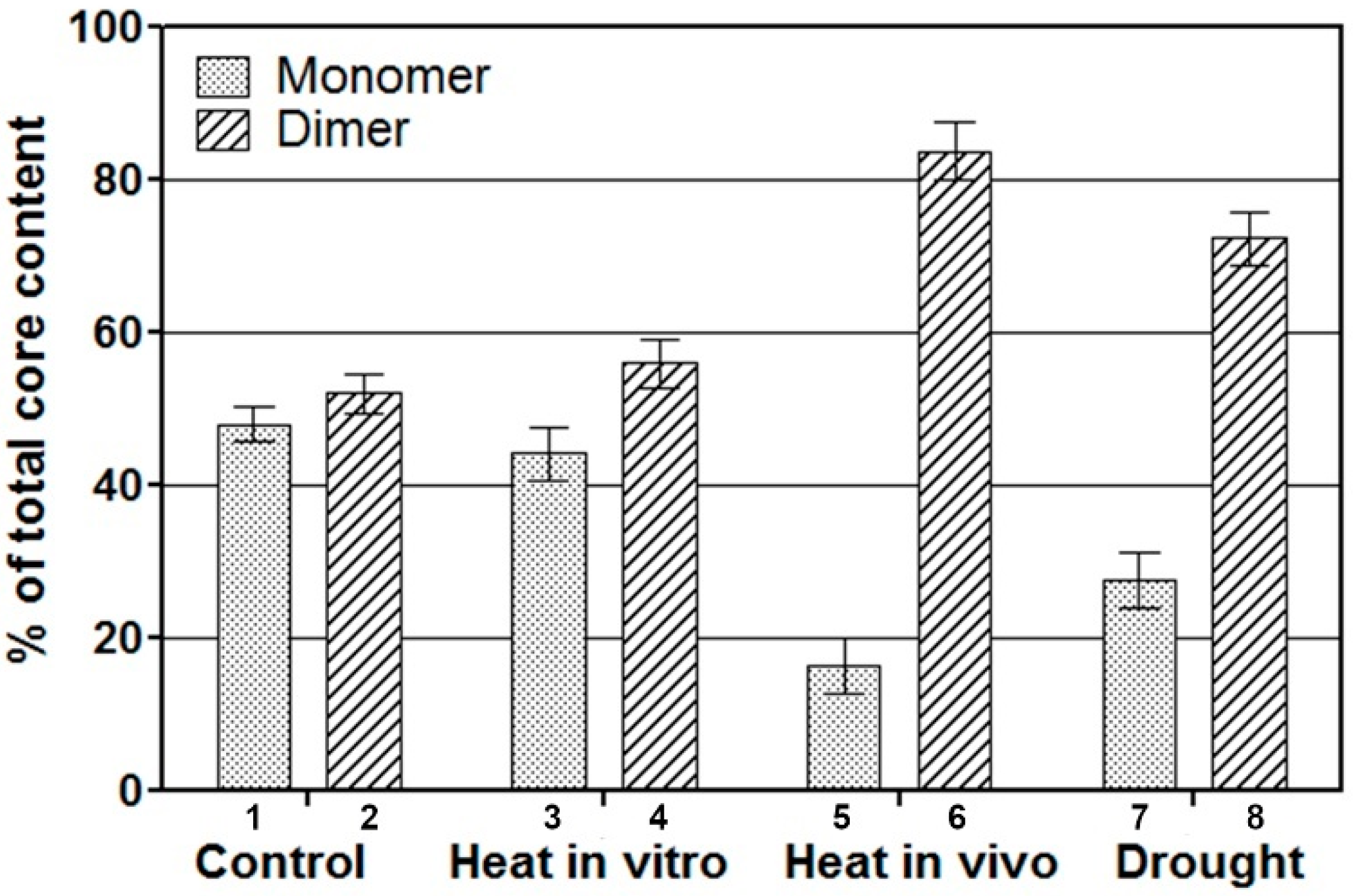
2.2. Distribution of PSII Core Populations Isolated from Stressed Pisum sativum
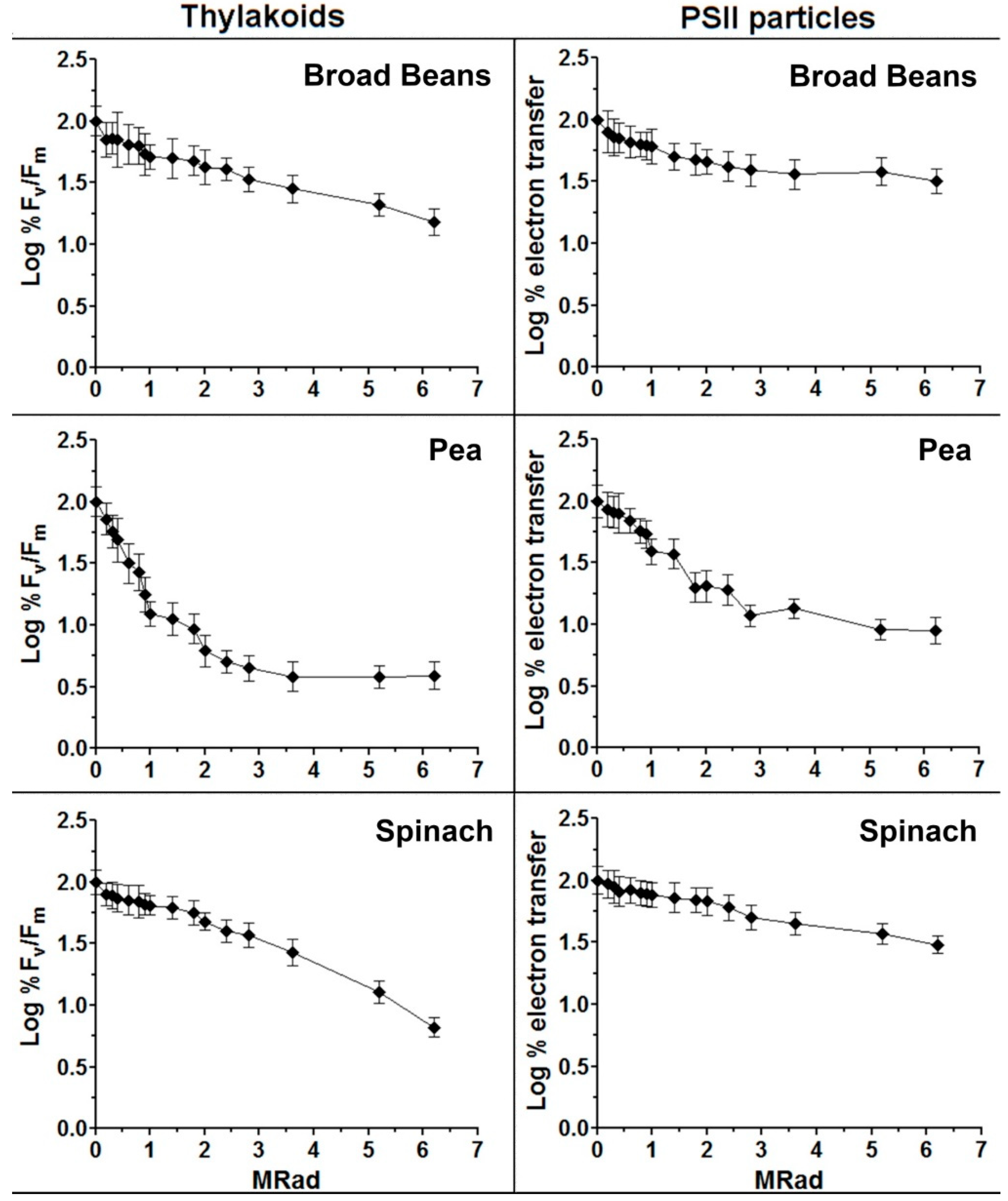
2.3. Radiation Target Analyses of Thylakoids and PSII Preparations
2.4. Radiation Target Analyses of Intact Plant Leaves under Normal and Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Plants and PSII Membrane Isolation
4.2. Radiation Inactivation Analyses
4.3. Photosystem II Activity, Chlorophylls, P700 and Cyt b559 Content
4.4. SDS-PAGE and Immunoblot Analyses
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.; Mattoo, A.K. D1-protein dynamics in photosystem II: The lingering enigma. Photosynth. Res. 2008, 98, 609–620. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Yuan, Q.; Feng, Y. Structure and function of the photosystem supercomplexes. Front. Plant Sci. 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Watanabe, A.; Li, A.; Kim, E.; Song, C.; Murata, K.; Song, D.; Minagawa, J.; Liu, Z. Structural insight into light harvesting for photosystem II in green algae. Nat. Plants 2019, 5, 1320–1330. [Google Scholar] [CrossRef]
- Pagliano, C.; Chimirri, F.; Saracco, G.; Marsano, F.; Barber, J. One-step isolation and biochemical characterization of highly active plant PSII monomeric core. Photosynth. Res. 2011, 108, 33–46. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A.; Polizzi, N.F.; Therien, M.J.; Beratan, D.N. Biochemistry and theory of proton-coupled electron transfer. Chem. Rev. 2014, 114, 3381–3465. [Google Scholar] [CrossRef]
- Zobnina, V.; Lambreva, M.D.; Rea, G.; Campi, G.; Antonacci, A.; Scognamiglio, V.; Giardi, M.T.; Polticelli, F. The plastoquinol–plastoquinone exchange mechanism in photosystem II: Insight from molecular dynamics simulations. Photosynth. Res. 2017, 131, 15–30. [Google Scholar] [CrossRef]
- Samborska, I.A.; Kalaji, H.M.; Sieczko, L.; Goltsev, V.; Borucki, W.; Jajoo, A. Structural and functional disorder in the photosynthetic apparatus of radish plants under magnesium deficiency. Funct. Plant Biol. 2018, 45, 668–679. [Google Scholar] [CrossRef]
- Gupta, R. The oxygen-evolving complex: A super catalyst for life on earth, in response to abiotic stresses. Plant Signal. Behav. 2020, 15, 1824721. [Google Scholar] [CrossRef]
- Kouřil, R.; Oostergetel, G.T.; Boekema, E.J. Fine structure of granal thylakoid membrane organization using cryo electron tomography. Biochim. Biophys. Acta 2011, 1807, 368–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pagliano, C.; Saracco, G.; Barber, J. Structural, functional and auxiliary proteins of photosystem II. Photosynth. Res. 2013, 116, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Daum, B.; Nicastro, D.; Austin, J., II; McIntosh, J.R.; Kühlbrandt, W. Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell 2010, 22, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Haniewicz, P.; Floris, D.; Farci, D.; Kirkpatrick, J.; Loi, M.C.; Buchel, C.; Bochtler, M.; Piano, D. Isolation of plant photosystem II complexes by fractional solubilization. Front. Plant Sci. 2015, 6, 1100. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 2016, 534, 69–74. [Google Scholar] [CrossRef]
- Grinzato, A.; Albanese, P.; Marotta, R.; Swuec, P.; Saracco, G.; Bolognesi, M.; Zanotti, G.; Pagliano, C. High-light versus low-light: Effects on paired photosystem II supercomplex structural rearrangement in pea plants. Int. J. Mol. Sci. 2020, 21, 8643. [Google Scholar] [CrossRef]
- Guskov, A.; Kern, J.; Gabdulkhakov, A.; Broser, M.; Zouni, A.; Saenger, W. Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Mol. Biol. 2009, 16, 334–342. [Google Scholar] [CrossRef]
- Broser, M.; Gabdulkhakov, A.; Kern, J.; Guskov, A.; Müh, F.; Saenger, W.; Zouni, A. Crystal structure of monomeric photosystem II from Thermosynechococcus elongatus at 3.6-Å resolution. J. Biol. Chem. 2010, 285, 26255–26262. [Google Scholar] [CrossRef]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamato, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef]
- Nixon, P.J.; Michoux, F.; Yu, J.; Boehm, M.; Komenda, J. Recent advances in understanding the assembly and repair of Photosystem II. Ann. Bot. 2010, 106, 1–16. [Google Scholar] [CrossRef]
- Danielsson, R.; Suorsa, M.; Paakkarinen, V.; Albertsson, P.A.; Styring, S.; Aro, E.M.; Mamedov, F. Dimeric and monomeric organization of photosystem II. Distribution of five distinct complexes in the different domains of the thylakoid membrane. J. Biol. Chem. 2006, 281, 14241–14249. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Inoue-Kashino, N.; Ozawa, S.; Takahashi, Y.; Kashino, Y.; Satoh, K. Photosystem II complex in vivo is a monomer. J. Biol. Chem. 2009, 284, 15598–15606. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Iwai, M.; Narikawa, R.; Ikeuchi, M. Is the photosystem II complex a monomer or a dimer? Plant Cell Physiol. 2009, 50, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Kempner, E.S. Novel predictions from radiation target analysis. Trends Biochem. Sci. 1993, 18, 236–239. [Google Scholar] [CrossRef]
- Kempner, E.S. Advances in radiation target analysis. Anal. Biochem. 1999, 276, 113–123. [Google Scholar] [CrossRef]
- Nugent, J.H.A.; Atkinson, Y.E. Estimation of the functional size of photosystem II. FEBS Lett. 1984, 170, 89–93. [Google Scholar] [CrossRef]
- Takahashi, M.; Mano, J.; Asada, K. Molecular sizes of the catalytic units of oxygen evolution and the reaction center in photosystem II of spinach thylakoids. Plant Cell Physiol. 1985, 26, 383–388. [Google Scholar]
- Takahashi, M.; Mano, J.; Asada, K. Functional structure of the oxygen-evolving unit of photosystem II as determined by radiation inactivation. Plant Cell Physiol. 1990, 31, 1191–1198. [Google Scholar]
- Boekema, E.J.; Hankamer, B.; Bald, D.; Kruip, J.; Nield, J.; Boonstra, A.F.; Barber, J.; Rogner, M. Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc. Natl. Acad. Sci. USA 1995, 92, 175–179. [Google Scholar] [CrossRef]
- Di Marco, G.; D’Ambrosio, N.; Giardi, M.T.; Massacci, A.; Tricoli, D. Photosynthetic properties of leaves of a yellow green mutant of wheat compared to its wild type. Photosynth. Res. 1989, 21, 117–122. [Google Scholar] [CrossRef]
- Franco, E.; Alessandrelli, S.; Masojídek, J.; Margonelli, A.; Giardi, M.T. Modulation of D1 protein turnover under cadmium and heat stresses monitored by [35S] methionine incorporation. Plant Sci. 1999, 144, 53–61. [Google Scholar] [CrossRef]
- Beauregard, G.; Potier, M. Temperature dependence of the radiation inactivation of proteins. Anal. Biochem. 1985, 150, 117–120. [Google Scholar] [CrossRef]
- Golub, M.; Hussein, R.; Ibrahim, M.; Hecht, M.; Wieland, D.C.F.; Martel, A.; Machado, B.; Zouni, A.; Pieper, J. Solution structure of the detergent–photosystem II core complex investigated by small-angle scattering techniques. J. Phys. Chem. B 2020, 124, 8583–8592. [Google Scholar] [CrossRef] [PubMed]
- Whitmarsh, J.; Eckert, H.; Schöneich, C.; Renger, G. Functional size of Photosystem II determined by radiation inactivation. Photosynth. Res. 1993, 38, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Panico, M.; Barber, J.; Morris, H.R. Purification and determination of intact molecular-mass by electrospray-ionization mass-spectrometry of the photosystem II reaction-center subunits. J. Biol. Chem. 1997, 272, 33153–33157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Panico, M.; Shipton, C.A.; Nilsson, F.; Morris, H.R.; Barber, J. Primary structure characterization of the photosystem II D1 and D2 subunits. J. Biol. Chem. 1997, 272, 33158–33166. [Google Scholar] [CrossRef]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Lipids in photosystem II: Interactions with protein and cofactors. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 509–519. [Google Scholar] [CrossRef]
- Hellmich, J.; Bommer, M.; Burkhardt, A.; Ibrahim, M.; Kern, J.; Meents, A.; Muh, F.; Dobbek, H.; Zouni, A. Native-like photosystem II superstructure at 2.44 Å resolution through detergent extraction from the protein crystal. Structure 2014, 22, 1607–1615. [Google Scholar] [CrossRef]
- Kruse, O.; Hankamer, B.; Konczak, C.; Gerle, C.; Morris, E.; Radunz, A.; Schmid, G.H.; Barber, J. Phosphatidylglycerol is involved in the dimerization of photosystem II. J. Biol. Chem. 2000, 275, 6509–6514. [Google Scholar] [CrossRef]
- Sakurai, I.; Hagio, M.; Gombos, Z.; Tyystjarvi, T.; Paakkarinen, V.; Aro, E.M.; Wada, H. Requirement of phosphatidylglycerol for maintenance of photosynthetic machinery. Plant Physiol. 2003, 133, 1376–1384. [Google Scholar] [CrossRef]
- Kim, E.H.; Razeghifard, R.; Anderson, J.M.; Chow, W.S. Multiple sites of retardation of electron transfer in Photosystem II after hydrolysis of phosphatidylglycerol. Photosynth. Res. 2007, 93, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Murata, N. The essential role of phosphatidylglycerol in photosynthesis. Photosynth. Res. 2007, 92, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Kansy, M.; Wilhelm, C.; Goss, R. Influence of thylakoid membrane lipids on the structure and function of the plant photosystem II core complex. Planta 2014, 240, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Saiki, M.; Yoshida, N.; Tomiyama, Y.; Mizushina, Y. Fatty acid distribution in triacylglycerols and phospholipids of broad beans (Vicia faba). Food Chem. 2009, 112, 924–928. [Google Scholar] [CrossRef]
- Luévano, A.; Kowaltowski, M.A.J. Phosphatidylglycerol-derived phospholipids have a universal, domain-crossing role in stress responses. Arch. Biochem. Biophys. 2015, 585, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Mazur, R.; Gieczewska, K.; Kowalewska, L.; Kuta, A.; Proboszcz, M.; Gruszecki, W.; Mostowska, A.; Garstka, M. Specific composition of lipid phases allows retaining an optimal thylakoid membrane fluidity in plant response to low-temperature treatment. Front. Plant Sci. 2020, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Botyanszka, L.; Chen, Y.E.; Allakhverdiev, S.I. Phenotyping of isogenic chlorophyll-less bread and durum wheat mutant lines in relation to photoprotection and photosynthetic capacity. Photosynth. Res. 2019, 139, 239–251. [Google Scholar] [CrossRef]
- Kieleczawa, J.; Coughlan, S.J.; Hind, G. Isolation and characterization of an alkaline phosphatase from pea thylakoids. Plant Physiol. 1992, 99, 1029–1036. [Google Scholar] [CrossRef]
- Giardi, M.T.; Komenda, J.; Masojidek, J. Role of protein phosphorylation on the sensitivity of photosystem II to strong illumination. Physiol. Plant. 1994, 92, 181–187. [Google Scholar] [CrossRef]
- Giardi, M.T.; Kucera, T.; Briantais, J.M.; Hodges, M. Decreased photosystem II core phosphorylation in a yellow-green mutant of wheat showing monophasic fluorescence induction curve. Plant Physiol. 1995, 109, 1059–10683. [Google Scholar] [CrossRef][Green Version]
- Berthold, D.A.; Babcock, G.T.; Yocum, C.F. A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes: EPR and electron-transport properties. FEBS Lett. 1981, 134, 231–234. [Google Scholar] [CrossRef]
- Giardi, M.T.; Cona, A.; Kucera, T.; Geiken, B.; Masojidek, J.; Mattoo, A.K. Long-term drought stress induces structural and functional reorganization of Photosystem II. Planta 1996, 199, 118–125. [Google Scholar] [CrossRef]
- Mattila, H.; Mishra, K.B.; Kuusisto, I.; Mishra, A.; Novotna, K.; Sebela, D.; Tyystjarvi, E. Effects of low temperature on photoinhibition and singlet oxygen production in four natural accessions of Arabidopsis. Planta 2020, 252, 19. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H. Chlorophyll and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–381. [Google Scholar]
- Geiken, B.; Masojidek, J.; Rizzuto, M.; Pompili, M.L.; Giardi, M.T. Incorporation of 35S-methionine in higher plants reveals that stimulation of the D1 reaction centre II protein turnover accompanies tolerance to heavy metal stress. Plant Cell Environ. 1988, 21, 1265–1273. [Google Scholar] [CrossRef]




| Ratio Wheat/Yellow-Green Wheat | |
|---|---|
| Total chlorophyll | 2.52 ± 0.40 |
| Chlorophyll a/b | 1.31 ± 0.12 |
| P700 | 2.00 ± 0.31 |
| Cyt f | 1.40 ± 0.09 |
| Electron transport | |
| on chlorophyll basis | 0.39 ± 0.06 |
| on leaf basis | 0.98 ± 0.05 |
| Fv/Fm Pea Leaves | ET Percentage of Control (from DPC to DCPIP) Pea PSII Particles | |
|---|---|---|
| Control | 0.873 ± 0.003 | 100 ± 3 |
| Heat stress 39 °C | 0.853 ± 0.009 | 97 ± 6 |
| Recovery (2 days at 25 °C) | 0.869 ± 0.004 | 99 ± 3 |
| Drought RWC 70% | 0.866 ± 0.015 | 99 ± 8 |
| Recovery (3 days after watering) | 0.861 ± 0.05 | 101 ± 8 |
| Thylakoids (Fv/Fm) | ||
| Plants | MW | |
| Spinach | 334 ± 39 | |
| Broad bean | 270 ± 31 | |
| Wheat | 253 ± 22 | |
| Yellow-green wheat | Nd | |
| Peas-MW 1 | 1300 ± 63 | |
| Peas-MW 2 | 291 ± 23 | |
| PSII particles (Electron Transfer) | ||
| MW | ||
| Electron Transfer | ||
| Plants | H2O → DCPIP | DPC → DCPIP |
| Spinach | 226 ± 26 | 177 ± 18 |
| Broad bean | 250 ± 22 | 186 ± 21 |
| Wheat-wild-type | 205 ± 16 | 171 ± 25 |
| Wheat-mutant | 232 ± 31 | 155 ± 15 |
| Peas-MW 1 | 1200 ± 68 | 548 ± 44 |
| Peas-MW 2 | 249 ± 27 | 172 ± 19 |
| PSII Cores (Electron Transfer DPC → DCPIP) | ||
| Peas | MW | |
| PSII Core monomer | 260 ± 27 | |
| PSII Core dimer | 483 ± 34 | |
| Intact Leaves | PSII Size Measured by RTA | R2 |
| Broad beans | 101 ± 18 | 0.94 |
| Spinach | 97 ± 18 | 0.96 |
| Peas | 75 ± 13 | 0.95 |
| Peas after heat stress | 160 ± 17 | 0.93 |
| Peas after recovery from heat stress | 80 ± 11 | 0.96 |
| Peas after drought | 129 ± 21 | 0.92 |
| Peas after recovery from drought | 122 ± 17 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giardi, M.T.; Antonacci, A.; Touloupakis, E.; Mattoo, A.K. Investigation of Photosystem II Functional Size in Higher Plants under Physiological and Stress Conditions Using Radiation Target Analysis and Sucrose Gradient Ultracentrifugation. Molecules 2022, 27, 5708. https://doi.org/10.3390/molecules27175708
Giardi MT, Antonacci A, Touloupakis E, Mattoo AK. Investigation of Photosystem II Functional Size in Higher Plants under Physiological and Stress Conditions Using Radiation Target Analysis and Sucrose Gradient Ultracentrifugation. Molecules. 2022; 27(17):5708. https://doi.org/10.3390/molecules27175708
Chicago/Turabian StyleGiardi, Maria Teresa, Amina Antonacci, Eleftherios Touloupakis, and Autar K. Mattoo. 2022. "Investigation of Photosystem II Functional Size in Higher Plants under Physiological and Stress Conditions Using Radiation Target Analysis and Sucrose Gradient Ultracentrifugation" Molecules 27, no. 17: 5708. https://doi.org/10.3390/molecules27175708
APA StyleGiardi, M. T., Antonacci, A., Touloupakis, E., & Mattoo, A. K. (2022). Investigation of Photosystem II Functional Size in Higher Plants under Physiological and Stress Conditions Using Radiation Target Analysis and Sucrose Gradient Ultracentrifugation. Molecules, 27(17), 5708. https://doi.org/10.3390/molecules27175708









