Spatial Patterning of Fluorescent Liquid Crystal Ink Based on Inkjet Printing
Abstract
:1. Introduction
2. Results
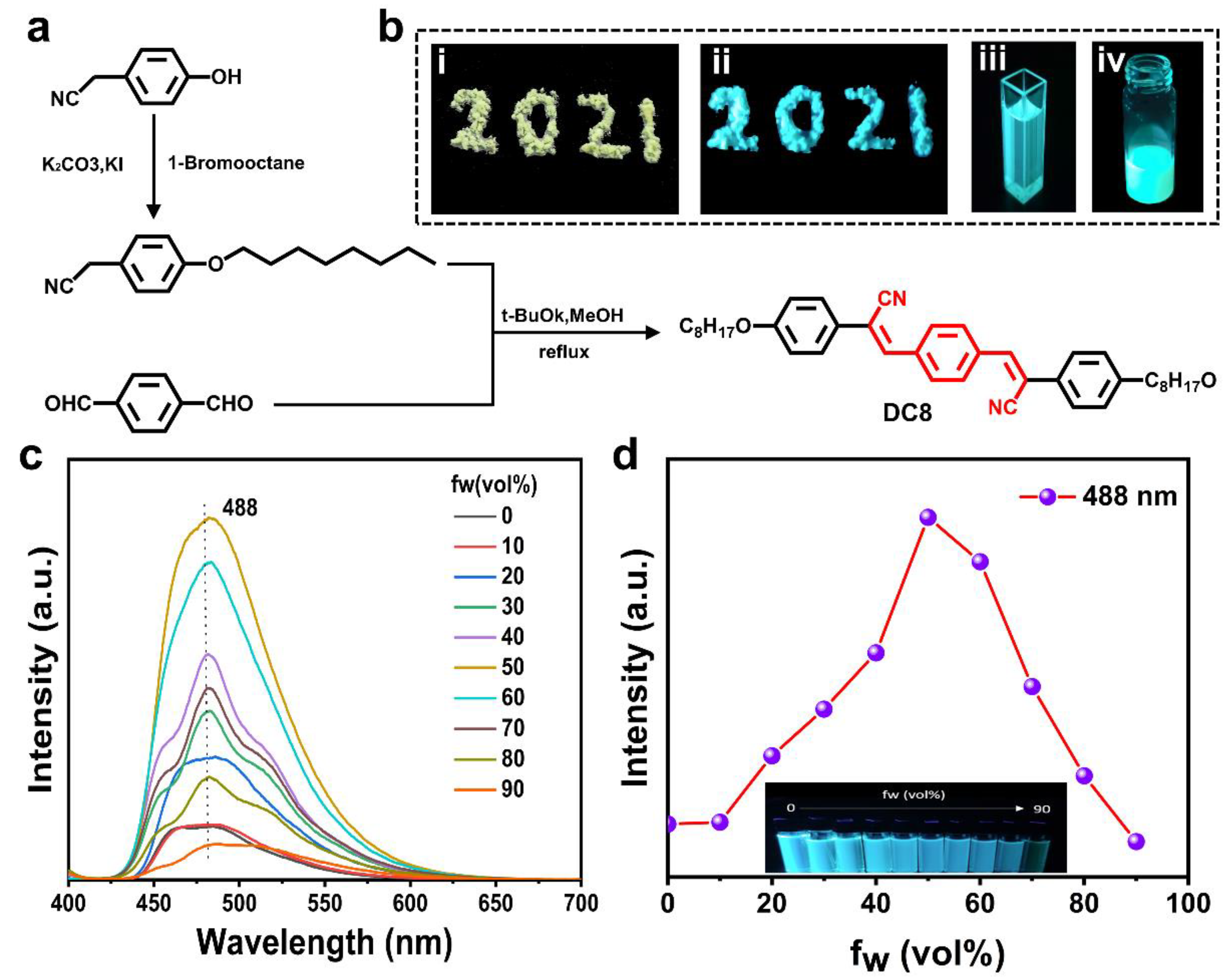
2.1. Synthesis and Characterization of Liquid Crystal Molecule DC8 with AIE Properties
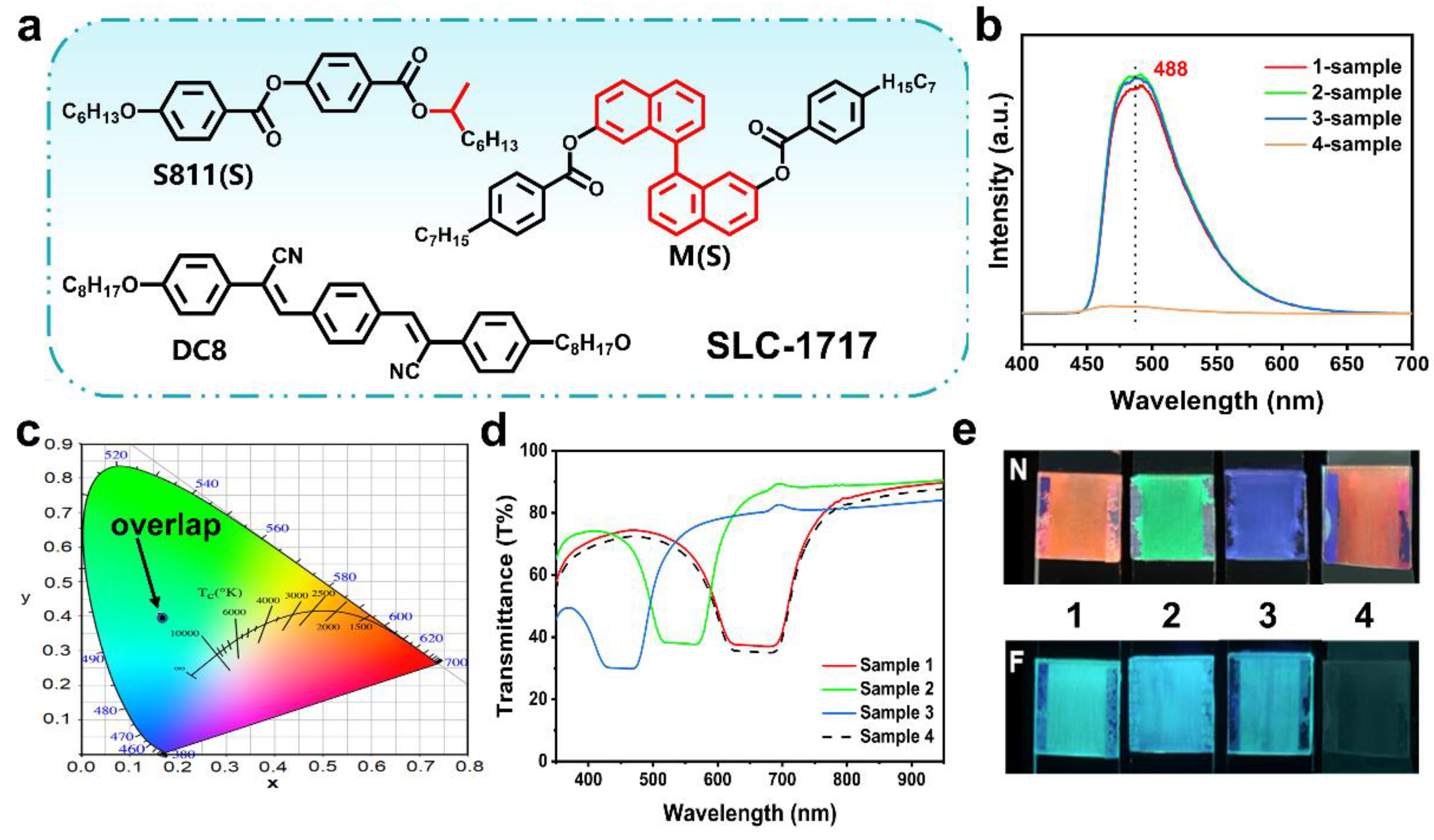
2.2. Construction of FCLC System with Both Fluorescence and Structural Color
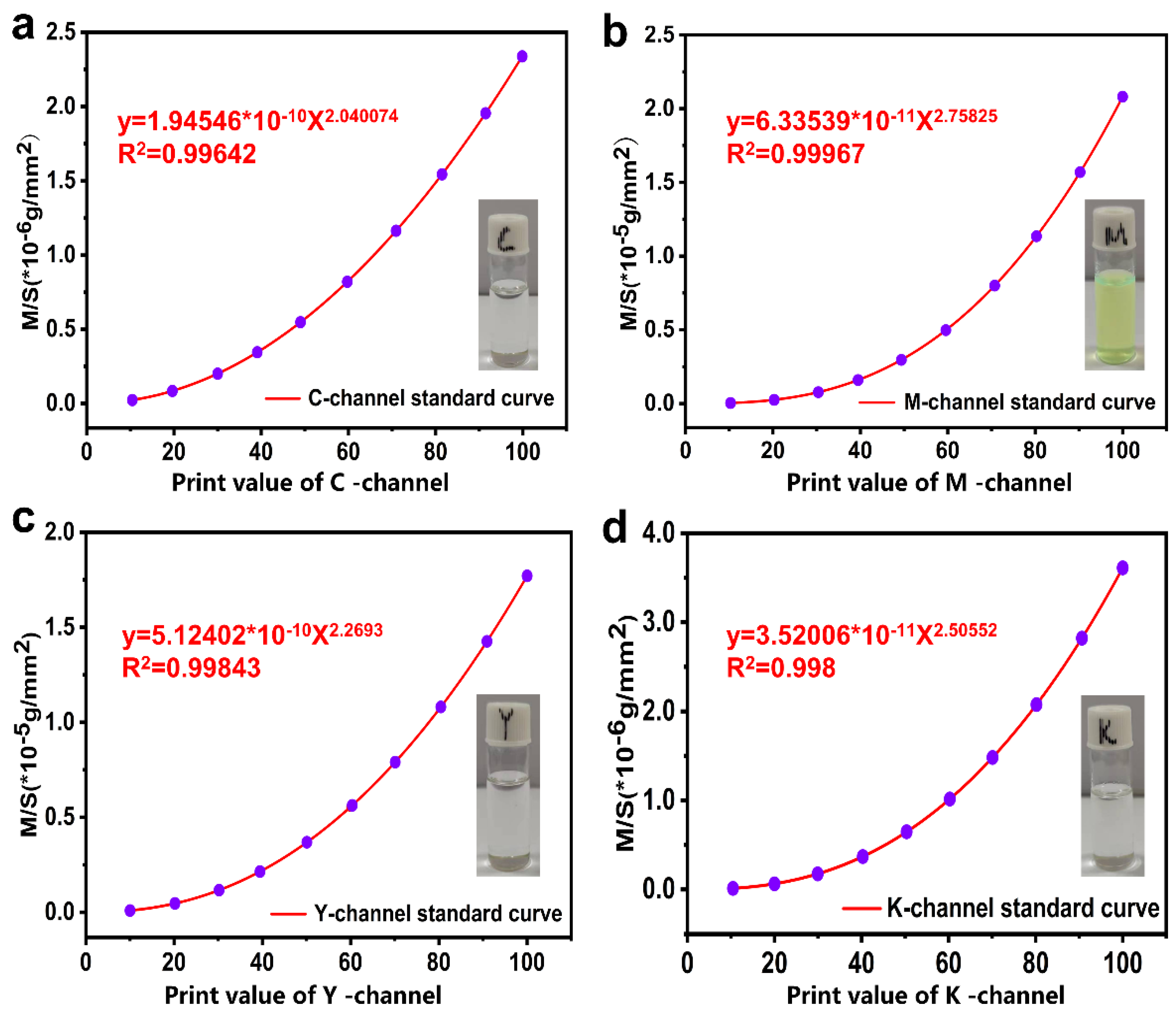
2.3. Preparation of the Standard Curve
2.4. Print Feasibility Assessment of Inkjet Printer
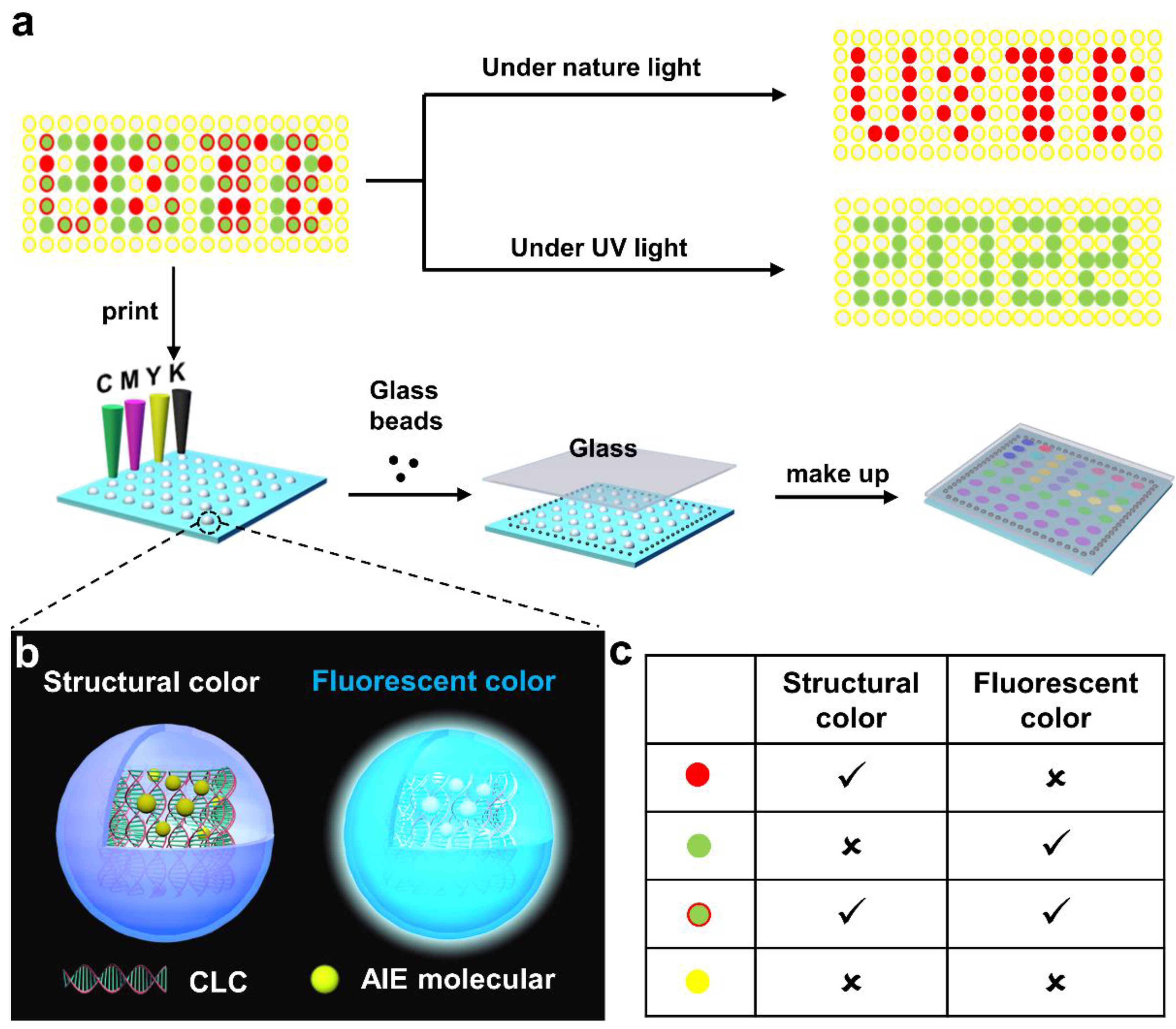
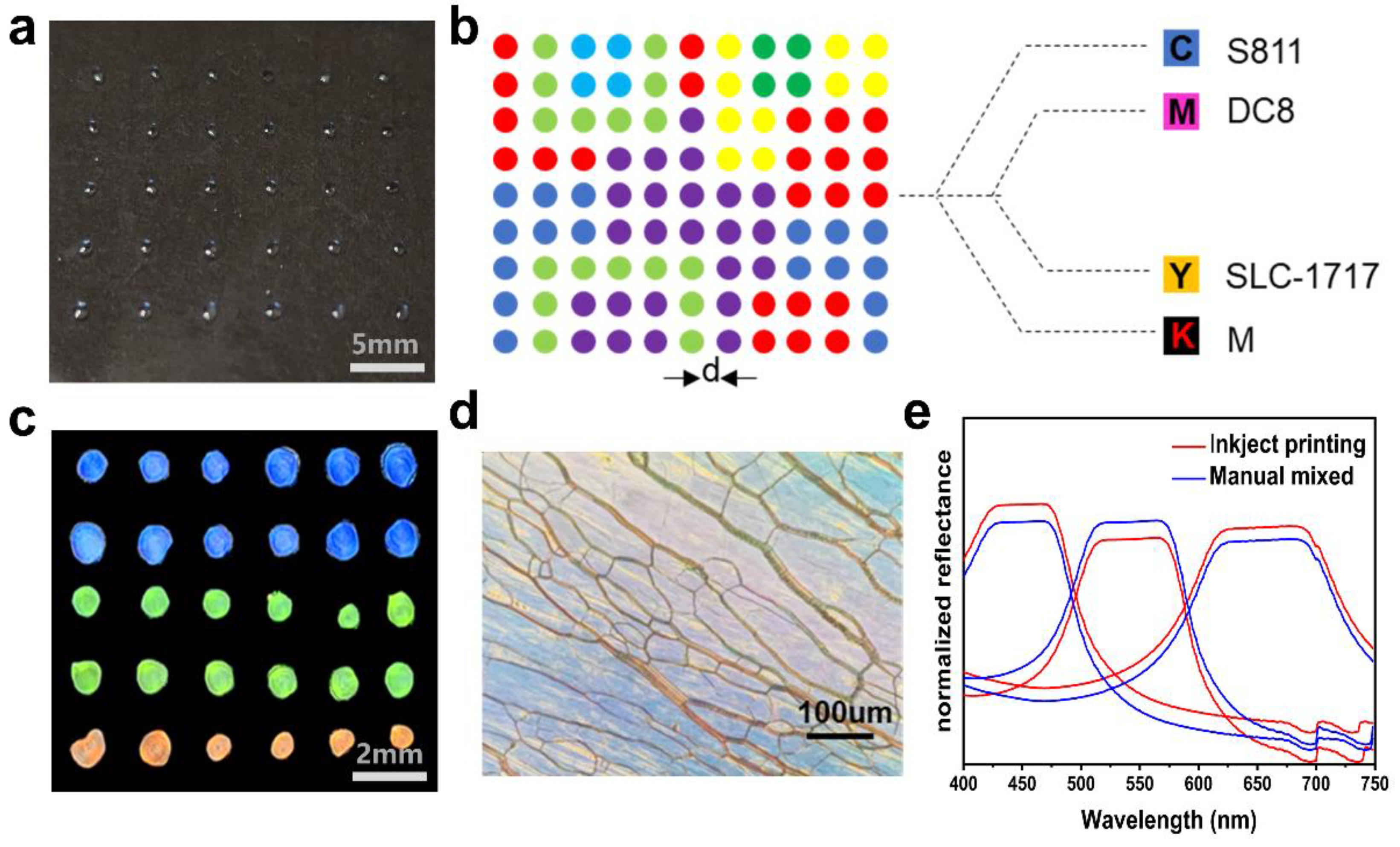
2.5. Spatial Patterning of Liquid CRYSTAL Inks with Different Components
3. Conclusions
4. Experiments
4.1. Materials
4.2. Preparation of the FCLC and CLC Mixtures
4.3. Functionalization of Glass Substrates
4.4. Preparation of LC Microdroplet Arrays
4.5. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, Y.; Zhao, X.; Gu, Z. Photonic crystals in bioassays. Adv. Funct. Mater. 2010, 20, 2970–2988. [Google Scholar] [CrossRef]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, Z.; Gu, H.; Zhu, C.; Gu, Z. Bio-inspired variable structural color materials. Chem. Soc. Rev. 2012, 41, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Dumanli, A.G.; Savin, T. Recent advances in the biomimicry of structural colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef]
- Tadepalli, S.; Slocik, J.M.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Bio-optics and bio-inspired optical materials. Chem. Rev. 2017, 117, 12705–12763. [Google Scholar] [CrossRef]
- Fu, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, eaar8580. [Google Scholar] [CrossRef]
- Spruell, J.M.; Hawker, C.J. Triggered structural and property changes in polymeric nanomaterials. Chem. Sci. 2011, 2, 18–26. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Broer, D.J.; Schenning, A.P. Stimuli-responsive photonic polymer coatings. Chem. Commun. 2014, 50, 15839–15848. [Google Scholar] [CrossRef]
- Hu, W.; Sun, J.; Wang, Q.; Zhang, L.; Yuan, X.; Chen, F.; Li, K.; Miao, Z.; Yang, D.; Yu, H.; et al. Humidity-Responsive Blue Phase Liquid-Crystalline Film with Reconfigurable and Tailored Visual Signals. Adv. Funct. Mater. 2020, 30, 2004610. [Google Scholar] [CrossRef]
- Ma, T.; Li, T.; Zhou, L.; Ma, X.; Yin, J.; Jiang, X. Dynamic wrinkling pattern exhibiting tunable fluorescence for anticounterfeiting applications. Nat. Commun. 2020, 11, 1811. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Li, C.; Liu, X.; Jiang, S.; Xu, Z.; Lee, R.; Zhu, M.; Xu, B.; Tian, W. Solid-state photoinduced luminescence switch for advanced anticounterfeiting and super-resolution imaging applications. J. Am. Chem. Soc. 2017, 139, 16036–16039. [Google Scholar] [CrossRef]
- Xie, M.; Xu, F.; Zhang, L.; Yin, J.; Jiang, X. Reversible surface dual-pattern with simultaneously dynamic wrinkled topography and fluorescence. ACS Macro Lett. 2018, 7, 540–545. [Google Scholar] [CrossRef]
- Lan, R.; Wang, Q.; Shen, C.; Huang, R.; Bao, J.; Zhang, Z.; Zhang, L.; Yang, H. Humidity-Induced Simultaneous Visible and Fluorescence Photonic Patterns Enabled by Integration of Covalent Bonds and Ionic Crosslinks. Adv. Funct. Mater. 2021, 31, 2106419. [Google Scholar] [CrossRef]
- Zhao, Z.; Deng, C.; Chen, S.; Lam, J.W.; Qin, W.; Lu, P.; Wang, Z.; Kwok, H.S.; Ma, Y.; Qiu, H. Full emission color tuning in luminogens constructed from tetraphenylethene, benzo-2, 1, 3-thiadiazole and thiophene building blocks. Chem. Commun. 2011, 47, 8847–8849. [Google Scholar] [CrossRef] [PubMed]
- Ilchishin, I.P.; Lisetski, L.N.; Mykytiuk, T.V. Reversible phototuning of lasing frequency in dye doped cholesteric liquid crystal and ways to improve it. Optical. Mater. Express 2011, 1, 1484–1493. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, W.; Zhou, T.; Peng, Q.; Zhai, D.; Li, H.; Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Conjugation-induced rigidity in twisting molecules: Filling the gap between aggregation-caused quenching and aggregation-induced emission. Adv. Mater. 2015, 27, 4496–4501. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, Y.; Kondo, K.; Akita, M.; Yoshizawa, M. Polyaromatic nanocapsules displaying aggregation-induced enhanced emissions in water. J. Am. Chem. Soc. 2015, 137, 98–101. [Google Scholar] [CrossRef]
- Zhao, D.; He, H.; Gu, X.; Guo, L.; Wong, K.S.; Lam, J.W.Y.; Tang, B.Z. Circularly Polarized Luminescence and a Reflective Photoluminescent Chiral Nematic Liquid Crystal Display Based on an Aggregation-Induced Emission Luminogen. Adv. Opt. Mater. 2016, 4, 534–539. [Google Scholar] [CrossRef]
- Ni, B.; Li, Y.; Liu, W.; Li, B.; Li, H.; Yang, Y. Circularly polarized luminescence from structurally coloured polymer films. Chem. Commun. 2021, 57, 2796–2799. [Google Scholar] [CrossRef]
- Qin, L.; Liu, X.; He, K.; Yu, G.; Yuan, H.; Xu, M.; Li, F.; Yu, Y. Geminate labels programmed by two-tone microdroplets combining structural and fluorescent color. Nat. Commun. 2021, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Additive manufacture of ceramics components by inkjet printing. Engineering 2015, 1, 113–123. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Li, K.; Li, T.; Zhang, T.; Li, H.; Li, A.; Li, Z.; Lai, X.; Hou, X.; Wang, Y.; Shi, L.; et al. Facile full-color printing with a single transparent ink. Sci. Adv. 2021, 7, eabh1992. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yuan, J.; Shen, S.; Gao, M.; Chesman, A.S.; Yin, H.; Cheng, J.; Zhang, Q.; Angmo, D. Perovskite and organic solar cells fabricated by inkjet printing: Progress and prospects. Adv. Funct. Mater. 2017, 27, 1703704. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Chen, Y.; Yang, X.; Ma, J.; Wang, J.; Wang, L.; Feng, W. Bioinspired Color-Changing Photonic Polymer Coatings Based on Three-Dimensional Blue Phase Liquid Crystal Networks. ACS Appl. Mater. Interfaces 2021, 13, 41102–41111. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet printing for pharmaceutical applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Kamal, W.; Li, M.; Lin, J.D.; Parry, E.; Jin, Y.; Elston, S.J.; Castrejón-Pita, A.A.; Morris, S.M. Spatially Patterned Polymer Dispersed Liquid Crystals for Image-Integrated Smart Windows. Adv. Opt. Mater. 2021, 10, 2101748. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, C.; Yang, W.; Guan, B.; Wang, J.; Ikeda, T.; Jiang, L. High-Resolution Erasable “Live” Patterns Based on Controllable Ink Diffusion on the 3D Blue-Phase Liquid Crystal Networks. Adv. Funct. Mater. 2022, 32, 2110985. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Zhang, X.; Wang, L.; Feng, W.; Li, Q. Beyond the visible: Bioinspired infrared adaptive materials. Adv. Mater. 2021, 33, 2004754. [Google Scholar] [CrossRef]
- Dou, S.; Xu, H.; Zhao, J.; Zhang, K.; Li, N.; Lin, Y.; Pan, L.; Li, Y. Bioinspired microstructured materials for optical and thermal regulation. Adv. Mater. 2021, 33, 2000697. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Cui, Y.; Wang, Q.; Zhou, H.; Wang, H.; Li, Y.; Yang, Z.; Cao, H.; Wang, D.; He, W. Spatial Patterning of Fluorescent Liquid Crystal Ink Based on Inkjet Printing. Molecules 2022, 27, 5536. https://doi.org/10.3390/molecules27175536
Zhang L, Cui Y, Wang Q, Zhou H, Wang H, Li Y, Yang Z, Cao H, Wang D, He W. Spatial Patterning of Fluorescent Liquid Crystal Ink Based on Inkjet Printing. Molecules. 2022; 27(17):5536. https://doi.org/10.3390/molecules27175536
Chicago/Turabian StyleZhang, Lei, Yongfeng Cui, Qi Wang, Huimin Zhou, Hao Wang, Yuzhan Li, Zhou Yang, Hui Cao, Dong Wang, and Wanli He. 2022. "Spatial Patterning of Fluorescent Liquid Crystal Ink Based on Inkjet Printing" Molecules 27, no. 17: 5536. https://doi.org/10.3390/molecules27175536
APA StyleZhang, L., Cui, Y., Wang, Q., Zhou, H., Wang, H., Li, Y., Yang, Z., Cao, H., Wang, D., & He, W. (2022). Spatial Patterning of Fluorescent Liquid Crystal Ink Based on Inkjet Printing. Molecules, 27(17), 5536. https://doi.org/10.3390/molecules27175536







