Catalytic Performance of Cobalt(II) Polyethylene Catalysts with Sterically Hindered Dibenzopyranyl Substituents Studied by Experimental and MLR Methods
Abstract
:1. Introduction
2. Results
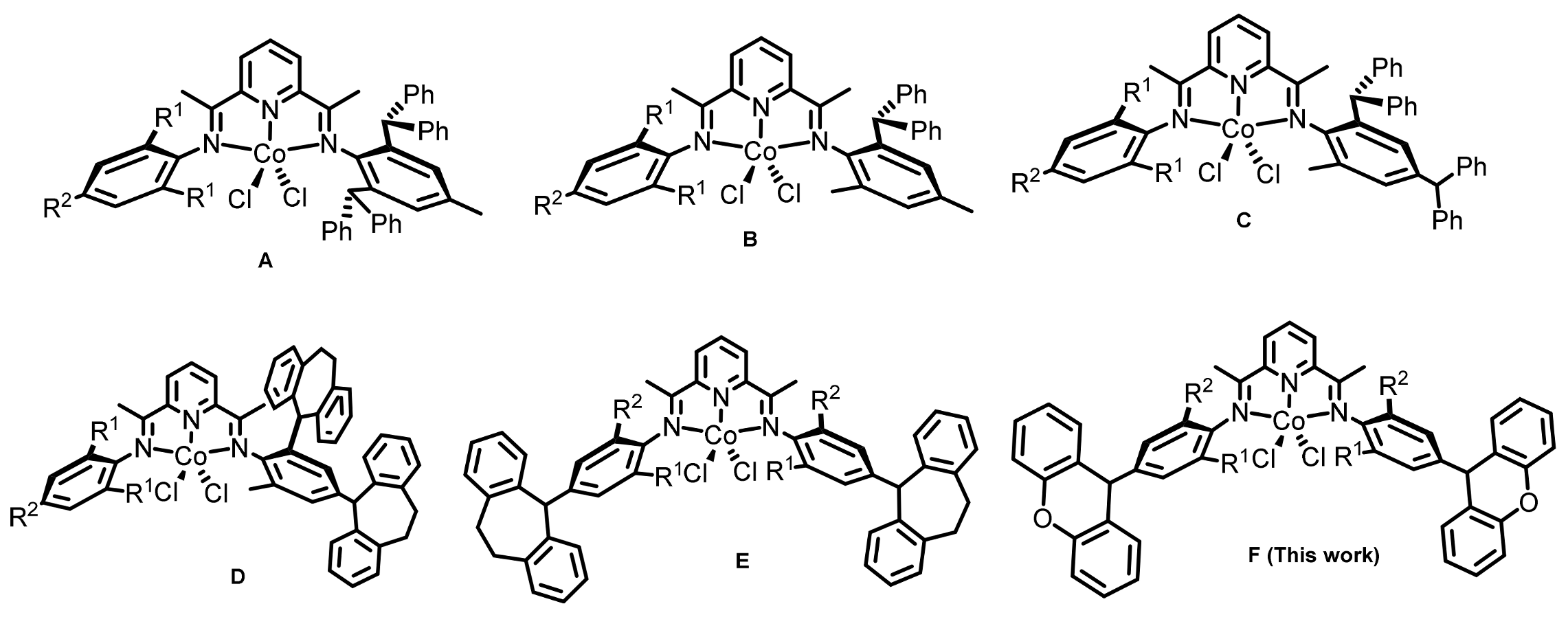
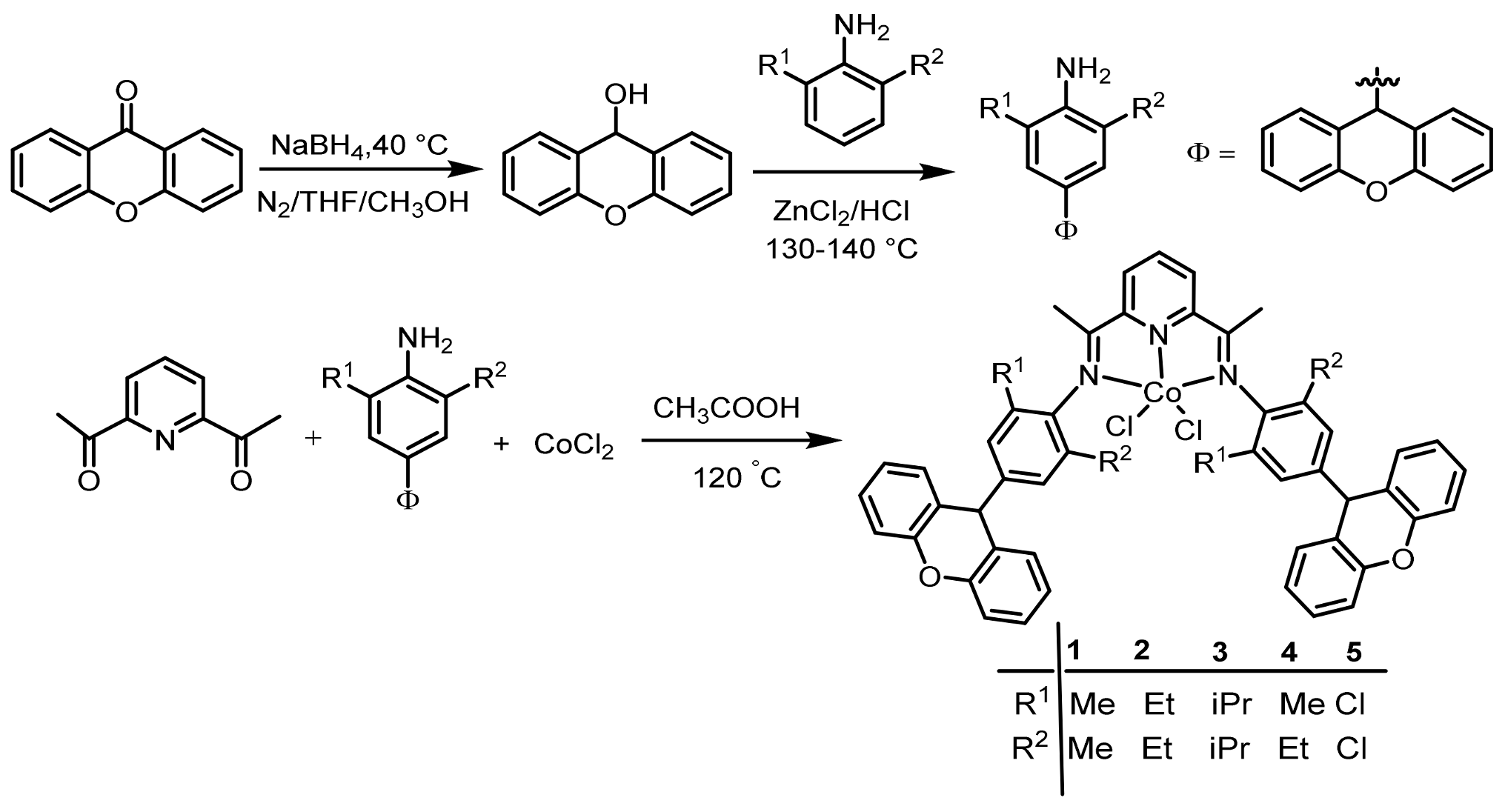
2.1. Synthesis and Characterization of Co1–Co5
2.2. Ethylene Polymerization
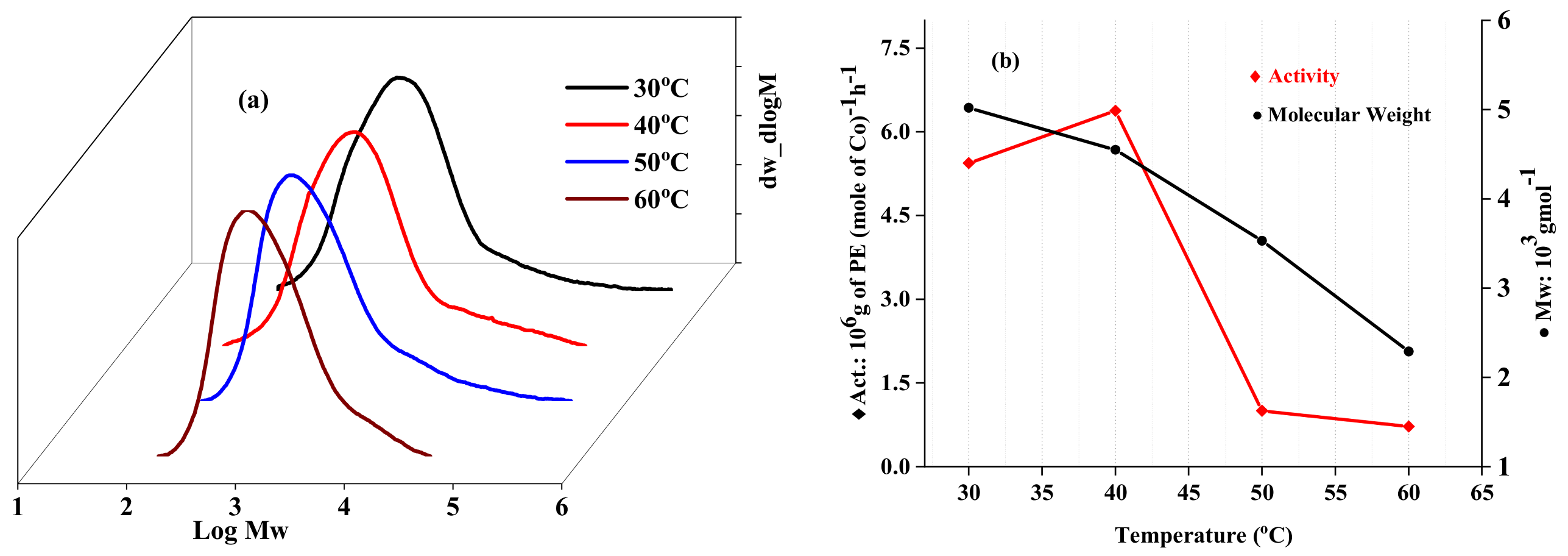
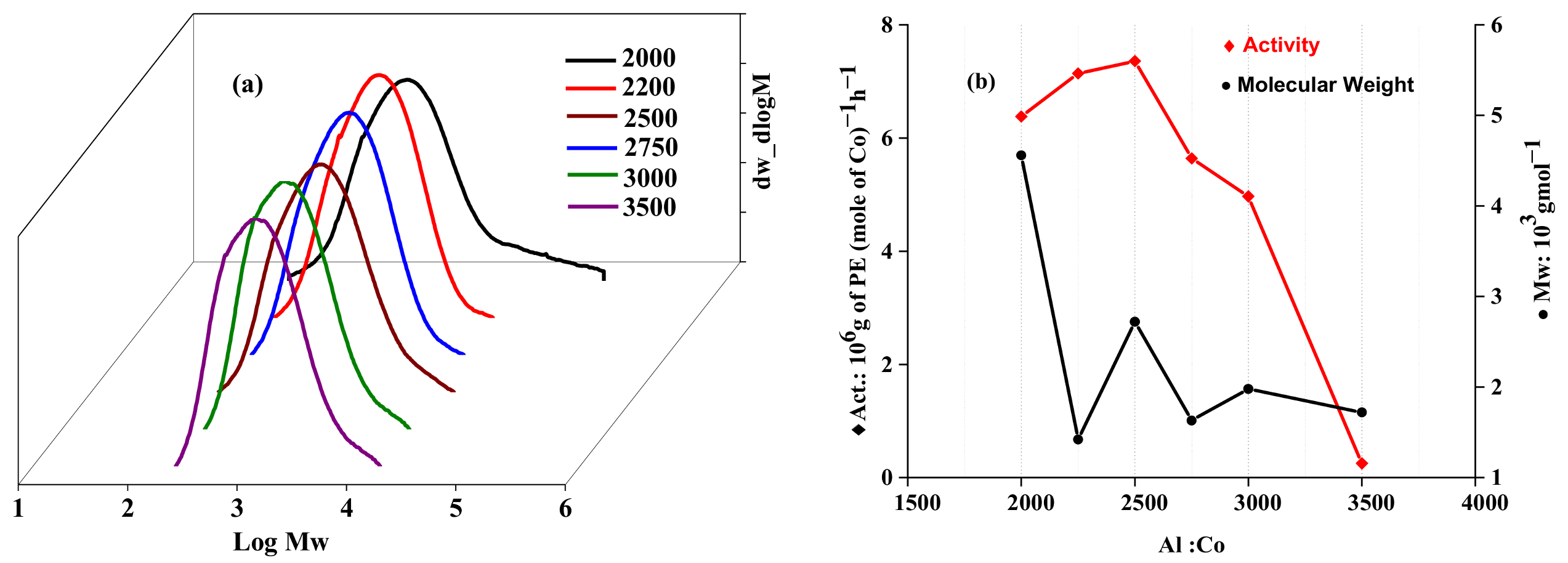
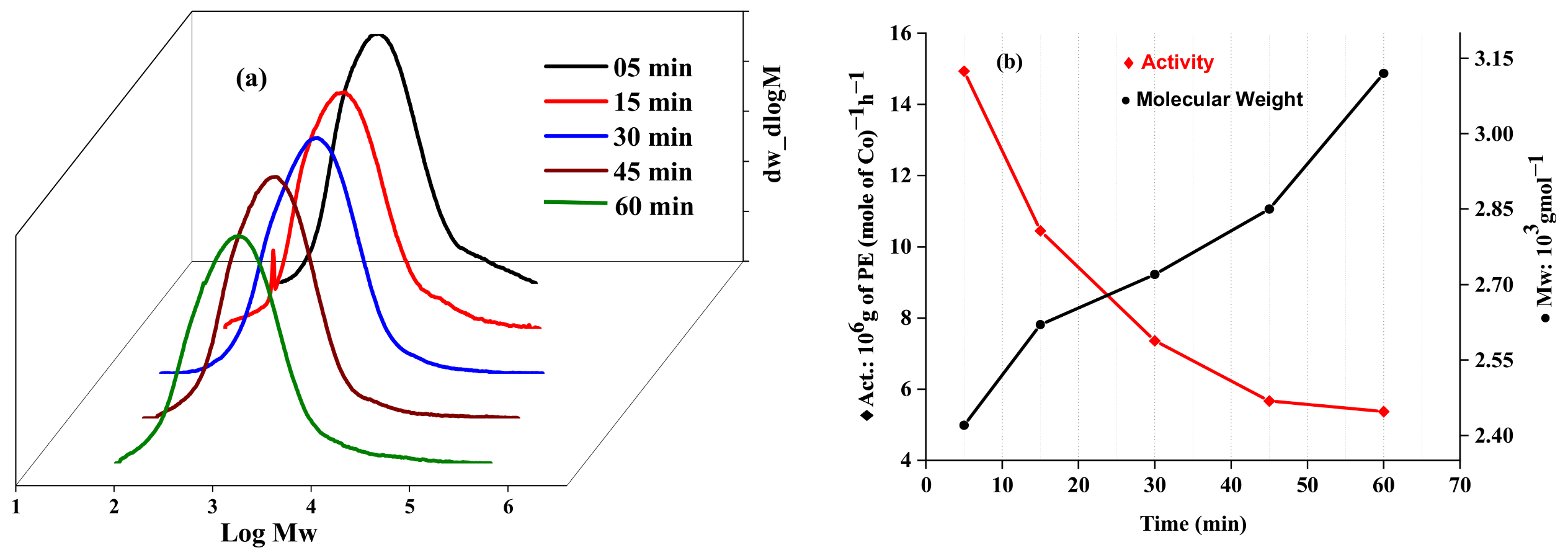
2.2.1. Catalytic Evaluation Using Co1 with MAO as a Cocatalyst
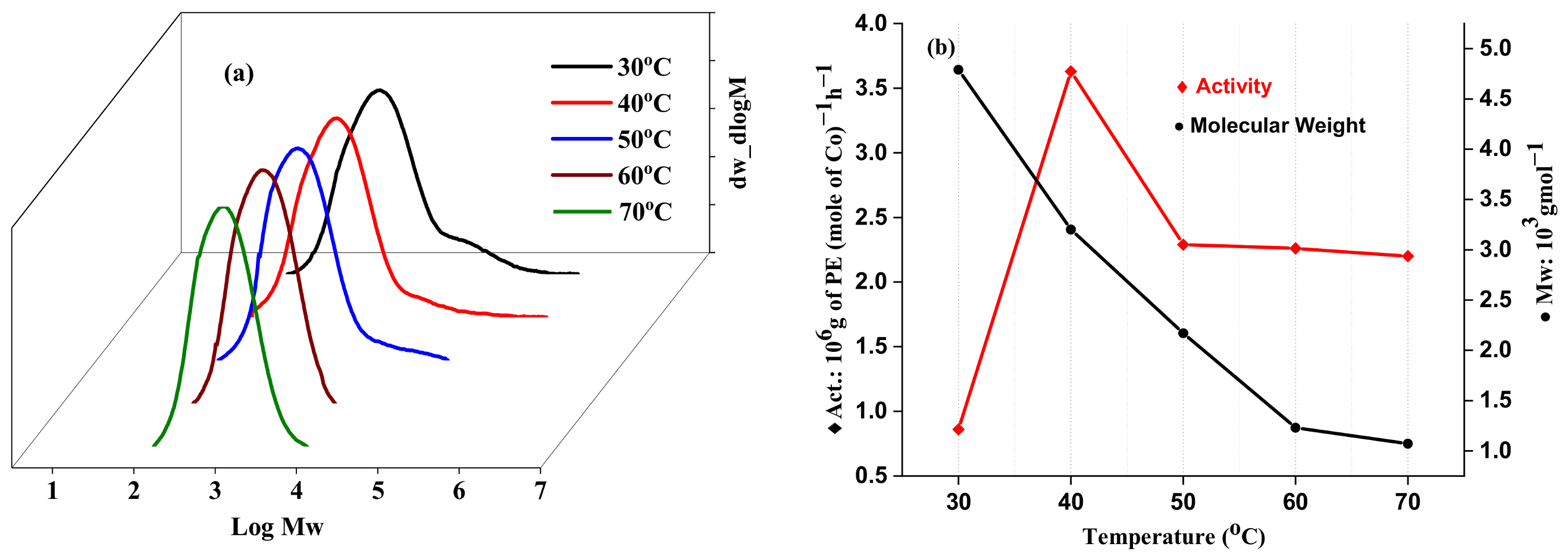
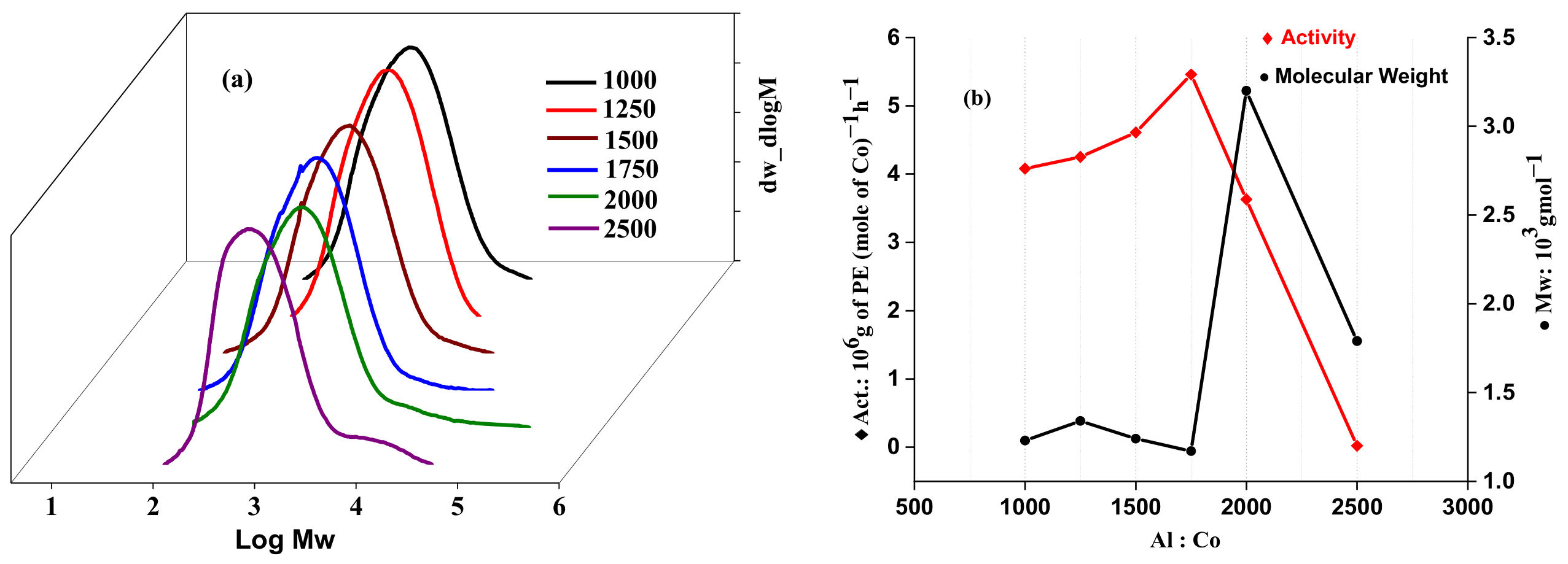
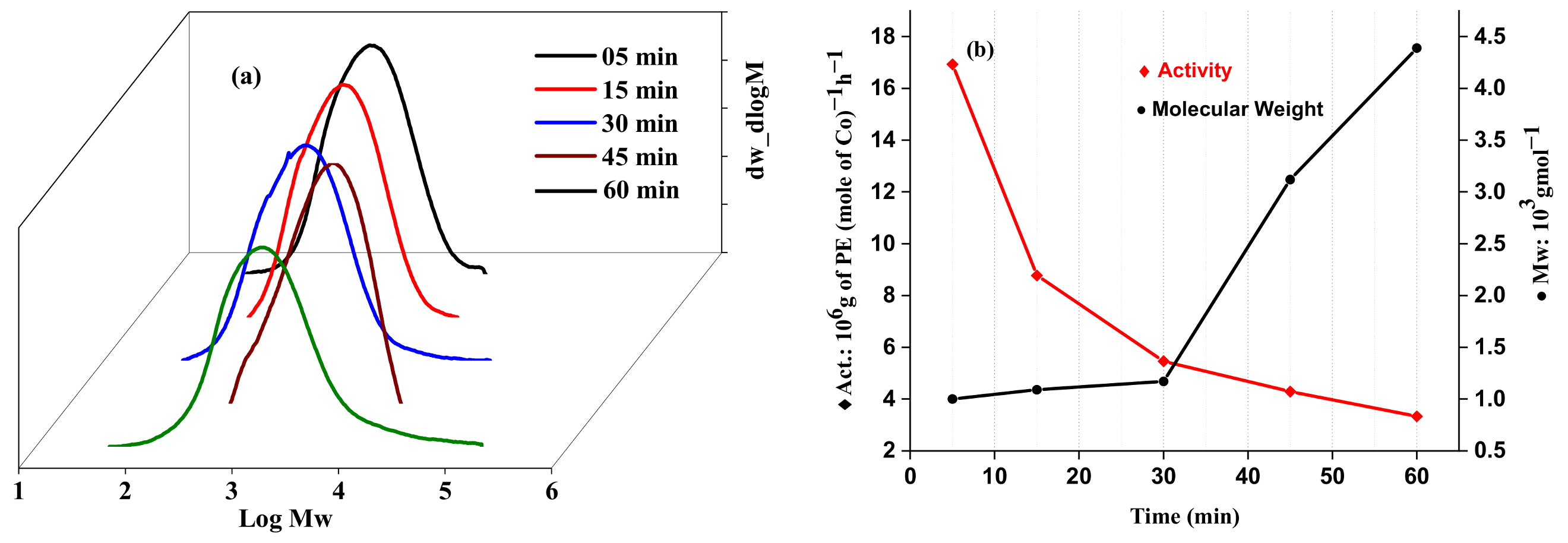
2.2.2. Catalytic Evaluation Using Co1 with MMAO as a Cocatalyst
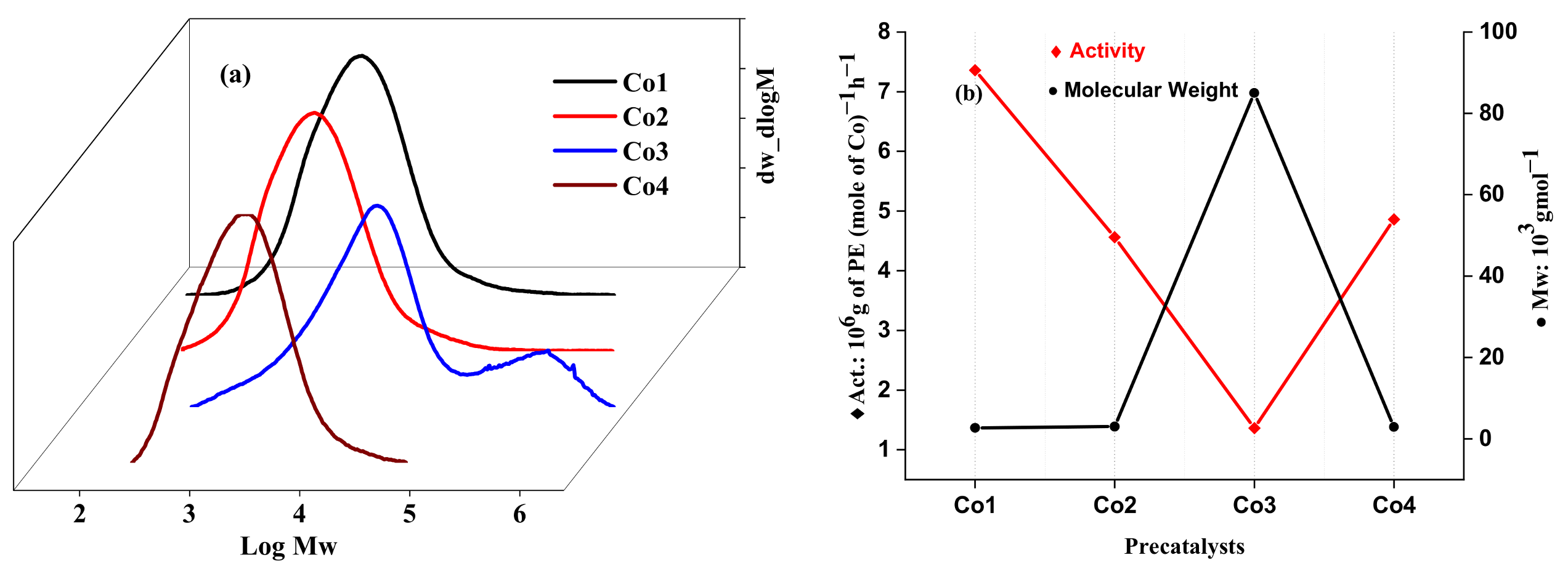
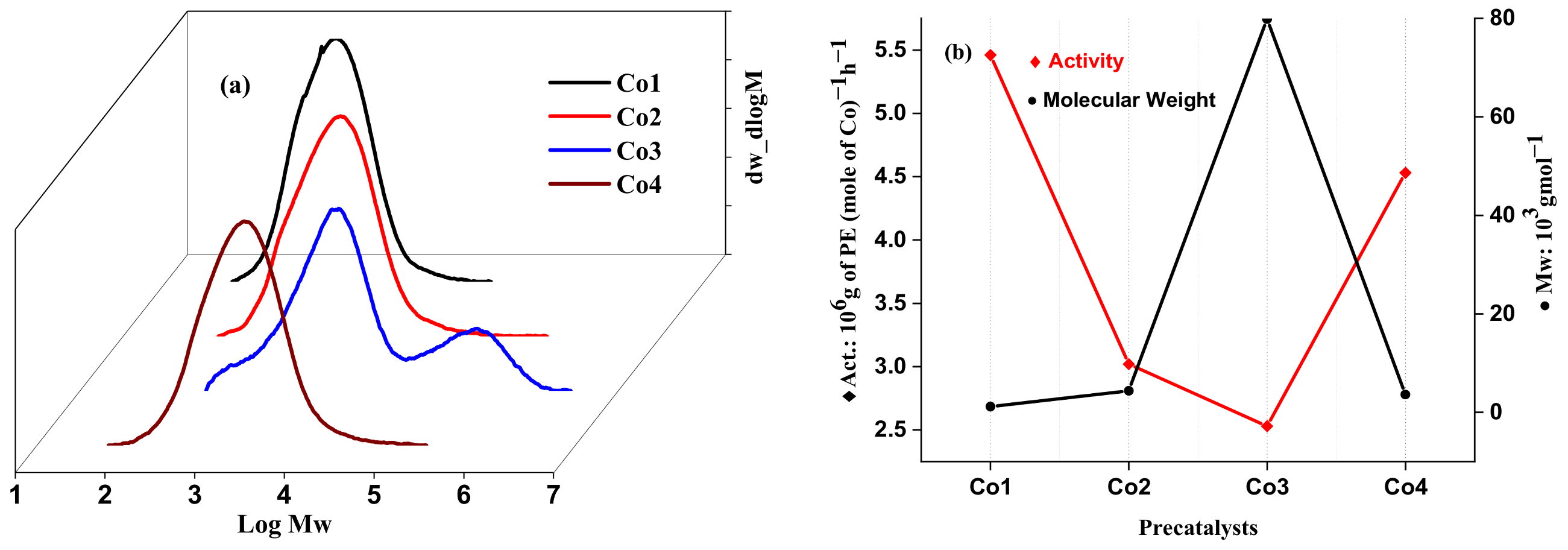
2.2.3. Catalytic Evaluation Using Co1–Co5 with MAO and MMAO as the Co-Catalysts
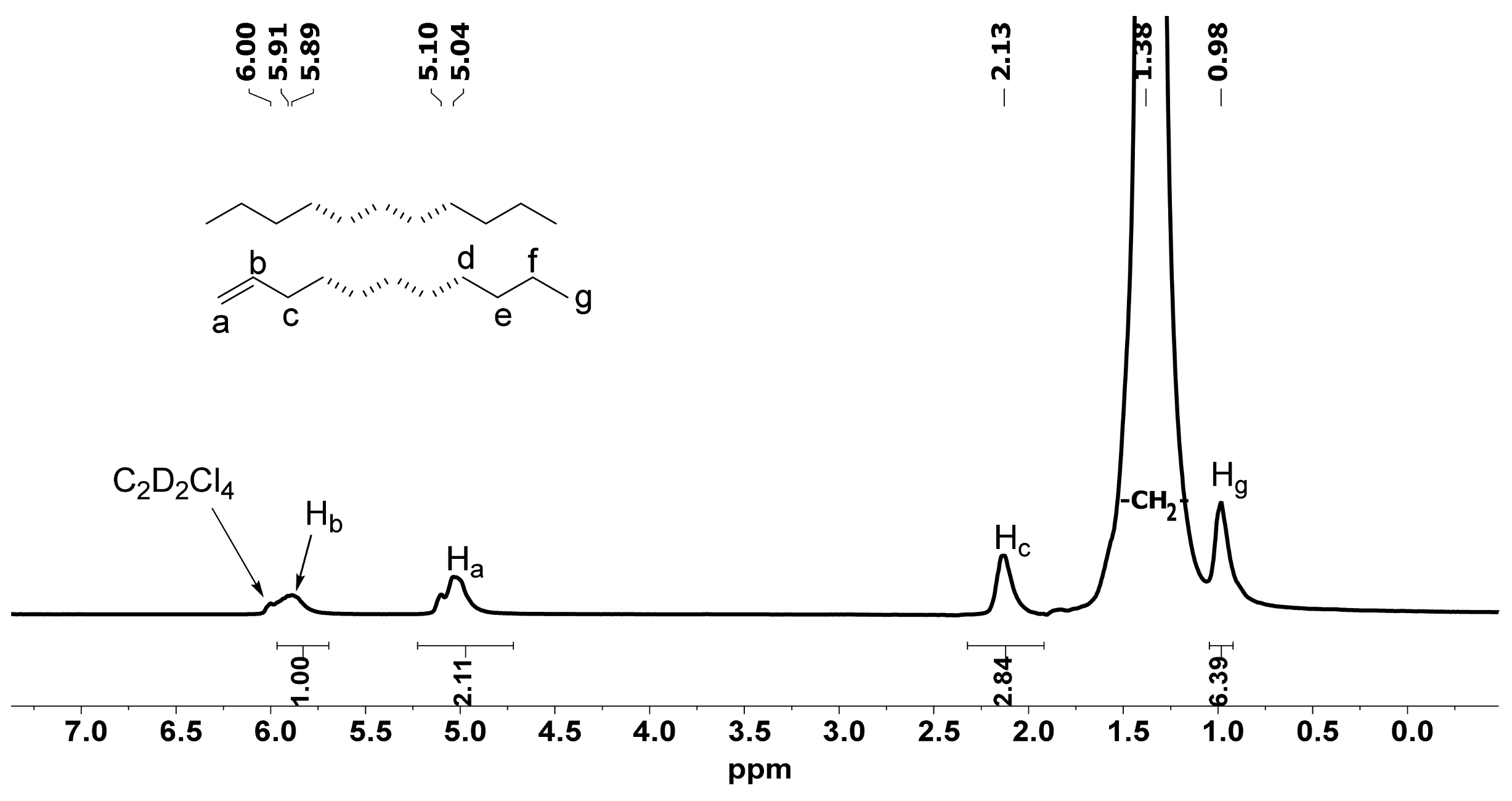
2.3. Microstructural Properties of Polyethylene
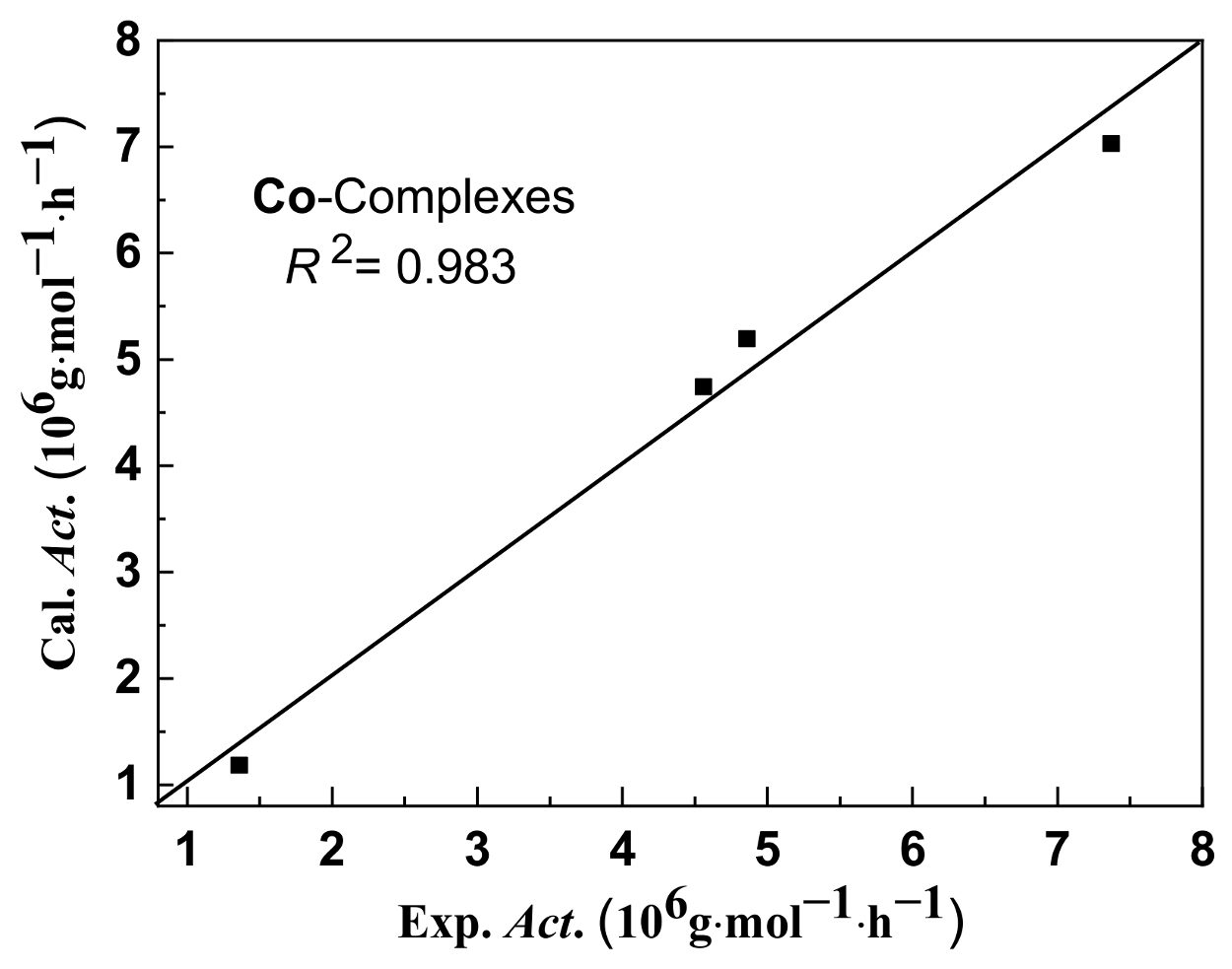
2.4. Interpretation of Catalytic Activity by MLR Analysis
3. Materials and Methods
3.1. General Consideration
3.2. General Synthesis of Bulky Anilines A1–A5
3.2.1. 2,6-Dimethyl-4-(dibenzopyranyl)aniline (A1)
3.2.2. 2,6-Diethyl-4-(dibenzopyranyl)aniline (A2)
3.2.3. 2,6-Diisopropyl-4-(dibenzopyranyl)aniline (A3)
3.2.4. 2-Methyl,6-ethyl-4-(dibenzopyranyl)aniline (A4)
3.2.5. 2,6-Chloro-4-(dibenzopyranyl)aniline (A5)
3.3. Synthesis of Cobalt Complexes Co1–Co5
3.3.1. Synthesis of 2,6-Bis[1-(4-dibenzopyranyl-2,6-dimethylphenylimino) ethyly]-pyridylcobalt Dichlorides (Co1)
3.3.2. Synthesis of 2,6-Bis[1-(4-dibenzopyranyl-2,6-diethylphenylimino) ethyly]-pyridylcobalt Dichlorides (Co2)
3.3.3. Synthesis of 2,6-Bis[1-(4-dibenzopyranyl-2,6-diisopropylphenylimino) ethyly]-pyridylcobalt Dichlorides (Co3)
3.3.4. Synthesis of 2,6-Bis[1-(4-dibenzopyranyl-2-methyl, 6-ethylphenylimino) ethyly]-pyridylcobalt Dichlorides (Co4)
3.3.5. Synthesis of 2,6-Bis[1-(4-dibenzopyranyl-2,6-chlorophenylimino) ethyly]-pyridylcobalt Dichlorides (Co5)
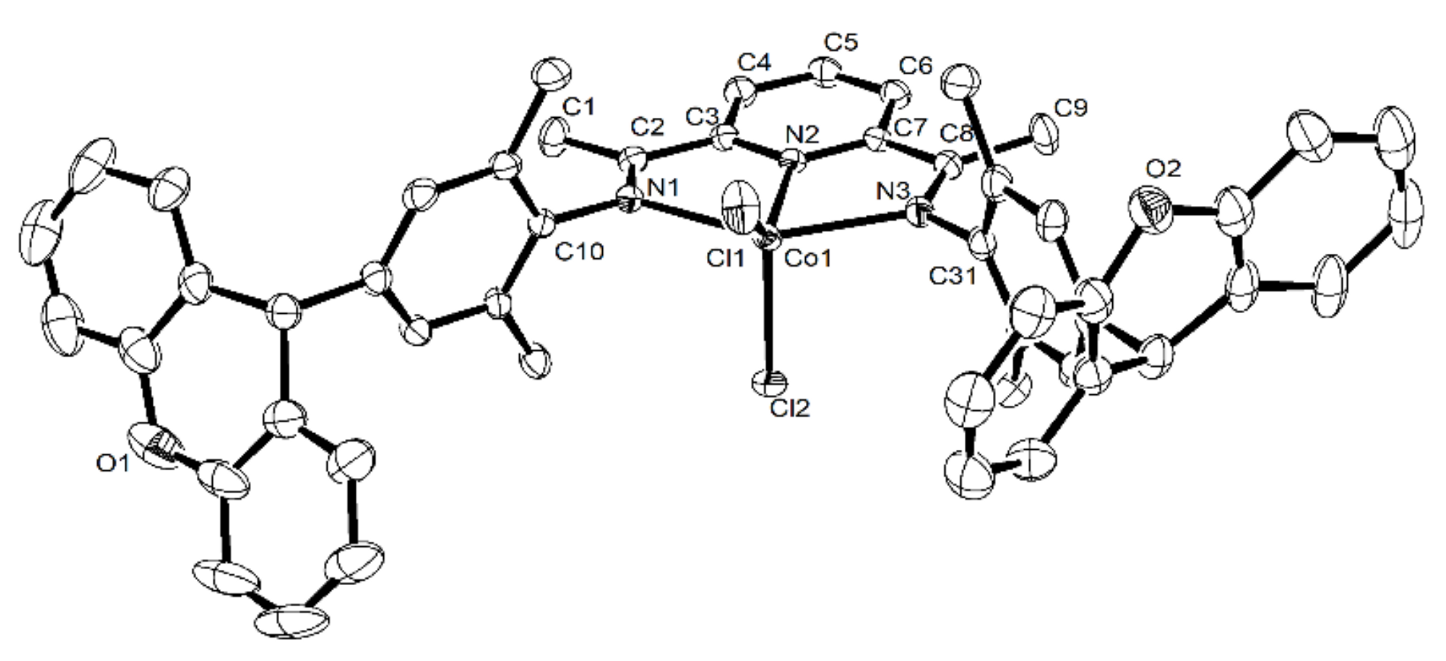
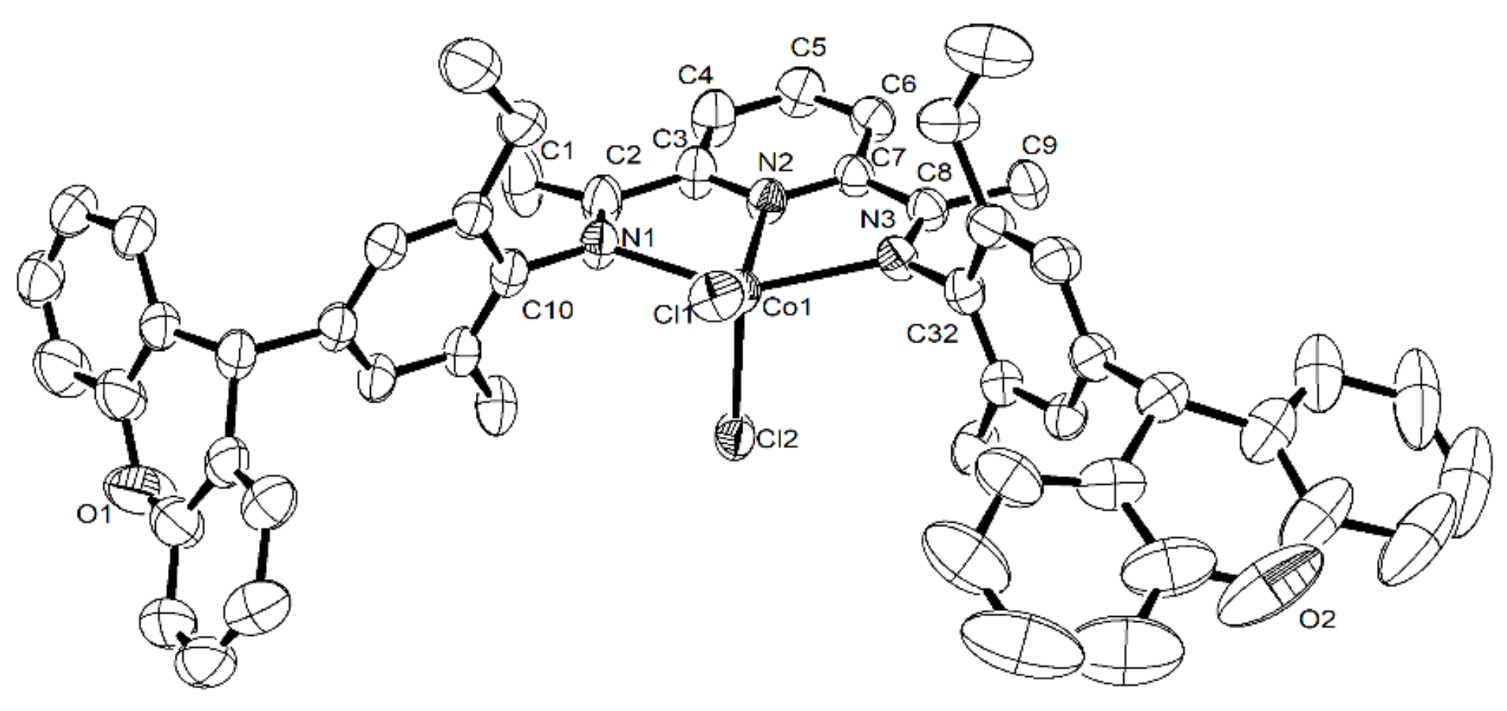
3.4. X-ray Crystallographic Studies
3.5. Ethylene Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Small, B.L.; Brookhart, M.; Bennett, A.M. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Gibson, V.C.; McTavish, S.J.; Solan, G.A.; White, A.J.; Williams, D.J.; Kimberley, B.S.; Maddox, P.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 7, 849–850. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd (II)-and Ni (II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Killian, C.M.; Tempel, D.J.; Johnson, L.K.; Brookhart, M. Living polymerization of α-olefins using NiII-α-diimine catalysts. Synthesis of new block polymers based on α-olefins. J. Am. Chem. Soc. 1996, 118, 11664–11665. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Görl, C.; Alt, H.G. Influence of the para-substitution in bis (arylimino) pyridine iron complexes on the catalytic oligomerization and polymerization of ethylene. J. Organomet. Chem. 2007, 692, 4580–4592. [Google Scholar] [CrossRef]
- Long, Z.; Wu, B.; Yang, P.; Li, G.; Liu, Y.; Yang, X.-J. Synthesis and characterization of para-nitro substituted 2,6-bis (phenylimino) pyridyl Fe (II) and Co (II) complexes and their ethylene polymerization properties. J. Organomet. Chem. 2009, 694, 3793–3799. [Google Scholar] [CrossRef]
- Tomov, A.K.; Gibson, V.C.; Britovsek, G.J.; Long, R.J.; van Meurs, M.; Jones, D.J.; Tellmann, K.P.; Chirinos, J.J. Distinguishing chain growth mechanisms in metal-catalyzed olefin oligomerization and polymerization systems: C2H4/C2D4 co-oligomerization/polymerization experiments using chromium, iron, and cobalt catalysts. Organometallics 2009, 28, 7033–7040. [Google Scholar] [CrossRef]
- Yu, J.; Huang, W.; Wang, L.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-methylphenylimino) ethyl]-6-[1-(arylimino) ethyl] pyridylcobalt (II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Dalton Trans. 2011, 40, 10209–10214. [Google Scholar] [CrossRef]
- He, F.; Zhao, W.; Cao, X.-P.; Liang, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2, 6-dibenzhydryl-4-chlorophenylimino) ethyl]-6-[1-aryliminoethyl] pyridyl cobalt dichlorides: Synthesis, characterization and ethylene polymerization behavior. J. Organomet. Chem. 2012, 713, 209–216. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Liang, T.; Redshaw, C.; Li, Y.; Sun, W.-H. Synthesis, characterization and catalytic behavior toward ethylene of 2-[1-(4, 6-dimethyl-2-benzhydrylphenylimino) ethyl]-6-[1-(arylimino) ethyl] pyridylmetal (iron or cobalt) chlorides. Dalton Trans. 2013, 42, 9188–9197. [Google Scholar] [CrossRef]
- Lai, J.; Zhao, W.; Yang, W.; Redshaw, C.; Liang, T.; Liu, Y.; Sun, W.-H. 2-[1-(2,4-Dibenzhydryl-6-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylcobalt(ii) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Polym. Chem. 2012, 3, 787–793. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Du, S.; Guo, C.Y.; Hao, X.; Sun, W.H. 2-(1-(2,4-Bis((di(4-fluorophenyl) methyl)-6-methylphenylimino) ethyl)-6-(1-(arylimino) ethyl) pyridylmetal (iron or cobalt) Complexes: Synthesis, Characterization, and Ethylene Polymerization Behavior. Macromol. Chem. Phys. 2014, 215, 1797–1809. [Google Scholar] [CrossRef]
- Yue, E.; Zeng, Y.; Zhang, W.; Sun, Y.; Cao, X.-P.; Sun, W.-H. Highly linear polyethylenes using the 2-(1-(2,4-dibenzhydrylnaphthylimino) ethyl)-6-(1-(arylimino) ethyl)-pyridylcobalt chlorides: Synthesis, characterization and ethylene polymerization. Sci. China Chem. 2016, 59, 1291–1300. [Google Scholar] [CrossRef]
- Mitchell, N.E.; Anderson, W.C., Jr.; Long, B.K. Mitigating chain-transfer and enhancing the thermal stability of co-based olefin polymerization catalysts through sterically demanding ligands. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3990–3995. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Mastroianni, S.; Solan, G.A.; Baugh, S.P.; Redshaw, C.; Gibson, V.C.; White, A.J.; Williams, D.J.; Elsegood, M.R. Oligomerisation of Ethylene by Bis (imino) pyridyliron and-cobalt Complexes. Chem. Eur. J. 2000, 6, 2221–2231. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis (imino) pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Xiao, L.; Gao, R.; Zhang, M.; Li, Y.; Cao, X.; Sun, W.-H. 2-(1H-2-Benzimidazolyl)-6-(1-(arylimino) ethyl) pyridyl iron (II) and cobalt (II) dichlorides: Syntheses, characterizations, and catalytic behaviors toward ethylene reactivity. Organometallics 2009, 28, 2225–2233. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Rios, I.G.; Mantovani, G.; Meli, A.; Segarra, A.M. Ethylene oligomerization, homopolymerization and copolymerization by iron and cobalt catalysts with 2,6-(bis-organylimino) pyridyl ligands. Coord. Chem. Rev. 2006, 250, 1391–1418. [Google Scholar] [CrossRef]
- Appukuttan, V.K.; Liu, Y.; Son, B.C.; Ha, C.-S.; Suh, H.; Kim, I. Iron and cobalt complexes of 2,3,7,8-tetrahydroacridine-4,5(1H, 6H)-diimine sterically modulated by substituted aryl rings for the selective oligomerization to polymerization of ethylene. Organometallics 2011, 30, 2285–2294. [Google Scholar] [CrossRef]
- Smit, T.M.; Tomov, A.K.; Britovsek, G.J.; Gibson, V.C.; White, A.J.; Williams, D.J. The effect of imine-carbon substituents in bis (imino) pyridine-based ethylene polymerisation catalysts across the transition series. Catal. Sci. Technol. 2012, 2, 643–655. [Google Scholar] [CrossRef]
- Sun, W.-H.; Kong, S.; Chai, W.; Shiono, T.; Redshaw, C.; Hu, X.; Guo, C.; Hao, X. 2-(1-(Arylimino) ethyl)-8-arylimino-5, 6, 7-trihydroquinolylcobalt dichloride: Synthesis and polyethylene wax formation. Appl. Catal. A Gen. 2012, 447, 67–73. [Google Scholar] [CrossRef]
- Du, S.; Zhang, W.; Yue, E.; Huang, F.; Liang, T.; Sun, W.H. α, α′-Bis (arylimino)-2,3:5,6-bis (pentamethylene) pyridylcobalt Chlorides: Synthesis, Characterization, and Ethylene Polymerization Behavior. Eur. J. Inorg. Chem. 2016, 2016, 1748–1755. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Controlling the molecular weights of polyethylene waxes using the highly active precatalysts of 2-(1-aryliminoethyl)-9-arylimino-5,6,7,8-tetrahydrocycloheptapyridylcobalt chlorides: Synthesis, characterization, and catalytic behavior. Dalton Trans. 2016, 45, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, W.; Sun, Y.; Hu, X.; Solan, G.A.; Sun, W.-H. Thermally stable and highly active cobalt precatalysts for vinyl-polyethylenes with narrow polydispersities: Integrating fused-ring and imino-carbon protection into ligand design. New J. Chem. 2016, 40, 8012–8023. [Google Scholar] [CrossRef]
- Bariashir, C.; Wang, Z.; Du, S.; Solan, G.A.; Huang, C.; Liang, T.; Sun, W.H. Cycloheptyl-fused NNO-ligands as electronically modifiable supports for M (II)(M = Co, Fe) chloride precatalysts; probing performance in ethylene oligo-/polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3980–3989. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Zhang, W.; Solan, G.A.; Liu, Q.; Liang, T.; Sun, W.-H. Enhancing thermostability of iron ethylene polymerization catalysts through N, N, N-chelation of doubly fused α, α′-bis (arylimino)-2, 3: 5, 6-bis (hexamethylene) pyridines. Catal. Sci. Technol. 2019, 9, 1933–1943. [Google Scholar] [CrossRef]
- Flisak, Z.; Sun, W.-H. Progression of Diiminopyridines: From Single Application to Catalytic Versatility. Catal. A 2015, 8, 4713–4724. [Google Scholar] [CrossRef]
- Zada, M.; Guo, L.; Ma, Y.; Zhang, W.; Flisak, Z.; Sun, Y.; Sun, W.-H. Activity and Thermal Stability of Cobalt (II)-Based Olefin Polymerization Catalysts Adorned with Sterically Hindered Dibenzocycloheptyl Groups. Molecules 2019, 24, 2007. [Google Scholar] [CrossRef]
- Ivanchev, S.S.; Yakimansky, A.V.; Rogozin, D.G. Quantum-chemical calculations of the effect of cycloaliphatic groups in α-diimine and bis (imino) pyridine ethylene polymerization precatalysts on their stabilities with respect to deactivation reactions. Polymer 2004, 45, 6453–6459. [Google Scholar] [CrossRef]
- Pelascini, F.; Peruch, F.; Lutz, P.J.; Wesolek, M.; Kress, J. Pyridine bis (imino) iron and cobalt complexes for ethylene polymerization: Influence of the aryl imino substituents. Eur. Polym. J. 2005, 41, 1288–1295. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zheng, Y.; Li, Y.-G.; Pan, L.; Li, Y.-S.; Hu, N.-H. Fe (II) and Co (II) pyridinebisimine complexes bearing different substituents on ortho-and para-position of imines: Synthesis, characterization and behavior of ethylene polymerization. J. Organomet. Chem. 2005, 690, 1233–1239. [Google Scholar] [CrossRef]
- Guo, L.; Zada, M.; Zhang, W.; Vignesh, A.; Zhu, D.; Ma, Y.; Liang, T.; Sun, W.-H. Highly linear polyethylenes tailored with 2, 6-bis [1-(p-dibenzo-cycloheptylarylimino) ethyl] pyridylcobalt dichlorides. Dalton Trans. 2019, 48, 5604–5613. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.-H.; Redshaw, C. Tailoring iron complexes for ethylene oligomerization and/or polymerization. Dalton Trans. 2013, 42, 8988–8997. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Strömberg, S. Iron and cobalt ethylene polymerization catalysts bearing 2, 6-bis (imino) pyridyl ligands: Synthesis, structures, and polymerization studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, W.; Hao, X.; Li, B.; Redshaw, C.; Li, Y.; Sun, W.-H. 2-(1-{2,6-Bis[bis(4-fluorophenyl) methyl]-4-methylphenylimino} ethyl)-6-[1-(arylimino) ethyl] pyridylcobalt dichlorides: Synthesis, characterization and ethylene polymerization behavior. J. Organomet. Chem. 2013, 731, 78–84. [Google Scholar] [CrossRef]
- Ahmed, S.; Yang, W.; Ma, Z.; Sun, W.-H. Catalytic activities of bis (pentamethylene) pyridyl (Fe/Co) complex analogues in ethylene polymerization by modeling method. J. Phys. Chem. A 2018, 122, 9637–9644. [Google Scholar] [CrossRef]
- Malik, A.A.; Yang, W.; Ma, Z.; Sun, W.-H. The Catalytic Activities of Carbocyclic Fused Pyridineimine Nickel Complexes Analogues in Ethylene Polymerization by Modeling Study. Catalysts 2019, 9, 520. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Wang, Z.; Solan, G.A.; Liang, T.; Sun, W.-H. Doubly fused N, N, N-iron ethylene polymerization catalysts appended with fluoride substituents; probing catalytic performance via a combined experimental and MLR study. Catal. Sci. Technol. 2021, 11, 4605–4618. [Google Scholar] [CrossRef]
- Kratzert, D.; Holstein, J.J.; Krossing, I. DSR: Enhanced modelling and refinement of disordered structures with SHELXL. J. Appl. Crystallogr. 2015, 48, 933–938. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar]
- Le, T.; Epa, V.C.; Burden, F.R.; Winkler, D.A. Quantitative structure–property relationship modeling of diverse materials properties. Chem. Rev. 2012, 112, 2889–2919. [Google Scholar]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Becke, A.D. A multicenter numerical integration scheme for polyatomic molecules. J. Chem. Phys. 1988, 88, 2547–2553. [Google Scholar] [CrossRef]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted abinitio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar]
- Bergner, A.; Dolg, M.; Küchle, W.; Stoll, H.; Preub, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Li, Z.; Bian, K.; Zhou, M. Excel for Windows95 Encyclopaedia; Electronics Industry Press: Beijing, China, 1997. [Google Scholar]

| Co1 | Co4 | |
|---|---|---|
| Bond Lengths [Å] | ||
| Co1–N1 | 2.223(3) | 2.239 (4) |
| Co1–N2 | 2.040(3) | 2.043(4) |
| Co1–N3 | 2.225(3) | 2.246(4) |
| Co1–Cl1 | 2.2457(10) | 2.2339(13) |
| Co1–Cl2 | 2.2650(9) | 2.2905(14) |
| Bond Angles [°] | ||
| N1–Co1–N2 | 75.04(10) | 74.65(15) |
| N1–Co1–N3 | 148.78(10) | 146.73(15) |
| N2–Co1–N3 | 75.12(10) | 74.46(14) |
| N1–Co1–Cl2 | 97.55(7) | 95.57(13) |
| N2–Co1–Cl2 | 103.83(7) | 104.87(13) |
| N3–Co1–Cl2 | 98.25(8) | 104.02(11) |
| N1–Co1–Cl1 | 98.68(7) | 101.17(12) |
| N2–Co1–Cl1 | 136.03(8) | 142.33(13) |
| N3–Co1–Cl1 | 96.39(8) | 95.72(11) |
| Cl2–Co1–Cl1 | 120.13(5) | 112.79(6) |
| Entry | Precat. | T, °C | t, min | Al/Co | PE, g | Activity b | Mwc | Mw/Mnc | Tm, oC d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Co1 | 30 | 30 | 2000 | 4.09 | 5.44 | 5.02 | 5.52 | 123.8 |
| 2 | Co1 | 40 | 30 | 2000 | 4.78 | 6.38 | 4.55 | 5.07 | 124.8 |
| 3 | Co1 | 50 | 30 | 2000 | 0.62 | 0.82 | 3.53 | 3.70 | 124.4 |
| 4 | Co1 | 60 | 30 | 2000 | 0.54 | 0.72 | 2.29 | 2.54 | 124.6 |
| 5 | Co1 | 70 | 30 | 2000 | - | - | - | - | - |
| 6 | Co1 | 40 | 30 | 2250 | 5.36 | 7.14 | 1.42 | 1.75 | 122.4 |
| 7 | Co1 | 40 | 30 | 2500 | 5.52 | 7.36 | 2.72 | 1.85 | 122.1 |
| 8 | Co1 | 40 | 30 | 2750 | 4.23 | 5.64 | 1.63 | 1.86 | 123.1 |
| 9 | Co1 | 40 | 30 | 3000 | 3.73 | 4.97 | 1.98 | 2.13 | 124.4 |
| 10 | Co1 | 40 | 30 | 3500 | 0.19 | 0.25 | 1.72 | 1.84 | 124.7 |
| 11 | Co1 | 40 | 5 | 2500 | 1.86 | 14.93 | 2.42 | 2.60 | 124.8 |
| 12 | Co1 | 40 | 15 | 2500 | 3.92 | 10.45 | 2.62 | 3.43 | 122.9 |
| 13 | Co1 | 40 | 45 | 2500 | 6.38 | 5.67 | 2.85 | 3.56 | 121.9 |
| 14 | Co1 | 40 | 60 | 2500 | 8.06 | 5.37 | 3.12 | 4.17 | 122.7 |
| 15 e | Co1 | 40 | 30 | 2500 | 3.99 | 5.32 | 2.04 | 2.42 | 121.9 |
| 16 f | Co1 | 40 | 30 | 2500 | 0.20 | 0.26 | 1.89 | 2.22 | 124.8 |
| Entry | Precat. | T, °C | t, min | Al/Co | PE, g | Activity b | Mwc | Mw/Mnc | Tm, °C d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Co1 | 30 | 30 | 2000 | 0.64 | 0.86 | 4.70 | 4.57 | 124.5 |
| 2 | Co1 | 40 | 30 | 2000 | 2.73 | 3.63 | 3.20 | 4.32 | 122.7 |
| 3 | Co1 | 50 | 30 | 2000 | 1.72 | 2.29 | 2.17 | 2.83 | 123.5 |
| 4 | Co1 | 60 | 30 | 2000 | 1.69 | 2.26 | 1.23 | 1.70 | 121.9 |
| 5 | Co1 | 70 | 30 | 2000 | 1.65 | 2.20 | 1.07 | 1.72 | 120.6 |
| 6 | Co1 | 40 | 30 | 1000 | 3.06 | 4.08 | 1.23 | 2.06 | 122.4 |
| 7 | Co1 | 40 | 30 | 1250 | 3.20 | 4.25 | 1.34 | 1.82 | 122.5 |
| 8 | Co1 | 40 | 30 | 1500 | 3.46 | 4.61 | 1.24 | 2.34 | 122.5 |
| 9 | Co1 | 40 | 30 | 1750 | 4.10 | 5.46 | 1.17 | 2.26 | 121.8 |
| 10 | Co1 | 40 | 30 | 2500 | 0.02 | 0.02 | 1.79 | 2.89 | 123.2 |
| 11 | Co1 | 40 | 5 | 1750 | 2.11 | 16.93 | 1.00 | 1.89 | 121.8 |
| 12 | Co1 | 40 | 15 | 1750 | 3.29 | 8.77 | 1.09 | 1.80 | 121.3 |
| 13 | Co1 | 40 | 45 | 1750 | 4.83 | 4.29 | 3.12 | 1.77 | 122.3 |
| 14 | Co1 | 40 | 60 | 1750 | 4.99 | 3.33 | 4.39 | 4.79 | 123.7 |
| 15 e | Co1 | 40 | 30 | 1750 | 3.10 | 4.13 | 1.00 | 1.90 | 121.5 |
| 16 f | Co1 | 40 | 30 | 1750 | 0.95 | 1.26 | 0.78 | 1.64 | 112.5 |
| Entry | Co-Cat. | Precat. | Al/Co | PE, g | Activity b | Mwc | Mw/Mnc | Tm, °C d |
|---|---|---|---|---|---|---|---|---|
| 1 | Co1 | MAO | 2500 | 5.52 | 7.36 | 2.72 | 1.85 | 122.1 |
| 2 | Co2 | MAO | 2500 | 3.42 | 4.56 | 3.04 | 3.35 | 125.7 |
| 3 | Co3 | MAO | 2500 | 1.02 | 1.36 | 85.02 | 15.22 | 134.9 |
| 4 | Co4 | MAO | 2500 | 3.65 | 4.86 | 2.95 | 2.33 | 124.6 |
| 5 | Co5 | MAO | 2500 | - | - | - | - | - |
| 6 | Co1 | MMAO | 1750 | 4.10 | 5.46 | 1.17 | 2.26 | 121.8 |
| 7 | Co2 | MMAO | 1750 | 2.30 | 3.06 | 4.38 | 3.04 | 125.4 |
| 8 | Co3 | MMAO | 1750 | 1.89 | 2.53 | 79.85 | 20.72 | 131.6 |
| 9 | Co4 | MMAO | 1750 | 3.40 | 4.53 | 3.61 | 4.28 | 123.5 |
| 10 | Co5 | MMAO | 1750 | - | - | - | - | - |
| Complex No. | Descriptors | Activity (106 g·mol−1·h−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| F | Q [e] | θ [°] | β [°] | ΔE [kcal/mol] | Δε1 [kcal/mol] | Δε2 [kcal/mol] | ||
| Co1 | 0.04 | 0.527 | 139.83 | 147.32 | 9.72 | 64.38 | 88.10 | 7.37 |
| Co2 | 0 | 0.507 | 129.32 | 141.01 | 6.61 | 71.53 | 88.79 | 4.56 |
| Co3 | 0.16 | 0.493 | 115.98 | 142.17 | 11.86 | 71.59 | 89.11 | 1.36 |
| Co4 | 0.02 | 0.520 | 132.99 | 144.12 | 7.10 | 70.03 | 90.86 | 4.86 |
| Co1 | Co4 | |
|---|---|---|
| CCDC No. | 2,182,734 | 2,182,735 |
| Empirical formula | C51H43Cl2CoN3O2 | C53H47Cl2CoN3O2 |
| Formula weight | 859.76 | 887.81 |
| Temperature/K | 170.01 (10) | 169.99 (15) |
| Crystal system | monoclinic | orthorhombic |
| Space group | P21/n | Pbca |
| a/Å | 16.0661 (2) | 15.6912 (2) |
| b/Å | 16.2364 (2) | 19.7010 (2) |
| c/Å | 19.4457 (3) | 30.6689 (3) |
| α/° | 90 | 90 |
| β/° | 113.773 (2) | 90 |
| γ/° | 90 | 90 |
| Volume/Å3 | 4642.11 (13) | 9480.75 (18) |
| Z | 4 | 8 |
| DCalcd (g/cm3) | 1.352 | 1.363 |
| μ/mm−1 | 5.359 | 5.263 |
| F(000) | 1956.0 | 4040.0 |
| Crystal size/mm3 | 0.5 × 0.39 × 0.21 | 2.2 × 1.1 × 0.6 |
| Radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2 θ range for data collection/° | 6.06 to 150.944 | 5.764 to 151.426 |
| Index ranges | −19 ≤ h ≤ 20, −20 ≤ k ≤ 16, −24 ≤ l ≤ 23 | −19 ≤ h ≤ 19, −23 ≤ k ≤ 11, −38 ≤ l ≤ 37 |
| Reflections collected | 33,571 | 74,220 |
| Independent reflections | 9252 [Rint = 0.0444, Rsigma = 0.0372] | 9599 [Rint = 0.0632, Rsigma = 0.0333] |
| Data/restraints/parameters | 9252/0/565 | 9599/0/583 |
| Goodness-of-fit on F2 | 1.021 | 1.272 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0592, wR2 = 0.1473 | R1 = 0.0950, wR2 = 0.2955 |
| Final R indexes [all data] | R1 = 0.0760, wR2 = 0.1623 | R1 = 0.1155, wR2 = 0.3223 |
| Largest diff. peak/hole/e Å−3 | 0.75/−0.83 | 0.85/−1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, A.A.; Meraz, M.M.; Yang, W.; Zhang, Q.; Sage, D.D.; Sun, W.-H. Catalytic Performance of Cobalt(II) Polyethylene Catalysts with Sterically Hindered Dibenzopyranyl Substituents Studied by Experimental and MLR Methods. Molecules 2022, 27, 5455. https://doi.org/10.3390/molecules27175455
Malik AA, Meraz MM, Yang W, Zhang Q, Sage DD, Sun W-H. Catalytic Performance of Cobalt(II) Polyethylene Catalysts with Sterically Hindered Dibenzopyranyl Substituents Studied by Experimental and MLR Methods. Molecules. 2022; 27(17):5455. https://doi.org/10.3390/molecules27175455
Chicago/Turabian StyleMalik, Arfa Abrar, Md Mostakim Meraz, Wenhong Yang, Qiuyue Zhang, Desalegn Demise Sage, and Wen-Hua Sun. 2022. "Catalytic Performance of Cobalt(II) Polyethylene Catalysts with Sterically Hindered Dibenzopyranyl Substituents Studied by Experimental and MLR Methods" Molecules 27, no. 17: 5455. https://doi.org/10.3390/molecules27175455
APA StyleMalik, A. A., Meraz, M. M., Yang, W., Zhang, Q., Sage, D. D., & Sun, W.-H. (2022). Catalytic Performance of Cobalt(II) Polyethylene Catalysts with Sterically Hindered Dibenzopyranyl Substituents Studied by Experimental and MLR Methods. Molecules, 27(17), 5455. https://doi.org/10.3390/molecules27175455










