Structural Investigation of Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin: A Spectroscopic Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
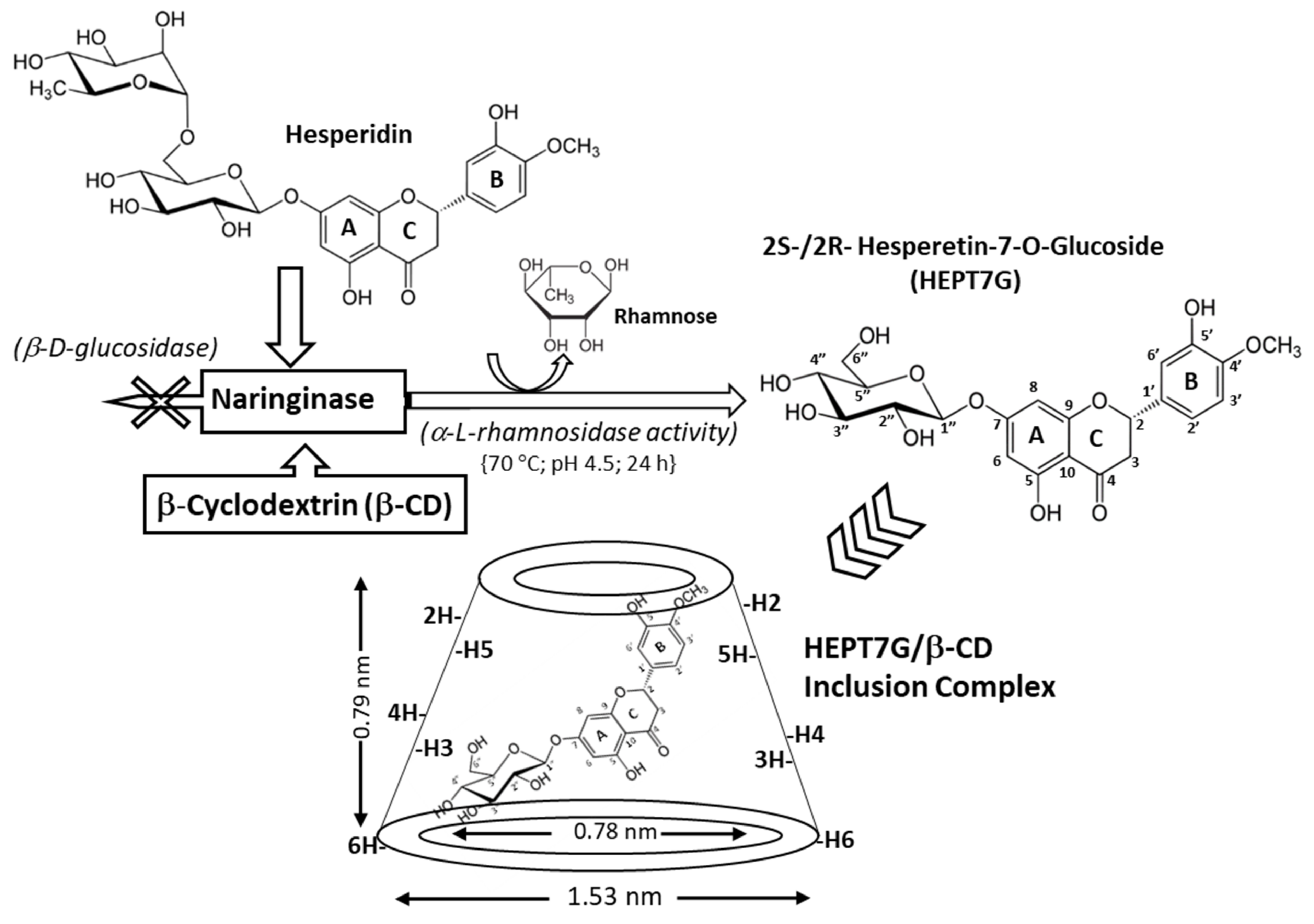
2.2. Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin
2.3. Characterization Methods of Hesperetin-7-O-Glucoside/β-Cyclodextrin Inclusion Complex
2.3.1. Vibrational Spectroscopy
2.3.2. Nuclear Magnetic Resonance Spectroscopy (NMR Analysis)
2.3.3. Thermal Analysis
2.3.4. UV–Visible Spectroscopy
2.3.5. X-Ray Powder Diffraction (PXRD)
2.3.6. Liquid Chromatography–Mass Spectrometry (LC-MS/MS)
2.3.7. Scanning Electron Microphotographs
2.3.8. Zeta Potential (ζ)
2.3.9. Solubility Determination and Phase–Solubility of HEPT7G/β CD Inclusion Complex
2.3.10. High-Performance Liquid Chromatography (HPLC)
3. Results and Discussion
3.1. Vibrational Spectral Change Analysis of Inclusion Complex Formation
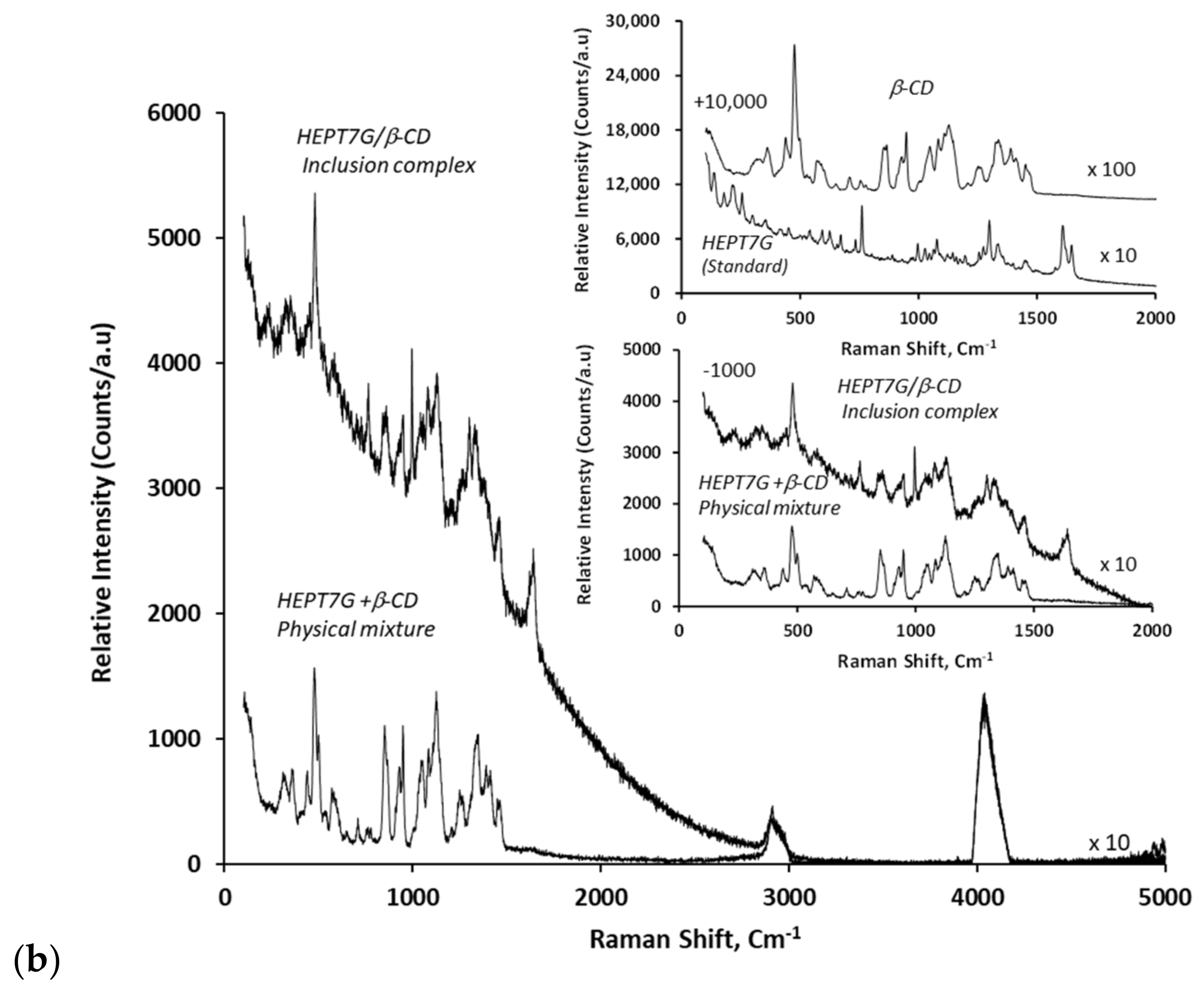
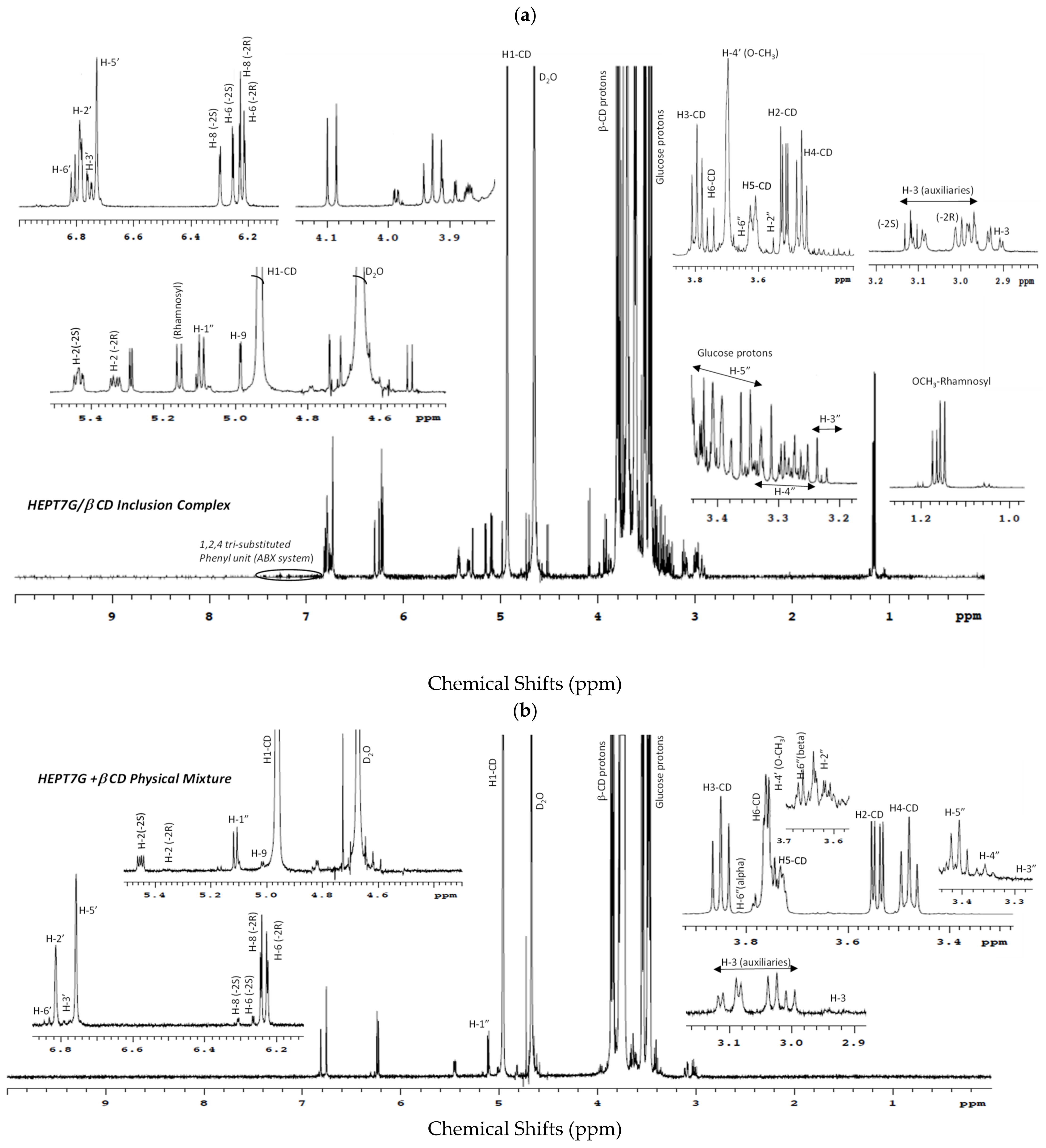
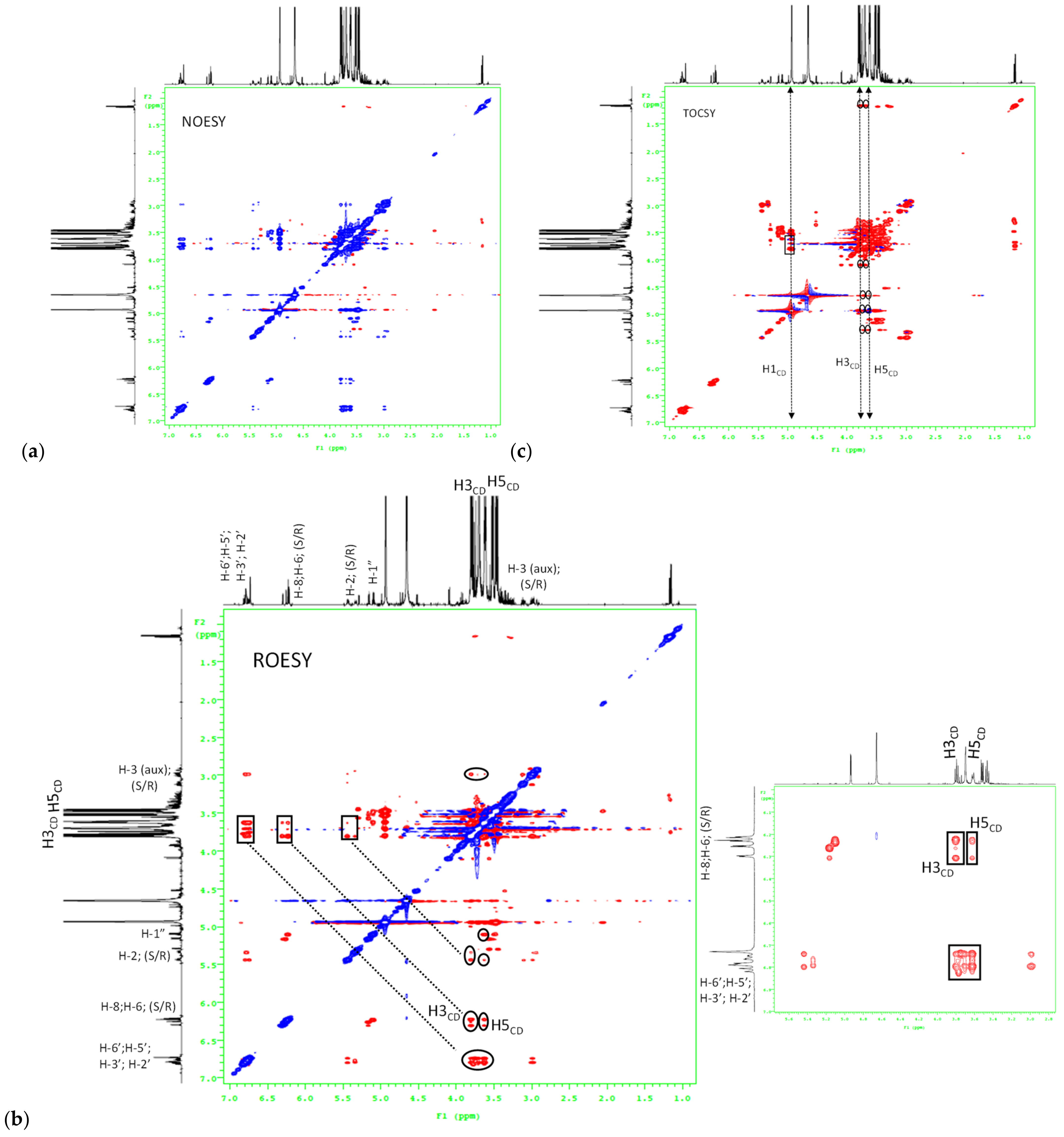
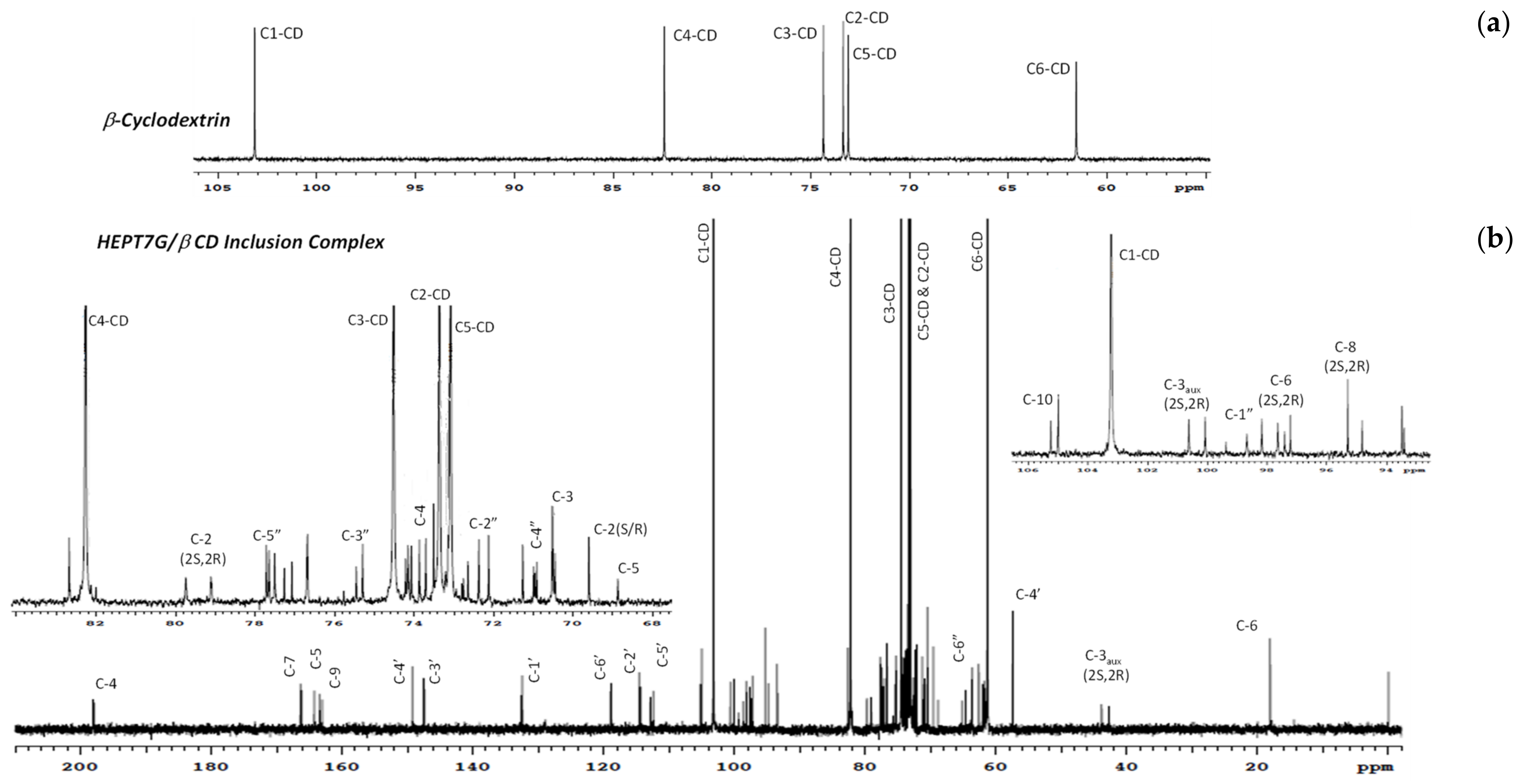
3.2. Inclusion Complex Formation Study by Nuclear Magnetic Resonance Analysis
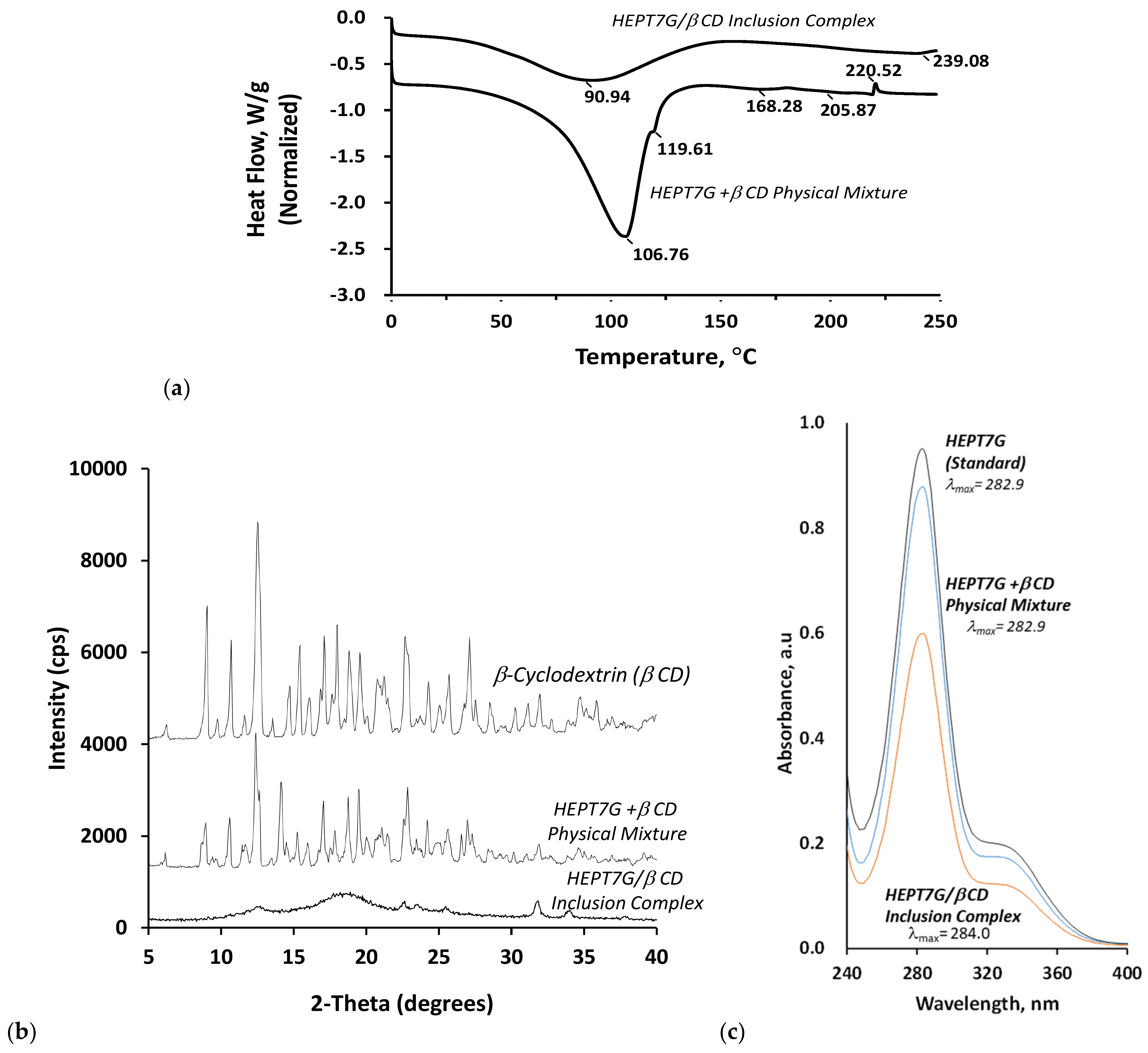
3.3. Thermal Analysis of the Complexation
3.4. X-Ray Crystallography Studies of Complexation
3.5. UV–Vis Spectroscopy Analysis of Complexation
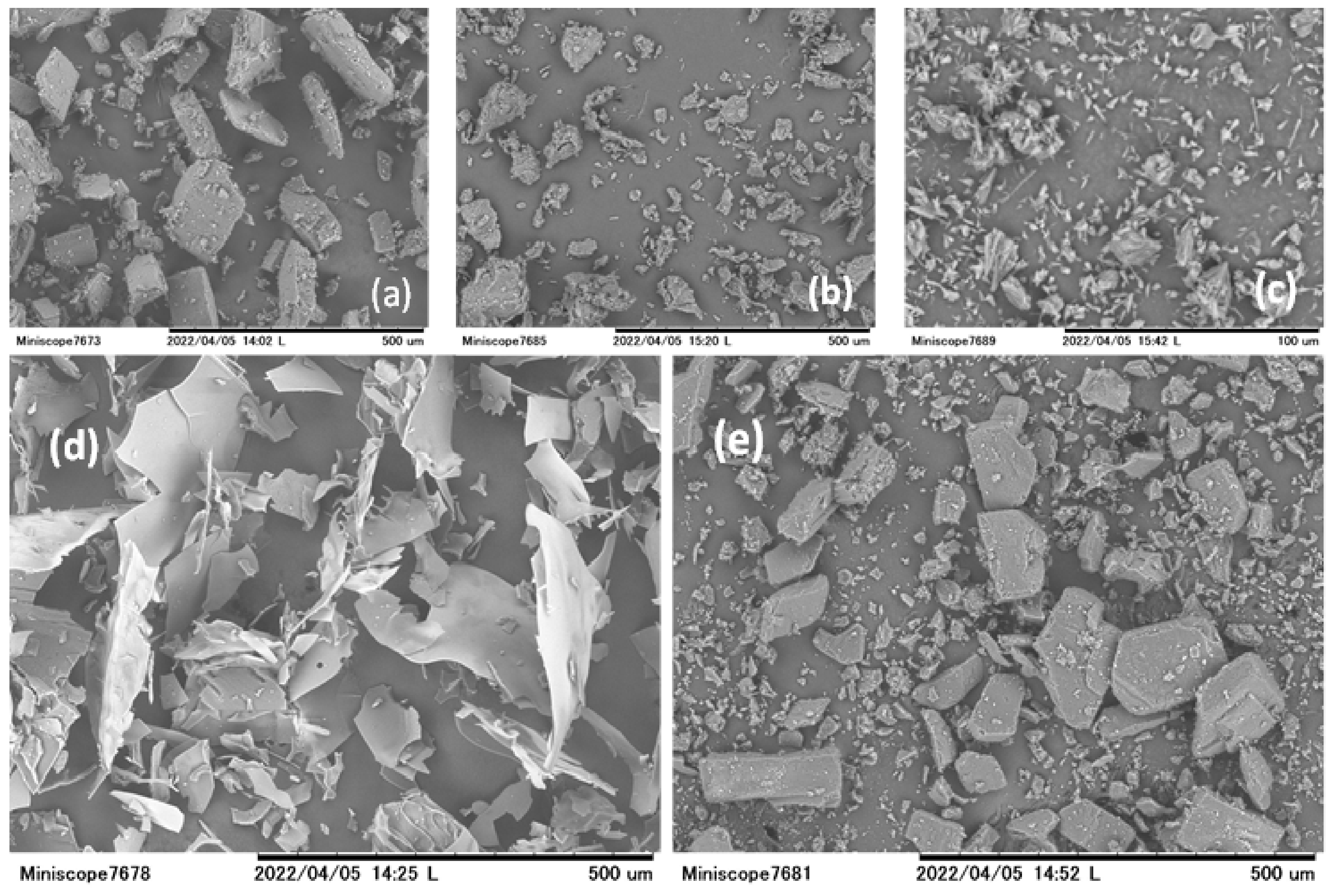
3.6. Scanning Electron Microscopic Analysis
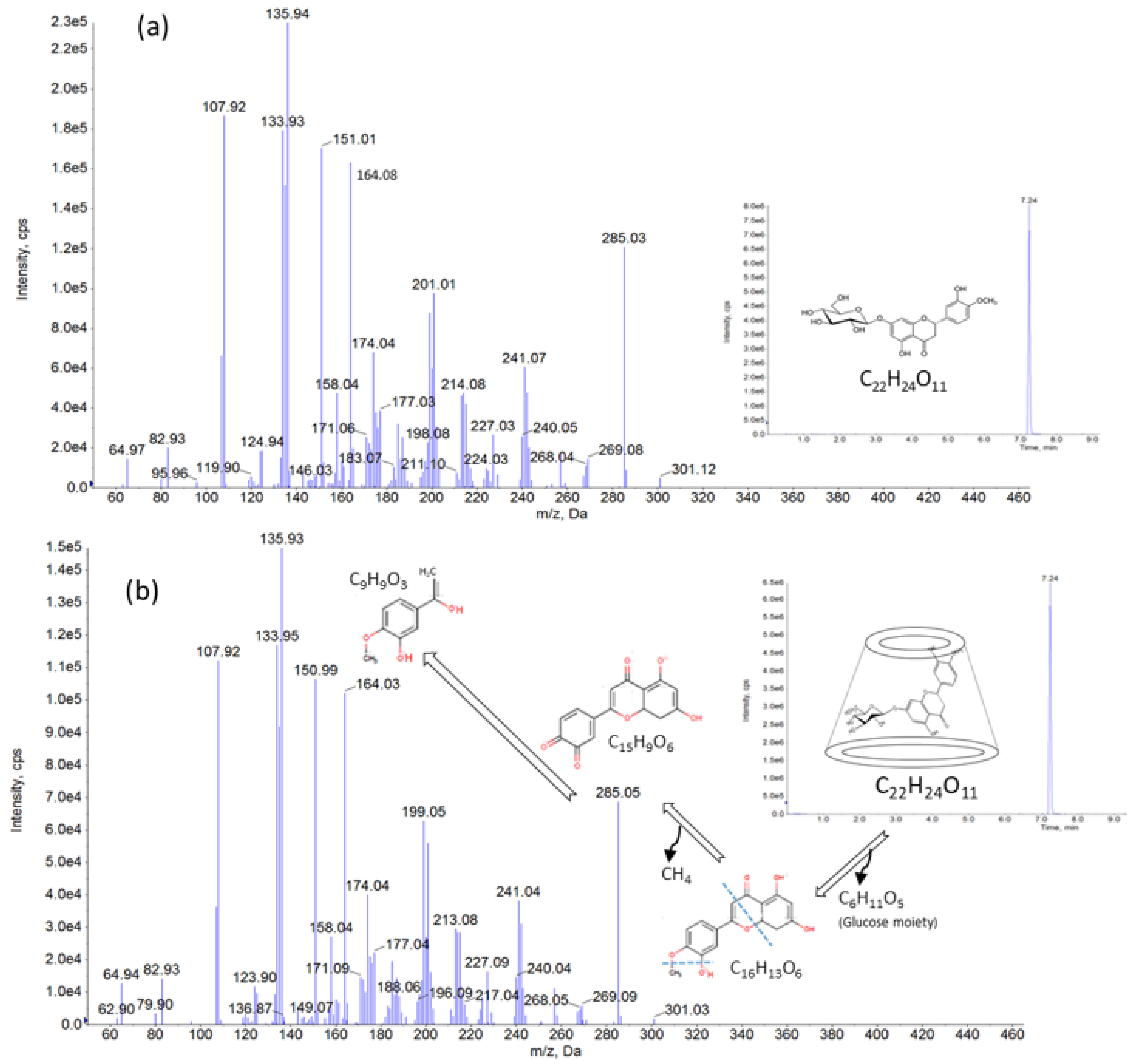
3.7. LC– MS/MS Study Results
3.8. The Zeta Potential Studies
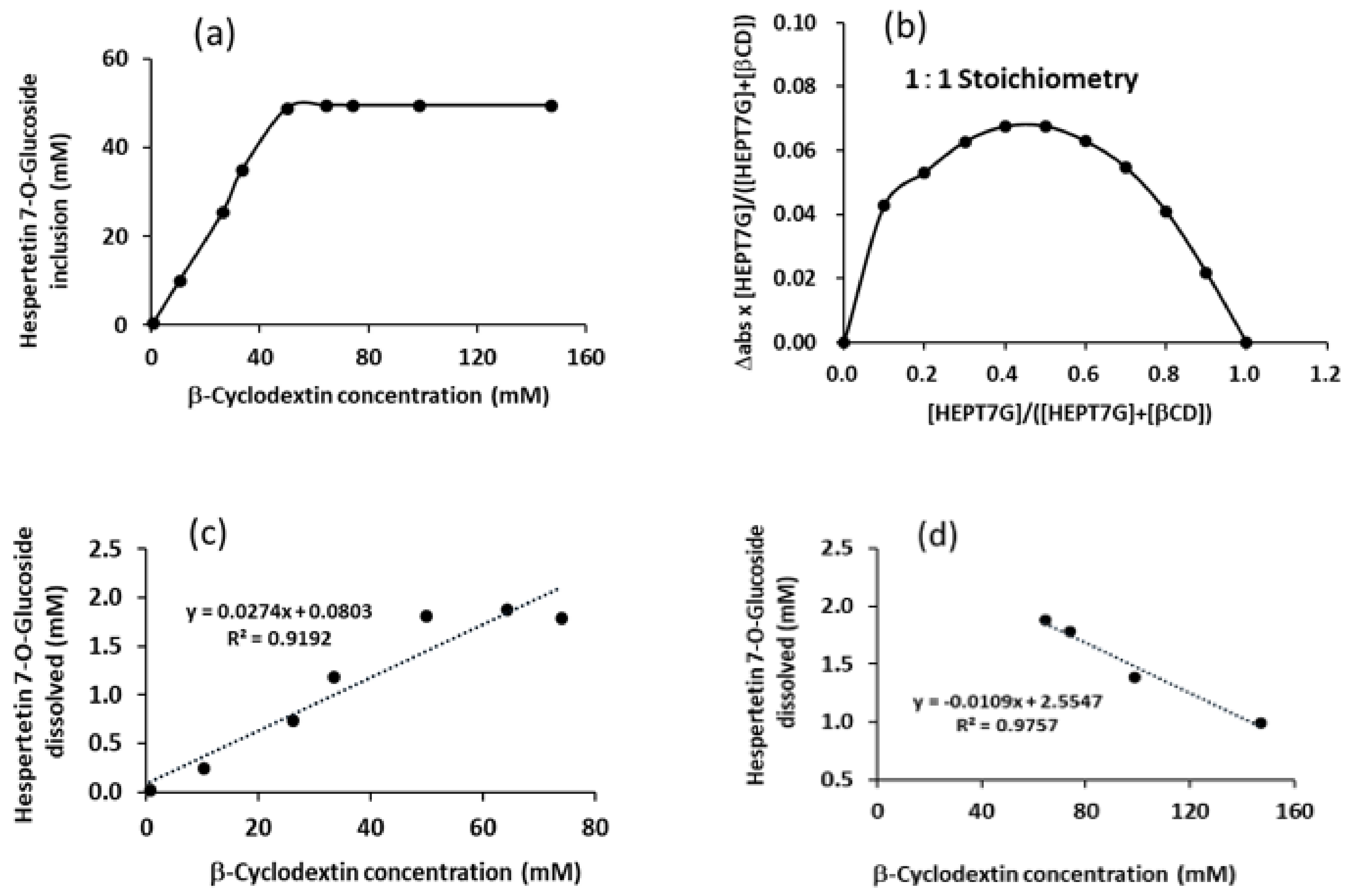
3.9. Solubility Measurement, Job’s Plot, and Phase–Solubility Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Ma, S.; Ren, D. Phytochemistry and bioactivity of Citrus flavonoids: A focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017, 16, 479–511. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, H.; Wen, X.; Ho, C.T.; Li, S. Citrus flavonoids and the intestinal barrier: Interactions and effects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 225–251. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Deng, W.; Fang, X.; Wu, J. Flavonoids function as antioxidants: By scavenging reactive oxygen species or by chelating iron? Radiat. Phys. Chem. 1997, 50, 271–276. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus Polyphenol Hesperidin Stimulates Production of Nitric Oxide in Endothelial Cells while Improving Endothelial Function and Reducing Inflammatory Markers in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef] [PubMed]
- Corciova, A.; Ciobanu, C.; Poiata, A.; Mircea, C.; Nicolescu, A.; Drobota, M.; Varganici, C.D.; Pinteala, T.; Marangoci, N.N. Antibacterial and antioxidant properties of hesperidin: β-cyclodextrin complexes obtained by different techniques. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 71–84. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Casimeer, S.C.; Ghidan, A.Y.; Al Antary, T.M.; Singaravelu, A. Exploration of Antioxidant, Antibacterial Activities of Green Synthesized Hesperidin Loaded PLGA Nanoparticles. Biointerface Res. Appl. Chem 2021, 11, 14520–14528. [Google Scholar]
- Galati, E.M.; Monforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Trovato, A.; Tripodo, M.M. Biological effects of hesperidin, a citrus flavonoid. (Note I): Antiinflammatory and analgesic activity. Farmaco 1994, 40, 709–712. [Google Scholar] [PubMed]
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Tanaka, T.; Ogawa, H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 1997, 18, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.Z.; Kong, L.L.; Chen, N.H. Hesperidin. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018; pp. 81–86. [Google Scholar]
- Souza, L.C.; de Gomes, M.G.; Goes, A.T.; Del Fabbro, L.; Carlos Filho, B.; Boeira, S.P.; Jesse, C.R. Evidence for the involvement of the serotonergic 5-HT1A receptors in the antidepressant-like effect caused by hesperidin in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 103–109. [Google Scholar] [CrossRef]
- Monforte, M.T.; Trovato, A.; Kirjavainen, S.; Forestieri, A.M.; Galati, E.M.; Curto, R.B.L. Biological effects of hesperidin, a Citrus flavonoid. (note II): Hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco 1995, 50, 595–599. [Google Scholar]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.-X.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent For Obesity. Drug Des. Dev. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Uehara, M.; Wu, J.; Wang, X.; Masuyama, R.; Suzuki, K.; Kanazawa, K.; Ishimi, Y. Hesperidin, a Citrus Flavonoid, Inhibits Bone Loss and Decreases Serum and Hepatic Lipids in Ovariectomized Mice. J. Nutr. 2003, 133, 1892–1897. [Google Scholar] [CrossRef]
- Horcajada-Molteni, M.-N.; Crespy, V.; Coxam, V.; Davicco, M.-J.; Rémésy, C.; Barlet, J.-P. Rutin Inhibits Ovariectomy-Induced Osteopenia in Rats. J. Bone Miner. Res. 2000, 15, 2251–2258. [Google Scholar] [CrossRef]
- Sa’Ayinzat, F.E.; Bawa, E.K.; Ogwu, D.; Ayo, J.O. Hesperidin-Sources, chemistry, extraction, measurement and biologic effects on reproduction in animals: A review. Int. J. Vet. Sci. Anim. Husb. 2021, 6, 1–8. [Google Scholar]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2016, 57, 613–631. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of silver nano-particles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Hesperidin Supplementation Alleviates Oxidative DNA Damage and Lipid Peroxidation in Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytother. Res. 2017, 31, 1539–1545. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: A randomized controlled trial (Citrus study). Eur. J. Nutr. 2021, 60, 1277–1288. [Google Scholar] [CrossRef]
- Dimpfel, W. Different anticonvulsive effects of hesperidin and its aglycone hesperetin on electrical activity in the rat hip-pocampus in-vitro. J. Pharm. Pharmacol. 2006, 58, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Yen, G.C. Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Wilson, L.; Jordan, M.A.; Nguyen, V.; Tang, J.; Smiyun, G. Hesperidin suppressed proliferations of both Human breast cancer and androgen-dependent prostate cancer cells. Phytotherapy Res. 2009, 24, S15–S19. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Kumar, A. Hesperidin pre-treatment attenuates NO-mediated cerebral ischemic reperfusion injury and memory dysfunction. Pharmacol. Rep. 2010, 62, 635–648. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shadboorestan, A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer 2015, 68, 29–39. [Google Scholar] [CrossRef]
- Aranganathan, S.; Selvam, J.P.; Nalini, N. Effect of hesperetin, a citrus flavonoid, on bacterial enzymes and carcino-gen-induced aberrant crypt foci in colon cancer rats: A dose-dependent study. J. Pharm. Pharmacol. 2008, 60, 1385–1392. [Google Scholar] [CrossRef]
- Kaur, G.; Tirkey, N.; Chopra, K. Beneficial effect of hesperidin on lipopolysaccharide-induced hepatotoxicity. Toxicology 2006, 226, 152–160. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tanabe, S.; Sugiyama, M.; Konishi, Y. Transepithelial transport of hesperetin and hesperidin in intestinal Caco-2 cell monolayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 33–41. [Google Scholar] [CrossRef]
- Loscalzo, L.M.; Wasowski, C.; Paladini, A.C.; Marder, M. Opioid receptors are involved in the sedative and antinociceptive effects of hesperidin as well as in its potentiation with benzodiazepines. Eur. J. Pharmacol. 2008, 580, 306–313. [Google Scholar] [CrossRef]
- Loscalzo, L.M.; Yow, T.T.; Wasowski, C.; Chebib, M.; Marder, M. Hesperidin induces antinociceptive effect in mice and its aglicone, hesperetin, binds to μ-opioid receptor and inhibits GIRK1/2 currents. Pharmacol. Biochem. Behav. 2011, 99, 333–341. [Google Scholar] [CrossRef]
- Kumar, A.; Lalitha, S.; Mishra, J. Possible nitric oxide mechanism in the protective effect of hesperidin against pentylene-tetrazole (PTZ)-induced kindling and associated cognitive dysfunction in mice. Epilepsy Behav. 2013, 29, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wasowski, C.; Loscalzo, L.M.; Higgs, J.; Marder, M. Chronic intraperitoneal and oral treatments with hesperidin induce central nervous system effects in mice. Phytother. Res. 2012, 26, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y.; et al. Hesperidin is a potential inhibitor against SARS-CoV-2 infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, K.; Manthey, J.A.; Cameron, R. Acid-catalyzed hydrolysis of hesperidin at elevated temperatures. Carbohydr. Res. 2000, 328, 141–146. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef]
- Hollman, P.C. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Habauzit, V.; Nielsen, I.-L.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Chee, W.; Barron, D.; Lebecque, P.; Davicco, M.-J.; Williamson, G.; et al. Increased bioavailability of hesperetin-7-glucoside compared with hesperidin results in more efficient prevention of bone loss in adult ovariectomised rats. Br. J. Nutr. 2009, 102, 976–984. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Chee, W.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.-N.; Williamson, G. Bioavailability Is Improved by Enzymatic Modification of the Citrus Flavonoid Hesperidin in Humans: A Randomized, Double-Blind, Crossover Trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef]
- Furtado, A.F.; Nunes, M.A.; Ribeiro, M.H. Hesperidinase encapsulation towards hesperitin production targeting improved bioavailability. J. Mol. Recognit. 2012, 25, 595–603. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Dew, T.P.; Lévèques, A.; Pereira-Caro, G.; Rein, M.; Teml, A.; Schäfer, C.; Hofmann, U.; Schwab, M.; Eichelbaum, M. Gastrointestinal absorption and metabolism of hesperetin-7-O-rutinoside and hesperetin-7-O-glucoside in healthy humans. Mol. Nutr. Food Res. 2015, 59, 1651–1662. [Google Scholar] [CrossRef]
- Wan, W.; Xia, N.; Zhu, S.; Liu, Q.; Gao, Y. A Novel and High-Effective Biosynthesis Pathway of Hesperetin-7-O-Glucoside Based on the Construction of Immobilized Rhamnosidase Reaction Platform. Front. Bioeng. Biotechnol. 2020, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2017, 98, 2422–2427. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Taulier, N.; Chalikian, T.V. Hydrophobic Hydration in Cyclodextrin Complexation. J. Phys. Chem. B 2006, 110, 12222–12224. [Google Scholar] [CrossRef] [PubMed]
- Koontz, J.L.; Marcy, J.E.; O’Keefe, S.F.; Duncan, S.E. Cyclodextrin inclusion complex formation and solid-state charac-terization of the natural antioxidants α-tocopherol and quercetin. J. Agric. Food Chem. 2009, 57, 1162–1171. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion com-plexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Hazra, S.; Kumar, G.S. Physicochemical properties of inclusion complexes of sanguinarine with natural cyclodextrins: Spec-troscopy, calorimetry and NMR studies. RSC Adv. 2015, 5, 1873–1882. [Google Scholar] [CrossRef]
- Cai, W.; Sun, T.; Shao, X.; Chipot, C. Can the anomalous aqueous solubility of β-cyclodextrin be explained by its hydration free energy alone? Phys. Chem. Chem. Phys. 2008, 10, 3236–3243. [Google Scholar] [CrossRef]
- Ficarra, R.; Tommasini, S.; Raneri, D.; Calabro, M.L.; Di Bella, M.R.; Rustichelli, C.; Gamberini, M.C.; Ficarra, P. Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J. Pharm. Biomed. Anal. 2002, 29, 1005–1014. [Google Scholar] [CrossRef]
- Tommasini, S.; Calabrò, M.; Stancanelli, R.; Donato, P.; Costa, C.; Catania, S.; Villari, V.; Ficarra, P. The inclusion complexes of hesperetin and its 7-rhamnoglucoside with (2-hydroxypropyl)-β-cyclodextrin. J. Pharm. Biomed. Anal. 2005, 39, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Corciova, A.; Ciobanu, C.; Poiata, A.; Nicolescu, A.; Drobota, M.; Varganici, C.D.; Pinteala, T.; Fifere, A.; Marangoci, N.; Mircea, C. Inclusion complexes of hesperidin with hydroxypropyl-β-cyclodextrin. Physico-chemical characterization and biological assessment. Dig. J. Nanomater. Biostruct. 2014, 9, 1623–1637. [Google Scholar]
- Sangpheak, W.; Kicuntod, J.; Schuster, R.; Rungrotmongkol, T.; Wolschann, P.; Kungwan, N.; Viernstein, H.; Mueller, M.; Pongsawasdi, P. Physical properties and biological activities of hesperetin and naringenin in complex with methylated β-cyclodextrin. Beilstein J. Org. Chem. 2015, 11, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellán, C.; Pérez-Abril, M.; Castillo, J.; Serrano, A.; Mercader, M.T.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of temperature, pH, β-and HP-β-cds on the solubility and stability of flavanones: Naringenin and hesperetin. LWT 2019, 108, 233–239. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, M.; Kumoi, K.; Ozeki, M. Method for producing flavonoid inclusion compound. U.S. Patent 10,519,182, 31 December 2019. [Google Scholar]
- Dos Santos, L.; Shanmugam, S.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Araújo, A.A.D.S. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Moriwaki, M.; Ozeki, M.; Timm, D. Structural elucidation of novel isoquercitrin-γ-cyclodextrin (IQC-γCD) molecular inclusion complexes of potential health benefits. Carbohydr. Polym. Technol. Appl. 2021, 2, 100046. [Google Scholar] [CrossRef]
- Fossen, T.; Andersen, Ø.M. Spectroscopic Techniques Applied to Flavonoids. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; Taylor and Francis CRC Press: Boca Raton, FL, USA, 2005; pp. 37–142. [Google Scholar] [CrossRef]
- Maltese, F.; Erkelens, C.; van der Kooy, F.; Choi, Y.H.; Verpoorte, R. Identification of natural epimeric flavanone gly-cosides by NMR spectroscopy. Food Chem. 2009, 116, 575–579. [Google Scholar] [CrossRef]
- Markham, K.R.; Ternai, B. 13C NMR of flavonoids—II: Flavonoids other than flavone and flavonol aglycones. Tetrahedron 1976, 32, 2607–2612. [Google Scholar] [CrossRef]
- Markham, K.R.; Chari, V.M. Carbon-13 NMR Spectroscopy of Flavonoids. In The Flavonoid; Springer: Boston, MA, USA; pp. 19–134.
- Yang, B.; Lin, J.; Chen, Y.; Liu, Y. Artemether/hydroxypropyl-β-cyclodextrin host–guest system: Characterization, phase-solubility and inclusion mode. Bioorganic Med. Chem. 2009, 17, 6311–6317. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.C.; Rousseau, A.; Fillet, M.; Lomsadze, K.; Salgado, A.; Crommen, J.; Chankvetadze, B. Capillary electrophoretic and nuclear magnetic resonance studies on the opposite affinity pattern of propranolol enantiomers towards various cyclodextrins. J. Sep. Sci. 2010, 33, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Ma, S.X.; Zhou, S.Y.; Chen, W.; Yuan, M.W.; Yin, Y.Q.; Yang, X.D. Preparation and characterization of inclu-sion complexes of naringenin with β-cyclodextrin or its derivative. Carbohydr. Polym. 2013, 98, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, E.; Bocian, W.; Poznański, J.; Sitkowski, J.; Sadlej-Sosnowska, N.; Kozerski, L. Complexation of steroid hormones: Prednisolone, ethinyloestradiol and estriol with β-cyclodextrin. An aqueous 1H NMR study. J. Chem. Soc. Perkin Trans. 2002, 2, 999–1004. [Google Scholar] [CrossRef]
- Correia, I.; Bezzenine, N.; Ronzani, N.; Platzer, N.; Beloeil, J.C.; Doan, B.T. Study of inclusion complexes of acridine with β-and (2, 6-di-O-methyl) -β-cyclodextrin by use of solubility diagrams and NMR spectroscopy. J. Phys. Org. Chem. 2002, 15, 647–659. [Google Scholar] [CrossRef]
- Calabrò, M.; Tommasini, S.; Donato, P.; Stancanelli, R.; Raneri, D.; Catania, S.; Costa, C.; Villari, V.; Ficarra, P. The rutin/β-cyclodextrin interactions in fully aqueous solution: Spectroscopic studies and biological assays. J. Pharm. Biomed. Anal. 2005, 36, 1019–1027. [Google Scholar] [CrossRef]
- Du, Z.P.; Suo, Q.L.; Zhang, X.Y.; Zhang, L.W.; Wei, X.H. Spectral studies of the interaction between sanguinarine and guano-sine. Chin. Chem. Lett. 2008, 19, 1465–1469. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Wu, Y.; Shuang, S.; Dong, C.; Choi, M.M. Luminescence and binding properties of two isoquinoline alkaloids chelerythrine and sanguinarine with ctDNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 95, 80–85. [Google Scholar] [CrossRef]
- US Department of health and Human Service, Food and Drug Administration (FDA). Guidance for Industry: Bioanalytical Method Validation, 2001; FDA: Silver Spring, MD, USA, 2001.
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef]
- Hadian, Z.; Maleki, M.; Abdi, K.; Atyabi, F.; Mohammadi, A.; Khaksar, R. Preparation and Characterization of Nanoparticle β-Cyclodextrin: Geraniol Inclusion Complexes. Iran J. Pharm. Res. 2018, 17, 39–51. [Google Scholar]
- Creteanu, A.; Pamfil, D.; Vasile, C.; Tantaru, G.; Ghiciuc, C.M.; Ochiuz, L.; Ghilan, A.; Macsim, A.M. Study on the Role of the Inclusion Complexes with 2-Hydroxypropyl-β-cyclodextrin for Oral Administration of Amiodarone. Int. J. Polym. Sci. 2019, 2019, 1695189. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–122. [Google Scholar]
- Del Valle, E. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Lula, I.S.; Sinisterra, R.D.; Bassani, V.L. Quercetin/β-cyclodextrin solid complexes prepared in aqueous solution followed by spray-drying or by physical mixture. Aaps Pharmscitech 2009, 10, 235–242. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Z.; Xiao, Z. Preparation, characterization, and thermal stability of β-cyclodextrin/soybean lecithin inclusion complex. Carbohydr. Polym. 2014, 101, 1027–1032. [Google Scholar] [CrossRef]
- Naidoo, K.J.; Chen, J.Y.J.; Jansson, J.L.; Widmalm, G.; Maliniak, A. Molecular properties related to the anomalous solubility of β-cyclodextrin. J. Phys. Chem. B 2004, 108, 4236–4238. [Google Scholar] [CrossRef]
- Szejtli, J. Utilization of cyclodextrins in industrial products and processes. J. Mater. Chem. 1997, 7, 575–587. [Google Scholar] [CrossRef]
- Gaffield, W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides: Determination of aglycone chirality in flavanone glycosides. Tetrahedron 1970, 26, 4093–4108. [Google Scholar] [CrossRef]
- Fujii, S.; Yamagata, Y.; Jin, G.Z.; Tomita, K.I. Novel molecular conformation of (R, S)-hesperetin in anhydrous crystal. Chem. Pharm. Bull. 1994, 42, 1143–1145. [Google Scholar] [CrossRef]
- Jadeja, Y.S.; Kapadiya, K.M.; Jebaliya, H.J.; Shah, A.K.; Khunt, R.C. Dihedral angle study in Hesperidin using NMR Spectroscopy. Org. Magn. Reson. 2017, 55, 589–594. [Google Scholar] [CrossRef]
- Moriwaki, M.; Kito, K.; Nakagawa, R.; Kapoor, M.P.; Matsumiya, Y.; Fukuhara, T.; Kamiya, U. Bioavailability comparison between a compound comprising hesperetin-7-glucoside with β-cyclodextrin and a mixture of hesperidin and dextrin in healthy adult human males. Biosci. Biotechnol. Biochem. 2021, 85, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
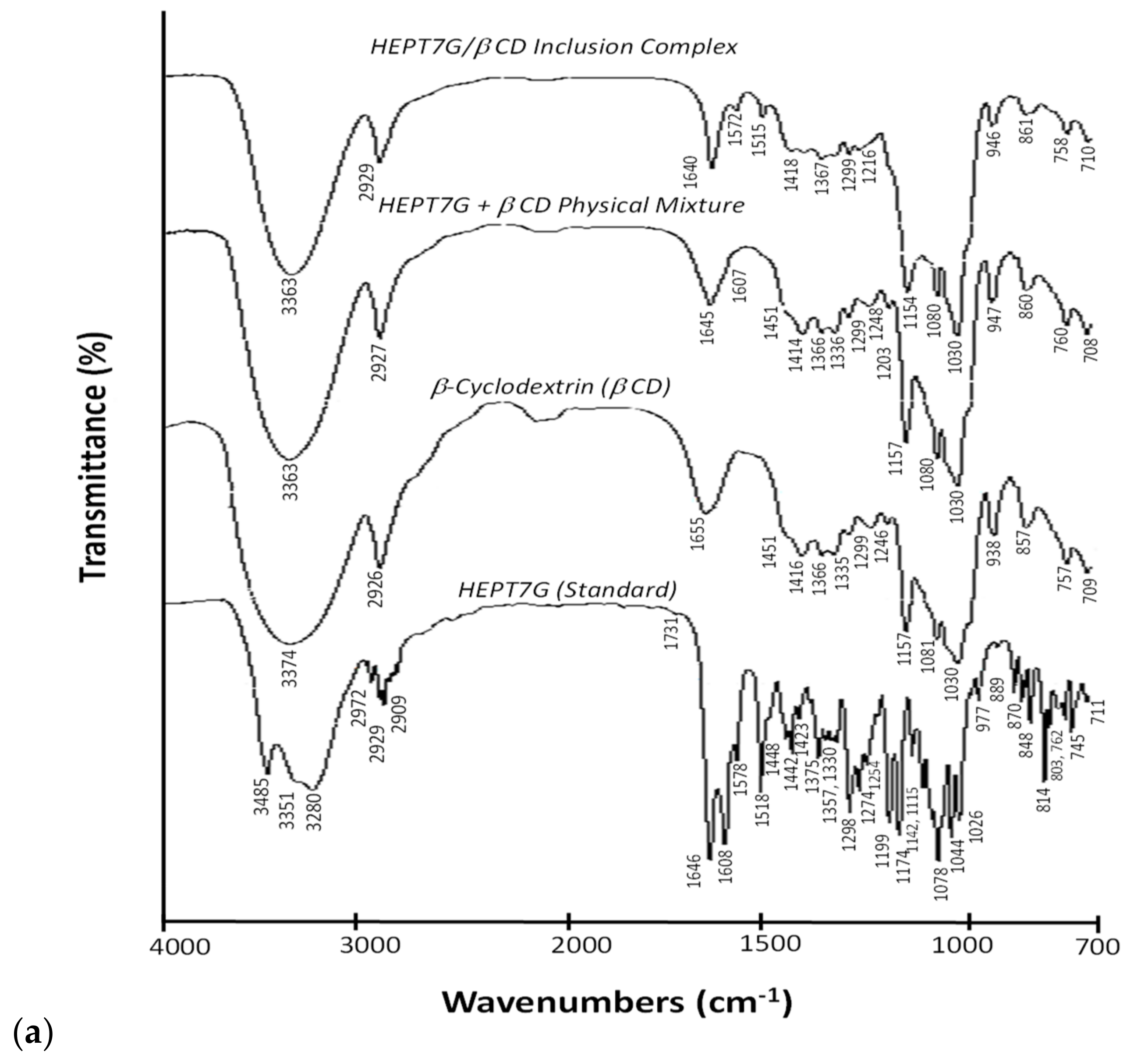
| FTIR Spectrum Wave Number (cm−1) | Description of Vibrational Regions | Raman Shift Wave Number (cm−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| β–CD | HEPT7G Standard | HEPT7G/βCD Inclusion Complex | HEPT7G+βCD Physical Mixture | Band Assignment of Vibrational Modes of Functional Groups | β–CD | HEPT7G Standard | HEPT7G/βCD Inclusion Complex | HEPT7G+βCD Physical Mixture |
| Vibrational band frequency region (4000–2500 cm−1) | ||||||||
| 3485 | Hydroxyl group (–OH) stretching vibration mode of hydrophobic activity of β-CD bridged system (symmetric and asymmetric phenolic). Primary –OH bonded to ring oxygen intermolecular or to each other and stretching vibration of secondary hydroxyl groups bonded to each other in a different way. | 4039 | 4035 | 4032 | 4040 | |||
| 3374 | 3351 | 3363 | 3363 | |||||
| 3260 | 3034 | |||||||
| 2972 | Aliphatic CH, CH2 stretching vibration mode (symmetric and asymmetric bonds); SP3 carbons. | 2975 | ||||||
| 2926 | 2929 | 2930 | 2928 | 2911 | 2928 | 2930 | 2938 | |
| 2909 | 2908 | 2905 | 2913 | |||||
| 2889, 2840 | ||||||||
| Vibrational band frequency region (1700–1200 cm−1) | ||||||||
| 1655 | Deformation band of adsorbed water, H-O-H; | |||||||
| 1646, 1608 | 1640 | 1645, 1607 | C-C vibration: stretching vibration of aromatic rings; C=O (carbonyls) and C=C conjugation in stretching mode; C=C bond of aromatic dihydroxphenyl ring. | 1646, 1609 | 1641, 1607 | 1646 | ||
| 1578, 1519 | 1579, 1515 | 1517 | 1576, 1485 | 1579, 1515 | ||||
| 1451 | 1448, 1442 | 1449 | 1451 | Distribution of C=C stretching bond of aromatic ring; asymmetric CH deformation vibrations. | 1451 | 1449 | 1460 | 1463, 1451 |
| 1416 | 1423 | 1418 | 1414 | C-O-H rocking stretching vibration or C-C-H, H-C-H and C-O-H modes of deformation; complex CH2OH-type vibrations. | 1412 | 1413 | 1404 | 1412, 1407 |
| 1366 | 1375, 1357 | 1367 | 1366 | 1390 | 1398, 1371 | 1390 | ||
| 1335 | 1329 | 1337 | 1336 | C-O-H bending vibration of heterocyclic bond or C-C stretching. | 1332 | 1334 | 1330 | 1349 |
| 1299 | 1298 | 1299 | 1300 | C-O-H bending vibration band; C-O-C of chromen structure. | 1298 | 1301 | ||
| 1274 | 1276 | 1274 | 1275 | |||||
| 1246 | 1254 | 1248 | 1253 | 1255 | 1256 | 1249 | ||
| Vibrational band frequency region (1200–1000 cm−1) | ||||||||
| 1202 | 1199 | 1200 | 1203 | Stretching vibration of C-O-H phenolic components. | 1211 | 1205, 1197, 1174 | 1204 | 1205 |
| 1157 | 1143 | 1155 | 1157 | C-O-C asymmetric stretching vibration coupled with bending C-O-H vibration mode. | 1158, 1145 | |||
| 1116 | 1127 | 1123 | 1129, 1120, 1115 | 1125, 1109 | ||||
| 1081 | 1078, 1044 | 1080 | 1080 | C-O-H stretching mode (alcoholic). | 1083 | 1078, 1068 | 1084 | |
| 1030 | 1026 | 1031 | 1030 | Overtones of C-H band with C-O-H stretching. | 1046 | 1045 | ||
| 1000 | 1000 | 1000 | 1010 | 1028 | ||||
| Vibrational band frequency region (1000–700 cm−1) | ||||||||
| 938 | 977 | 946 | 947 | Carbohydrates isolated components: Skeletal vibration involving linkage to fingerprint region. | 946 | 997, 976, 959 | 995, 949 | 947 |
| 889 | 885 | 926 | 929 | 927 | 928 | |||
| 858 | 871 | 861 | 860 | Wagging-type vibration modes of hydroxyl bonds directly linked at sugar rings. | 865 | 866 | 865 | |
| 848 | 853 | 851 | 850 | |||||
| 815, 803 | 803 | |||||||
| 758 | 762 | 758 | 760 | Fingerprint region vibration of the C=O (carbonyls), and C-C bonds of the β-CD and glucopyranosyl ring structure. | 776 | 760 | 764 | 776 |
| 746 | 754 | 734 | 762 | |||||
| 709 | 711 | 710 | 708 | 708 | 696, 672 | 708 | ||
| 651 | 655, 624 | 645 | ||||||
| 571 | 594, 542 | 560 | ||||||
| 475, 440 | 499, 452, 413 | 480 | 498, 477; 439 | |||||
| 361 | 353 | 375,351 | 357 | |||||
| 320 | 321, 299 | 319, 310 | ||||||
| 255, 215 | 252 | 256 | ||||||
| 1H NMR Description | Chemical Shifts, ppm | ||||||
|---|---|---|---|---|---|---|---|
| β–Cyclodextrin Protons | Peaks | β–CD | HEPT7G+βCD Physical Mixture (PM) | Δδ = δCD − δPM (ppm) | HEPT7G/βCD Inclusion complex (IC) | Δδ = δIC−δPM (ppm) | Δδ = δIC−δCD (ppm) |
| H1 | [d] | 4.954 (JH = 3.8) | 4.962 (JH = 3.8) | 0.008 | 4.934 (JH = 3.8) | −0.028 | −0.01 |
| H2 | [dd] | 3.533 (JH = 10.0) | 3.535 (JH = 10.0) | 0.002 | 3.518 (JH = 10.0) | −0.017 | −0.015 |
| H3 | [t] | 3.848 (JH = 9.6) | 3.851 (JH = 9.5) | 0.003 | 3.794 (JH = 9.6) | −0.057 | −0.054 |
| H4 | [t] | 3.467 (JH = 9.6) | 3.47 (JH = 9.4) | 0.003 | 3.463 (JH = 9.5) | −0.007 | −0.004 |
| H5 | [dt] | 3.747 (JH = 8.6) | 3.745 (JH = 8.3) | −0.002 | 3.619 (JH = 8.5) | −0.126 | −0.128 |
| H6 | [dd] | 3.778 (JH = 13.9) | 3.775 (JH = 12.7) | −0.003 | 3.75 (JH = 12.3) | −0.025 | −0.028 |
| 1H NMR Description | Chemical Shifts, ppm | 13C NMR Description | Chemical Shifts, ppm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flavanone ring Protons | Peaks | HEPT7G/βC Inclusion Complex (IC) | HEPT7G+βCD Physical Mixture (PM) | Δδ = δIC−δPM (ppm) | HEPT7G Standard | Hesperidin | Flavanone ring Carbons | Peaks | HEPT7G/βCD Inclusion Complex (IC) |
| H-8 (–2S) | [d] | 6.299 (JH = 2.4) | 6.307(JH = 2.6) | −0.008 | 6.177 | 6.286 | C-2 | [d], [s] | 69.606 and 79.427 |
| H-8 (–2R) | [d] | 6.229 (JH = 2.3) | 6.244(JH = 2.6) | −0.015 | nd | nd | C-3 | [d], [d] | 43.302 and 70.492 |
| H-6 (–2S) | [d] | 6.253 (JH = 2.1) | 6.267(JH = 2.0) | −0.014 | 6.177 | 6.275 | C-4 | [d], [s] | 73.178 and 197.99 |
| H-6 (–2R) | [d] | 6.213 (JH = 2.0) | 6.227(JH = 2.0) | −0.014 | nd | nd | C-5 | [d], [s] | 68.878 and 164.256 |
| H-3 | [dd] | 2.919 ((JH = 17.3) | Very Small | nd | 2.810 | 2.921 | C-6 | [d], [d] | 18.082 and 97.429 |
| H-3 (auxiliary) (–2S) | [m]/[dd] | 3.106 (JH = 17.1) | 3.099 (JH = 17.5) | 0.007 | nd | nd | C-7 | [s] | 166.287 |
| H-3 (auxiliary) (–2R) | [m]/[dd] | 2.985 (JH = 19.2) | 3.017 (JH = 17.2) | −0.032 | nd | nd | C-8 | [d] | 95.060 |
| H-2 (–2S) | [dd] | 5.436 (JH = 7.9) | 5.452(JH = 8.4) | −0.016 | 5.448 | 5.551 | C-9 | [s] | 163.245 |
| H-2 (–2R) | [dd] | 5.283 (JH = 10.2) | Very Small | nd | nd | nd | C-10 | [s] | 105.080 |
| H-2′ | [d] | 6.785 (JH = 4.4) | 6.812 (JH = 3.8) | −0.027 | 6.690 | 7.108 | C-1′ | [s] | 132.526 |
| H-3′ | [dd] | 6.755(JH = 8.3) | 6.779 (JH = 7.9) | −0.024 | 6.690 | 7.094 | C-2′ | [d] | 114.460 |
| H-4′ (O-CH3 group) | [s] | 3.696 | 3.756 | −0.060 | 3.789 | 3.905 | C-3′ | [d], [d] | 75.373 and 147.469 |
| H-5′ | [d] | 6.729 (JH = 2.0) | 6.754 (JH = 1.3) | −0.025 | 6.690 | nd | C-4′ | [s], [s] | 57.475 and 149.263 |
| H-6′ | [d] | 6.812 (JH = 8.2) | 6.839 (JH = 7.8) | −0.027 | 6.690 | 7.094 | C-5′ | [d] | 112.632 |
| C-6′ | [d] | 118.897 | |||||||
| * Flavanone glucose protons: | 10 protons: | Flavanone glucose carbons: | |||||||
| H-1″ | [t] | 5.089 to 5.109; [5.009] | 4.908 to 5.119; [5.014] | 0.085 | 5.077 to 5.090 | 5.203 to 5.216 | C-1″ | [d] | 98.745 |
| H-2″ | [m] | 3.221 to 3.252; [3.237] | 3.202 to 3.232; [3.217] | 0.020 | 3.186 to 3.241 | 3.294 to 3.358 | C-2″ | [d] | 72.493 |
| H-3″ | [m] | 3.545 to 3.595; [3.570] | 3.584 to 3.620; [3.602] | −0.032 | 3.491 to 3.549 | 3.580 to 3.635 | C-3″ | [d] | 75.373 |
| H-4″ | [m] | 3.259 to 3.312; [3.286] | 3.341 to 3.372; [3.357] | −0.071 | 3.378 to 3.410 | 3.380 to 3.413 | C-4″ | [d] | 70.957 |
| H-5″ | [m] | 3.325 to 3.423; [3.374] | 3.391 to 3.430; [3.411] | −0.037 | 3.450 to 3.505 | 3.511 to 3.541 | C-5″ | [d] | 76.875 |
| H-6″ | [m] | 3.647 to 3.678; [3.663] | 3.633 to 3.681; [3.657] | 0.006 | 3.629 to 3.658 | 3.695 to 3.736 | C-6″ | [t] | 65.200 |
| 13C NMR Description | Chemical Shifts, ppm | ||
|---|---|---|---|
| β–Cyclodextrin Carbons | β–CD | HEPT7G/βCD Inclusion complex (IC) | Δδ = δIC−δCD (ppm) |
| C1 | 103.141 | 103.225 | 0.084 |
| C2 | 73.353 | 73.361 | 0.008 |
| C3 | 74.357 | 74.511 | 0.154 |
| C4 | 82.412 | 82.266 | − 0.146 |
| C5 | 73.101 | 73.085 | − 0.016 |
| C6 | 61.560 | 61.322 | − 0.238 |
| β-Cyclodextrin | HEPT7G+βCD Physical Mixture (PM) | HEPT7G/βCD Inclusion Complex (IC) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2θ (Degree) | d-Value (nm) | Rel. Intensity (I/I0) | 2θ (Degrees) | d-Value (nm) | Rel. Intensity (I/I0, %) | 2θ (Degrees) | d-Value (nm) | Rel. Intensity (I/I0, %) |
| 6.233 | 1.41684 | 6.7 | 6.143 | 1.43753 | 10.9 | 12.685 | 0.69727 | 30.2 |
| 9.017 | 0.97994 | 62.7 | 8.924 | 0.99006 | 33.4 | 22.629 | 0.39262 | 50.6 |
| 10.682 | 0.82754 | 45.7 | 10.578 | 0.83563 | 36.7 | 23.577 | 0.37703 | 24.6 |
| 12.503 | 0.70735 | 100.0 | 12.396 | 0.71344 | 100.0 | 25.478 | 0.34932 | 22.5 |
| 14.667 | 0.60344 | 18.5 | 14.144 | 0.62563 | 63.4 | 31.796 | 0.2812 | 100.0 |
| 15.292 | 0.57895 | 13.8 | 15.284 | 0.57924 | 6.4 | 33.996 | 0.26349 | 50.3 |
| 15.415 | 0.57435 | 39.2 | 17.033 | 0.52014 | 42.0 | 37.838 | 0.23757 | 18.6 |
| 17.104 | 0.51799 | 46.3 | 18.686 | 0.47448 | 20.4 | |||
| 17.653 | 0.502 | 13.9 | 18.848 | 0.47043 | 12.1 | |||
| 18.925 | 0.46853 | 21.2 | 19.512 | 0.45457 | 29.0 | |||
| 19.586 | 0.45287 | 30.8 | 21.052 | 0.42165 | 20.8 | |||
| 21.205 | 0.41864 | 24.8 | 22.842 | 0.38899 | 53.8 | |||
| 22.702 | 0.39136 | 42.2 | 24.187 | 0.36767 | 19.1 | |||
| 24.266 | 0.36649 | 20.0 | 24.769 | 0.35915 | 4.9 | |||
| 25.657 | 0.34691 | 23.7 | 25.426 | 0.35002 | 11.4 | |||
| 27.102 | 0.32874 | 45.3 | 26.961 | 0.33044 | 30.6 | |||
| 28.543 | 0.31246 | 12.3 | 28.629 | 0.31154 | 6.2 | |||
| 30.985 | 0.28838 | 4.7 | 31.89 | 0.28039 | 11.9 | |||
| 31.946 | 0.27992 | 15.7 | 34.608 | 0.25897 | 9.2 | |||
| 34.74 | 0.25802 | 12.1 | 36.915 | 0.24329 | 4.5 | |||
| 35.864 | 0.25018 | 10.2 | ||||||
| 36.923 | 0.24325 | 4.9 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, M.P.; Moriwaki, M.; Minoura, K.; Timm, D.; Abe, A.; Kito, K. Structural Investigation of Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin: A Spectroscopic Assessment. Molecules 2022, 27, 5395. https://doi.org/10.3390/molecules27175395
Kapoor MP, Moriwaki M, Minoura K, Timm D, Abe A, Kito K. Structural Investigation of Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin: A Spectroscopic Assessment. Molecules. 2022; 27(17):5395. https://doi.org/10.3390/molecules27175395
Chicago/Turabian StyleKapoor, Mahendra P., Masamitsu Moriwaki, Katsuhiko Minoura, Derek Timm, Aya Abe, and Kento Kito. 2022. "Structural Investigation of Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin: A Spectroscopic Assessment" Molecules 27, no. 17: 5395. https://doi.org/10.3390/molecules27175395
APA StyleKapoor, M. P., Moriwaki, M., Minoura, K., Timm, D., Abe, A., & Kito, K. (2022). Structural Investigation of Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin: A Spectroscopic Assessment. Molecules, 27(17), 5395. https://doi.org/10.3390/molecules27175395





