Nanostructured Electrospun Polycaprolactone—Propolis Mats Composed of Different Morphologies for Potential Use in Wound Healing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Propolis Extract Chemical Composition Analysis
2.2. Effect of Storage Time on PCL Molecular Weight and Spun Solution Viscosity
2.3. Morphology and Fibre Diameter in the Electrospun Mats
2.4. Thermal Behaviour of Electrospun Mats
2.5. Wettability Analysis
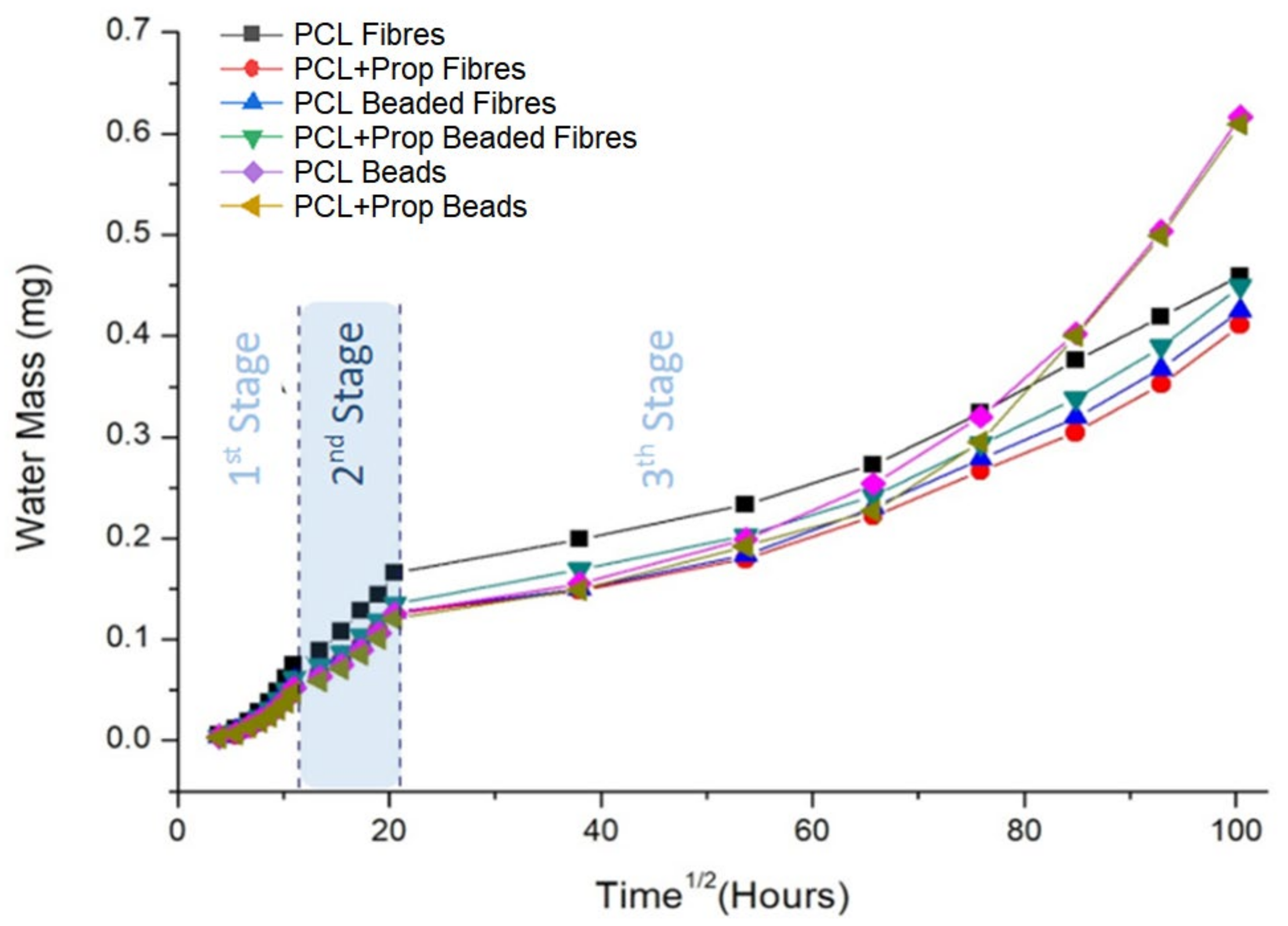
2.6. Water Vapour Transpiration Rate (WVTR) Analysis
2.7. Propolis Release Analysis
2.8. In vitro Wound-Healing Assay
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Propolis Extract Characterisation
3.2.2. Polymeric Solutions
3.2.3. Viscosimetry and Gel Permeation Chromatography (GPC) Analyses
3.2.4. Scanning Electron Microscopy (SEM)
3.2.5. Thermal Characterisation
3.2.6. Wettability Analysis
3.2.7. Water Vapour Transpiration Rate Analysis
3.2.8. Propolis Release Assay
3.2.9. Scratch Wound Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, M.E.; Shalaby, E.S.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. Technol. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Mancipe, J.M.A.; Lobianco, F.A.; Dias, M.L.; Thiré, R.M.S.M. Electrospinning: New strategies for the treatment of skin melanoma. Mini-Rev. Med. Chem. 2022, 22, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.; Choonara, Y.E. Advanced strategies for tissue engineering in regenerative medicine: A biofabrication and biopolymer perspective. Molecules 2021, 26, 2518. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.E.; Abu Ali, O.A.; Saleh, D.I.; Abu-Saied, M.; Ahmed, M.; Abdel-Fattah, E.; Mansour, S.; Kenawy, E.-R. Facile modification of polycaprolactone nanofibers with hydroxyapatite doped with thallium ions for wound and mucosal healing applications. J. Mater. Res. Technol. 2021, 15, 2909–2917. [Google Scholar] [CrossRef]
- Viana, V.R.; Ferreira, W.H.; Azero, E.G.; Dias, M.L.; Andrade, C.T. Optimization of electrospinning conditions by Box-Behnken design to prepare poly(vinyl alcohol)/chitosan crosslinked nanofibers. J. Mater. Sci. Chem. Eng. 2020, 8, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug–delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Zhao, H.; Chi, H. Electrospun bead-on-string fibers: Useless or something of value. In Novel Aspects of Nanofibers; Tong, L., Ed.; IntechOpen: London, UK, 2018; pp. 87–102. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Ding, X.; Tian, L.; Hu, J.; Yang, X.; Ramakrishna, S. The control of beads diameter of bead-on-string electrospun nanofibers and the corresponding release behaviors of embedded drugs. Mater. Sci. Eng. C 2017, 74, 471–477. [Google Scholar] [CrossRef]
- Steipel, R.T.; Gallovic, M.D.; Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Electrospray for generation of drug delivery and vaccine particle applied in vitro and in vivo. Mater. Sci. Eng. C 2019, 105, 110070. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Souto, E.B.; Durazzo, A.; Lucarini, M.; Novellino, E.; Tewari, D.; Wang, D.; Atanasov, A.G.; Santini, A. Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol. 2020, 2, 53–63. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Mondal, D.; May, G.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Pavelić, S.K. Bioactives from Bee Products and Accompanying Extracellular Vesicles as Novel Bioactive Components for Wound Healing. Molecules 2021, 26, 3770. [Google Scholar] [CrossRef]
- Stefano, C.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Oliveira, R.N.; McGuinness, G.B.; Ramos, M.E.T.; Kajiyama, C.E.; Thiré, R.M.S.M. Properties of PVA Hydrogel Wound-Care Dressings Containing UK Propolis. Macromol. Symp. 2016, 368, 122–127. [Google Scholar] [CrossRef]
- Atik, D.S.; Bölük, E.; Altay, F.; Torlak, E.; Kaplan, A.A.; Kopuk, B.; Palabiyik, I. Particle morphology and antimicrobial properties of electrosprayed propolis. Food Packag. Shelf Life. 2022, 33, 100881. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Wang, G.; Tao, Y.; Chang, X.; Xia, H.; Gui, S. Reviewing Propolis with Multi-components in the Treatment of Oral Diseases through Multi-pathways and multi-targets. Mini-Rev. Med. Chem. 2021, 21, 1406–1420. [Google Scholar] [CrossRef]
- Oliveira, R.N.; McGuinness, G.B.; Rouze, R.; Quilty, B.; Cahill, P.; Soares, G.D.A.; Thiré, R.M.S.M. PVA hydrogels loaded with a Brazilian propolis for burn wound healing applications. J. Appl. Polym. Sci. 2015, 132, 42129. [Google Scholar] [CrossRef]
- Stojko, M.; Włodarczyk, J.; Sobota, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Dobrzyński, P.; Starczynowska, G.; Orchel, A.; Stojko, J.; et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics 2020, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Alberti, T.B.; Coelho, D.S.; de Prá, M.; Maraschin, M.; Veleirinho, B. Electrospun PVA nanoscaffolds associated with propolis nanoparticles with wound healing activity. J. Mater. Sci. 2020, 55, 9712–9727. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Oliveira, R.N.; McGuinness, G.B. Electrospun poly(ɛ-caprolactone)/propolis fiber morphology: A process optimization study. J. Appl. Polym. Sci. 2022, 139, 52131. [Google Scholar] [CrossRef]
- Atari, M.; Mohammadalizadeh, Z.; Kharazi, A.Z.; Javanmard, S.H. The effect of different solvent systems on physical properties of electrospun poly(glycerol sebacate)/poly(ɛ-caprolactone) blend. Polym.-Plast. Technol. Mater. 2022, 61, 789–802. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, M.; Guidoin, R.; Li, Y.; Wang, F.; Brochu, G.; Zhang, Z.; Wang, L. Potential of a facile sandwiched electrospun scaffolds loaded with ibuprofen as an anti-adhesion barrier. Mater. Sci. Eng. C 2021, 118, 111451. [Google Scholar] [CrossRef] [PubMed]
- Shahrousvand, M.; Haddadi-Asl, V.; Shahrousvand, M. Step-by-step design of poly((ɛ-caprolactone)/chitosan/melilotus Officinalis extract electrospun nanofibers for wound dressing applications. Int. J. Biol. Macromol. 2021, 180, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Mancipe, J.M.A.; Dias, M.L.; Thiré, R.M.S.M. Morphological evaluation of electrospun polycaprolactone fibers depending on the type of solvent. Rev. Matéria 2019, 24, e12400. [Google Scholar] [CrossRef]
- Van der Schueren, L.; de Schoenmaker, B.; Kalaoglu, Ö.I.; De Clerck, K. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Malinová, L.; Stolínová, M.; Lubasová, D.; Martinová, L.; Brožek, J. Electrospinning of polyesteramides based on ε-caprolactam and ε-caprolactone from solution. Eur. Polym. J. 2017, 49, 3135–3143. [Google Scholar] [CrossRef]
- Lavielle, N.; Popa, A.-M.; de Geus, M.; Hébraud, A.; Schlatter, G.; Thöny-Meyer, L.; Rossi, R.M. Controlled formation of poly (ε-caprolactone) ultrathin electrospun nanofibres in a hydrolytic degradation-assisted process. Eur. Polym. J. 2013, 49, 1331–1336. [Google Scholar] [CrossRef]
- Dulnik, J.; Dennis, P.; Sajkiewicz, P.; Kolbuk, D.; Choinska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Effect of the dissolution time into an acid hydrolytic solvent to tailor electrospun nanofibrous polycaprolactone scaffolds. Eur. Polym. J. 2017, 87, 74–187. [Google Scholar] [CrossRef] [Green Version]
- Ramos, C.; Lanno, G.M.; Laidmäe, I.; Meos, A.; Harmas, R.; Kogermann, K. High humidity electrospinning of porous fibers for tuning the release of drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 880–892. [Google Scholar] [CrossRef]
- Elnaggar, M.A.; El-Fawal, H.A.; Allam, N.K. Biocompatible PCL-nanofibers scaffold with immobilized fibronectin and laminin for neuronal tissue regeneration. Mater. Sci. Eng. C 2021, 119, 111550. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Monavari, M.; Koenen, B.; Boccaccini, A.R. Biomimetic biohybrid nanofibers containing bovine serum albumin as a bioactive moiety for wound dressing. Mater. Sci. Eng. C 2021, 123, 111965. [Google Scholar] [CrossRef] [PubMed]
- Matos, F.D.A. Introdução à Fitoquímica Experimental, 3rd ed.; Edições UFC: Fortaleza, Brazil, 2009; pp. 45–70. [Google Scholar]
- Oliveira, R.N.; Moreira, A.P.D.; Thiré, R.M.S.M.; Quilty, B.; Passos, T.M.; Simon, P.; Mancini, M.C.; McGuinness, G.B. Absorbent polyvinyl alcohol–sodium carboxymethyl cellulose hydrogels for propolis delivery in wound healing applications. Polym. Eng. Sci. 2017, 57, 1224–1233. [Google Scholar] [CrossRef]
- Ibrahim, N.; Zakaria, A.J.; Ismail, Z.; Ahmad, Y.; Mohd, K.S. Application of GCMS and FTIR fingerprinting in discriminating two species of Malaysian stingless bees propolis. Int. J. Eng. Technol. 2018, 7, 106–112. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.D.; Passos, T.M.; Quilty, B.; Thiré, R.M.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean propolis from the Adriatic Sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.T.; Araujo-Filho, H.G.; Barreto, A.S.; Quintans-Junior, L.J.; Quintans, J.S.; Barreto, R.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants; Springer: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant properties of plant-derived phenolic compounds and their effect on skin fibroblast cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Shirvani, B.; Aroon, M.A.; Nazari, T. Physico-chemical properties of hybrid electrospun nanofibers containing polyvinylpyrrolidone (PVP), propolis and aloe vera. Mater. Res. Express 2018, 5, 125404. [Google Scholar] [CrossRef]
- Canevarolo, S.V., Jr. Polymer Science: A Textbook for Engineers and Technologists, 1st ed.; Hanser Publications: Cincinnati, OH, USA, 2020; pp. 149–188. [Google Scholar]
- Dias, J.; Antunes, F.; Bartolo, P. Influence of the rheological behavior in electrospun PCL nanofibres production for tissue engineering applications. Chem. Eng. Trans. 2013, 32, 1015–1020. [Google Scholar] [CrossRef]
- Gurler, E.B.; Ergul, N.M.; Ozbek, B.; Ekren, N.; Oktar, F.N.; Haskoylu, M.E.; Oner, E.T.; Eroglu, M.S.; Ozbeyli, D.; Korkut, V.; et al. Encapsulated melatonin in polycaprolactone (PCL) microparticles as a promising graft material. Mater. Sci. Eng. C 2019, 100, 798–808. [Google Scholar] [CrossRef]
- Zhang, M.; Kiratiwongwan, T.; Shen, W. Oxygen-releasing polycaprolactone/calcium peroxide composite microspheres. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of polymeric microparticles by electrospray: The impact of experimental parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Sutjarittangtham, K.; Tunkasiri, T.; Chantawannakul, P.; Intatha, U.; Eitssayeam, S. Mechanical improved antibacterial polycaprolactone/propolis electrospun fiber mat by adding bacterial nanocellulose. J. Comput. Theor. Nanosci. 2015, 12, 798–803. [Google Scholar] [CrossRef]
- Abel, S.B.; Liverani, L.; Boccaccini, A.R.; Abraham, G.A. Effect of benign solvents composition on poly (ε-caprolactone) electrospun fiber properties. Mater. Lett. 2019, 245, 86–89. [Google Scholar] [CrossRef]
- Sailema-Palarte, G.P.; Vidaurre, A.; Campillo-Fernandez, A.J.; Castilla-Cortazar, I. A Comparative study on poly(ε-caprolactone) film degradation at extreme pH values. Polym. Degrad. Stab. 2016, 130, 118–125. [Google Scholar] [CrossRef]
- Pedrosa, M.C.G.; dos Anjos, S.A.; Mavropoulos, E.; Bernardo, P.L.; Granjeiro, J.M.; Rossi, A.M.; Dias, M.L. Structure and biological compatibility of polycaprolactone/zinc-hydroxyapatite electrospun nanofibers for tissue regeneration. J. Bioact. Compat. Polym. 2021, 36, 314–333. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L. The effect of molecular weight on the crystallization kinetics of polycaprolactone. Polym. Adv. Technol. 2006, 17, 474–478. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Alagesan, T.; Parthasarathy, V.; Tung, K.L.; Anbarasan, R. Non-isothermal crystallization kinetics and degradation kinetics studies on barium thioglycolate end-capped poly(ε-caprolactone). J. Therm. Anal. Calorim. 2019, 135, 3129–3140. [Google Scholar] [CrossRef]
- Karizmeh, M.S.; Poursamar, S.A.; Kefayat, A.; Farahbakhsh, Z.; Rafienia, M. An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Mater. Sci. Eng. C 2022, 135, 112667. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.L.M.M.; Ramalho, B.S.; Picciani, P.H.S.; Baptista, L.S.; Martinez, A.M.B.; Dias, M.L. Effect of three different amines on the surface properties of electrospun polycaprolactone mats. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 1258–1270. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Liu, K.; Yang, L.; Zhang, B.; Luo, Q.; Luo, R.; Wang, Y. Nanoparticles-stacked superhydrophilic coating supported synergistic antimicrobial ability for enhanced wound healing. Mater. Sci. Eng. C 2022, 132, 112535. [Google Scholar] [CrossRef]
- Jia, W.; Kharraz, J.A.; Choi, P.J.; Guo, J.; Deka, B.J.; An, A.K. Superhydrophobic membrane by hierarchically structured PDMS-POSS electrospray coating with cauliflower-shaped beads for enhanced MD performance. J. Membr. Sci. 2020, 597, 117638. [Google Scholar] [CrossRef]
- De Oliveira, J.M.; Mei, L.H.I. Nonionic reactive surfactants in emulsion polymerization of vinyl acetate-vinyl neodecanoate latexes: Influence on the water barrier properties. Polímeros 2009, 19, 23–30. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist wound healing with commonly available dressing. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Yu, S.-H.; Mi, F.-L.; Wu, C.-W.; Shyu, S.-S.; Peng, C.-K.; Chao, A.-C. Preparation and characterization on mechanical and antibacterial properties of chitosan/cellulose blends. Carbohydr. Polym. 2004, 57, 435–440. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Zhang, C.; Liu, D.; Meng, S.; Zhang, W.; Meng, S. Effect to polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure. Pharmaceutics 2019, 11, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, T.N.; Gonçalves, R.P.; Rocha, C.L.; Archanjo, B.S.; Barboza, C.A.G.; Pierre, M.B.R.; Reynaud, F.; Picciani, P.H.S. Controlling burst effect with PLA/PVA coaxial electrospun scaffolds loaded with BMP-2 for bone guided regeneration. Mater. Sci. Eng. C 2019, 97, 602–612. [Google Scholar] [CrossRef]
- Maver, T.; Kurečič, M.; Pivec, T.; Maver, U.; Gradišnik, L.; Gašparič, P.; Kaker, B.; Bratuša, A.; Hribernik, S.; Kleinschek, K.S. Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose 2020, 27, 4487–4508. [Google Scholar] [CrossRef]
- Zupančič, S.; Preem, L.; Kristl, J.; Putrinš, M.; Tenson, T.; Kocbek, P.; Kogermann, K. Impact of PCL nanofiber mat structural properties on hydrophilic drug release and antibacterial activity on periodontal pathogens. Eur. J. Pharm. Sci. 2018, 122, 347–358. [Google Scholar] [CrossRef]
- Reshmi, C.R.; Suja, P.S.; Manaf, O.; Sanu, P.P.; Sujith, A. Nanochitosan enriched poly ε-caprolactone electrospun wound dressing membranes: A fine tuning of physicochemical properties, hemocompatibility and curcumin release profile. Int. J. Biol. Macromol. 2018, 108, 1261–1272. [Google Scholar] [CrossRef]
- Jacob, A.; Parolia, A.; Pau, A.; Amalraj, F.D. The effects of Malaysian propolis and Brazilian red propolis on connective tissue fibroblasts in the wound healing process. BMC Complementary Altern. Med. 2015, 15, 294. [Google Scholar] [CrossRef] [Green Version]
- Rojczyk, E.; Klama-Baryla, A.; Łabuś, W.; Wilemska-Kucharzewska, K.; Kucharzewski, M. Historical and modern research on propolis and its application in wound healing and other fields of medicine and contributions by Polish studies. J. Ethnopharmacol. 2020, 262, 113159. [Google Scholar] [CrossRef]
- Behyari, M.; Imani, R.; Keshvari, H. Evaluation of skin fibroin nanofibrous dressing incorporation niosomal propolis, for potential use in wound healing. Fibers Polym. 2021, 22, 2090–2101. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Ebadi, P.; Fazeli, M. Evaluation of the potential in vitro effects of propolis and honey on wound healing in human dermal fibroblast cells. S. Afr. J. Bot. 2021, 137, 414–422. [Google Scholar] [CrossRef]
- Rosseto, H.C.; Toledo, L.D.A.S.D.; de Francisco, L.M.B.; Esposito, E.; Lim, Y.; Valacchi, G.; Cortesi, R.; Bruschi, M.L. Nanostructured lipid systems modified with waste material of propolis for wound healing: Design, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2017, 158, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Tayfeh-Ebrahimi, R.; Amniattalab, A.; Mohammadi, R. Evaluation of Effect of Biologically Synthesized Ethanolic Extract of Propolis-Loaded Poly (-Lactic-co-Glycolic Acid) Nanoparticles on Wound Healing in Diabetic Rats. Int. J. Low. Extrem. Wounds 2022, 1–11, Online First. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Propolis antibacterial and antioxidant synergisms with gentamicin and honey. J. Appl. Microbiol. 2022, 132, 2733–2745. [Google Scholar] [CrossRef]
- Alanazi, S.; Alenzi, N.; Fearnley, J.; Harnett, W.; Watson, D.G. Temperate propolis has anti-inflammatory effects and is a potent inhibitor of nitric oxide formation in macrophages. Metabolites 2020, 10, 413. [Google Scholar] [CrossRef]
- Lesmana, R.; Zulhendri, F.; Fearnley, J.; Irsyam, I.A.; Rasyid, R.P.; Abidin, T.; Abdulah, R.; Suwantika, A.; Paradkar, A.; Budiman, A.S.; et al. The Suitability of Propolis as a Bioactive Component of Biomaterials. Front. Pharmacol. 2022, 13, 930515. [Google Scholar] [CrossRef]
- Bonadies, I.; Cimino, F.; Guarino, V. In vitro degradation of zein nanofibres for propolis release in oral treatments. Mater. Res. Express 2019, 6, 75407. [Google Scholar] [CrossRef]
- Filho, G.R.; Monteiro, D.S.; Meireles, C.D.S.; de Assunção, R.M.N.; Cerqueira, D.; Barud, H.S.; Ribeiro, S.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. In Epidermal Cells Methods in Molecular Biology; Turksen, K., Ed.; Humana: New York, NY, USA, 2019; Volume 2109, pp. 225–229. [Google Scholar] [CrossRef]
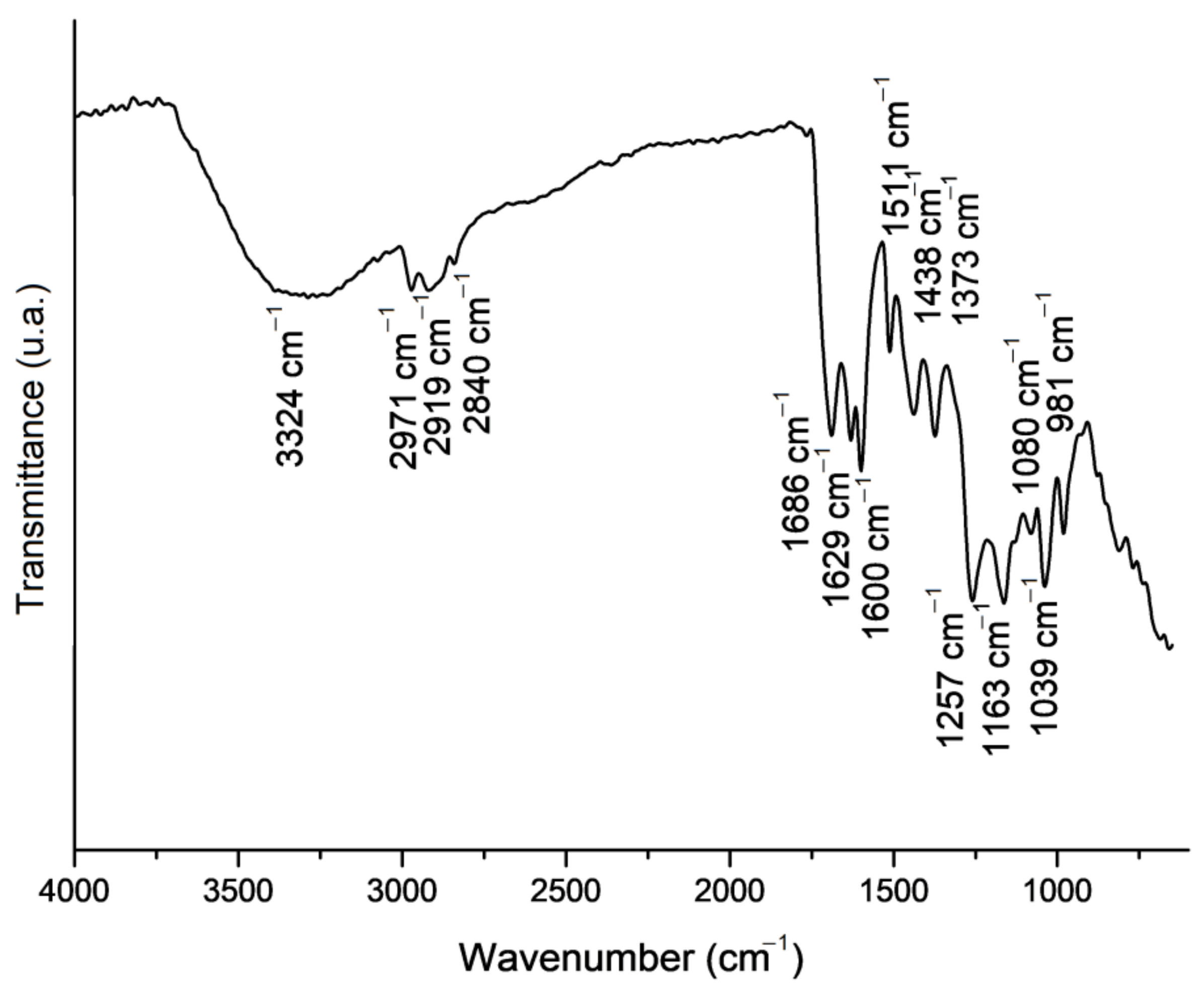
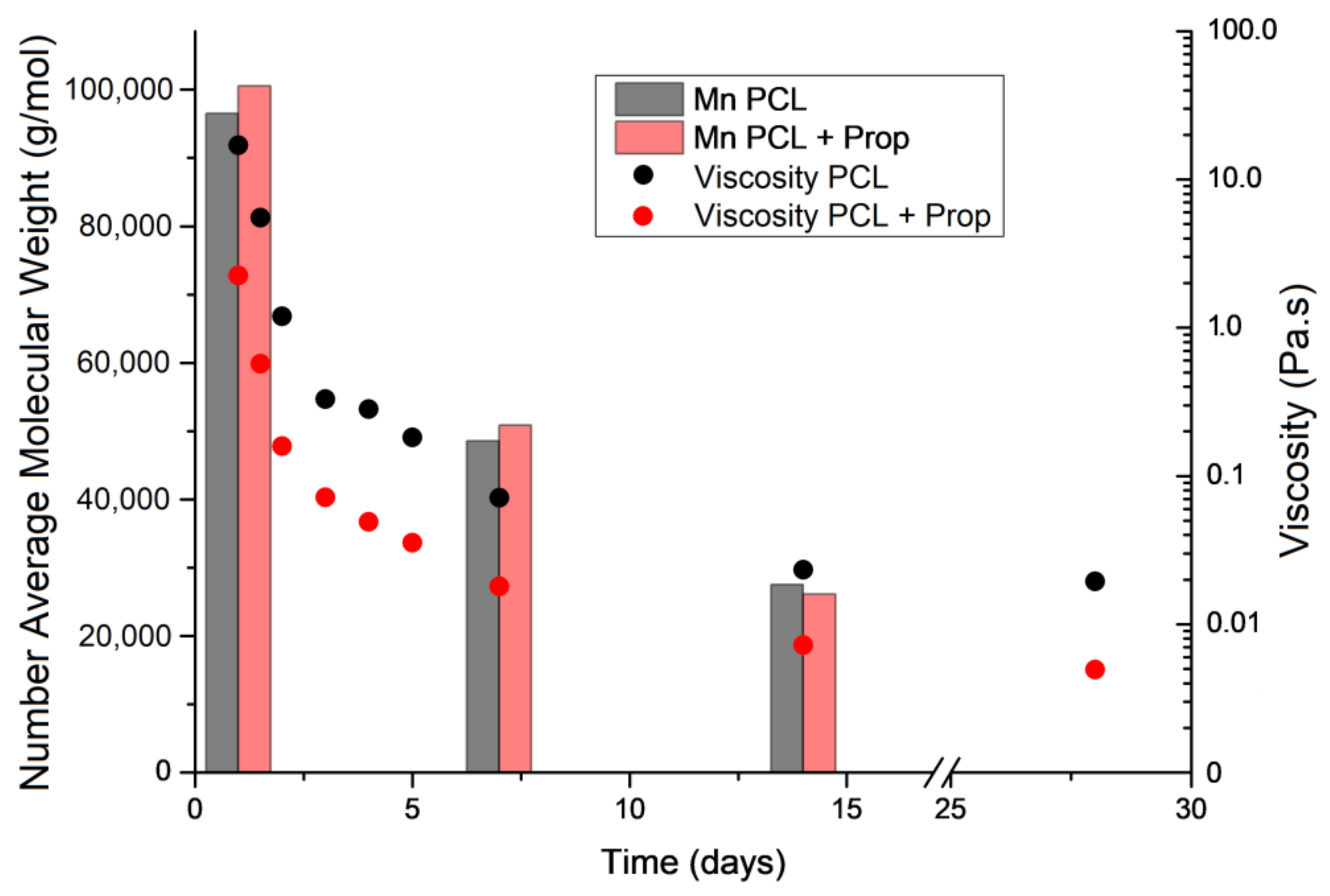

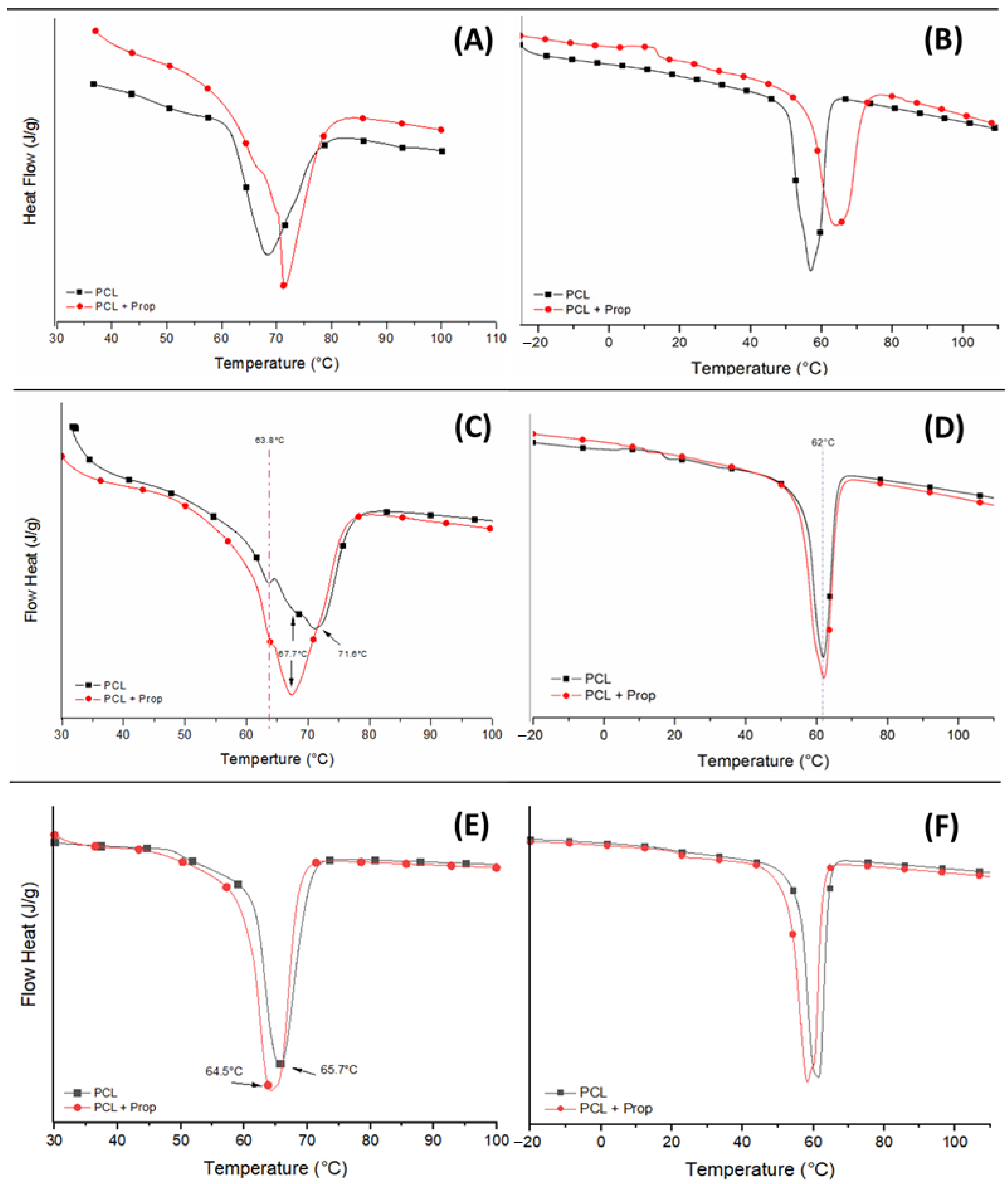
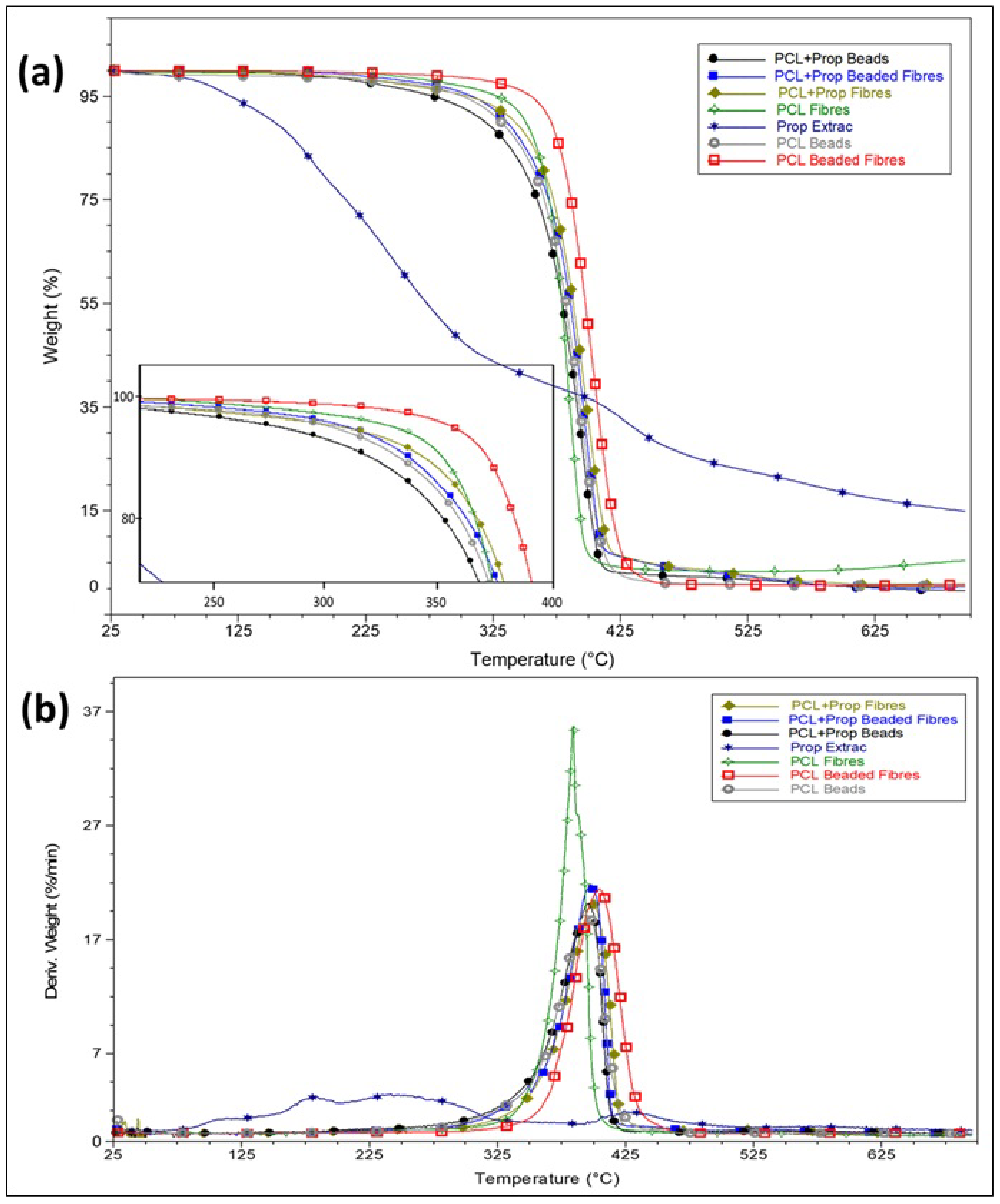
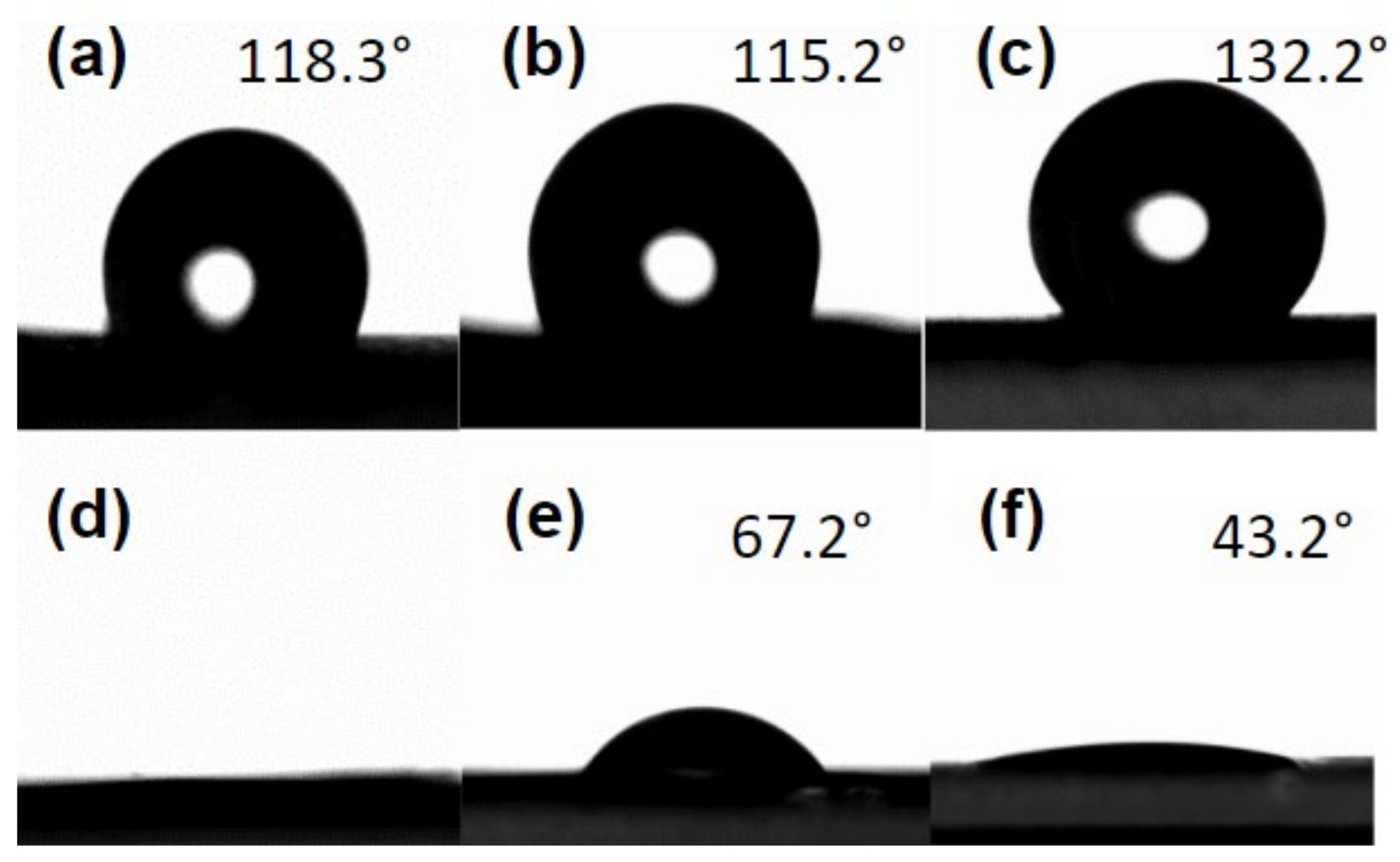

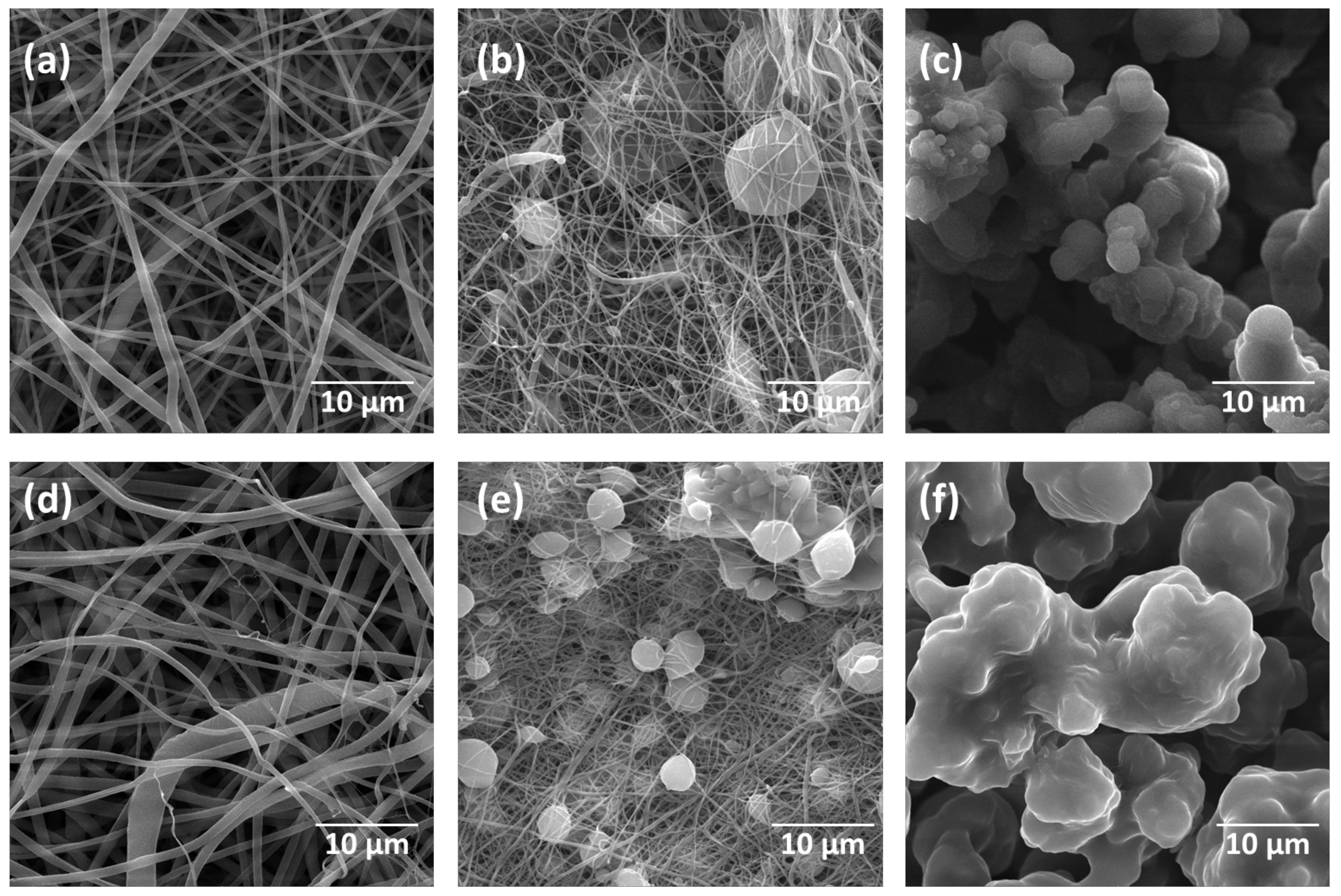


| Variable | Fibres | Beaded Fibres | Beads |
|---|---|---|---|
| PCL Concentration, % (w/v) | 30 | 30 | 30 |
| Distance needle tip/collector, cm | 10 | 10 | 10 |
| Flow Rate, mL/h | 2.0 | 1.0 | 1.0 |
| Voltage, kV | 10 | 15 | 15 |
| Storage at 35 °C, days | 0 | 7 | 14 |
| Solution Sample | Storage Time (Days) | Morphology | Diameter | |
|---|---|---|---|---|
| Fibre (nm) | Bead (μm) | |||
| PCL | 0 | Fibres | 280.5 ± 70.1 | |
| 7 | Beaded Fibres | 190.7 ± 83.7 | 5.6 ± 2.4 | |
| 14 | Beads | 2.9 ± 0.5 | ||
| PCL + Prop | 0 | Fibres | 208.3 ± 72.6 | |
| 7 | Beaded Fibres | 159.2 ± 52.6 | 2.6 ± 0.7 | |
| 14 | Beads | 1.9 ± 0.5 | ||
| Samples | First Heat Cycle | Second Heat Cycle | |||||
|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/mg) | Xc (%) | Tm (°C) | ΔHm (J/mg) | Xc (%) | ||
| PCL | Fibres | 68.4 | 26.4 | 17.4 | 57.0 | 27.1 | 17.9 |
| Beaded Fibres | 71.1 | 51.2 | 33.8 | 62.0 | 38.3 | 25.2 | |
| Beads | 65.7 | 65.8 | 43.4 | 61.4 | 54.9 | 36.2 | |
| PCL + Prop | Fibres | 71.4 | 31.3 | 20.6 | 64.3 | 28.0 | 18.5 |
| Beaded Fibres | 67.3 | 54.4 | 35.9 | 62.0 | 42.0 | 28.0 | |
| Beads | 64.5 | 65.5 | 43.2 | 58.0 | 54.0 | 36.0 | |
| Samples | J = Δm/Δt (mg/min) | Thickness (mm) | Water Vapour Transpiration Rate (g/m2 per day) | |
|---|---|---|---|---|
| PCL | Fibres | 0.0107 | 0.09 | 2179.84 |
| Beaded Fibres | 0.0081 | 0.56 | 1650.16 | |
| Beads | 0.0088 | 0.48 | 1792.77 | |
| PCL + Prop | Fibres | 0.0082 | 0.24 | 1670.53 |
| Beaded Fibres | 0.0087 | 0.39 | 1772.39 | |
| Beads | 0.0086 | 0.14 | 1263.08 | |
| Samples | Diameters | |||
|---|---|---|---|---|
| Fibre (nm) | Bead (um) | |||
| Before | After | Before | After | |
| PCL Fibres | 280.5 ± 70.1 | 811.1 ± 381.0 | ||
| PCL Beaded Fibres | 190.7 ± 83.7 | 461.2 ± 220.9 | 5.6 ± 2.4 | 6.6 ± 2.6 |
| PCL Beads | 2.9 ± 0.5 | 5.5 ± 1.6 | ||
| PCL + Prop Fibres | 208.3 ± 72.6 | 1098.5 ± 478.3 | ||
| PCL + Prop Beaded Fibres | 159.2 ± 52.6 | 349.8 ± 109.9 | 2.6 ± 0.7 | 2.9 ± 0.9 |
| PCL + Prop Beads | 1.9 ± 0.5 | |||
| Variable | Value | |
|---|---|---|
| PCL Concentration | 30 | % (w/v) |
| Distance needle tip/collector | 10 | cm |
| Flow Rate | 1.0–2.0 | mL/h |
| Voltage | 10–15 | kV |
| Molecular Weight | 26,500–100,517 | g/mol |
| Humidity | 50–70 | % |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Figueiredo, A.C.; Anaya-Mancipe, J.M.; de Barros, A.O.d.S.; Santos-Oliveira, R.; Dias, M.L.; Thiré, R.M.d.S.M. Nanostructured Electrospun Polycaprolactone—Propolis Mats Composed of Different Morphologies for Potential Use in Wound Healing. Molecules 2022, 27, 5351. https://doi.org/10.3390/molecules27165351
de Figueiredo AC, Anaya-Mancipe JM, de Barros AOdS, Santos-Oliveira R, Dias ML, Thiré RMdSM. Nanostructured Electrospun Polycaprolactone—Propolis Mats Composed of Different Morphologies for Potential Use in Wound Healing. Molecules. 2022; 27(16):5351. https://doi.org/10.3390/molecules27165351
Chicago/Turabian Stylede Figueiredo, Agnes Chacor, Javier Mauricio Anaya-Mancipe, Aline Oliveira da Silva de Barros, Ralph Santos-Oliveira, Marcos Lopes Dias, and Rossana Mara da Silva Moreira Thiré. 2022. "Nanostructured Electrospun Polycaprolactone—Propolis Mats Composed of Different Morphologies for Potential Use in Wound Healing" Molecules 27, no. 16: 5351. https://doi.org/10.3390/molecules27165351
APA Stylede Figueiredo, A. C., Anaya-Mancipe, J. M., de Barros, A. O. d. S., Santos-Oliveira, R., Dias, M. L., & Thiré, R. M. d. S. M. (2022). Nanostructured Electrospun Polycaprolactone—Propolis Mats Composed of Different Morphologies for Potential Use in Wound Healing. Molecules, 27(16), 5351. https://doi.org/10.3390/molecules27165351







