Abstract
Plants of the Veratrum genus have been used throughout history for their emetic properties, rheumatism, and for the treatment of high blood pressure. However, inadvertent consumption of these plants, which resemble wild ramps, induces life-threatening side effects attributable to an abundance of steroidal alkaloids. Several of the steroidal alkaloids from Veratrum spp. have been investigated for their ability to antagonize the Hedgehog (Hh) signaling pathway, a key pathway for embryonic development and cell proliferation. Uncontrolled activation of this pathway is linked to the development of various cancers; most notably, basal cell carcinoma and acute myeloid leukemia. Additional investigation of Veratrum spp. may lead to the identification of novel alkaloids with the potential to serve as chemotherapeutics. V. parviflorum is a relatively uncommon species of Veratrum that resides in the southeastern regions of North America. The phytochemical profile of this plant remains largely unexplored; however, bioactive steroidal alkaloids, including cyclopamine, veratramine, veratridine, and verazine were identified in its extract. The structural elucidation and bioactivity assessment of steroidal alkaloids in lesser abundance within the extract of V. parviflorum may yield potent Hh pathway inhibitors. This review seeks to consolidate the botanical and phytochemical information regarding V. parviflorum.
1. Introduction
Most modern therapeutics have originated from a treasure trove of secondary metabolites extracted from natural products of terrestrial or marine origin. An estimated 70,000 plant species have been used throughout history for medicinal purposes and more than 3000 plants are reported to contain compounds with anticancer properties [1,2]. The Veratrum californicum derived the steroidal alkaloid, cyclopamine; it was first isolated in 1965 and later identified as an inhibitor of the protein Smoothened (Smo), which is a critical protein in the Hedgehog signaling pathway [3,4]. Since this discovery, a new class of Food and Drug Administration (FDA) approved chemotherapeutics called Hedgehog pathway inhibitors have been developed for the treatment of cancers; most prominently, for basal cell carcinoma and acute myeloid leukemia [5]. Plants from the genus Veratrum, including V. viride, V. album, V. nigrum, and V. californicum have been extensively studied; they are found to be rich sources for unique steroidal alkaloids (>100 alkaloids/plant), with approximately 20% of these secondary metabolites being characterized [6]. Here, we present a consolidated review of the morphological, ecological, and phytochemical information regarding the sparsely studied Veratrum spp., V. parviflorum.
1.1. Background
1.1.1. Veratrum Genus
The Veratrum genus is comprised of perennial flowering herbs located predominantly in the Northern hemisphere [7]. These plants are found throughout temperate regions of North America and northern temperate to arctic regions in Eurasia [7]. Depending on the taxonomic treatment, the number of species varies between 17–45 species. These can be divided into four species complexes: V. album L., V. nigrum L., V. mackii Regal, and V. viride Aiton [7,8]. Wide variability in taxonomic treatment may be attributed to a dissimilarity in morphology, including leaves, tepals, and perigonal nectaries, and habitats, including rocky tundra, bogs, meadows, riverbanks, swamps, and deciduous forest slopes [7]. Veratrum may be further divided into two major sections based on gynoecia characteristics: Veratrum sect. Veratrum [Clade B] and Veratrum sect. Fuscoveratrum [Clade C] (Figure 1).
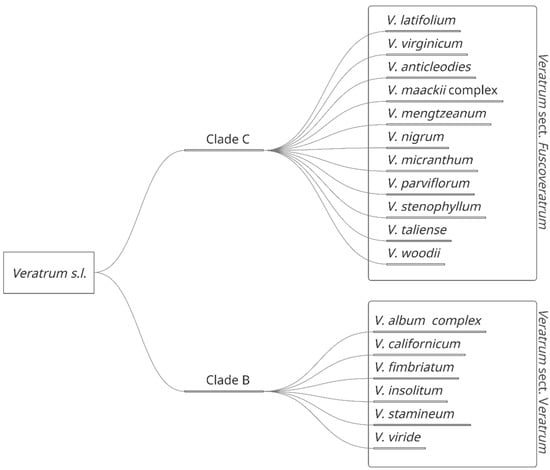
Figure 1.
Veratrum spp. separated by classification in Veratrum sect. Fuscoveratrum and Veratrum sect. Veratrum.
1.1.2. Medicinal Relevance
Traditional medicines have utilized Veratum spp. plants as a source of therapeutically active compounds for centuries [6,9]. Chinese medicine utilized V. nigrum in a medicinal concoction, referred to as Li-lu, to treat conditions including aphasia resulting from apoplexy, wind-type dysentery, jaundice, scabies, and chronic malaria [10]. The roots and rhizome of V. album subsp. lobelianum are described in the first Pharmacopoeia Rossica as a traditional Russian medicine that is made into a tincture or ointment for the treatment of head lice, scabies, neuralgic and rheumatic pain, eczema, or fevers [11]. This plant has also been used as an antiparasitic in cattle against hypodermatosis [11]. V. album has seen widespread use in Eurasia [12]. The Greeks used a powdered form of V. album to induce sneezing and for psychological diseases such as depression and epilepsy [12,13]. An alcohol extract of V. album’s roots was used in Italy as an antirheumatic [12]. In Iranian folk tradition, V. album root, pulverized into a paste, was used to relive headache and neuralgic pain [12]. In North America, Native American tribes, including the Shoshone, Bella Coola, Cherokee, Gitksan, Haisla, Hanaksiala, Iroquois, Kitasoo, Okanagan-Colville, Oweekeno, Quinault, Salish Thompson, and Tsimishian used the crushed roots of V. viride as an antirheumatic to treat snake bite wounds, to make a tea for venereal diseases, and as an analgesic for sore throats and colds [6,12]. Table 1 presents a comparison of several Veratrum spp., including the identified steroidal alkaloids and traditional medical applications.

Table 1.
A comparison of the identified steroidal alkaloids and traditional medicinal applications for several Veratrum species.
2. Veratrum parviflorum
2.1. Taxonomy and Physical Characteristics
Veratrum (Melanthium) parviflorum, commonly known as mountain bunchflower, has a complex history regarding its classification in the Veratrum and Melanthium genera due to variation in the morphological constraints set by botanists [7,16]. To provide a more defined taxonomy of Veratrum spp., the nuclear ribosomal internal transcribed spacers (ITS) were analyzed and correlated to traditional taxonomic classifications, including flower color and geographical location [7]. The strict and bootstrap consensus trees were almost identical, except for V. parviflorum. The strict consensus suggested that V. parviflorum formed a subclade and was sister to V. latifolium, V. virginicum, and V. woodii; whereas the bootstrap consensus suggested that this species falls outside of the clade, forming a polytomy with the V. maackii and V. micranthum complexes [7].
V. parviflorum is identified in nature using defined morphological traits. The stem is slender and 2 to 5 feet tall [15,17]. A pseudostem is formed by the overlapping sheaths of the leaves, which are broad (2–4 inches wide), petiolate, obscurely plicate, and have a blue tint adaxially [7,16,18]. The tepals are pale green to olive green, narrowly rhombic oblanceolate, with entire margins, gradually attenuated at base, filaments adnate, gland bilobed, diffuse, and dark (Figure 2) [7].

Figure 2.
V. parviflorum in situ before blooming (left). In the later stages of growth, a stem protrudes from the base of the plant and blooms with pale green flowers (right) [19].
2.2. Geographic Location and Herbivory
V. parviflorum is found in the southeastern regions of North America [15,16,17,18,20]. This species grows in rich deciduous forests (800–2030 m) in the mid-Appalachians, including parts of Alabama, Georgia, Kentucky, North Carolina, South Carolina, Tennessee, Virginia, and West Virginia [15,16]. The states of Alabama, Kentucky, and West Virginia have classified the conservation status of V. parviflorum as critically imperiled (S1), imperiled (S2), and vulnerable (S3), respectively [18,20]. This plant is most easily discovered in the spring when it reaches peak germination [21]. Unfortunately, V. parviflorum growth season coincides with wild ramps (Allium tricoccum); furthermore, it has led to the accidental ingestion of V. parviflorum, resulting in cardiac and gastrointestinal toxicity [15]. Despite inducing toxic effects when ingested by humans, white-tailed deer have been observed to consume the entire inflorescences of this species [16,22].
2.3. Toxicity
Cases of Veratrum poisoning are found extensively within the literature; however, V. parviflorum has only been implicated in one case of poisoning in the United States [15]. Symptoms of Veratrum poisoning generally include nausea, vomiting, diarrhea, hypotension, bradycardia, hypopnea, paresthesia, or death if medical attention is not received [15,23,24,25,26,27,28,29,30,31,32]. Treatment for Veratrum poisoning is generally symptomatic and supportive; it may include the administration of atropine, intravenous fluids, vasopressors, activated charcoal, and promethazine [15,23,24,25,26,27,28,29,30,32]. Although digoxin immune Fab has been used for treating symptoms of cardiotoxicity similar to those observed in cases of Veratrum poisoning, it has been suggested that medical providers should not unnecessarily administer DigiFabTM as they do not bind steroidal alkaloids extracted from V. viride [32]. Furthermore, MultigentTM digoxin immunoassay reagent antibodies demonstrated cross-reactivity with the alkaloids; this resulted in false-positive tests [32]. Table 2 presents a summary of treatments for several cases of Veratrum poisoning.

Table 2.
A summary of several cases of Veratrum poisoning, including the causative plant, symptoms, and treatment.
The cardiotoxic effects from consuming Veratrum spp. are primarily due to the steroidal alkaloids produced by the plant [33]. These steroidal alkaloids are present throughout the plant; however, the roots and rhizomes contain higher concentrations than the leaves [31,34]. Veratrum steroidal alkaloids are recognized for their tendency to bind to the type 2 receptor site of voltage-gated sodium ion channels in vertebrate organisms [33,35]. Once bound, the resting membrane potential is depolarized, causing excitable membranes to fire repetitively [33,35,36]. Symptoms including bradycardia and hypotension are caused by the alkaloids interacting with cardiac receptors in the left ventricle posterior wall and the baroreceptor area of the coronary sinus; while depolarization in the vagus nerve can induce bradycardia, hypotension, and dyspnea [33,37]. Additional symptoms resulting from depolarization of the nerve cells may comprise of paresthesia, numbness, and vomiting [33]. The triad of symptoms, including bradycardia, hypotension, and dyspnea, caused by Veratrum poisoning is referred to as a Bezold–Jarisch reflex [6,33,38,39].
Veratrum steroidal alkaloids are recognized as antagonistic to the Hedgehog signaling pathway (Figure 3) [40,41,42]. In the late 1950s, sheep herders in the south-central and southwestern alpine meadows of Idaho observed that 1–25% of their lambs were born with cyclopean-type developmental defects [43]. These malformations were originally thought to be congenital; however, further investigations revealed that they resulted from pregnant ewes feeding on V. californicum between the 10th and 15th days of gestation [43,44]. The Veratrum steroidal alkaloids cyclopamine, jervine, cycloposine, and veratrosine were identified as the causative teratogenic agents [45]. Veratrum steroidal alkaloids exert teratogenic effects via antagonism of the Hedgehog signaling pathway by directly binding to the transmembrane protein Smoothened (Smo) [4,40]. The binding of the small molecule to Smo takes place in the extracellular pocket of Smo, inhibiting activation by membrane sterols [46].
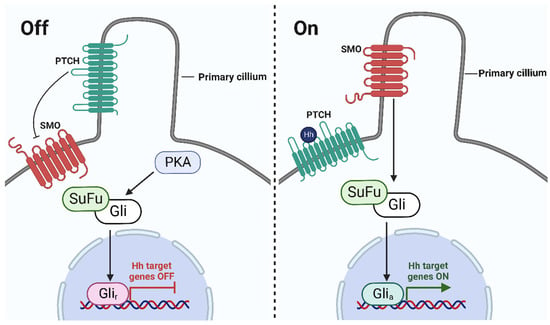
Figure 3.
Schematic of the Hedgehog signaling pathway. In the absence of Hedgehog (Hh) ligands (left), Patched (PTCH) inhibits the G protein-coupled receptor, SMO. Protein kinase A (PKA) phosphorylates glioma-associated (Gli) transcription factors, which then undergo proteolytic cleavage from the suppressor of fused (SuFu) to generate the repressor form (Glir). Glir hinders transcription of the Hh genes and turns the pathway off. In the presence of Hh ligands (right), PTCH is bound by the Hh ligand, resulting in the phosphorylation of SMO. Gli transcription factors dissociate from SuFu and generate the activator form (Glia). Glia promotes the transcription of the Hh genes and turns the pathway on. In the presence of a Hh pathway inhibitor such as cyclopamine, SMO will remain inactivated and PKA will phosphorylate Gli transcription factors; thus, this will generate the Glir that inhibits transcription [46]. (Graphic created with BioRender.com.)
2.4. Phytochemistry
There is a lack of published information regarding the steroidal alkaloid content in V. parviflorum. Currently, only four alkaloids have been identified in an extract of V. parviflorum root and rhizome [15]. The four alkaloids, including cyclopamine, veratramine, verazine, and veratridine, are not novel to the Veratrum genus and have been investigated for a variety of bioactive properties. Challenges to the structural elucidation of Veratrum steroidal alkaloids are largely due to the complexity of the molecules and presence of isomers. Many alkaloids cannot be differentiated by chemical formula and require full characterization, unless a commercially available standard can be purchased. A variety of instrumentation may be used to identify the structures of unknown steroidal alkaloids, including nuclear magnetic resonance (NMR) spectroscopy, infrared spectroscopy, ultraviolet spectroscopy, and liquid chromatography mass spectrometry [4,14,15,34,41].
2.4.1. Cyclopamine
Cyclopamine (Figure 4a) was isolated from V. grandiflorum in 1965 and was the first molecule identified to inhibit the Hedgehog signaling pathway [3,4,6,47]. Since its discovery, cyclopamine has also been identified in V. californicum and V. parviflorum [6,14,15,42,45]. Cyclopamine is classified as a jervanine-type steroidal alkaloid with a C-nor-D-homosteroidal skeleton, where the C and D rings of the steroidal backbone are five- and six-membered rings, respectively [6]. Jervanine-type alkaloids feature a tetrahydrofuran E-ring that links the nitrogen containing F-ring to the D-ring through a spiro-carbon at the cyclic ether [6]. This compound has been observed to inhibit Hedgehog signaling in Shh Light II cells, inhibit the growth of breast cancer, induce apoptosis in human prostate cancer, increase the expression of death receptor 5 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistant gastric cancer cells, induce apoptosis and COX-2 overexpression via PKC activation in HEL and TF1a human erythroleukemia cell lines, and induce growth inhibition in human carcinogenesis of cholangiocarcinoma cell lines [42,48,49,50,51,52]. Cyclopamine shows promise as a human chemotherapy; however, it has limited use due to its low solubility in aqueous solutions (~5 µg/mL), and instability in acidic conditions [53,54]. To addresses these limitations, semi-synthetic approaches have been undertaken to increase potency and solubility [54,55]. The cyclopamine derivative KAAD-cyclopamine included the addition of a 3-keto,N-aminoethyl aminocaproyl dihydrocinnamoyl (KAAD) functional group to the F-ring nitrogen; this resulted in a 10-20 fold increase in potency [53,55]. A cyclopamine-tartrate salt was developed to increase the solubility of the compound in water [53,56]. The cyclopamine-tartrate salt was more soluble in water at about 5 mg/mL, had a higher LD50 of 62.5 mg/kg of body weight compared to that of cyclopamine, which is 43.5 mg/kg, and had a lower tumor area value in Krt6a-cre: Ptch1neo/neo mice [56]. Cyclopamine has been used as a molecular scaffold for the semi-synthetic derivative patidegib (Figure 5), which is currently undergoing phase III clinical trials. Patidegib, formerly known as saridegib and IPI-926, has received orphan drug approval for the treatment of nevoid basal cell carcinoma [5,57].
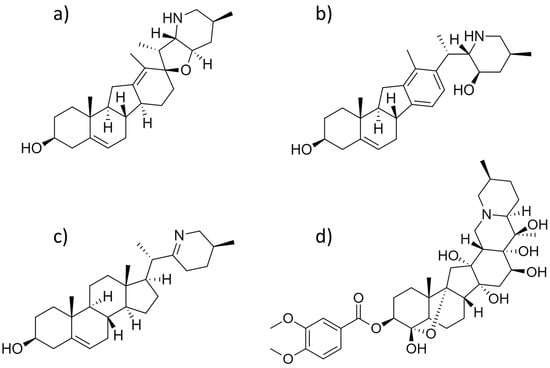
Figure 4.
Chemical structures of (a) cyclopamine, (b) veratramine, (c) verazine, and (d) veratridine.
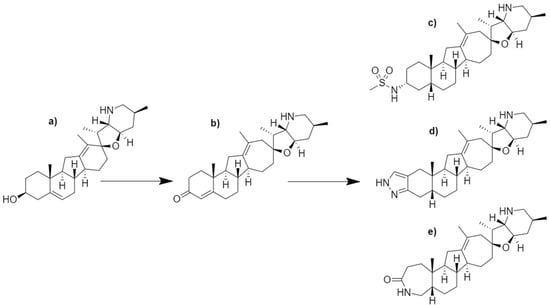
Figure 5.
Chemical structures of (a) cyclopamine and its semi-synthetic (b–e) analogs. Initial modifications led to the production of the (b) α/β-unsaturated ketone analog with improved chemical stability and aqueous solubility. Successive studies produced three lead compounds: (c) methyl sulfonamide analog, (d) pyrazole analog, and the (e) lactam analog. Compound (c) was named saridegib, now known as patidegib.
Patidegib was developed through a series of structure–activity relationship (SAR) studies focused on the improvement of potency, aqueous solubility, and chemical stability for cyclopamine [54,58]. In acidic environments, cyclopamine readily converts to veratramine due to the acid-catalyzed opening of the spirotetrahydrofuran E-ring and aromatization of the D-ring [54]. Veratramine possesses the ability to cause neurotoxic effects and hemolysis; thus, removing the potential degradation of cyclopamine, or its analogues, was advantageous to furthering the modification of the cyclopamine skeleton [54,59,60,61]. Tremblay et al. (2008) describe two modifications, a D-ring expansion and the formation of an α/β-unsaturated ketone, to cyclopamine [54]. These modifications not only improved chemical stability in simulated gastric fluid from 60% remaining to 98% remaining following a 60 min incubation, but also maintained Hh inhibitory properties equivalent to cyclopamine [54]. This analog (Figure 5b) was administered to CD-1 mice orally and intravenously resulting in an observed 80% oral bioavailability and elimination half-life of 3.2 h. A successive study by Tremblay et al. (2009) sought to improve upon the previous study by modifying the A-ring system [58]. Although the cyclopamine analog developed in 2008 exhibited improved chemical stability and aqueous solubility, the α/β-unsaturated ketone in the A-ring was observed to be readily metabolized [58]. Through exploration of SAR surrounding the A-ring, three lead compounds emerged with improved bioactivity and metabolic stability (Figure 5c–e). A cis-ring fusion system showed an improved bioactivity while the addition of the sulfonamide (Figure 5c), pyrazole (Figure 5d), and lactam (Figure 5e) functionalities increased metabolic stability. All three lead compounds outperformed the previous analog (Figure 5b); however, the sulfonamide containing analog, now known as patidegib, outperformed the other compounds regarding efficacy and pharmacokinetics [58].
2.4.2. Veratramine
Veratramine (Figure 4b) has been identified in V. parviflorum, V. viride, V. oxysepalum, V. nigrum L., V. californicum, and V. grandiflorum [15,42,62,63,64,65]. Similar to cyclopamine, veratramine contains a C-nor-D-homosteroidal skeleton; however, it is further categorized as a veratramine-type alkaloid [6]. In comparison to jervanine-type alkaloids, those of the veratranine-type feature an aromatized D-ring and lack a tetrahydrofuran E-ring that connects the piperidine ring to the D-ring. Veratramine is a lipid-soluble alkaloid that exhibits a range of bioactivities [66]. This alkaloid was observed to cause DNA damage in the cerebellum and cerebral cortex of mice in a dose-dependent trend through the generation of reactive oxygen species; moreover, it inhibited Hedgehog signaling in Shh light II cells, reduced the growth, proliferation, and migration of the PC-3 human metastatic prostate cancer cell line, and induced autophagy-mediated apoptosis by inhibiting PI3K/Akt/mTOR signaling in HepG2 cells [14,42,66,67,68,69]. One study regarding the metabolism of veratramine in male Sprague–Dawley rats suggested that elimination of the alkaloid primarily occurred through phenyl mono-oxidation, hydroxylation, and methylation [66]. The phenyl-oxidation metabolite of veratramine was proposed to lead to the formation of reactive oxygen species that oxidize DNA and proteins [66].
2.4.3. Verazine
Verazine (Figure 4c) is a precursor to steroidal alkaloids, including cyclopamine and veratramine, found across the Melanthiaceae and Solanaceae plant families [6,15,70,71,72,73,74]. This compound is classified as a verazine-type steroidal alkaloid in the cyclopentanophenanthrene skeleton ring system [6]. The cyclopentanophenanthrene skeleton features a ring scaffold typical of cholesterol, where the C-ring and D-ring are six- and five-membered, respectively [6]. Verazine-type alkaloids are differentiated from additional cyclopentanophenanthrene skeleton alkaloids by the presence of an imine-containing ring [6]. The importance of verazine to the biosynthesis of Veratrum steroidal alkaloids has promoted efforts to elucidate its biosynthetic production from cholesterol [74]. Augustin et al. identified cholesterol 22-hydroxylase (CYP90B27), 22-hydroxycholesterol, 26-hydroxylase/oxidase (CYP94N1), 22-hydroxycholesterol-26-al transaminase (GABAT1), and 22-hydroxy-26-aminocholesterol 22-oxidase (CYP90G1) as the four enzymes that transform cholesterol into verazine [74]. Although these efforts illustrated how verazine forms, proceeding steps in the biosynthetic formation of additional steroidal alkaloids remain largely unexplored. Kaneko et al. performed a series of studies that observed the conversion of products within the biosynthetic pathway; however, the mechanisms in which these conversions take place remain unknown [75,76,77,78,79,80,81,82]. Verazine has not been studied for potential anticancer properties, however, the alkaloid does exhibit antifungal and melanogenesis inhibitory properties [72,73]. The growth of Candida albicans and Trichophyton rubrum was inhibited at minimum inhibitory concentrations of 6.2 µg/mL and 3.1 µg/mL, respectively [74]. Furthermore, melanogenesis in B16 F1 mouse melanoma cells were inhibited with an IC50 of <1 µg/mL [72]. Verazine also showed inhibitory activity for Sc7 yeast; however, it proved to be cytotoxic in an M-109 cell line with an IC50 of 12.5 µg/mL [70].
2.4.4. Veratridine
Veratridine (Figure 4d) has been identified in V. album, V. viride, V. parviflorum, and Schoenocaulon officinale [15,31,36,83]. This compound is classified as a cevanine-type alkaloid with a C-nor-D-homosteroidal skeleton [6]. The cevanine alkaloids are defined by the presence of a six-membered E-ring, being highly hydroxylated, and a hemiketal linkage between C4 and C9 [6]. This compound is primarily recognized as one of the major alkaloids contributing to the cardiotoxic effects from Veratrum poisoning [15,27,31,84]. Veratridine has been identified as an agonist of voltage-gated sodium ion channels [85]. The compound binds to the type 2 receptor of voltage-gated sodium ion channels, leading to membrane depolarization and repetitive firing of the nerve [33,35,36,85]. Unlike cyclopamine and veratramine, veratridine has not been observed to inhibit Hedgehog signaling through the antagonism of Smo. Belgacem and Borodinsky used veratridine to study the effects of a Ca2+ spike on Gli transcriptional activity [86]. Veratridine selectively inhibits voltage-gated Na+ ion channels, resulting in an increase of Ca2+ spike activity and diminished Gli levels; in turn, this downregulates Sonic hedgehog (Shh) signaling [86]. In contrast, if voltage-gated Na+ and Ca2+ ion channels were blocked, Gli transcriptional activity would increase, resulting in the upregulation of Shh signaling [86]. These results suggested that Shh signaling may be selectively regulated by a Veratrum steroidal alkaloid in mechanisms other than Smo antagonism.
3. Conclusions
Veratrum spp. have been investigated for their potential to inhibit the growth of cancers, such as basal cell carcinoma and acute myeloid leukemia, resulting from aberrant activation of the Hedgehog signaling pathway. Although species including V. viride provide precedent for the phytochemistry of V. parviflorum, only four alkaloids (cyclopamine, veratramine, verazine, and veratridine) with known bioactivities have been identified in V. parviflorum. However, over forty-three prominent peaks can be observed in the chromatogram of its ethanolic extract [15]. A combined effort to characterize these unknown alkaloids and assess their capability to antagonize Hh signaling may yield a compound suitable for further study.
Although these compounds have undergone extensive study over the last century, there are gaps in current research that limit the development of Veratrum steroidal alkaloids. Few alkaloids may be found in online databases with up-to-date spectral data. With each Veratrum spp. containing >100 alkaloids and approximately 20% of those alkaloids receiving full characterization, reducing the likelihood of repeated discovery would make the natural product drug discovery process more efficient. Lu et al. utilized ITS2 sequence and metabolite profiling of Veratrum steroidal alkaloids to distinguish species [87]. A continuation of such efforts may prove fruitful for the rapid identification of alkaloids within Veratrum biomass extracts. An additional deficiency in current Veratrum research is with respect to the biosynthesis of the structurally complex alkaloids. There may be a demand in the future for low-cost production of cyclopamine if the semi-synthetic drug patidegib receives full approval by the FDA. The chemical synthesis of cyclopamine is relatively complex and low yield. Giannis et al. reported a diastereoselective and biomimetic synthetic procedure for cyclopamine with over twenty steps and a 1% overall yield [88]. Furthermore, efforts to cultivate Veratrum proved challenging due to the slow growth rate, temperature requirements, and low germination rates [6]. Only four enzymes that catalyze the biosynthesis of cyclopamine have been characterized, leaving the majority of the pathway unknown [74]. Next-generation sequencing technology has been applied to V. nigrum to identify candidate genes involved in the biosynthesis of the Veratrum steroidal alkaloid jervine [89]. Such technologies may be beneficial for works dedicated to cyclopamine biosynthesis.
Author Contributions
Conceptualization, J.T.S. and O.M.M.; methodology, J.T.S. and O.M.M.; validation, J.T.S. and O.M.M.; formal analysis, J.T.S. and O.M.M.; investigation, J.T.S. and O.M.M.; resources, O.M.M.; data curation, O.M.M.; writing—original draft preparation, J.T.S.; writing—review and editing, J.T.S. and O.M.M.; visualization, J.T.S.; supervision, O.M.M.; project administration, O.M.M.; funding acquisition, O.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: NIH Grants #P20GM103408, P20GM109095, and 1C06RR020533; NSF Grants #0619793 and #0923535; Murdock Charitable Trust; and IGEM HERC funding of the Food and Dairy Innovation Center at Boise State University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuruppu, A.I.; Paranagama, P.; Goonasekara, C.L. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm. J. 2019, 27, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Taipale, J.; Cooper, M.K.; Beachy, P.A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002, 16, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Masamune, T.; Mori, Y.; Takasugi, M.; Murai, A.; Ohuchi, S.; Sato, N.; Katsui, N. 11-Deoxojervine, a new alkaloid from Veratrum species. Bull. Chem. Soc. Jpn. 1965, 38, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.; Martinelli, G.; Papayannidis, C.; Cortes, J.E. Hedgehog Pathway Inhibitors: A New Therapeutic Class for the Treatment of Acute Myeloid Leukemia. Blood Cancer Discov. 2020, 1, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.M.; McDougal, O.M. Medicinal history of North American Veratrum. Phytochem. Rev. 2014, 13, 671–694. [Google Scholar] [CrossRef]
- Zomlefer, W.B.; Whitten, W.M.; Williams, N.H.; Judd, W.S. An overview of Veratrum sl (Liliales: Melanthiaceae) and an infrageneric phylogeny based on ITS sequence data. Syst. Bot. 2003, 28, 250–269. [Google Scholar]
- Liao, W.-J.; Yuan, Y.-M.; Zhang, D.-Y. Biogeography and evolution of flower color in Veratrum (Melanthiaceae) through inference of a phylogeny based on multiple DNA markers. Plant Syst. Evol. 2007, 267, 177–190. [Google Scholar] [CrossRef]
- Li, H.-J.; Jiang, Y.; Li, P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat. Prod. Rep. 2006, 23, 735–752. [Google Scholar] [CrossRef]
- Li, H.l.; Tang, J.; Liu, R.; Lin, M.; Wang, B.; Lv, Y.; Huang, H.; Zhang, C.; Zhang, W. Characterization and identification of steroidal alkaloids in the Chinese herb Veratrum nigrum L. by high-performance liquid chromatography/electrospray ionization with multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 869–879. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Alm, T. Norwegian and Sámi Ethnobotany of Veratrum album (Melanthiaceae). SIDA Contrib. Bot. 2002, 20, 611–619. [Google Scholar]
- Rätsch, C.; Hofmann, A. The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications; Inner Traditions—Bear & Company: Rochester, VT, USA, 2005. [Google Scholar]
- Dirks, M.L.; Seale, J.T.; Collins, J.M.; McDougal, O.M. Review: Veratrum californicum Alkaloids. Molecules 2021, 26, 5934. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Turner, M.; Farrell, N.; Zomlefer, W.B.; McDougal, O.M.; Morgan, B.W. Hikers poisoned: Veratrum steroidal alkaloid toxicity following ingestion of foraged Veratrum parviflorum. Clin. Toxicol. 2018, 56, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Zomlefer, W.B. The genera of Melanthiaceae in the southeastern United States. Harv. Pap. Bot. 1997, 2, 133–177. [Google Scholar]
- Watson, S. Contributions to American Botany: Revision of the North American Liliaceae; Descriptions of Some New Species of North American Plants. Proc. Am. Acad. Arts Sci. 1878, 14, 213–303. [Google Scholar] [CrossRef]
- Thompson, Y.; D’Angelo, E.; Karathanasis, A.; Sandefur, B.C. Plant community composition as a function of geochemistry and hydrology in three Appalachian wetlands. Ecohydrology 2012, 5, 389–400. [Google Scholar] [CrossRef]
- Brundage, S.; Lady Bird Johnson Wildflower Center. Veratrum parviflorum. Available online: https://www.wildflower.org/gallery/result.php?id_image=66638 (accessed on 30 March 2022).
- Annable, C. Melanthium Parviflorum; NatureServe: Arlington, VI, USA, 2022. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Germination Ecophysiology of Herbaceous Plant Species in a Temperate Region. Am. J. Bot. 1988, 75, 286–305. [Google Scholar] [CrossRef]
- Atwood, E.L. White-tailed deer foods of the United States. J. Wildl. Manag. 1941, 5, 314–332. [Google Scholar] [CrossRef]
- Jaffe, A.M.; Gephardt, D.; Courtemanche, L. Poisoning due to ingestion of Veratrum viride (false hellebore). J. Emerg. Med. 1990, 8, 161–167. [Google Scholar] [CrossRef]
- Prince, L.; Stork, C. Prolonged cardiotoxicity from poison lilly (Veratrum viride). Vet. Hum. Toxicol. 2000, 42, 282–285. [Google Scholar] [PubMed]
- Zagler, B.; Zelger, A.; Salvatore, C.; Pechlaner, C.; Giorgi, F.D.; Wiedermann, C.J. Dietary poisoning with Veratrum album—A report of two cases. Wien. Klin. Wochenschr. 2005, 117, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.D.; Price, J.H.; Holstege, C.P. Intoxication with a Ramp (Allium tricocca) Mimicker. Wilderness Environ. Med. 2010, 21, 61–63. [Google Scholar] [CrossRef]
- Gilotta, I.; Brvar, M. Accidental Poisoning with Veratrum album Mistaken for Wild Garlic (Allium ursinum). Clin. Toxicol. 2010, 48, 949–952. [Google Scholar] [CrossRef]
- Melnik, E.V.; Belova, M.V.; Potskhveriya, M.M.; Simonova, A.Y.; Tyurin, I.A.; Ramenskaya, G.V. Veratrum Alkaloid Determination in Four Cases of Veratrum Aqua Poisonings. J. Anal. Toxicol. 2022, 46, 42–47. [Google Scholar] [CrossRef]
- Festa, M.; Andreetto, B.; Ballaris, M.A.; Panio, A.; Piervittori, R. A Case of Veratrum Poisoning. Minerva Anestesiol. 1996, 62, 195–196. [Google Scholar]
- Grobosch, T.; Binscheck, T.; Martens, F.; Lampe, D. Accidental Intoxication with Veratrum album. J. Anal. Toxicol. 2008, 32, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, Y.; Pepin, G. LC-EI-MS Determination of Veratridine and Cevadine in Two Fatal Cases of Veratrum album Poisoning. J. Anal. Toxicol. 2001, 25, 481–485. [Google Scholar] [CrossRef]
- Bechtel, L.; Lawrence, D.; Haverstick, D.; Powers, J.; Wyatt, S.; Croley, T.; Holstege, C. Ingestion of False Hellebore Plants Can Cross-React with a Digoxin Clinical Chemistry Assay. Clin. Toxicol. 2010, 48, 435–442. [Google Scholar] [CrossRef]
- Schep, L.J.; Schmierer, D.M.; Fountain, J.S. Veratrum Poisoning. Toxicol. Rev. 2006, 25, 73–78. [Google Scholar] [CrossRef]
- Turner, M.W.; Rossi, M.; Campfield, V.; French, J.; Hunt, E.; Wade, E.; McDougal, O.M. Steroidal alkaloid variation in Veratrum californicum as determined by modern methods of analytical analysis. Fitoterapia 2019, 137, 104281. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wang, G.K. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal. 2003, 15, 151–159. [Google Scholar] [CrossRef]
- Ulbricht, W. Effects of Veratridine on Sodium Currents and Fluxes. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 1998; Volume 133, pp. 1–54. [Google Scholar]
- Jarisch, A.; Richter, H. Die Kreislaufwirkung des Veratrins. Archiv f. experiment. Pathol. Pharmakol. 1939, 193, 347–354. [Google Scholar] [CrossRef]
- Schaefer, H. Elektrophysiologie der Herznerven. Rev. Physiol. Biochem. Pharmacol. 1950, 46, 71–125. [Google Scholar]
- von Bezold, A. Uber die Physiologischen Wirkungen des Essigsauren Veratrins. Unters. Aus. Dem. Physiol. Lab. Wurzbg. 1867, 1, 75–156. [Google Scholar]
- Cooper, M.K.; Porter, J.A.; Young, K.E.; Beachy, P.A. Teratogen-Mediated Inhibition of Target Tissue Response to Shh Signaling. Science 1998, 280, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, F.; Li, X.; Xu, S.; Huang, W.; Ye, Y. Three New Alkaloids from Veratrum grandiflorum Loes with Inhibition Activities on Hedgehog Pathway. Bioorganic Med. Chem. Lett. 2016, 26, 4735–4738. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.W.; Cruz, R.; Elwell, J.; French, J.; Mattos, J.; McDougal, O.M. Native V. californicum Alkaloid Combinations Induce Differential Inhibition of Sonic Hedgehog Signaling. Molecules 2018, 23, 2222. [Google Scholar] [CrossRef] [PubMed]
- Binns, W.; James, L.F.; Shupe, J.L.; Thacker, E.J. Cyclopian-Type Malformation in Lambs. Arch. Environ. Health 1962, 5, 106–108. [Google Scholar] [CrossRef]
- Binns, W.; James, L.F.; Shupe, J.L. Toxicosis of Veratrum californicum in Ewes and its Relationship to a Congenital Deformity in Lambs. Ann. N. Y. Acad. Sci. 1964, 111, 571–576. [Google Scholar] [CrossRef]
- Keeler, R.F.; Binns, W. Teratogenic Compounds of Veratrum californicum (Durand) V. Comparison of Cyclopian Effects of Steroidal Alkaloids from the Plant and Structurally Related Compounds from Other Sources. Teratology 1968, 1, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, I.; Liang, J.; Hedeen, D.; Roberts, K.J.; Zhang, Y.; Ha, B.; Latorraca, N.R.; Faust, B.; Dror, R.O.; Beachy, P.A.; et al. Smoothened Stimulation by Membrane Sterols Drives Hedgehog Pathway Activity. Nature 2019, 571, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Gaffield, W.; Kapur, R.P.; Roelink, H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 1998, 125, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, M.; Kalnytska, A.; Palagani, V.; Kossatz, U.; Manns, M.P.; Malek, N.P.; Wilkens, L.; Plentz, R.R. Inhibition of Hedgehog Signaling Attenuates Carcinogenesis In Vitro and Increases Necrosis of Cholangiocellular Carcinoma. Hepatology 2013, 57, 1035–1045. [Google Scholar] [CrossRef]
- Na, Y.J.; Lee, D.H.; Kim, J.L.; Kim, B.R.; Park, S.H.; Jo, M.J.; Jeong, S.; Kim, H.J.; Lee, S.Y.; Jeong, Y.A.; et al. Cyclopamine Sensitizes TRAIL-Resistant Gastric Cancer Cells to TRAIL-Induced Apoptosis Via Endoplasmic Reticulum Stress-Mediated Increase of Death Receptor 5 and Survivin Degradation. Int. J. Biochem. Cell. Biol. 2017, 89, 147–156. [Google Scholar] [CrossRef]
- Shaw, G.; Price, A.M.; Ktori, E.; Bisson, I.; Purkis, P.E.; McFaul, S.; Oliver, R.T.; Prowse, D.M. Hedgehog Signalling in Androgen Independent Prostate Cancer. Eur. Urol. 2008, 54, 1333–1343. [Google Scholar] [CrossRef]
- Zhang, X.; Harrington, N.; Moraes, R.C.; Wu, M.-F.; Hilsenbeck, S.G.; Lewis, M.T. Cyclopamine Inhibition of Human Breast Cancer Cell Growth Independent of Smoothened (Smo). Breast Cancer Res. Treat. 2009, 115, 505–521. [Google Scholar] [CrossRef]
- Ghezali, L.; Leger, D.Y.; Limami, Y.; Cook-Moreau, J.; Beneytout, J.L.; Liagre, B. Cyclopamine and Jervine Induce COX-2 Overexpression in Human Erythroleukemia Cells but Only Cyclopamine has a Pro-Apoptotic Effect. Exp. Cell. Res. 2013, 319, 1043–1053. [Google Scholar] [CrossRef]
- Lee, S.T.; Welch, K.D.; Panter, K.E.; Gardner, D.R.; Garrossian, M.; Chang, C.-W.T. Cyclopamine: From Cyclops Lambs to Cancer Treatment. J. Agric. Food Chem. 2014, 62, 7355–7362. [Google Scholar] [CrossRef]
- Tremblay, M.R.; Nevalainen, M.; Nair, S.J.; Porter, J.R.; Castro, A.C.; Behnke, M.L.; Yu, L.-C.; Hagel, M.; White, K.; Faia, K.; et al. Semisynthetic Cyclopamine Analogues as Potent and Orally Bioavailable Hedgehog Pathway Antagonists. J. Med. Chem. 2008, 51, 6646–6649. [Google Scholar] [CrossRef]
- Taipale, J.; Chen, J.K.; Cooper, M.K.; Wang, B.; Mann, R.K.; Milenkovic, L.; Scott, M.P.; Beachy, P.A. Effects of Oncogenic Mutations in Smoothened and Patched can be Reversed by Cyclopamine. Nature 2000, 406, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Gu, D.; He, M.; Liu, H.; Sheng, T.; Xie, G.; Li, C.-X.; Zhang, X.; Wainwright, B.; Garrossian, A.; et al. Tumor 61 Shrinkage by Cyclopamine Tartrate through Inhibiting Hedgehog Signaling. Chin. J. Cancer 2011, 30, 472–481. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Orphan Drug Designations and Approvals. Patidegib. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=587917 (accessed on 30 March 2022).
- Tremblay, M.R.; Lescarbeau, A.; Grogan, M.J.; Tan, E.; Lin, G.; Austad, B.C.; Yu, L.-C.; Behnke, M.L.; Nair, S.J.; Hagel, M.; et al. Discovery of a Potent and Orally Active Hedgehog Pathway Antagonist (IPI-926). J. Med. Chem. 2009, 52, 4400–4418. [Google Scholar] [CrossRef]
- Badria, F.A.; McChesney, J.D.; Halim, A.F.; Zaghloul, A.M.; El Sayed, K.A. Time course and inhibition of stavaroside K, veratramine and cevine-induced hemolysis by other pregnane glycosides and Veratrum alkaloids. Pharmazie 1995, 50, 421–423. [Google Scholar]
- Nagata, R.; Izumi, K. Veratramine-induced behavior associated with serotonergic hyperfunction in mice. Jpn. J. Pharmacol. 1991, 55, 129–137. [Google Scholar] [CrossRef]
- Thron, C.D.; McCann, F.V. Pharmacological tests of the mechanism of the periodic rhythm caused by veratramine in the sinoatrial node of the guinea pig. Gen. Pharmacol. 1999, 32, 81–89. [Google Scholar] [CrossRef]
- El Sayed, K.A.; McChesney, J.D.; Halim, A.F.; Zaghloul, A.M.; Lee, I.-S. A Study of Alkaloids in Veratrum viride Aiton. Int. J. Pharmacogn. 1996, 34, 161–173. [Google Scholar] [CrossRef]
- Saito, K. Veratramine, a New Alkaloid of White Hellebore (Veratrum grandifiorum Loes. fil.). Bull. Chem. Soc. Jpn. 1940, 15, 22–27. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Liu, Y. Hypotensive Effect and Toxicology of Total Alkaloids and Veratramine from Roots and Rhizomes of Veratrum nigrum L. in Spontaneously Hypertensive Rats. Pharmazie 2008, 63, 606–610. [Google Scholar]
- Yu, Y.; Li, H.; Jiang, Y. Separation and Preparation of Five Cyclopamine Analogs from Rhizomes of Veratrum oxysepalum Turcz. by Two-Step HighSpeed Counter-Current Chromatography. Sep. Sci. Technol. 2014, 49, 2748–2755. [Google Scholar] [CrossRef]
- Cong, Y.; Guo, J.; Tang, Z.; Lin, S.; Zhang, Q.; Li, J.; Cai, Z. Metabolism Study of Veratramine Associated with Neurotoxicity by Using HPLC–MSn. J. Chromatogr. Sci. 2014, 53, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.W.; Cruz, R.; Mattos, J.; Baughman, N.; Elwell, J.; Fothergill, J.; Nielsen, A.; Brookhouse, J.; Bartlett, A.; Malek, P.; et al. Cyclopamine Bioactivity by Extraction Method from Veratrum californicum. Bioorg. Med. Chem. 2016, 24, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; El Sayed, K.A. The Veratrum Alkaloids Jervine, Veratramine, and their Analogues as Prostate Cancer Migration and Proliferation Inhibitors: Biological Evaluation and Pharmacophore Modeling. Med. Chem. Res. 2013, 22, 4775–4786. [Google Scholar] [CrossRef]
- Yin, L.; Xia, Y.; Xu, P.; Zheng, W.; Gao, Y.; Xie, F.; Ji, Z. Veratramine suppresses human HepG2 liver cancer cell growth in vitro and in vivo by inducing autophagic cell death. Oncol. Rep. 2020, 44, 477–486. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Bahler, B.D.; Malone, S.; Werkhoven, M.C.; van Troon, F.; David, I.; Wisse, J.H.; Bursuker, I.; Neddermann, K.M.; Mamber, S.W. DNA-damaging steroidal alkaloids from Eclipta alba from the suriname rainforest. J. Nat. Prod. 1998, 61, 1202–1208. [Google Scholar] [CrossRef]
- Colmenares, A.P.; Alarcón, L.; Rojas, L.B.; Mitaine-Offer, A.-C.; Pouységu, L.; Quideau, S.; Paululat, T.; Usubillaga, A.; Lacaille-Dubois, M.-A. New steroidal alkaloids from Solanum hypomalacophyllum. Nat. Prod. Commun. 2010, 5, 1743–1746. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, S.-J.; Kang, S.-H.; Kim, C.-H.; Jung, M.-H.; Jin, M.-H. Three melanogenesis inhibitors from the roots of Veratrum nigrum. Korean J. Pharmacogn. 2002, 33, 399–403. [Google Scholar]
- Kusano, G.; Takahashi, A.; Sugiyama, K.; Nozoe, S. Antifungal properties of Solanum alkaloids. Chem. Pharm. Bull. 1987, 35, 4862–4867. [Google Scholar] [CrossRef]
- Augustin, M.M.; Ruzicka, D.R.; Shukla, A.K.; Augustin, J.M.; Starks, C.M.; O’Neil-Johnson, M.; McKain, M.R.; Evans, B.S.; Barrett, M.D.; Smithson, A.; et al. Elucidating steroid alkaloid biosynthesis in Veratrum californicum: Production of verazine in Sf9 cells. Plant J. 2015, 82, 991–1003. [Google Scholar] [CrossRef]
- Kaneko, K.; Kawamura, N.; Kuribayashi, T.; Tanaka, M.; Mitsuhashi, H.; Koyama, H. Structures of two cevanine alkaloids, shinonomenine and veraflorizine, and a cevanidane alkaloid, procevine, isolated from illuminated Veratrum. Tetrahedron Lett. 1978, 19, 4801–4804. [Google Scholar] [CrossRef]
- Kaneko, K.; Kawamura, N.; Mitsuhashi, H.; Ohsaki, K. Two new Veratrum alkaloids, hosukinidine and epirubijervine from illuminated Veratrum plant. Chem. Pharm. Bull. 1979, 27, 2534–2536. [Google Scholar] [CrossRef][Green Version]
- Kaneko, K.; Mitsuhashi, H.; Hirayama, K.; Ohmori, S. 11-Deoxojervine as a precursor for jervine biosynthesis in Veratrum grandiflorum. Phytochemistry 1970, 9, 2497–2501. [Google Scholar] [CrossRef]
- Kaneko, K.; Mitsuhashi, H.; Hirayama, K.; Yoshida, N. Biosynthesis of C-nor-D-homo-steroidal alkaloids from acetate-1-14C, cholesterol-4-14C and cholesterol-26-14C in Veratrum grandiflorum. Phytochemistry 1970, 9, 2489–2495. [Google Scholar] [CrossRef]
- Kaneko, K.; Seto, H.; Motoki, C.; Mitsuhashi, H. Biosynthesis of rubijervine in Veratrum grandiflorum. Phytochemistry 1975, 14, 1295–1301. [Google Scholar] [CrossRef]
- Kaneko, K.; Tanaka, M.W.; Mitsuhashi, H. Origin of nitrogen in the biosynthesis of solanidine by Veratrum grandiflorum. Phytochemistry 1976, 15, 1391–1393. [Google Scholar] [CrossRef]
- Kaneko, K.; Tanaka, M.W.; Mitsuhashi, H. Dormantinol, a possible precursor in solanidine biosynthesis, from budding Veratrum grandiflorum. Phytochemistry 1977, 16, 1247–1251. [Google Scholar] [CrossRef]
- Kaneko, K.; Watanabe, M.; Taira, S.; Mitsuhashi, H. Conversion of solanidine to jerveratrum alkaloids in Veratrum grandiflorum. Phytochemistry 1972, 11, 3199–3202. [Google Scholar] [CrossRef]
- Maison, G.L.; Gotz, E.; Stutzman, J.W. Relative Hypotensive Activity of Certain Veratrum Alkaloids. J. Pharmacol. Exp. Ther. 1951, 103, 74–78. [Google Scholar]
- Taniguchi, M.; Minatani, T.; Miyazaki, H.; Tsuchihashi, H.; Zaitsu, K. A highly sensitive quantification method for 12 plant toxins in human serum using liquid chromatography tandem mass spectrometry with a quick solid-phase extraction technique. J. Pharm. Biomed. Anal. 2021, 192, 113676. [Google Scholar] [CrossRef]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Belgacem, Y.H.; Borodinsky, L.N. Inversion of Sonic hedgehog action on its canonical pathway by electrical activity. Proc. Natl. Acad. Sci. USA 2015, 112, 4140–4145. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wang, S.; Yin, Z.; Chen, Q.; He, X.; Wang, Q.; Hu, Q.; Gu, Y.; Tang, H.; Xie, H. Identification of Veratrum Species in Pimacao Based on ITS2 Sequences and Steroidal Alkaloids by a Pseudo-Targeted Metabolomics Method. Front. Plant Sci. 2022, 13, 831562. [Google Scholar] [CrossRef] [PubMed]
- Giannis, A.; Heretsch, P.; Sarli, V.; Stößel, A. Synthesis of Cyclopamine Using a Biomimetic and Diastereoselective Approach. Angew. Chem. Int. Ed. Engl. 2009, 48, 7911–7914. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, M.; Ciura, J.; Tyrka, M. Representational Difference Analysis of Transcripts Involved in Jervine Biosynthesis. Life 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).