Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 1: Recovery and Concentration of Indole Contained in Wash Oil by Solvent Extraction)
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Experimental Method
2.2.1. Batch Equilibrium Extraction
2.2.2. Batch Equilibrium Re-Extraction
2.3. Material System and Experimental Conditions
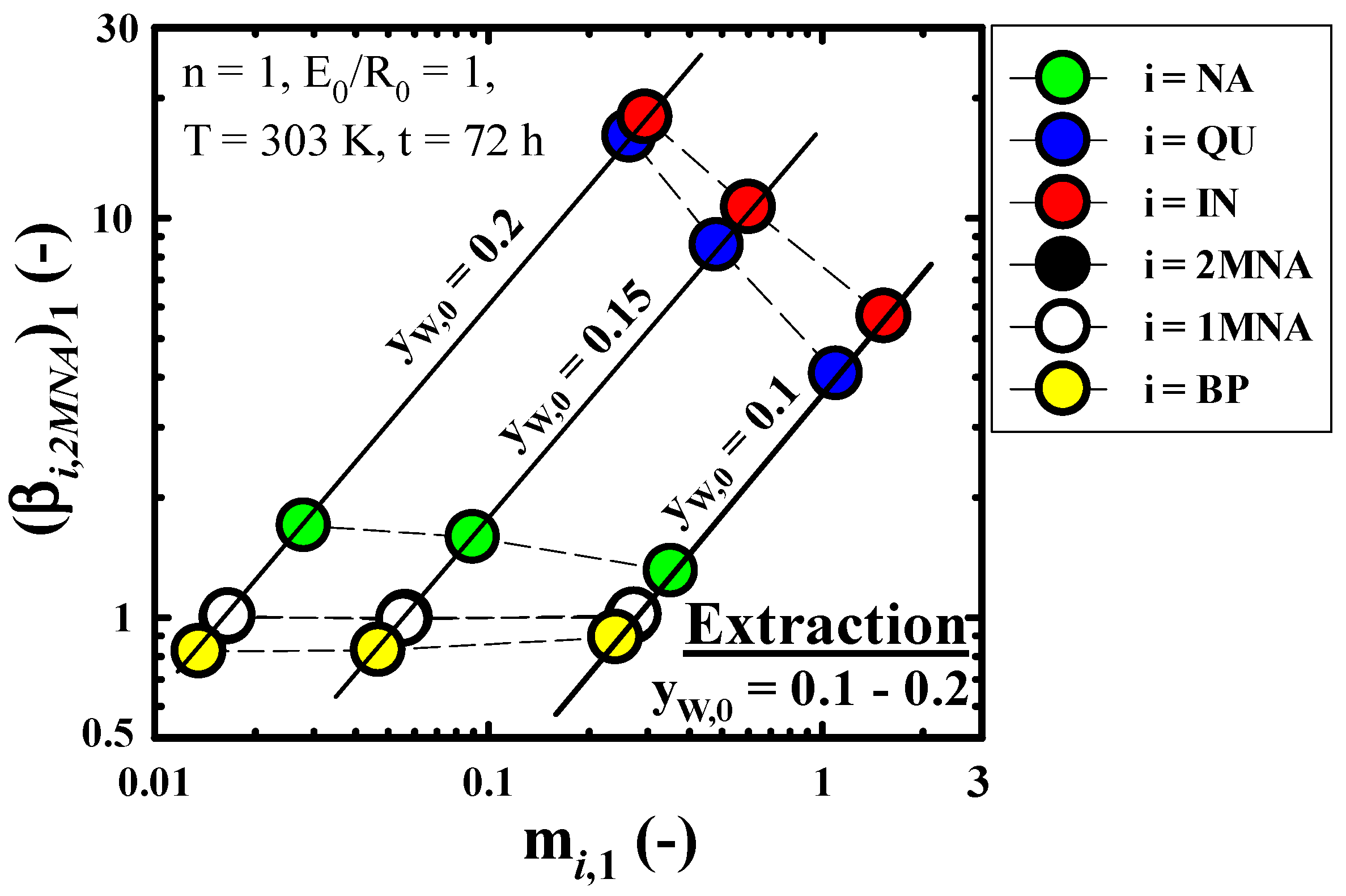
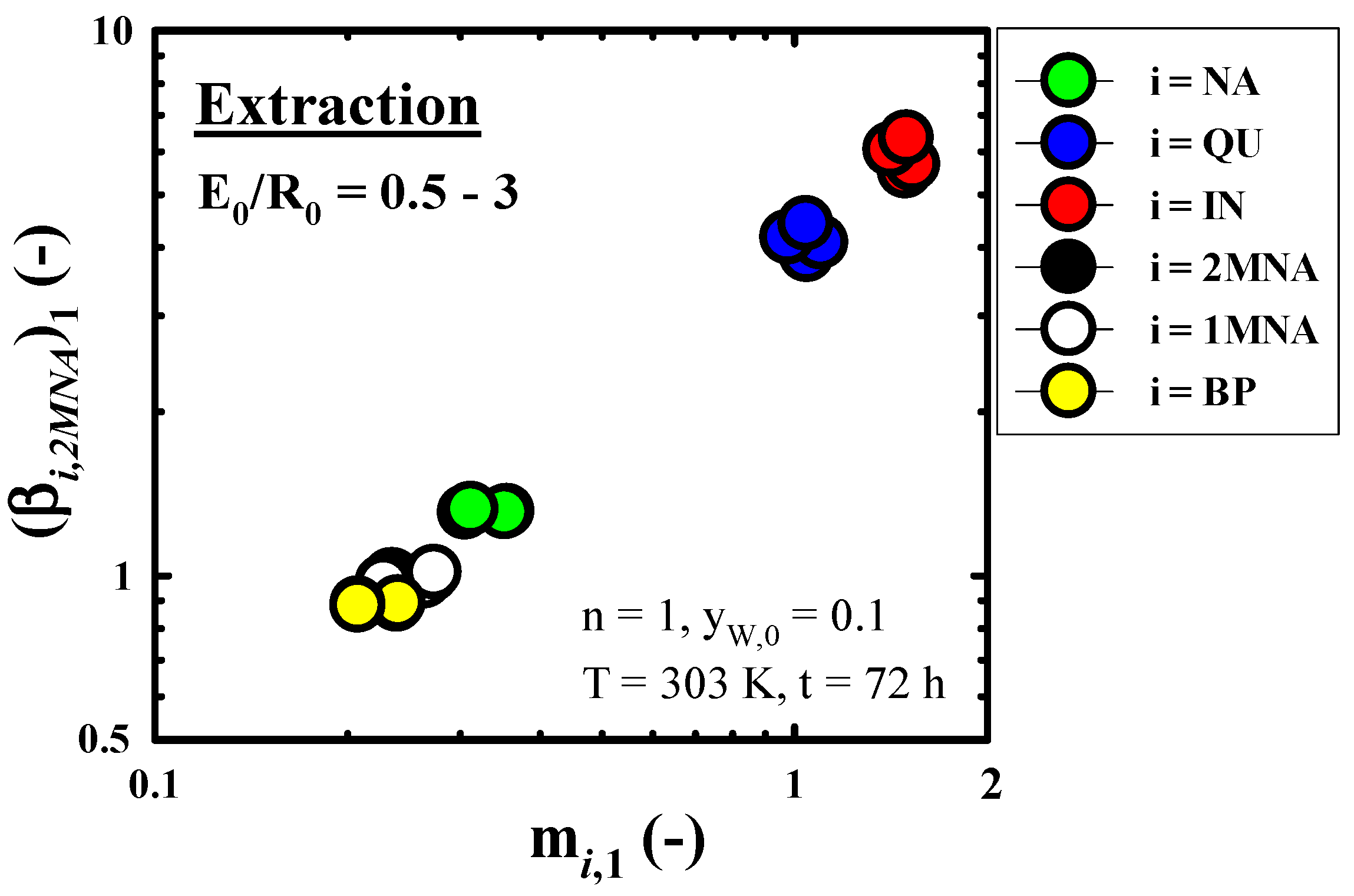
2.3.1. Batch Equilibrium Extraction
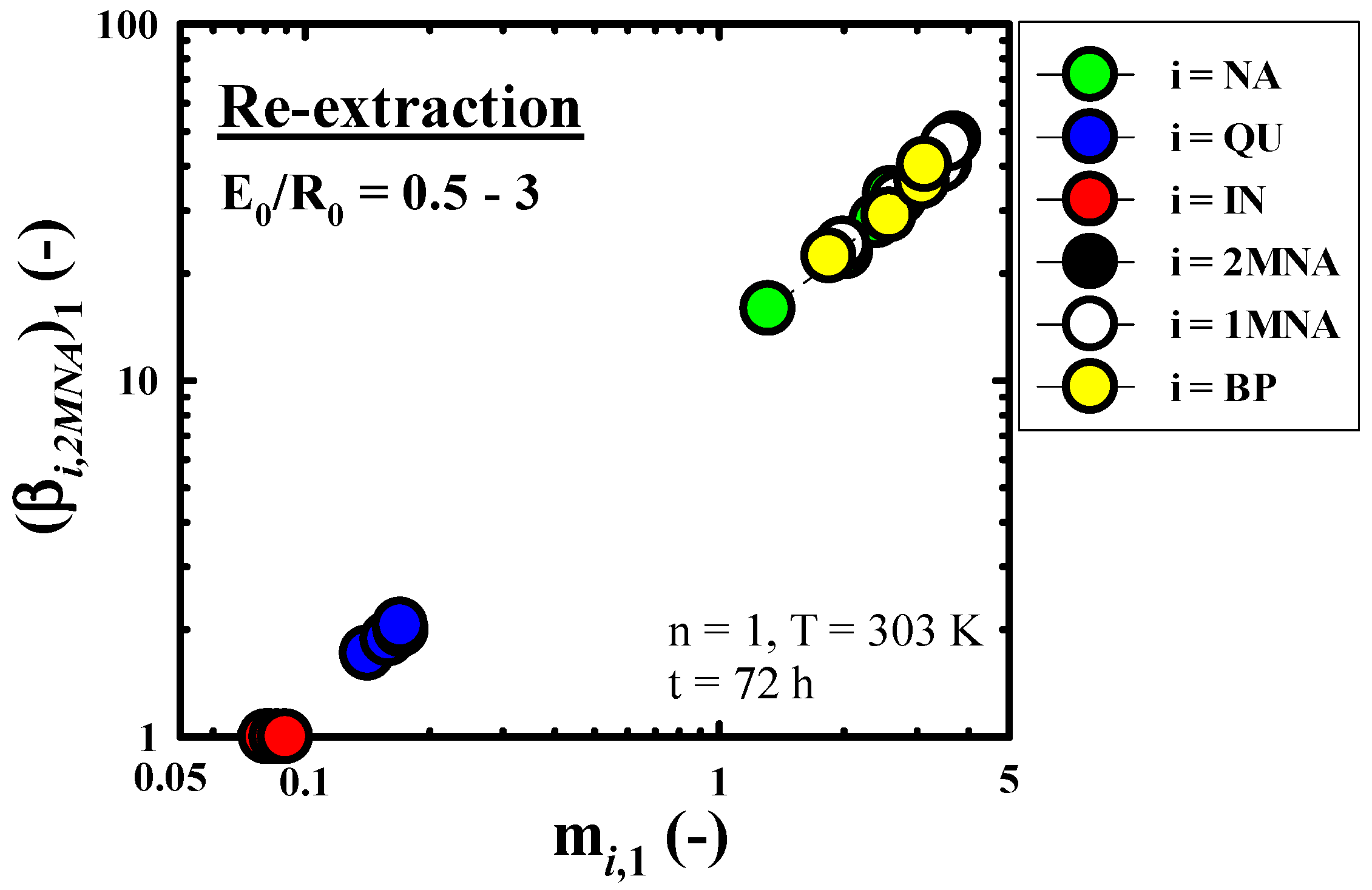
2.3.2. Batch Equilibrium Re-Extraction
3. Results and Discussion
3.1. Definition Equation
3.2. Confirmation of Equilibrium Arrival Time
3.3. Batch Equilibrium Extraction
3.3.1. Gas Chromatogram of Extraction Feed (Wash Oil)
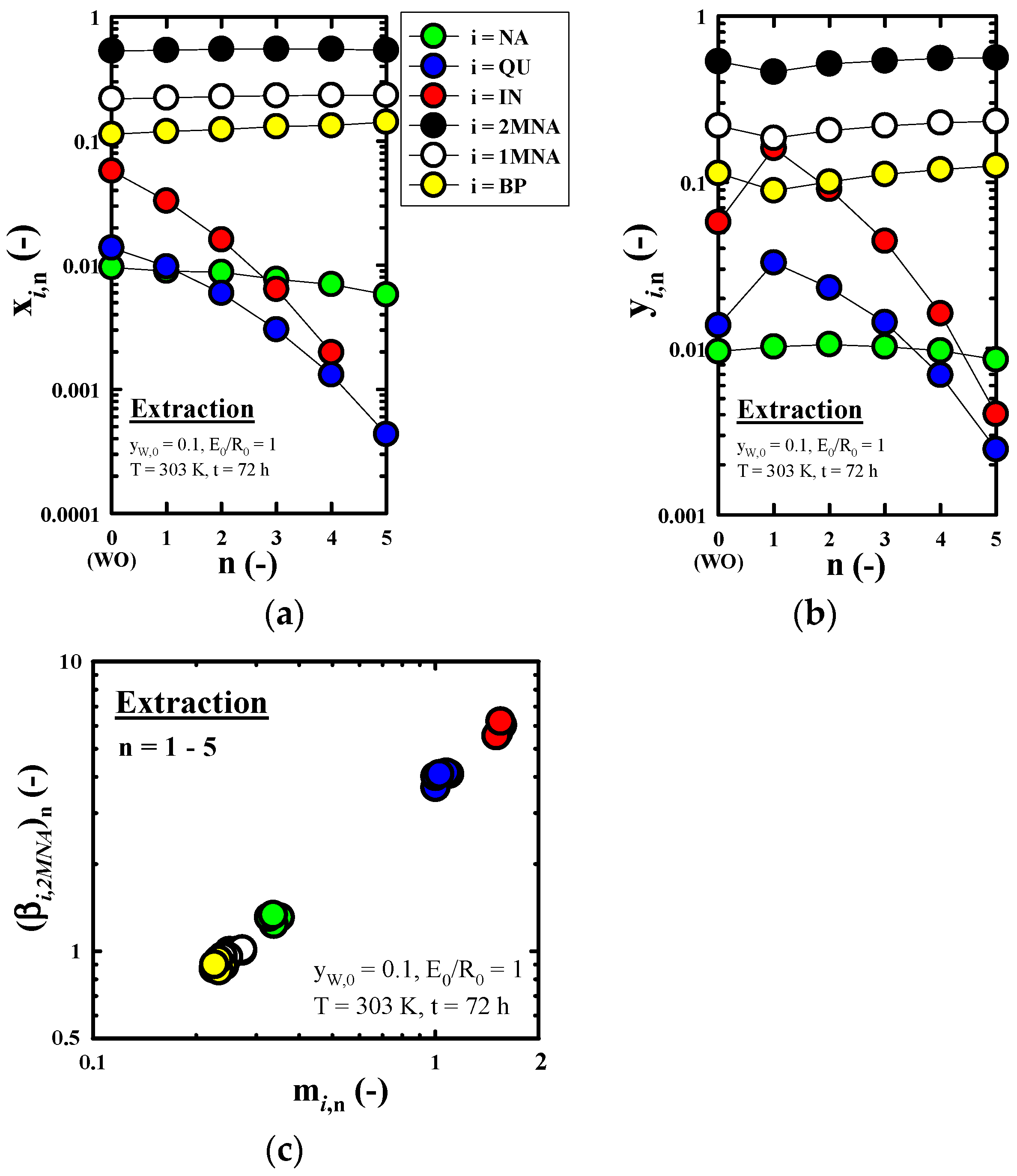
3.3.2. Recovery Performance of IN Contained in Wash Oil
3.4. Batch Equilibrium Re-Extraction
3.4.1. Gas Chromatogram of Re-Extraction Feed (Mixed Extract Phase)
3.4.2. Concentration Performance of IN Contained in Mixed Extract Phase
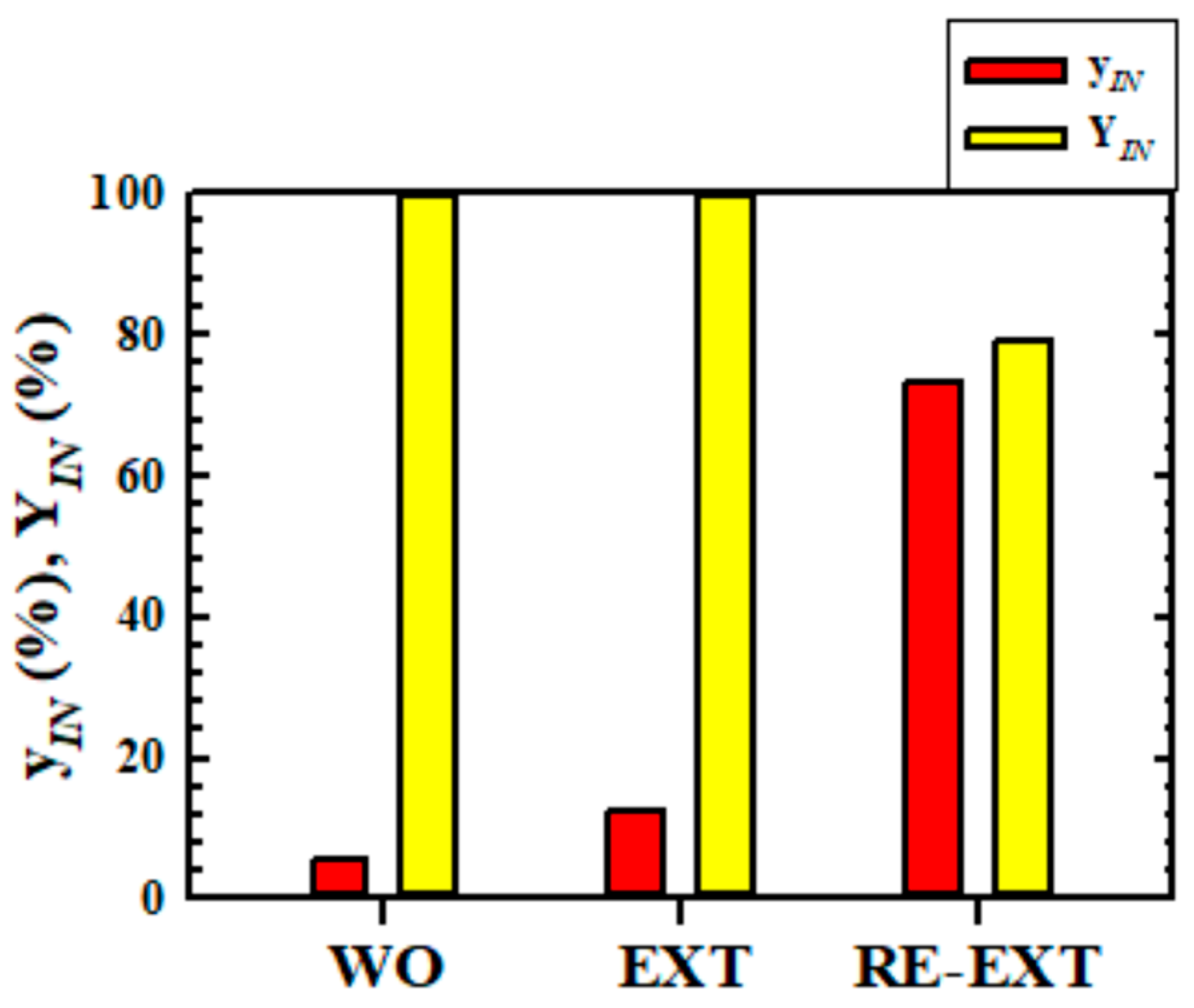
3.5. Changes of Composition and Yield of IN According as Each Operation
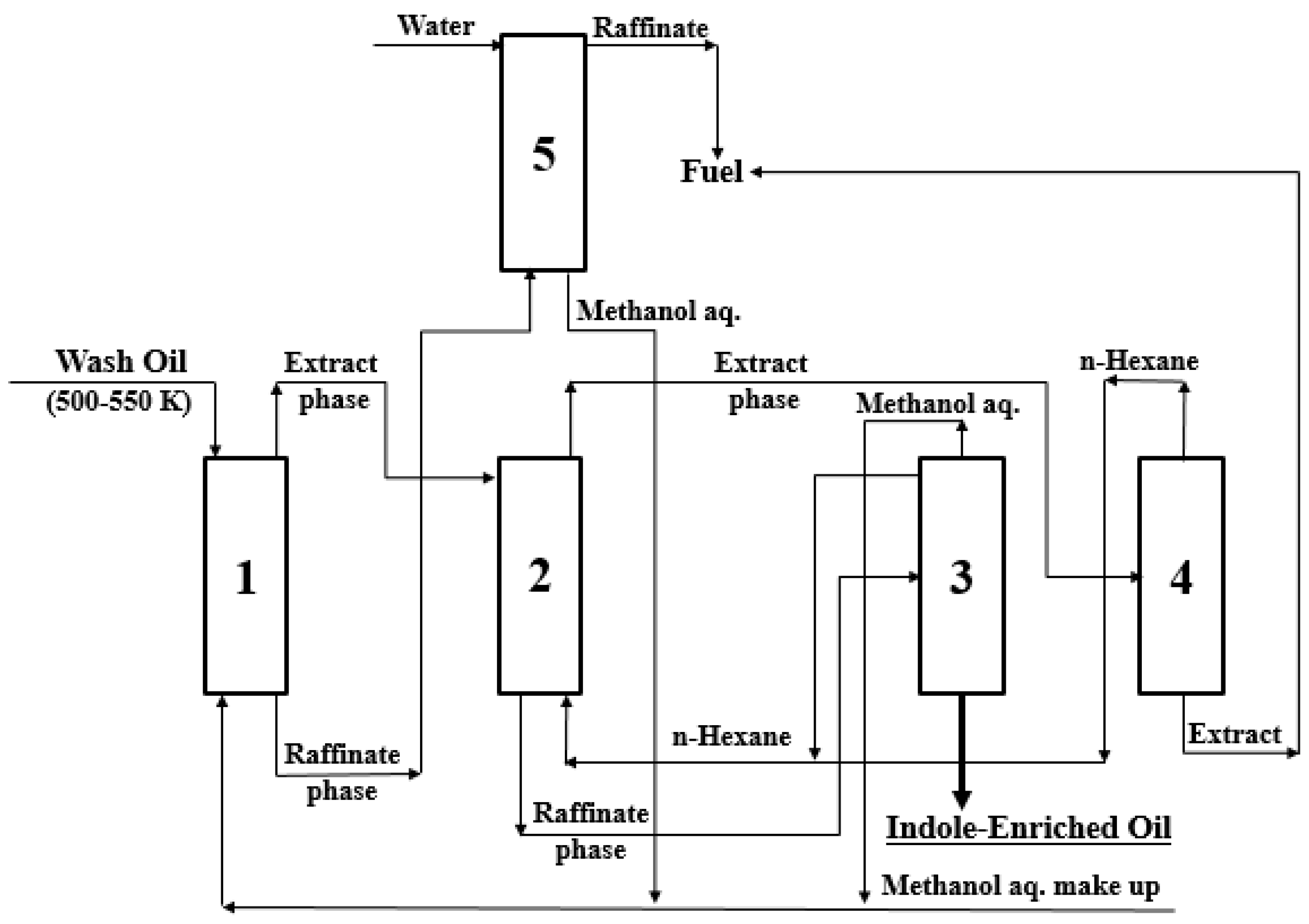
3.6. Recovery and Concentration Process of IN Containing in Wash Oil
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, S.J. Upgrading of wash oil through reduction of nitrogen-containing compounds. Processes 2021, 9, 1869. [Google Scholar] [CrossRef]
- Egashira, R.; Nagai, M. Separation of nitrogen heterocyclic compounds contained in coal tar absorption oil fraction by solvent extraction. J. Jpn. Pet. Inst. 2000, 43, 339–345. [Google Scholar] [CrossRef][Green Version]
- Kim, S.J. Separation and purification of indole in model coal tar fraction of 9 compounds system. Polycycl. Aromat. Compd. 2019, 39, 60–72. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, H.C.; Kim, Y.S.; Jeong, H.J. Liquid membrane permeation of nitrogen heterocyclic compounds contained in model coal tar fraction. Bull. Korean Chem. Soc. 2010, 31, 1143–1148. [Google Scholar] [CrossRef][Green Version]
- Egashira, R.; Salim, C. Solvent extraction of nitrogen heterocyclic compounds contained in coal tar absorption oil fraction. −Improvement of separation performance by addition of aluminum chloride to solvent. J. Jpn. Pet. Inst. 2001, 44, 178–182. [Google Scholar] [CrossRef][Green Version]
- Ukegawa, K.; Matsumura, A.; Kodera, Y.; Kondo, T.; Nakayama, T.; Tanabe, H.; Yoshida, S.; Mito, Y. Solvent extraction of nitrogen compounds from a coal tar fraction. (Part 1). Effect of extraction conditions on the extraction rate and the selectivities of nitrogen compounds. J. Jpn. Pet. Inst. 1990, 33, 250–254. [Google Scholar] [CrossRef][Green Version]
- Xu, D.; Zhang, M.; Gao, J.; Zhang, L.; Zhou, S.; Wang, Y. Separation of heterocyclic nitrogen compounds from coal tar fractions via ionic liquids: COSMO-SAC screening and experimental study. Chem. Eng. Commun. 2019, 206, 1199–1217. [Google Scholar] [CrossRef]
- Jiao, T.; Zhuang, X.; He, H.; Zhao, L.; Li, C.; Chen, H.; Zhang, S. An ionic liquid extraction process for the separation of indole from wash oil. Green Chem. 2015, 17, 3783–3790. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, D.; Gao, J.; Zhou, S.; Zhao, L.; Zhang, Z. Extraction and mechanism for the separation of neutral N-compounds from coal tar by ionic liquids. Fuel 2017, 194, 27–35. [Google Scholar] [CrossRef]
- Sakanishi, K.; Obata, H.; Mochida, I.; Sakaki, T. Removal and recovery of quinoline bases from methylnaphthalene oil in a semicontinuous supercritical CO2 separation apparatus with a fixed bed of supported aluminum sulfate. Ind. Eng. Chem. Res. 1995, 34, 4118–4124. [Google Scholar] [CrossRef]
- Sakanishi, K.; Obata, H.; Mochida, I.; Sakaki, T. Capture and recovery of indole from methylnaphthalene oil in a continuous supercritical CO2 extraction apparatus over a fixed bed of anion-exchange resin. Ind. Eng. Chem. Res. 1996, 35, 335–337. [Google Scholar] [CrossRef]
- Kodera, Y.; Ukegawa, K.; Mito, Y.; Komoto, M.; Ishikawa, E.; Nakayama, T. Solvent extraction of nitrogen compounds from coal liquids. Fuel 1991, 70, 765–769. [Google Scholar] [CrossRef]
- Uemasu, I. Effect of methanol-water mixture solvent on concentration of indole in coal tar using β-cyclodextrin as complexing agent. J. Jpn Pet. Inst. 1991, 34, 371–374. [Google Scholar] [CrossRef][Green Version]
- Uemasu, I.; Nakayama, T. Concentration of indole in coal tar using α-cyclodextrin as the host for inclusion complexation. J. Inclus. Phenom. Molec. Recogn. Chem. 1989, 7, 327–331. [Google Scholar] [CrossRef]
- Mochida, I.; Fei, Y.Q.; Sakanishi, K. Capture and recovery of basic nitrogen species in coal tar pitch, using nickel sulfate as adsorbent. Chem. Lett. 1990, 515–518. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sato, Y.; Ebina, T.; Yokoyama, C.; Takahasi, S.; Mito, Y.; Tanabe, H.; Nishiguchi, N.; Nagaoka, K. Separation of high purity indole from coal tar by high pressure crystallization. Fuel 1991, 70, 565–566. [Google Scholar] [CrossRef]



| Compound | Physical Property | Composition (wt%) | ||
|---|---|---|---|---|
| b.p.*[K] | m.p.**[K] | Extraction | Re-Extraction | |
| Naphthalene (NA, C10H8) | 491 | 351–353 | 0.960 | 0.128 |
| Quinoline (QU, C9H7N) | 511 | 257 | 1.379 | 0.270 |
| Indole (IN, C8H7N) | 526 | 325 | 5.753 | 1.075 |
| 2-Methylnaphthalene (2MNA, C11H10) | 514–515 | 307–309 | 53.251 | 7.198 |
| 1-Methylnaphthalene (1MNA, C11H10) | 513–516 | 251 | 21.854 | 2.785 |
| Biphenyl (BP, C12H10) | 528 | 342 | 11.339 | 1.404 |
| Others | 5.464 | 87.140 (with solvent) | ||
| Extraction | Re-Extraction | ||
|---|---|---|---|
| Feed: Wash Oil, Raffinate Phase * | Feed: Mixed Extract Phase **, Raffinate Phase *** | ||
| Solvent: aqueous methanol solution | Solvent: n-hexane | ||
| Liquid-liquid contact time, t (h) | 12–96 | Liquid-liquid contact time, t (h) | 72 |
| Number of equilibrium extraction, n (-) | 1–5 | Number of equilibrium extraction, n (-) | 1–5 |
| Operating temperature, T (K) | 303 | Operating temperature, T (K) | 303 |
| Volume of fresh solvent added to each stage (E0−E4), (mL) | 320 | Volume of fresh solvent added toeach stage (E0−E4), (mL) | 320 |
| Volume fraction of water in solvent in initial state, yw,0 (-) | 0.1–0.2 | Volume ratio of fresh solvent to feed in initial state, E0/R0 (-) | 0.5–3 |
| Volume ratio of fresh solvent to feed in initial state, E0/R0 (-) | 0.5–3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J. Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 1: Recovery and Concentration of Indole Contained in Wash Oil by Solvent Extraction). Molecules 2022, 27, 5331. https://doi.org/10.3390/molecules27165331
Kim SJ. Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 1: Recovery and Concentration of Indole Contained in Wash Oil by Solvent Extraction). Molecules. 2022; 27(16):5331. https://doi.org/10.3390/molecules27165331
Chicago/Turabian StyleKim, Su Jin. 2022. "Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 1: Recovery and Concentration of Indole Contained in Wash Oil by Solvent Extraction)" Molecules 27, no. 16: 5331. https://doi.org/10.3390/molecules27165331
APA StyleKim, S. J. (2022). Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 1: Recovery and Concentration of Indole Contained in Wash Oil by Solvent Extraction). Molecules, 27(16), 5331. https://doi.org/10.3390/molecules27165331





