Electrochemical Characteristics of Shewanella loihica PV-4 on Reticulated Vitreous Carbon (RVC) with Different Potentials Applied
Abstract
:1. Introduction
2. Results
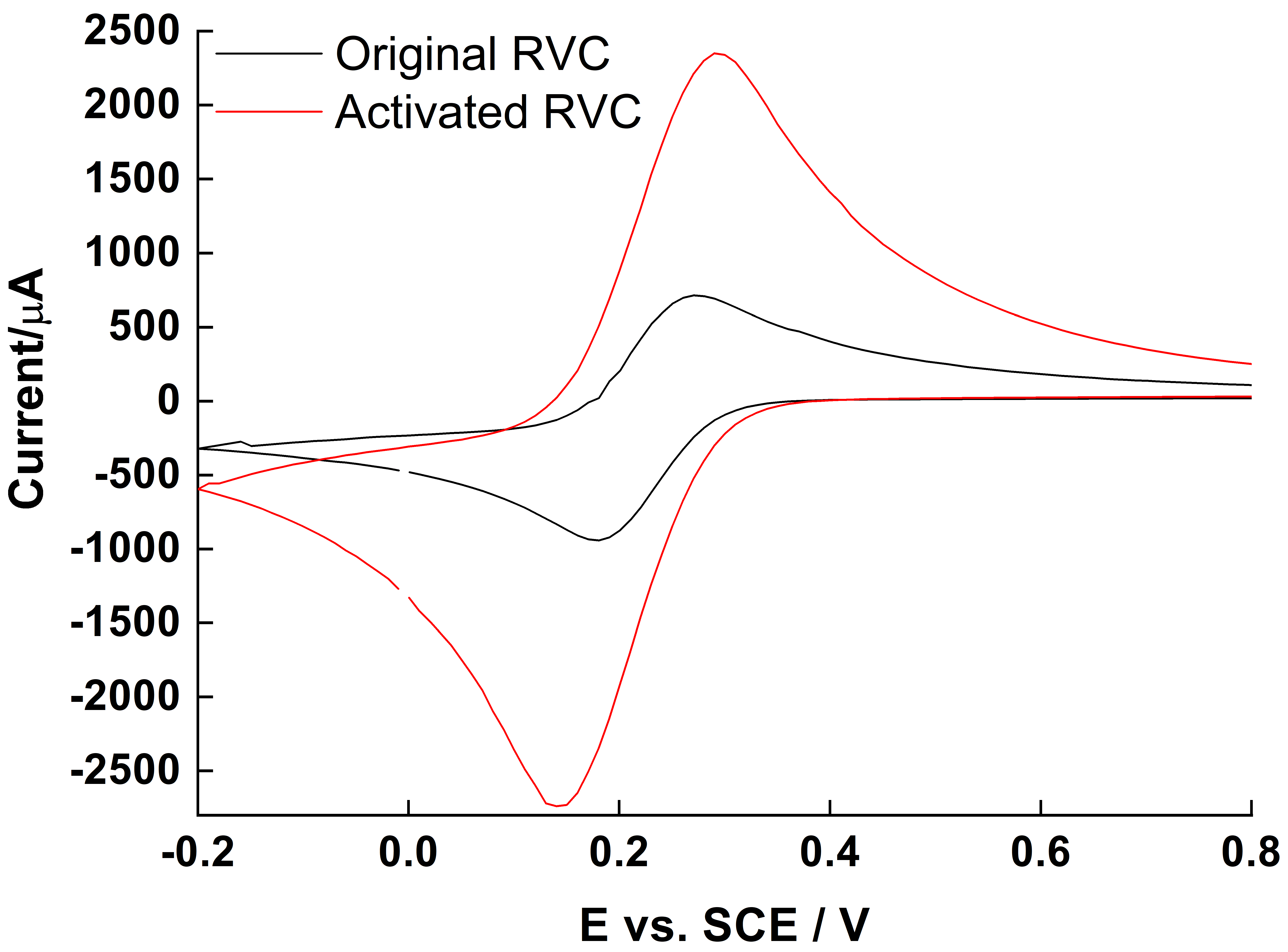
2.1. Oxidation of RVC
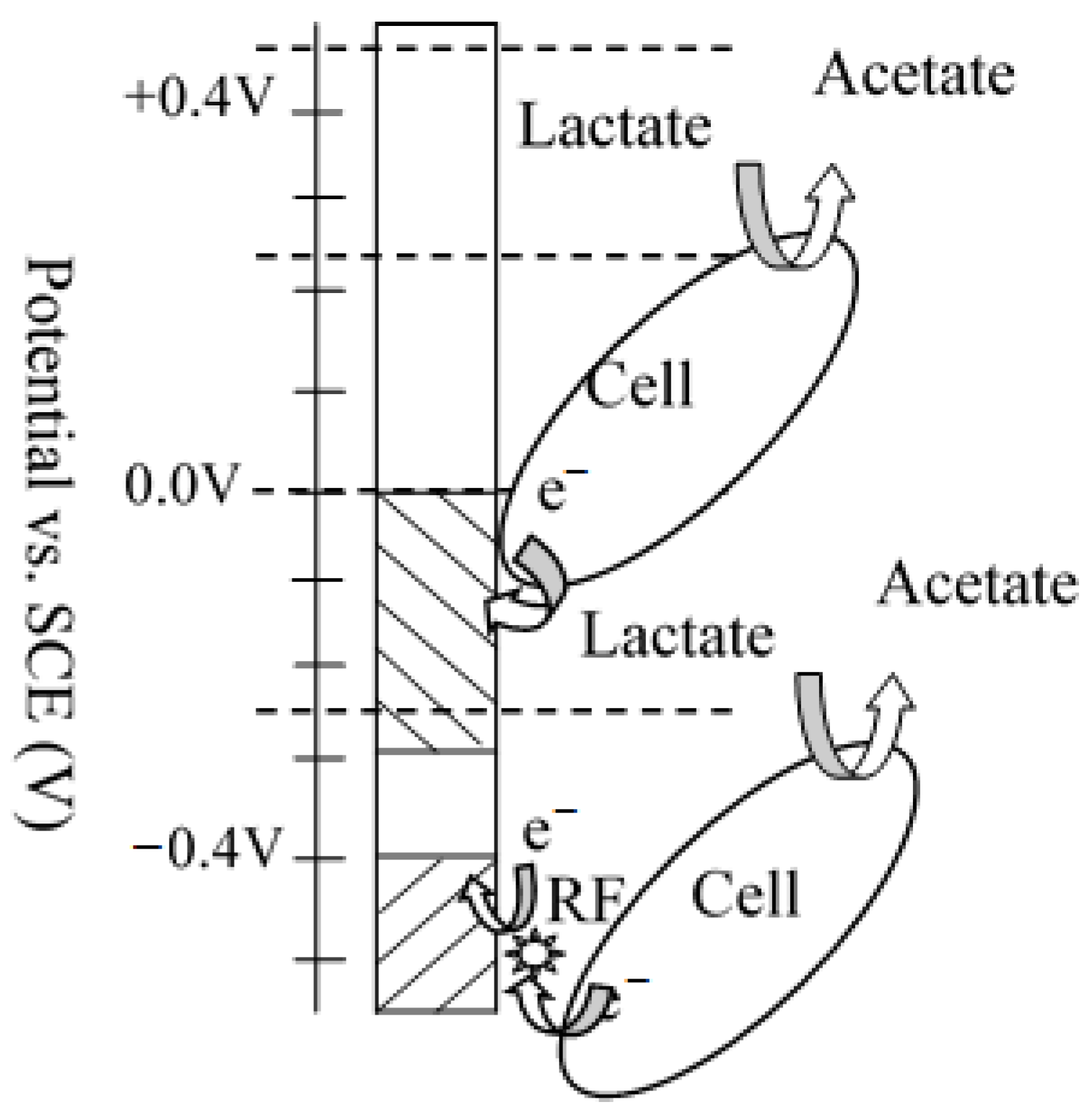
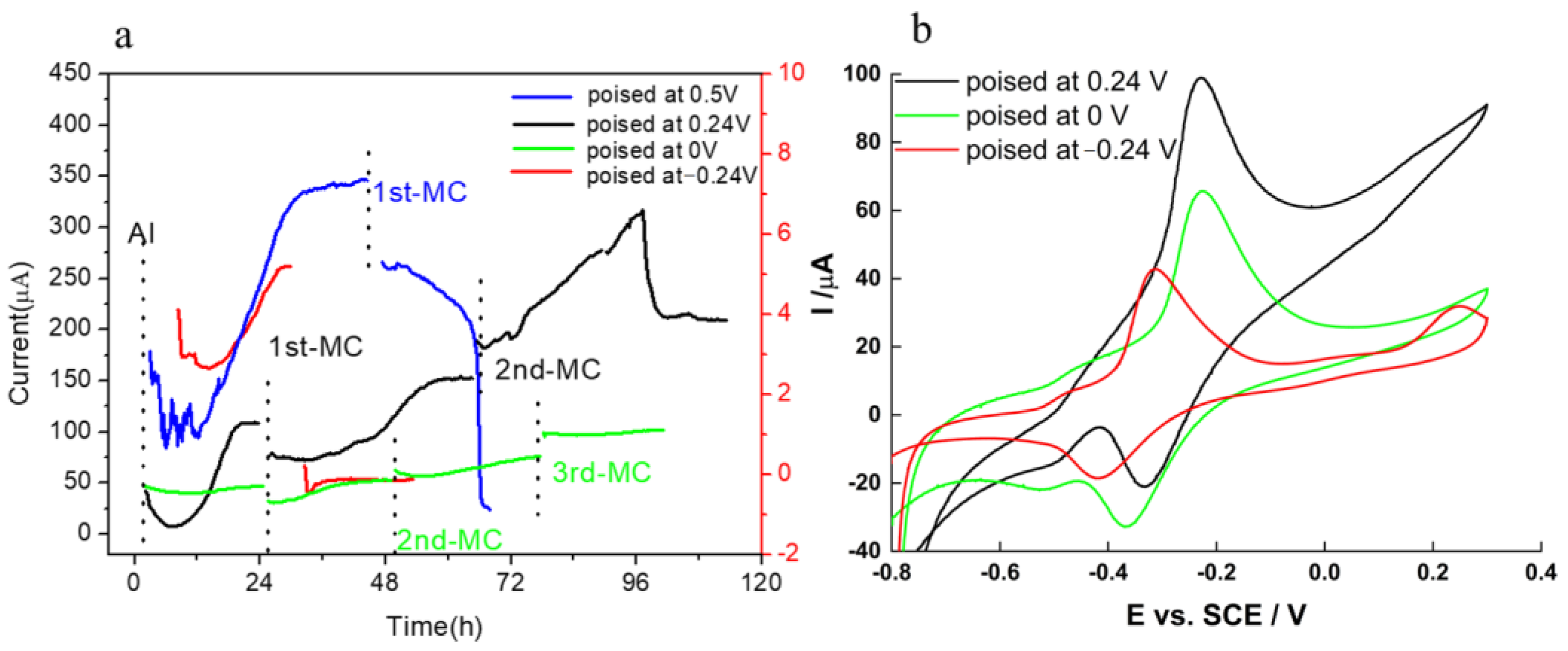
2.2. Potential Setup and Chronoamperometry (CA)
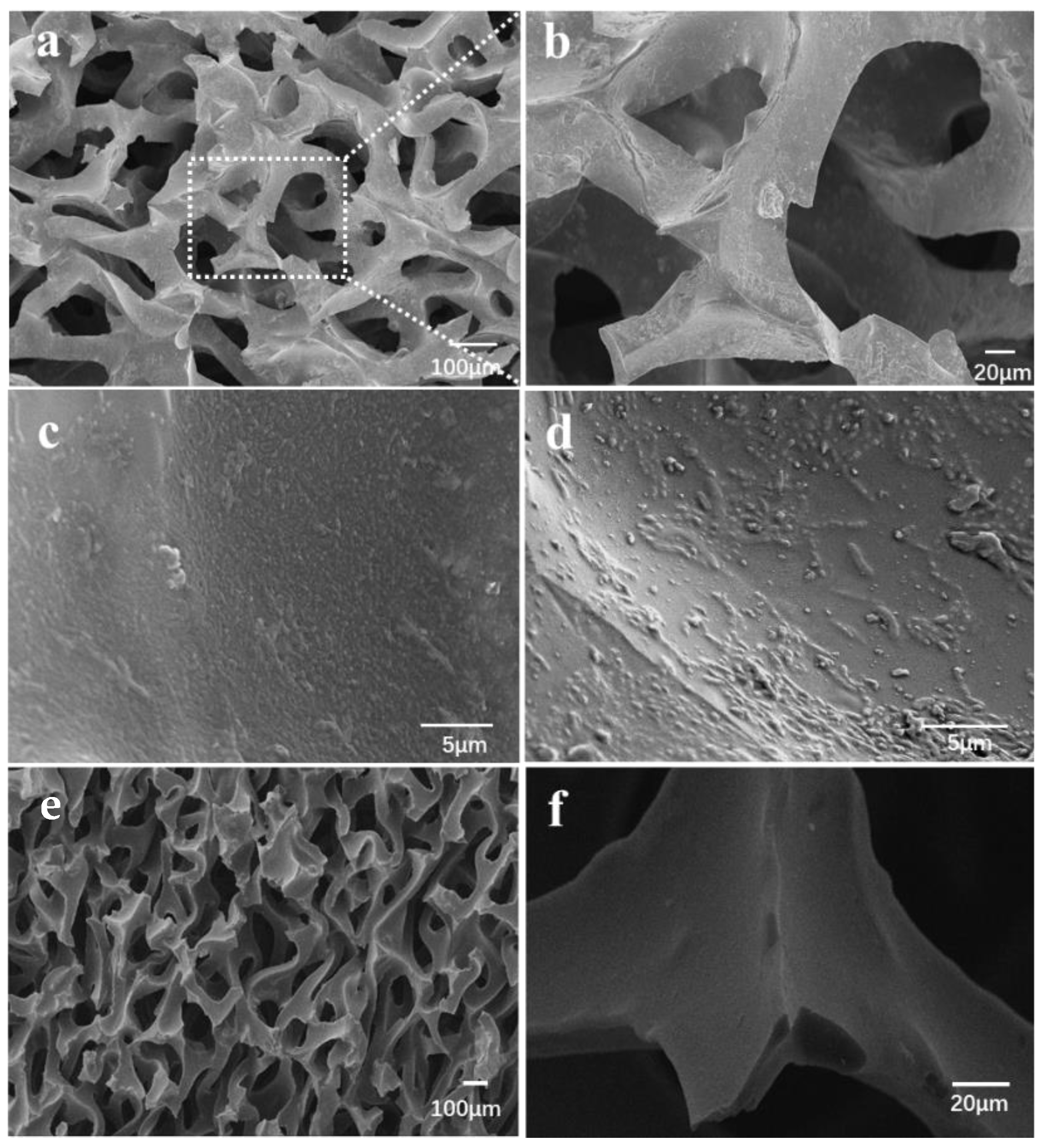
2.3. SEM Images
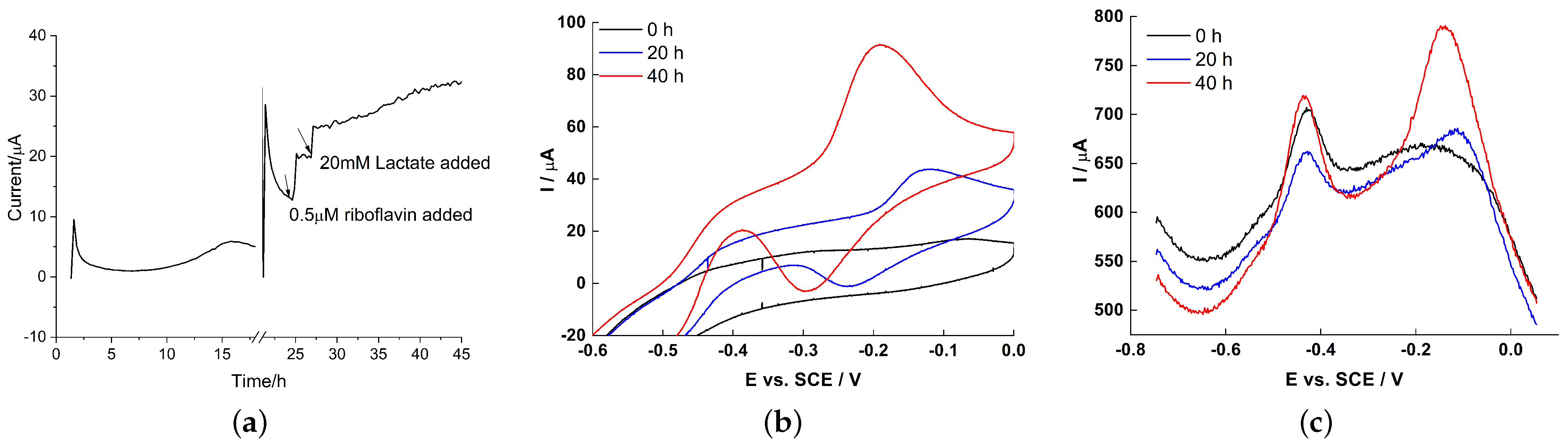
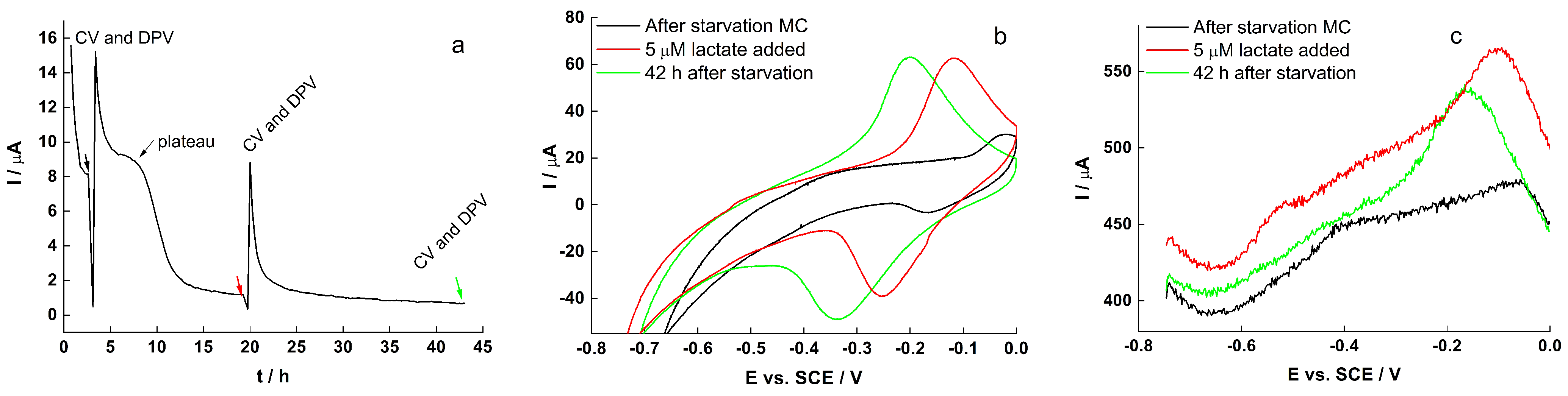
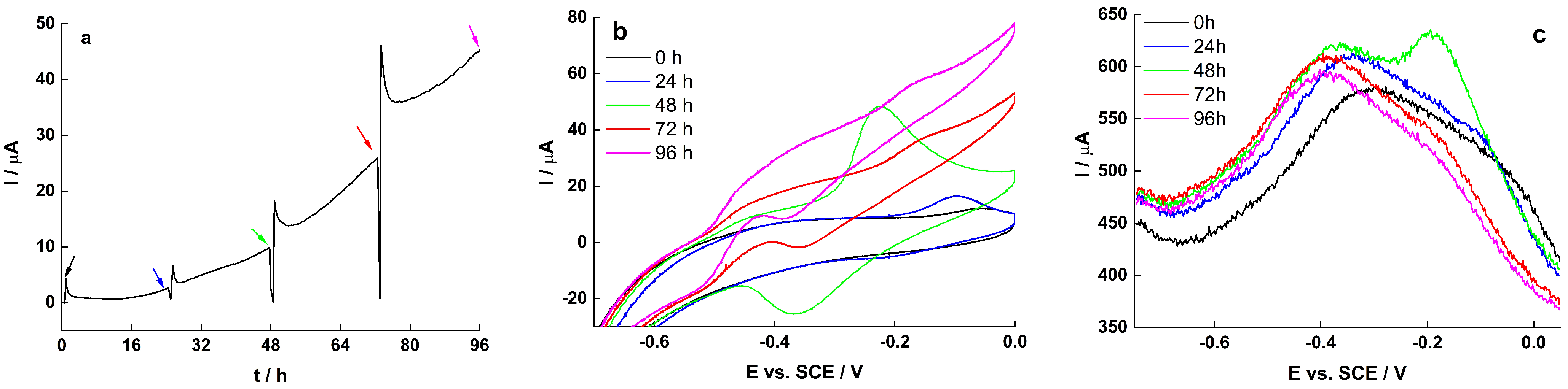
2.4. Electrochemical Characteristics of Biofilm
3. Material and Methods
3.1. Electrode Preparation
3.2. Assembly of Electrochemical Cells
3.3. Bacterial and Growth Medium
3.4. Growth in Electrochemical Cells
3.5. Fluorescence Spectroscopy
3.6. Scanning Electron Microscopy (SEM) Sample Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ueki, T. Cytochromes in extracellular electron transfer in Geobacter. Appl. Environ. Microb. 2021, 87, e03109-20. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, X.; Nealson, K.H.; Rensing, C.; Qin, S.; Zhou, S. Dissecting the structural and conductive functions of nanowires in Geobacter sulfurreducens electroactive biofilms. mBio 2022, 13, e0382221. [Google Scholar] [CrossRef]
- Clarke, T.A. Plugging into bacterial nanowires: A comparison of model electrogenic organisms. Curr. Opin. Microbiol. 2022, 66, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.W.; Karbelkar, A.A.; El-Naggar, M.Y. Nature’s conductors: What can microbial multi-heme cytochromes teach us about electron transport and biological energy conversion? Curr. Opin. Chem. Biol. 2018, 47, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, X.; Yin, J.; Xu, S.; Zhang, Y.; Xie, D.; Li, Z. Geobacter sulfurreducens adapts to low electrode potential for extracellular electron transfer. Electrochim. Acta 2016, 191, 743–749. [Google Scholar] [CrossRef]
- Chen, S.; Patil, S.A.; Brown, R.K.; Schröder, U. Strategies for optimizing the power output of microbial fuel cells: Transitioning from fundamental studies to practical implementation. Appl. Energy 2019, 233–234, 15–28. [Google Scholar] [CrossRef]
- Doyle, L.E.; Marsili, E. Weak electricigens: A new avenue for bioelectrochemical research. Bioresour. Technol. 2018, 258, 354–364. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Benjamin, E.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. J. Power Source 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Drendel, G.; Mathews, E.; Semenec, L.; Franks, A. Microbial fuel cells, related technologies, and their applications. Appl. Sci. 2018, 8, 2384. [Google Scholar] [CrossRef] [Green Version]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Gul, H.; Raza, W.; Lee, J.; Azam, M.; Ashraf, M.; Kim, K.H. Progress in microbial fuel cell technology for wastewater treatment and energy harvesting. Chemosphere 2021, 281, 130828. [Google Scholar] [CrossRef] [PubMed]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energy 2019, 12, 3390. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.J.; Zhou, M.H.; Liu, M.M.; Yang, W.L.; Gu, T.Y. Microbial fuel cells for biosensor applications. Biotechnol. Lett. 2015, 37, 2357–2364. [Google Scholar] [CrossRef]
- Guo, J.; Yang, G.; Zhuang, Z.; Mai, Q.; Zhuang, L. Redox potential-induced regulation of extracellular polymeric substances in an electroactive mixed community biofilm. Sci. Total Environ. 2021, 797, 149207. [Google Scholar] [CrossRef]
- Caizán-Juanarena, L.; Sleutels, T.; Borsje, C.; ter Heijne, A. Considerations for application of granular activated carbon as capacitive bioanode in bioelectrochemical systems. Renew. Energy 2020, 157, 782–792. [Google Scholar] [CrossRef]
- Huang, X.; Duan, C.S.; Duan, W.Y.; Sun, F.Y.; Cui, H.W.; Zhang, S.; Chen, X. Role of electrode materials on performance and microbial characteristics in the constructed wetland coupled microbial fuel cell (CW-MFC): A review. J. Clean. Prod. 2021, 301, 126951. [Google Scholar] [CrossRef]
- Mier, A.A.; Olvera-Vargas, H.; Mejía-López, M.; Longoria, A.; Verea, L.; Sebastian, P.J.; Arias, D.M. A review of recent advances in electrode materials for emerging bioelectrochemical systems: From biofilm-bearing anodes to specialized cathodes. Chemosphere 2021, 283, 131138. [Google Scholar] [CrossRef]
- Mouhib, M.; Antonucci, A.; Reggente, M.; Amirjani, A.; Boghossian, A.A. Enhancing bioelectricity generation in microbial fuel cells and biophotovoltaics using nanomaterials. Nano Res. 2019, 12, 2184–2199. [Google Scholar] [CrossRef]
- Rogulski, Z.; Lewdorowicz, W.; Tokarz, W.; Czerwiński, A. Applications of Reticulated Vitreous Carbon (RVC) in the electrochemical power sources. Pol. J. Chem. 2004, 78, 1357–1370. [Google Scholar]
- Rogulski, Z.; Chotkowski, M.; Czerwinski, A. New generation of the zinc—Manganese dioxide cell. J. New Mater. Elect. Syst. 2006, 9, 333–338. [Google Scholar]
- Tsutsumi, H.; Yamashita, S.; Oishi, T. Application of polyaniline/poly(p-styrenesulfonic acid) composite prepared by post-polymerization technique to positive active material for a rechargeable lithium battery. Synth. Met. 1997, 85, 1361–1362. [Google Scholar] [CrossRef]
- Gyenge, E.; Jung, J.; Mahato, B. Electroplated reticulated vitreous carbon current collectors for lead-acid batteries: Opportunities and challenges. J. Power Source 2003, 113, 388–395. [Google Scholar] [CrossRef]
- Fuentes-Albarran, C.; Del Razo, A.; Juarez, K.; Alvarez-Gallegos, A. Influence of NaCl, Na2SO4 and O2 on power generation from microbial fuel cells with non-catalyzed carbon electrodes and natural inocula. Sol. Energy 2012, 86, 1099–1107. [Google Scholar] [CrossRef]
- Friedrich, J.M.; Reade, G.; Walsh, F.C. Reticulated vitreous carbon as an electrode material. J. Electroanal. Chem. 2004, 561, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Bagal, R.; Bahir, M.; Lenka, N.; Patro, T.U. Polymer derived porous carbon foam and its application in bone tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2022. [Google Scholar] [CrossRef]
- LaBelle, E.V.; May, H.D. Energy efficiency and productivity enhancement of microbial electrosynthesis of acetate. Front. Microbiol. 2017, 8, 756. [Google Scholar] [CrossRef] [Green Version]
- Flexer, V.; Jourdin, L. Purposely designed hierarchical porous electrodes for high rate microbial electrosynthesis of acetate from carbon dioxide. Acc. Chem. Res. 2020, 53, 311–321. [Google Scholar] [CrossRef]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Huang, Y.; Xia, A.; Qian, F.; Wei, C.Y. A rapid inoculation method for microalgae biofilm cultivation based on microalgae-microalgae co-flocculation and zeta-potential adjustment. Bioresour. Technol. 2019, 278, 272–278. [Google Scholar] [CrossRef]
- Eddie, B.J.; Malanoski, A.P.; Onderko, E.L.; Phillips, D.A.; Glaven, S.M. Marinobacter atlanticus electrode biofilms differentially regulate gene expression depending on electrode potential and lifestyle. Biofilm 2021, 3, 100051. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, X.; Yan, Y.; Li, T.; An, J.; Liao, C.; Li, N.; Wang, X. Electrode potential regulates phenol degradation pathways in oxygen-diffused microbial electrochemical system. Chem. Eng. J. 2020, 38, 1122663. [Google Scholar] [CrossRef]
- Ikeda, S.; Takamatsu, Y.; Tsuchiya, M.; Suga, K.; Watanabe, K. Shewanella oneidensis MR-1 as a bacterial platform for electro-biotechnology. Essays Biochem. 2021, 65, 355–364. [Google Scholar] [PubMed]
- Strong, W.R.; Knauff, A.R.; Fravel, B.W.; Samide, M.J. Introduction of ion-exchange moieties to reticulated vitreous carbon by direct chemical modification. Carbon 2006, 44, 1936–1941. [Google Scholar] [CrossRef]
- Nakamura, R.; Ishii, K.; Hashimoto, K. Electronic absorption spectra and redox properties of C type cytochromes in living microbes. Angew. Chem. Int. Edit. 2009, 48, 1606–1608. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.Y.; Tang, J.H.; Chen, M.; Liu, X.; Zhou, S.G. Two modes of riboflavin-mediated extracellular electron transfer in Geobacter uraniireducens. Front. Microbiol. 2018, 9, 2886. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Ellington, A.D. Optimization of the biological component of a bioelectrochemical cell. Bioelectrochemistry 2007, 70, 165–172. [Google Scholar] [CrossRef]
- Teravest, M.A.; Angenent, L.T. Oxidizing electrode potentials decrease current production and coulombic efficiency through cytochromec inactivation in Shewanella oneidensis MR-1. ChemElectroChem 2014, 1, 2000–2006. [Google Scholar] [CrossRef]
- Sultana, S.T.; Babauta, J.T.; Beyenal, H. Electrochemical biofilm control: A review. Biofouling 2015, 31, 745–758. [Google Scholar] [CrossRef]
- Kim, N.; Choi, Y.; Jung, S.; Kim, S. Development of microbial fuel cells using Proteus vulgaris. Bull. Korean Chem. Soc. 2000, 21, 44–48. [Google Scholar]
- Su, L.; Yin, T.; Du, H.; Zhang, W.; Fu, D. Synergistic improvement of Shewanella loihica PV-4 extracellular electron transfer using a TiO2@TiN nanocomposite. Bioelectrochemistry 2020, 134, 107519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jain, A.; Marsili, E. Electrochemical characteristics of Shewanella loihica PV-4 on CNTs-modified graphite surfaces. Electrochim. Acta 2013, 102, 252–258. [Google Scholar] [CrossRef]

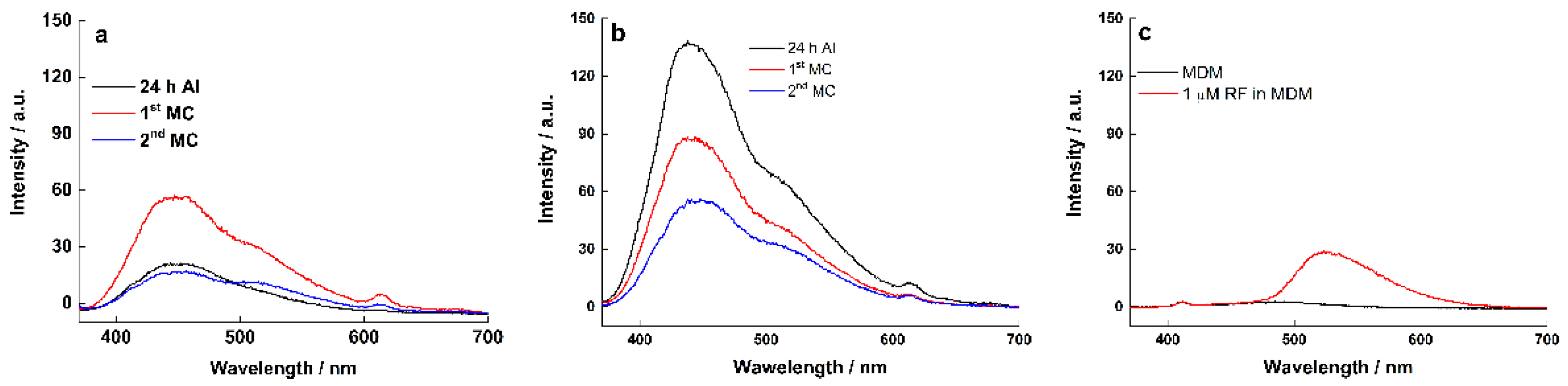
| Biofilm Age | Graphite Electrode (Peak Area) | RVC Electrode (Peak Area) | Graphite Electrode (Peak Area) | RVC Electrode (Peak Area) |
|---|---|---|---|---|
| Wavelength (nm) | 440 | 440 | 510 | 510 |
| 24 h AI | 2275 | 11,838 | 710 | 3372 |
| 1st MC | 5304 | 7550 | 2126 | 1869 |
| 2nd MC | 1847 | 4965 | 1038 | 1666 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, X.; Marsili, E. Electrochemical Characteristics of Shewanella loihica PV-4 on Reticulated Vitreous Carbon (RVC) with Different Potentials Applied. Molecules 2022, 27, 5330. https://doi.org/10.3390/molecules27165330
Wang S, Zhang X, Marsili E. Electrochemical Characteristics of Shewanella loihica PV-4 on Reticulated Vitreous Carbon (RVC) with Different Potentials Applied. Molecules. 2022; 27(16):5330. https://doi.org/10.3390/molecules27165330
Chicago/Turabian StyleWang, Shixin, Xiaoming Zhang, and Enrico Marsili. 2022. "Electrochemical Characteristics of Shewanella loihica PV-4 on Reticulated Vitreous Carbon (RVC) with Different Potentials Applied" Molecules 27, no. 16: 5330. https://doi.org/10.3390/molecules27165330
APA StyleWang, S., Zhang, X., & Marsili, E. (2022). Electrochemical Characteristics of Shewanella loihica PV-4 on Reticulated Vitreous Carbon (RVC) with Different Potentials Applied. Molecules, 27(16), 5330. https://doi.org/10.3390/molecules27165330







