Anti-Inflammatory Constituents of Antrodia camphorata on RAW 264.7 Cells Induced by Polyinosinic-Polycytidylic Acid
Abstract
:1. Introduction
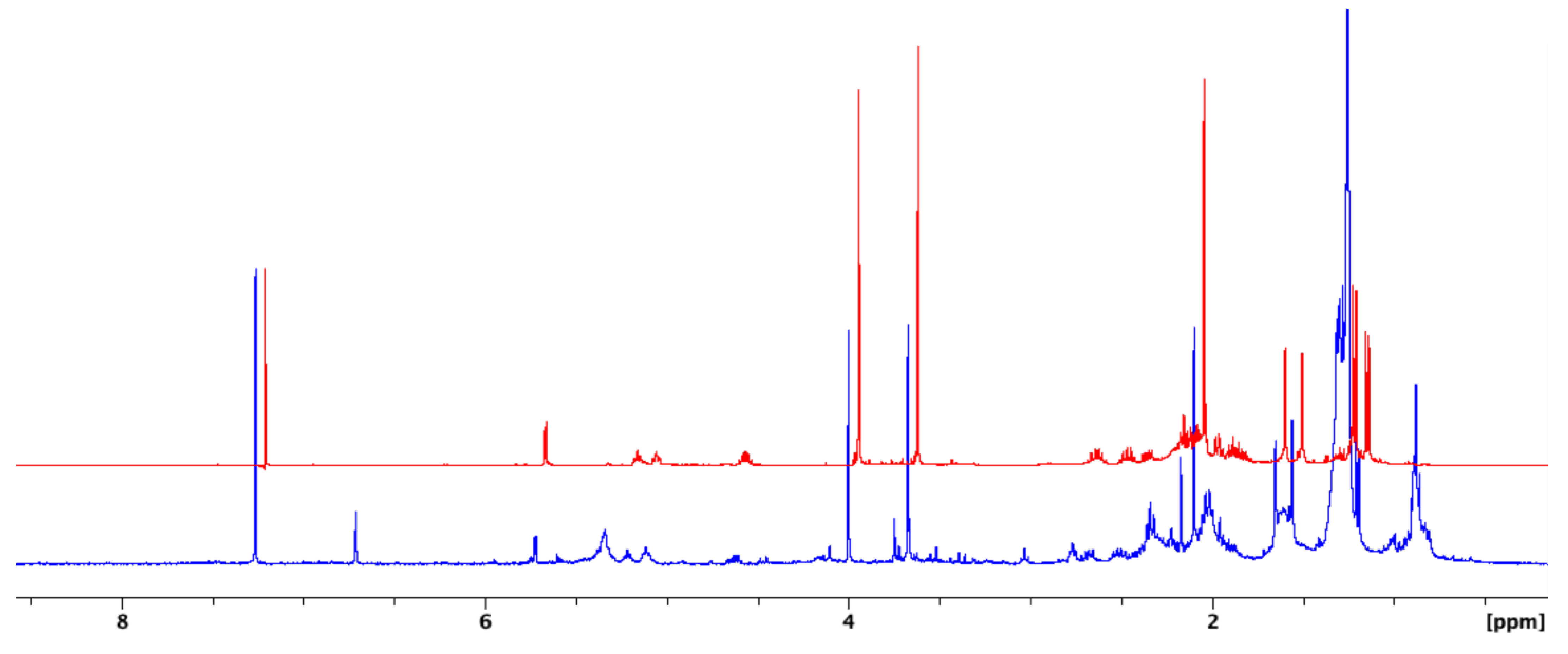
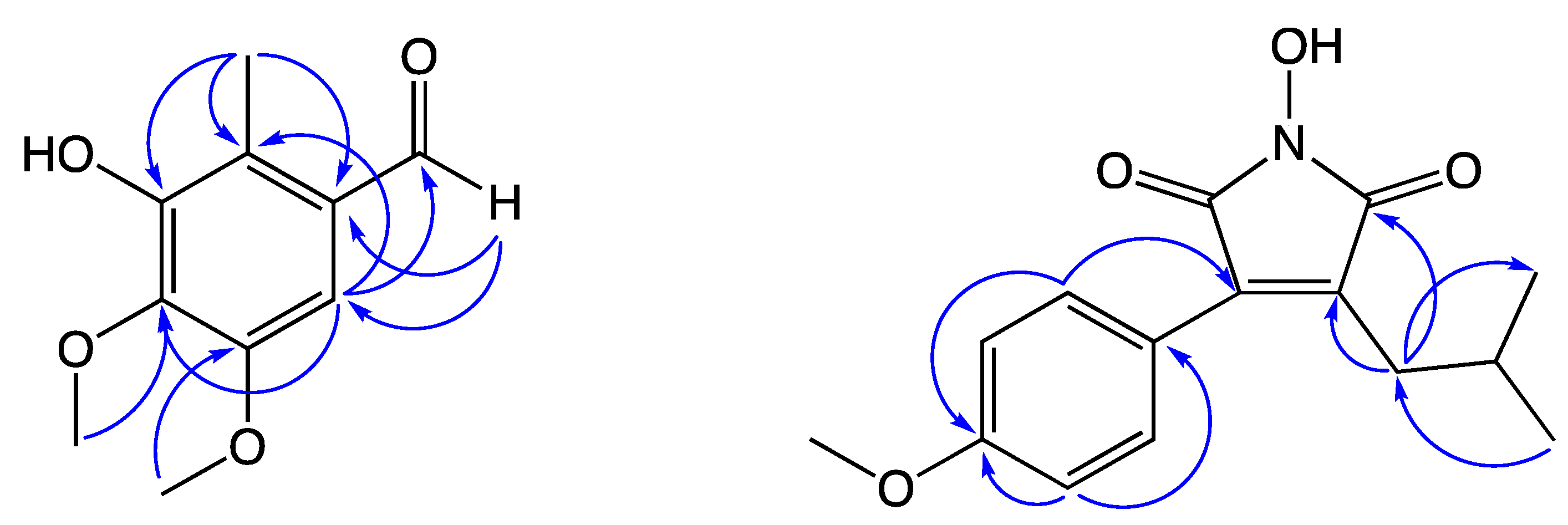
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemicals
3.3. Extraction and Isolation
3.4. NO Production and Cell Viability of RAW 264.7 Macrophages Induced by Poly I:C
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, W. Anti-inflammatory effect of wogonin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Molecules 2015, 20, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, W. Anti-inflammatory effect of quercetin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Sun, G.; Zhu, Z. Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect. Antivir. Ther. 2018, 23, 611–615. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, J.; Zhou, H.; Li, L.; Ding, Y.; Li, J.; Zhou, B.; Jiang, H.; Zhong, N.; Hu, W.; et al. Cappariloside A shows antiviral and better anti-inflammatory effects against influenza virus via regulating host IFN signaling, in vitro and vivo. Life Sci. 2018, 200, 115–125. [Google Scholar] [CrossRef]
- Hao, W.; Wang, L.; Li, S. FKBP5 regulates RIG-I-mediated NF-κB activation and influenza A virus infection. Viruses 2020, 12, 672. [Google Scholar] [CrossRef]
- Baranwal, M.; Gupta, Y.; Dey, P.; Majaw, S. Antiinflammatory phytochemicals against virus-induced hyperinflammatory responses: Scope, rationale, application, and limitations. Phytother. Res. 2021, 35, 6148–6169. [Google Scholar] [CrossRef]
- Singla, R.K.; He, X.; Chopra, H.; Tsagkaris, C.; Shen, L.; Kamal, M.A.; Shen, B. Natural products for the prevention and control of the COVID-19 pandemic: Sustainable bioresources. Front. Pharmacol. 2021, 12, 758159. [Google Scholar] [CrossRef]
- Isidoro, C.; Chang, A.C.F.; Sheen, L.Y. Natural products as a source of novel drugs for treating SARS-CoV2 infection. J. Tradit. Complement. Med. 2022, 12, 1–5. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Shokoohinia, Y.; Kiyani, N.; Stage, K.; Mohammadi, P.; Gravandi, M.M.; Farzaei, M.H.; Echeverría, J. Phytochemicals: Potential Therapeutic interventions against coronavirus-associated lung injury. Front. Pharmacol. 2020, 11, 588467. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complementary Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea—An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.J.; Gokila Vani, M.; Chen, C.Y.; Hsiao, W.W.; Li, J.; Lin, Z.X.; Chu, F.H.; Yen, G.C.; Wang, S.Y. A mechanistic and empirical review of antcins, a new class of phytosterols of formosan fungi origin. J. Food Drug Anal. 2020, 28, 38–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, Y.; Li, B.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef]
- Senthil Kumar, K.J.; Gokila Vani, M.; Hsieh, H.W.; Lin, C.C.; Wang, S.Y. Antcins from Antrodia cinnamomea and Antrodia salmonea inhibit angiotensin-converting enzyme 2 (ACE2) in epithelial cells: Can be potential candidates for the development of SARS-CoV-2 prophylactic agents. Plants 2021, 10, 1736. [Google Scholar] [CrossRef]
- Graziani, V.; Scognamiglio, M.; Belli, V.; Esposito, A.; D’Abrosca, B.; Chambery, A.; Russo, R.; Panella, M.; Russo, A.; Ciardiello, F.; et al. Metabolomic approach for a rapid identification of natural products with cytotoxic activity against human colorectal cancer cells. Sci. Rep. 2018, 8, 5309. [Google Scholar] [CrossRef] [Green Version]
- Tu, P.C.; Chan, C.J.; Liu, Y.C.; Kuo, Y.H.; Lin, M.K.; Lee, M.S. Bioactivity-guided fractionation and NMR-based identification of the immunomodulatory isoflavone from the roots of Uraria crinita (L.) Desv. ex DC. Foods 2019, 8, 543. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, Y.J.; Park, W. Berberine modulates hyper-inflammation in mouse macrophages stimulated with polyinosinic-polycytidylic acid via calcium-CHOP/STAT pathway. Sci. Rep. 2021, 11, 11298. [Google Scholar] [CrossRef]
- Gao, X.; Chen, P.K.S.; Lui, G.C.Y.; Hui, D.S.C.; Chu, I.M.T.; Sun, X.; Tsang, M.S.M.; Chan, B.C.L.; Lam, C.W.; Wong, C.K. Interleukin-38 ameliorates poly(I:C) induced lung inflammation: Therapeutic implications in respiratory viral infections. Cell Death Dis. 2021, 12, 53. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-J.; Lee, J.Y.; Kim, D.-H.; Kang, M.S.; Park, W. Anti-inflammatory effect of baicalein on polyinosinic–polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses 2018, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-W.; Pan, J.-H.; Liu, R.H.; Kuo, Y.-H.; Sheen, L.-Y.; Chiang, B.-H. The 4-acetylantroquinonol B isolated from mycelium of Antrodia cinnamomea inhibits proliferation of hepatoma cells. J. Sci. Food Agric. 2010, 90, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-Y.; Chiang, B.-H. 4-Acetylantroquinonol B from Antrodia cinnamomea enhances immune T function of dendritic cells against liver cancer stem cells. Biomed. Pharmacother. 2019, 109, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Yen, I.-C.; Tu, Q.-W.; Chang, T.-S.; Lin, P.-H.; Li, Y.-F.; Li, S.-Y. 4-Acetylantroquinonol B ameliorates nonalcoholic steatohepatitis by suppression of ER stress and NLRP3 inflammasome activation. Biomed. Pharmacother. 2021, 138, 111504. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Hsu, C.-C.; Lee, A.-S.; Wang, S.-W.; Lin, K.-T.; Chang, W.-L.; Peng, H.-C.; Huang, W.-C.; Chung, C.-H. 4-Acetylantroquinonol B inhibits lipopolysaccharide-induced cytokine release and alleviates sepsis through of MAPK and NFκB suppression. BMC Complement. Altern. Med. 2018, 18, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Bamodu, O.A.; Huang, W.C.; Zucha, M.A.; Lin, Y.-K.; Wu, A.T.H.; Huang, C.-C.; Lee, W.-H.; Yuan, C.-C.; Hsiao, M.; et al. 4-Acetylantroquinonol B suppresses autophagic flux and improves cisplatin sensitivity in highly aggressive epithelial cancer through the PI3K/Akt/mTOR/p70S6K signaling pathway. Toxicol. Appl. Pharmacol. 2017, 325, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Satriyo, P.B.; Su, C.M.; Ong, J.R.; Huang, W.-C.; Fong, I.-H.; Lin, C.-C.; Aryandono, T.; Haryana, S.M.; Deng, L.; Huang, C.-C.; et al. 4-Acetylantroquinonol B induced DNA damage response signaling and apoptosis via suppressing CDK2/CDK4 expression in triple negative breast cancer cells. Toxicol. Appl. Pharmacol. 2021, 422, 115493. [Google Scholar] [CrossRef]
- Huang, T.-F.; Wang, S.-W.; Lai, Y.-W.; Liu, S.-C.; Chen, Y.-J.; Hsueh, T.Y.; Lin, C.-C.; Lin, C.-H.; Chung, C.-H. 4-Acetylantroquinonol B suppresses prostate cancer growth and angiogenesis via VEGF/PI3K/ERK/mTOR-dependent signaling pathway in subcutaneous xenograft and in vivo angiogenesis models. Int. J. Mol. Sci. 2022, 23, 1446. [Google Scholar] [CrossRef]
- Li, B.; Lu, Y.-Y.; Lo, J.-Y.; Qiao, X.; Ye, M. Chemical constituents from the dish-cultured Antrodia camphorata and their cytotoxic activities. J. Asian Nat. Prod. Res. 2021, 23, 666–674. [Google Scholar] [CrossRef]
- Lee, T.-H.; Lee, C.-K.; Tsou, W.-L.; Liu, S.-Y.; Kuo, M.-T.; Wen, W.-C. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Med. 2007, 73, 1412–1415. [Google Scholar] [CrossRef] [Green Version]
- Chien, S.-C.; Chen, M.-L.; Kuo, H.-T.; Tsai, Y.-C.; Lin, B.-F.; Kuo, Y.-H. Anti-inflammatory activities of new succinic and maleic derivatives from the fruiting body of Antrodia camphorata. J. Agric. Food Chem. 2008, 56, 7017–7022. [Google Scholar] [CrossRef]
- Wu, M.-D.; Cheng, M.J.; Wang, B.C.; Yech, Y.J.; Lai, J.T.; Kuo, Y.-H.; Yuan, K.-F.; Chen, I.S. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages. J. Nat. Prod. 2008, 71, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
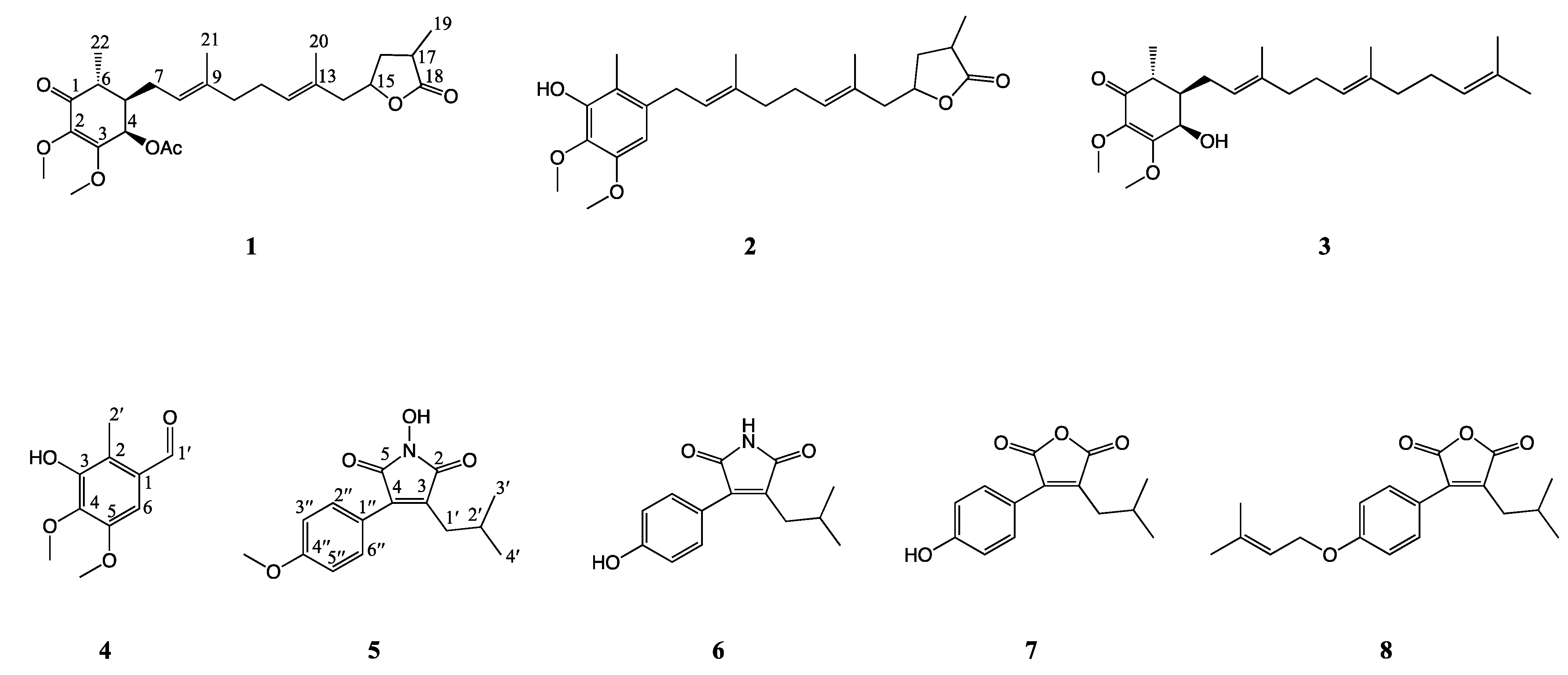
| Compounds | IC50 (μM) 1 |
|---|---|
| 1 | 0.57 ± 0.06 |
| 2 | 2.96 ± 0.77 |
| 3 | 10.80 ± 1.32 |
| 4 | 10.11 ± 1.76 |
| 5 | 11.30 ± 3.34 |
| 6 | 27.14 ± 4.61 |
| 7 | >50 |
| 8 | >50 |
| Indomethacin 2 | 67.10 ± 2.23 |
| Position | 13C NMR δC | 1H NMR δH (Multiplicity) | HMBC |
|---|---|---|---|
| 1 | 129.7 | ||
| 2 | 121.4 | ||
| 3 | 148.0 | ||
| 4 | 140.1 | ||
| 5 | 150.1 | ||
| 6 | 105.0 | 7.02 (s) | C-2, C-4, C-5, C-1′ |
| 1′ | 191.2 | 10.25 (s) | C-1, C-6 |
| 2′ | 9.93 | 2.51 (s) | C-1, C-2, C-3 |
| 3-OH | 6.03 (s) | C-2, C-3 |
| Position | 13C NMR δC | 1H NMR δH (Multiplicity) | HMBC |
|---|---|---|---|
| 2 | 167.8 | ||
| 3 | 136.3 | ||
| 4 | 135.9 | ||
| 5 | 167.1 | ||
| 1′ | 33.1 | 2.51 (d, 7.4) | C-2, C-3, C-3′, C-4′ |
| 2′ | 28.2 | 2.05 (m) | C-1′, C-3′, C-4′ |
| 3′, 4′ | 22.9 | 0.90 (d, 6.7) | C-1′, C-2′ |
| 1″ | 121.2 | ||
| 2″, 6″ | 131.5 | 7.47 (d, 8.6) | C-4, C-3″, C-4″, C-5″ |
| 3″, 5″ | 115.9 | 6.92 (d, 8.6) | C-1″, C-4″ |
| 4″ | 157.4 | ||
| 4″-OCH3 | 65.9 | 4.00 (s) | |
| OH | 5.55 (brs) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, P.-C.; Jiang, W.-P.; Lin, M.-K.; Huang, G.-J.; Li, Y.-J.; Kuo, Y.-H. Anti-Inflammatory Constituents of Antrodia camphorata on RAW 264.7 Cells Induced by Polyinosinic-Polycytidylic Acid. Molecules 2022, 27, 5320. https://doi.org/10.3390/molecules27165320
Tu P-C, Jiang W-P, Lin M-K, Huang G-J, Li Y-J, Kuo Y-H. Anti-Inflammatory Constituents of Antrodia camphorata on RAW 264.7 Cells Induced by Polyinosinic-Polycytidylic Acid. Molecules. 2022; 27(16):5320. https://doi.org/10.3390/molecules27165320
Chicago/Turabian StyleTu, Ping-Chen, Wen-Ping Jiang, Ming-Kuem Lin, Guan-Jhong Huang, Yi-Jen Li, and Yueh-Hsiung Kuo. 2022. "Anti-Inflammatory Constituents of Antrodia camphorata on RAW 264.7 Cells Induced by Polyinosinic-Polycytidylic Acid" Molecules 27, no. 16: 5320. https://doi.org/10.3390/molecules27165320
APA StyleTu, P.-C., Jiang, W.-P., Lin, M.-K., Huang, G.-J., Li, Y.-J., & Kuo, Y.-H. (2022). Anti-Inflammatory Constituents of Antrodia camphorata on RAW 264.7 Cells Induced by Polyinosinic-Polycytidylic Acid. Molecules, 27(16), 5320. https://doi.org/10.3390/molecules27165320






