Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Origin of Straight-Chain Aliphatic Aldehydes
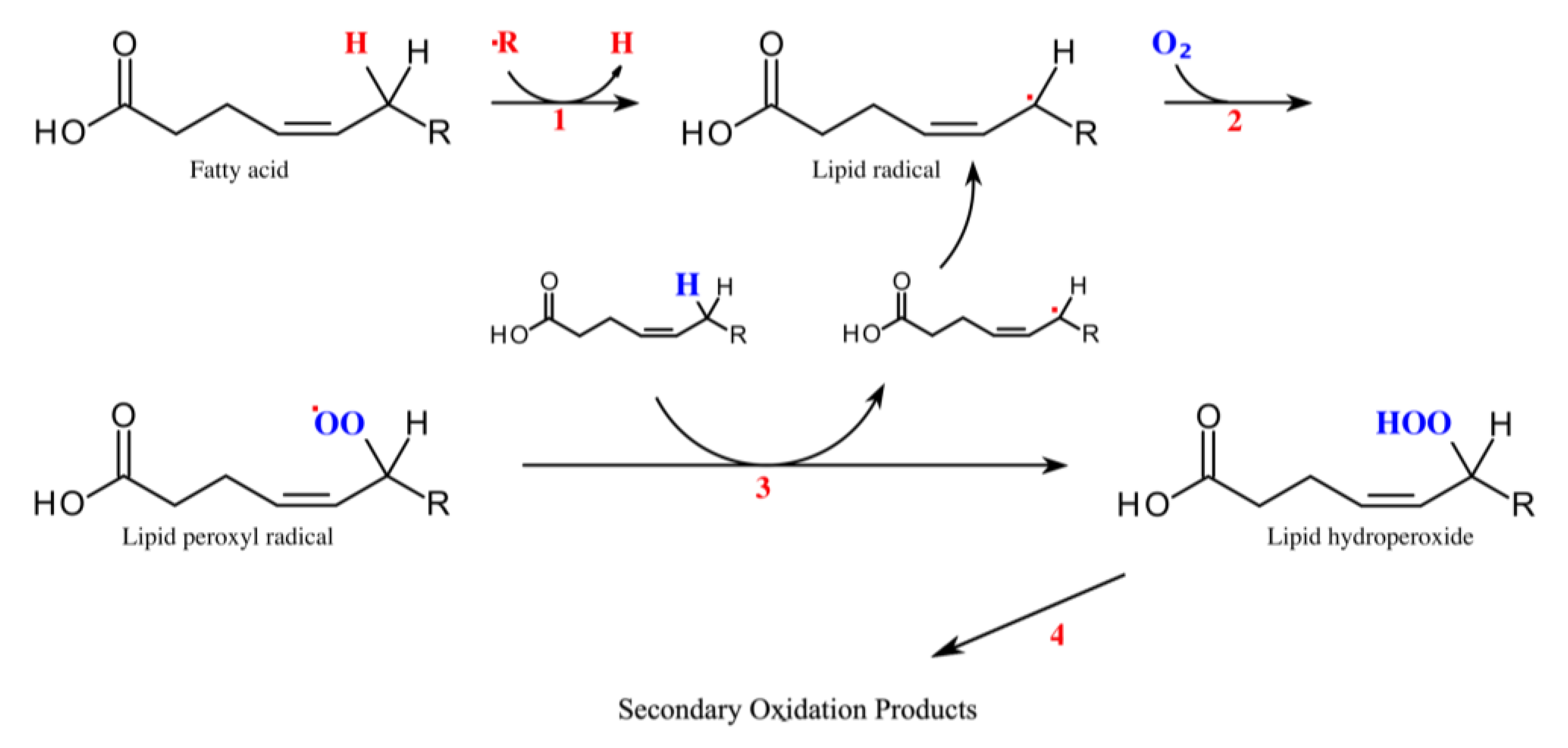
4. Detection Methods for Exhaled Aldehydes
5. Aldehyde Exhalation and Lung Cancer
| Author/Study | Method | Cancer Stage * | Histologic Type | Substance | Patients/ Controls [n] | Concentration Ratio Sick/Healthy |
|---|---|---|---|---|---|---|
| Fuchs et al. (2010) [35] Breath gas aldehydes as biomarkers of lung cancer | GC-MS | >T3 | NSCLC | Pentanal Hexanal Octanal Nonanal | 12/24 | 9.5 - 4.7 7.2 |
| Poli et al. (2010) [36] Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME–GC/MS | GC-MS | Stage 1 & 2 | NSCLC | Butanal Pentanal Hexanal Heptanal Octanal Nonanal | 40/38 | 2.4 2.2 3.7 2.3 2.0 3.6 |
| Baumbach et al. (2011) [39] Significant different volatile biomarker during bronchoscopic ion mobility spectrometry investigation of patients suffering lung carcinoma | IMS | - | - | Nonanal | 19 | - |
| Ulanowska et al. (2011) [41] The application of statistical methods using VOCs to identify patients with lung cancer | GC-MS | - | SCLC, NSCLC, Others | Propanal Pentanal Hexanal | 137/143 | 1.1 5.9 4.5 |
| Buszewski et al. (2012) [37] Identification of volatile lung cancer markers by gas chromatography–mass spectrometry: comparison with discrimination by canines | GC-MS | - | SCLC, NSCLC | Butanal | 29/44 | - |
| Handa et al. (2014) [38] Exhaled Breath Analysis for Lung Cancer Detection Using Ion Mobility Spectrometry | IMS | ≥Stage 1 | NSCLC | Hexanal | 50/39 | - |
| Heptanal Nonanal | ||||||
| Corradi et al. (2015) [40] Exhaled breath analysis in suspected cases of non-small-cell lung cancer: a cross-sectional study | GC-MS | ≥Stage 1 | NSCLC | Heptanal | 71/67 | 1.3 |
| Schallschmidt et al. (2016) [49] Comparison of volatile organic compounds from lung cancer patients and healthy controls—challenges and limitations of an observational study | GC-MS | ≥Stage 1 | - | Propanal Butanal Pentanal Hexanal Decanal | 37/23 | 3.3 2.0 1.5 1.1 2.7 |
| Wang et al. (2022) [42] Identification of lung cancer breath biomarkers based on perioperative breathomics testing: A prospective observational study | TOF-MS | - | SCLC, NSCLC | Pentanal Hexanal Heptanal Octanal Nonanal Decanal | 157/368 | - |
6. Aldehyde Exhalation and Inflammatory/Infectious Lung Diseases
7. Aldehyde Exhalation and Mechanical Lung Injury
| Author/Study | Method | Substance | Patients/ Controls [n] | Concentration Ratio Sick/Healthy |
|---|---|---|---|---|
| Corradi et al. (2003) [57] Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease | LC-MS | Hexanal Heptanal | 20/32 | 3.6 1.9 |
| Ruszkiewicz et al. (2019) [60] Diagnosis of COVID-19 by analysis of breath with gas chromatography- ion mobility spectrometry—a feasibility study | SIFT-MS | Ethanal Heptanal Octanal | 27/63 | - |
| Berna et al. (2021) [63] Reproducible breath metabolite changes in children with SARS-CoV-2 infection | TOF-MS | Heptanal Octanal Nonanal | 15/10 12/12 | - |
| Grassin-Delyle et al. (2021) [64] Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study | TOF-MS | Nonanal | 28/12 | - |
| Müller-Wirtz et al. (2021) [18] Volutrauma Increases Exhaled Pentanal in Rats: A Potential Breath Biomarker for Ventilator-Induced Lung Injury | MCC-IMS | Pentanal | 150 # | - * |
| Müller-Wirtz et al. (2021) [75] Differential Response of Pentanal and Hexanal Exhalation to Supplemental Oxygen and Mechanical Ventilation in Rats | MCC-IMS | Pentanal Hexanal | 30 # | - * |
| Müller-Wirtz et al. (2021) [4] Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients | MCC-IMS | Pentanal | 12 | - |
8. Aldehyde Exhalation from Non-Pulmonary Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Yoshino, K.; Sano, M.; Jujita, M.; Tomita, I. Production of aliphatic aldehydes on peroxidation of various types of lipids. Chem. Pharm. Bull. 1991, 39, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Shestivska, V.; Rutter, A.V.; Sulé-Suso, J.; Smith, D.; Španěl, P. Evaluation of peroxidative stress of cancer cells in vitro by real-time quantification of volatile aldehydes in culture headspace. Rapid Commun. Mass Spectrom. 2017, 31, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Müller-Wirtz, L.M.; Kiefer, D.; Ruffing, S.; Brausch, T.; Hüppe, T.; Sessler, D.I.; Volk, T.; Fink, T.; Kreuer, S.; Maurer, F. Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients. Molecules 2021, 26, 3089. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, J.; Trefz, P.; Wex, K.; Sabel, B.; Schubert, J.K.; Miekisch, W. Electrochemical sensor system for breath analysis of aldehydes, CO and NO. J. Breath Res. 2015, 9, 016008. [Google Scholar] [CrossRef]
- Sinharoy, P.; Mcallister, S.L.; Vasu, M.; Gross, E.R. Environmental Aldehyde Sources and the Health Implications of Exposure. Aldehyde Dehydrog. 2019, 1193, 35–52. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Clemente, S.M.; Martínez-Costa, O.H.; Monsalve, M.; Samhan-Arias, A.K. Targeting Lipid Peroxidation for Cancer Treatment. Molecules 2020, 25, 5144. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Wieczorek, T.; Drabińska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 034001. [Google Scholar] [CrossRef]
- McCartney, M.M.; Thompson, C.J.; Klein, L.R.; Ngo, J.H.; Seibel, J.D.; Fabia, F.; Simms, L.A.; Borras, E.; Young, B.S.; Lara, J.; et al. Breath carbonyl levels in a human population of seven hundred participants. J. Breath Res. 2020, 14, 046005. [Google Scholar] [CrossRef]
- Huang, J.; Kumar, S.; Hanna, G.B. Investigation of C3-C10 aldehydes in the exhaled breath of healthy subjects using selected ion flow tube-mass spectrometry (SIFT-MS). J. Breath Res. 2014, 8, 037104. [Google Scholar] [CrossRef]
- WikiCommons Lipid Peroxidation. Available online: https://commons.wikimedia.org/wiki/File:Mechanismus_der_Lipidperoxidation.svg (accessed on 11 April 2022).
- Buszewski, B.; Grzywinski, D.; Ligor, T.; Stacewicz, T.; Bielecki, Z.; Wojtas, J. Detection of volatile organic compounds as biomarkers in breath analysis by different analytical techniques. Bioanalysis 2013, 5, 2287–2306. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Sauer, S.; Kliem, M. Mass spectrometry tools for the classification and identification of bacteria. Nat. Rev. Microbiol. 2010, 8, 74–82. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Fink, T.; Baumbach, J.I.; Kreuer, S. Ion mobility spectrometry in breath research. J. Breath Res. 2014, 8, 027104. [Google Scholar] [CrossRef]
- Müller-Wirtz, L.M.; Kiefer, D.; Maurer, F.; Floss, M.A.; Doneit, J.; Hüppe, T.; Shopova, T.; Wolf, B.; Sessler, D.I.; Volk, T.; et al. Volutrauma Increases Exhaled Pentanal in Rats: A Potential Breath Biomarker for Ventilator-Induced Lung Injury. Anesth. Analg. 2021, 133, 263–273. [Google Scholar] [CrossRef]
- Ruzsanyi, V.; Baumbach, J.I.; Sielemann, S.; Litterst, P.; Westhoff, M.; Freitag, L. Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. J. Chromatogr. A 2005, 1084, 145–151. [Google Scholar] [CrossRef]
- Olarve, R.S.; Dela Torre, H.M.; Foronda, J.R.; Santos, M.G.; Sajor, N.J.; Lopez, T.B.; Haygood, K.J.; Santos, G.N. Aldehyde Gas Detection using Nanostructured Zno-based Gas Sensor fabricated via Horizontal Vapor Phase Growth Technique. J. Phys. Conf. Ser. 2021, 2015, 012102. [Google Scholar] [CrossRef]
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.D.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath Res. 2021, 15, 034001. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Dama, E.; Colangelo, T.; Fina, E.; Cremonesi, M.; Kallikourdis, M.; Veronesi, G.; Bianchi, F. Biomarkers and Lung Cancer Early Detection: State of the Art. Cancers 2021, 13, 3919. [Google Scholar] [CrossRef]
- Sun, J.; Chen, X.; Wang, Y. Comparison of the diagnostic value of CEA combined with OPN or DKK1 in non-small cell lung cancer. Oncol. Lett. 2020, 20, 3046–3052. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, P.; Wu, R.; Lu, K.; Zhou, H. Identifying the Best Marker Combination in CEA, CA125, CY211, NSE, and SCC for Lung Cancer Screening by Combining ROC Curve and Logistic Regression Analyses: Is It Feasible? Dis. Markers 2018, 2018, 2082840. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in lung cancer screening: Achievements, promises and challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef]
- Kubik, A.; Parkin, D.M.; Khlat, M.; Erban, J.; Polak, J.; Adamec, M. Lack of benefit from semi-annual screening for cancer of the lung: Follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int. J. cancer 1990, 45, 26–33. [Google Scholar] [CrossRef]
- Fontana, R.S.; Sanderson, D.R.; Woolner, L.B.; Taylor, W.F.; Miller, W.E.; Muhm, J.R. Lung cancer screening: The Mayo program. J. Occup. Med. 1986, 28, 746–750. [Google Scholar] [CrossRef]
- Bradley, S.H.; Grice, A.; Neal, R.D.; Abraham, S.; Rodriguez Lopez, R.; Shinkins, B.; Callister, M.E.J.; Hamilton, W.T. Sensitivity of chest X-ray for detecting lung cancer in people presenting with symptoms: A systematic review. Br. J. Gen. Pract. 2019, 69, E827–E835. [Google Scholar] [CrossRef]
- Team, T.N.L.S.T.R. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C.; et al. Screening for Lung Cancer with Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. J. Am. Med. Assoc. 2021, 325, 971–987. [Google Scholar] [CrossRef]
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 11 April 2022).
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B 2010, 878, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Handa, H.; Usuba, A.; Maddula, S.; Rg, J.; Baumbach, I.; Mineshita, M.; Miyazawa, T. Exhaled Breath Analysis for Lung Cancer Detection Using Ion Mobility Spectrometry. PLoS ONE 2014, 9, e114555. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, J.I.; Maddula, S.; Sommerwerck, U.; Besa, V.; Kurth, I.; Bödeker, B.; Teschler, H.; Freitag, L.; Darwiche, K. Significant different volatile biomarker during bronchoscopic ion mobility spectrometry investigation of patients suffering lung carcinoma. Int. J. Ion. Mobil. Spectrom. 2011, 14, 159–166. [Google Scholar] [CrossRef]
- Corradi, M.; Poli, D.; Banda, I.; Bonini, S.; Mozzoni, P.; Pinelli, S.; Alinovi, R.; Andreoli, R.; Ampollini, L.; Casalini, A.; et al. Exhaled breath analysis in suspected cases of non-small-cell lung cancer: A cross-sectional study. J. Breath Res. 2015, 9, 27101. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The application of statistical methods using VOCs to identify patients with lung cancer. J. Breath Res. 2011, 5, 046008. [Google Scholar] [CrossRef]
- Wang, P.; Huang, Q.; Meng, S.; Mu, T.; Liu, Z.; He, M.; Li, Q.; Zhao, S.; Wang, S.; Qiu, M. Identification of lung cancer breath biomarkers based on perioperative breathomics testing: A prospective observational study. EClinicalMedicine 2022, 47, 101384. [Google Scholar] [CrossRef]
- Callol-Sanchez, L.; Munoz-Lucas, M.A.; Gomez-Martin, O.; Maldonado-Sanz, J.A.; Civera-Tejuca, C.; Gutierrez-Ortega, C.; Rodriguez-Trigo, G.; Jareno-Esteban, J. Observation of nonanoic acid and aldehydes in exhaled breath of patients with lung cancer. J. Breath Res. 2017, 11, 26004. [Google Scholar] [CrossRef]
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects—Confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644. [Google Scholar] [CrossRef]
- Ghosh, S.; Kim, K.-H.; Sohn, J.R. Some insights into analytical bias involved in the application of grab sampling for volatile organic compounds: A case study against used Tedlar bags. Sci. World J. 2011, 11, 2160–2177. [Google Scholar] [CrossRef]
- Berna, A.Z.; Schaber, C.L.; Bollinger, L.B.; Mwale, M.; Mlotha-Mitole, R.; Trehan, I.; Odom John, A.R. Comparison of breath sampling methods: A post hoc analysis from observational cohort studies. Analyst 2019, 144, 2026. [Google Scholar] [CrossRef]
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Clinical Medicine Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2021, 10, 32. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Jung, C.; Bremser, W.; Walles, T.; Neudecker, J.; Leschber, G.; Frese, S.; Nehls, I. Comparison of volatile organic compounds from lung cancer patients and healthy controls-challenges and limitations of an observational study. J. Breath Res. 2016, 10, 046007. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Lange, M.; Szabo, C.; Traber, D.L.; Horvath, E.; Hamahata, A.; Nakano, Y.; Traber, L.D.; Cox, R.A.; Schmalstieg, F.C.; Herndon, D.N.; et al. Time profile of oxidative stress and neutrophil activation in ovine acute lung injury and sepsis. Shock 2012, 37, 468–472. [Google Scholar] [CrossRef]
- Chow, C.-W.W.; Herrera Abreu, M.T.; Suzuki, T.; Downey, G.P.; Abreu, M.T.H.; Suzuki, T.; Downey, G.P. Oxidative stress and acute lung injury. Am. J. Respir. Cell Mol. Biol. 2003, 29, 427–431. [Google Scholar] [CrossRef]
- Boshuizen, M.; Leopold, J.H.; Zakharkina, T.; Knobel, H.H.; Weda, H.; Nijsen, T.M.E.; Vink, T.J.; Sterk, P.J.; Schultz, M.J.; Bos, L.D.J.; et al. Levels of cytokines in broncho-alveolar lavage fluid, but not in plasma, are associated with levels of markers of lipid peroxidation in breath of ventilated ICU patients. J. Breath Res. 2015, 9, 036010. [Google Scholar] [CrossRef]
- Fazleen, A.; Wilkinson, T. Early COPD: Current evidence for diagnosis and management. Ther. Adv. Respir. Dis. 2020, 14. [Google Scholar] [CrossRef]
- Jung, T.; Vij, N. Early Diagnosis and Real-Time Monitoring of Regional Lung Function Changes to Prevent Chronic Obstructive Pulmonary Disease Progression to Severe Emphysema. J. Clin. Med. 2021, 10, 5811. [Google Scholar] [CrossRef]
- O’Reilly, P.; Bailey, W. Clinical use of exhaled biomarkers in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 403. [Google Scholar]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in Exhaled Breath Condensate of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1380–1386. [Google Scholar] [CrossRef]
- Corradi, M.; Folesani, G.; Andreoli, R.; Manini, P.; Bodini, A.; Piacentini, G.; Carraro, S.; Zanconato, S.; Baraldi, E. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am. J. Respir. Crit. Care Med. 2003, 167, 395–399. [Google Scholar] [CrossRef]
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef]
- Ruszkiewicz, D.M.; Sanders, D.; O’Brien, R.; Hempel, F.; Reed, M.J.; Riepe, A.C.; Bailie, K.; Brodrick, E.; Darnley, K.; Ellerkmann, R.; et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—A feasibility study. eClinicalMedicine 2020, 29, 100609. [Google Scholar] [CrossRef]
- Jegerlehner, S.; Suter-Riniker, F.; Jent, P.; Bittel, P.; Nagler, M. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings: Antigen tests in real-life clinical settings. Int. J. Infect. Dis. 2021, 109, 118–122. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 2020, CD013705. [Google Scholar] [CrossRef]
- Berna, A.Z.; Akaho, E.H.; Harris, R.M.; Congdon, M.; Korn, E.; Neher, S.; M’Farrej, M.; Burns, J.; John, A.R.O. Reproducible breath metabolite changes in children with SARS-CoV-2 infection. ACS Infect. Dis. 2021, 7, 2596–2603. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Roquencourt, C.; Moine, P.; Saffroy, G.; Carn, S.; Heming, N.; Fleuriet, J.; Salvator, H.; Naline, E.; Couderc, L.-J.; et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. eBioMedicine 2021, 63, 103154. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Negrini, D.; Rocco, P.R.M.; MacÊdo Rocco, P.R. Mechanisms of ventilator-induced lung injury in healthy lungs. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Negrini, D.; Passi, A.; Moriondo, A. The role of proteoglycans in pulmonary edema development. Intensiv. Care Med. 2008, 34, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, N.E.; Hubmayr, R.D. Cellular stress failure in ventilator-injured lungs. Am. J. Respir. Crit. Care Med. 2005, 171, 1328–1342. [Google Scholar] [CrossRef]
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest 2016, 150, 1109–1117. [Google Scholar] [CrossRef]
- Sun, Z.-T.; Yang, C.-Y.; Miao, L.-J.; Zhang, S.-F.; Han, X.-P.; Ren, S.-E.; Sun, X.-Q.; Cao, Y.-N. Effects of mechanical ventilation with different tidal volume on oxidative stress and antioxidant in lung. J. Anesth. 2015, 29, 346–351. [Google Scholar] [CrossRef]
- Ottolenghi, S.; Sabbatini, G.; Brizzolari, A.; Samaja, M.; Chiumello, D. Hyperoxia and oxidative stress in anesthesia and critical care medicine. Minerva Anestesiol. 2020, 86, 64–75. [Google Scholar] [CrossRef]
- Shosholcheva, M.; Jankulovski, N.; Kartalov, A.; Kuzmanovska, B.; Miladinova, D. Synergistic Effect of Hyperoxia and Biotrauma On Ventilator-Induced Lung Injury. Pril. Makedon. Akad. Nauk. I Umet. Oddel. Med. Nauk. 2017, 38, 91–96. [Google Scholar] [CrossRef][Green Version]
- Wolthuis, E.K.; Choi, G.; Dessing, M.C.; Bresser, P.; Lutter, R.; Dzoljic, M.; van der Poll, T.; Vroom, M.B.; Hollmann, M.; Schultz, M.J. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 2008, 108, 46–54. [Google Scholar] [CrossRef]
- Wrigge, H.; Zinserling, J.; Stüber, F.; von Spiegel, T.; Hering, R.; Wetegrove, S.; Hoeft, A.; Putensen, C. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology 2000, 93, 1413–1417. [Google Scholar] [CrossRef]
- Faller, S.; Strosing, K.M.; Ryter, S.W.; Buerkle, H.; Loop, T.; Schmidt, R.; Hoetzel, A. The volatile anesthetic isoflurane prevents ventilator-induced lung injury via phosphoinositide 3-kinase/Akt signaling in mice. Anesth. Analg. 2012, 114, 747–756. [Google Scholar] [CrossRef]
- Müller-Wirtz, L.M.; Kiefer, D.; Knauf, J.; Floss, M.A.; Doneit, J.; Wolf, B.; Maurer, F.; Sessler, D.I.; Volk, T.; Kreuer, S.; et al. Differential Response of Pentanal and Hexanal Exhalation to Supplemental Oxygen and Mechanical Ventilation in Rats. Molecules 2021, 26, 2752. [Google Scholar] [CrossRef]
- Dandona, P.; Thusu, K.; Cook, S.; Snyder, B.; Makowski, J.; Armstrong, D.; Nicotera, T. Oxidative damage to DNA in diabetes mellitus. Lancet 1996, 347, 444–445. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Mackenzie, H.A.; Veselkov, K.A.; Hoare, J.M.; Lovat, L.B.; Španěl, P.; Smith, D.; Hanna, G.B. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann. Surg. 2015, 262, 981–990. [Google Scholar] [CrossRef]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; De Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2012, 100, 144–150. [Google Scholar] [CrossRef]
- Markar, S.R.; Chin, S.-T.; Romano, A.; Wiggins, T.; Antonowicz, S.; Paraskeva, P.; Ziprin, P.; Darzi, A.; Hanna, G.B. Breath Volatile Organic Compound Profiling of Colorectal Cancer Using Selected Ion Flow-tube Mass Spectrometry. Ann. Surg. 2019, 269, 903–910. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Tietje, O.; Wong, C. Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Res. Treat. 2006, 99, 19–21. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin. Chim. Acta 2014, 436, 59–67. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Rahbari-Oskoui, F.; Wong, C. Volatile Markers of Breast Cancer in the Breath. Breast J. 2003, 9, 184–191. [Google Scholar] [CrossRef]
- Trefz, P.; Obermeier, J.; Lehbrink, R.; Schubert, J.K.; Miekisch, W.; Fischer, D.-C. Exhaled volatile substances in children suffering from type 1 diabetes mellitus: Results from a cross-sectional study. Sci. Rep. 2019, 9, 15707. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J.K.; Staude, H.; Fischer, D.-C.; Miekisch, W. Exhaled volatile substances mirror clinical conditions in pediatric chronic kidney disease. PLoS ONE 2017, 12, e0178745. [Google Scholar] [CrossRef]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; Zima, M.; Vetvicka, V. Biological Markers of Oxidative Stress in Cardiovascular Diseases: After so Many Studies, What do We Know? Immunol. Investig. 2018, 47, 823–843. [Google Scholar] [CrossRef]
| Aldehyde | Chain Length | Structural Formula |
|---|---|---|
| Ethanal | C2 | 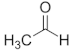 |
| Propanal | C3 | 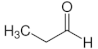 |
| Butanal | C4 | 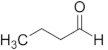 |
| Pentanal | C5 | 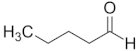 |
| Hexanal | C6 | 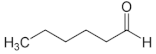 |
| Heptanal | C7 | 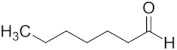 |
| Octanal | C8 | 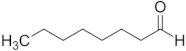 |
| Nonanal | C9 | 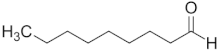 |
| Decanal | C10 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floss, M.A.; Fink, T.; Maurer, F.; Volk, T.; Kreuer, S.; Müller-Wirtz, L.M. Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review. Molecules 2022, 27, 5258. https://doi.org/10.3390/molecules27165258
Floss MA, Fink T, Maurer F, Volk T, Kreuer S, Müller-Wirtz LM. Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review. Molecules. 2022; 27(16):5258. https://doi.org/10.3390/molecules27165258
Chicago/Turabian StyleFloss, Maximilian Alexander, Tobias Fink, Felix Maurer, Thomas Volk, Sascha Kreuer, and Lukas Martin Müller-Wirtz. 2022. "Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review" Molecules 27, no. 16: 5258. https://doi.org/10.3390/molecules27165258
APA StyleFloss, M. A., Fink, T., Maurer, F., Volk, T., Kreuer, S., & Müller-Wirtz, L. M. (2022). Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review. Molecules, 27(16), 5258. https://doi.org/10.3390/molecules27165258







