Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects
Abstract
:1. Introduction
2. Chemical Constituents of ST
2.1. Volatile Oil
2.1.1. Monoterpenes
2.1.2. Sesquiterpenes
2.1.3. Aldehydes, Ketones and Quinones
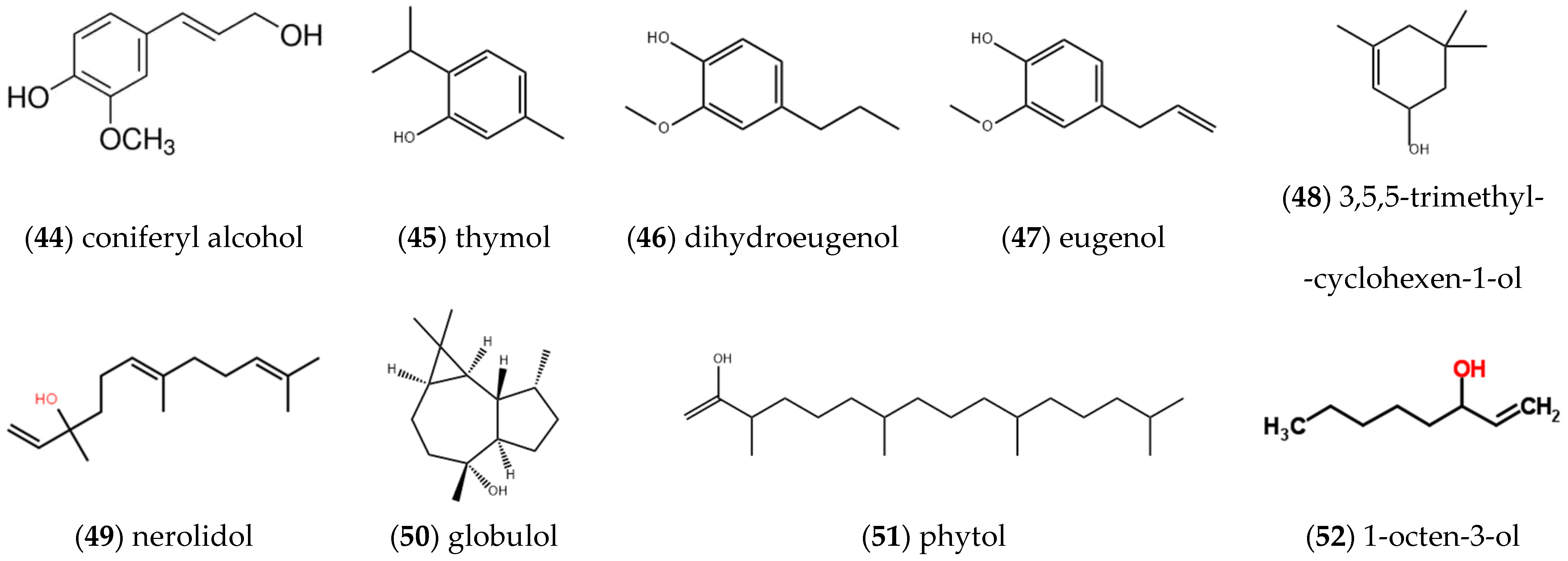
2.1.4. Alcohols and Phenolic Compounds
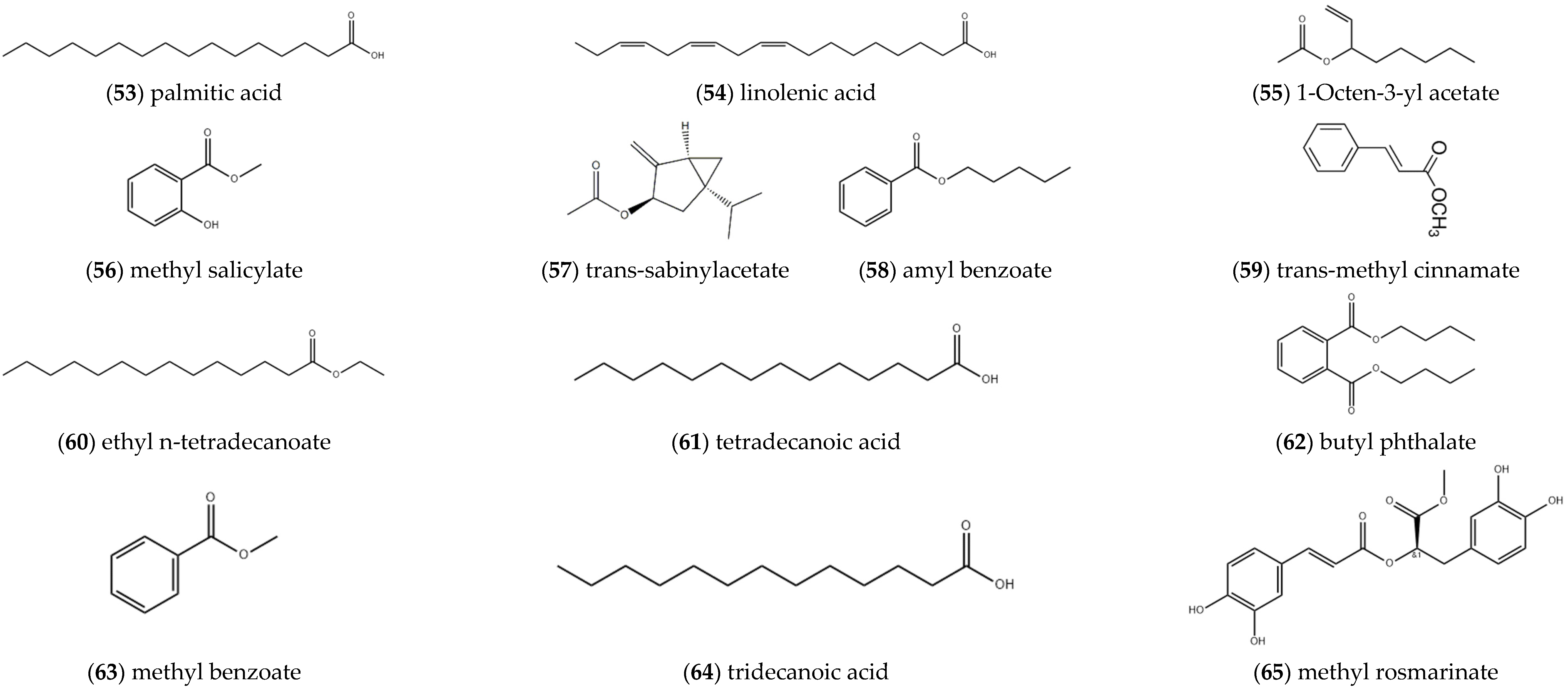
2.1.5. Carboxylic Acids and Esters
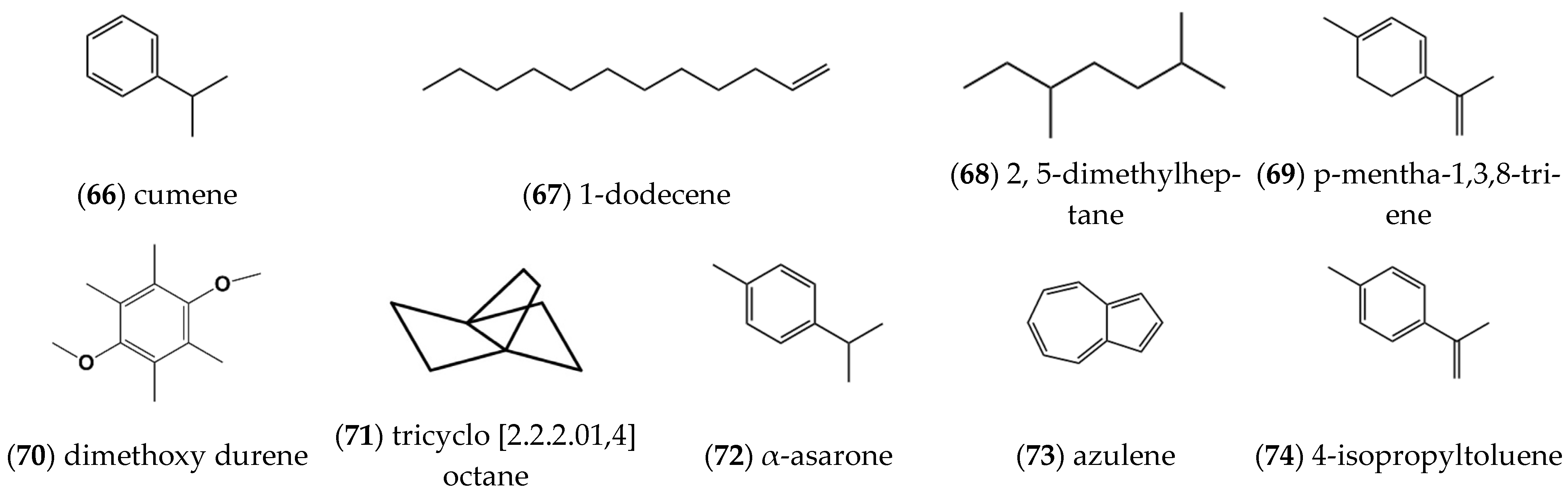
2.1.6. Alkenes and Alkanes
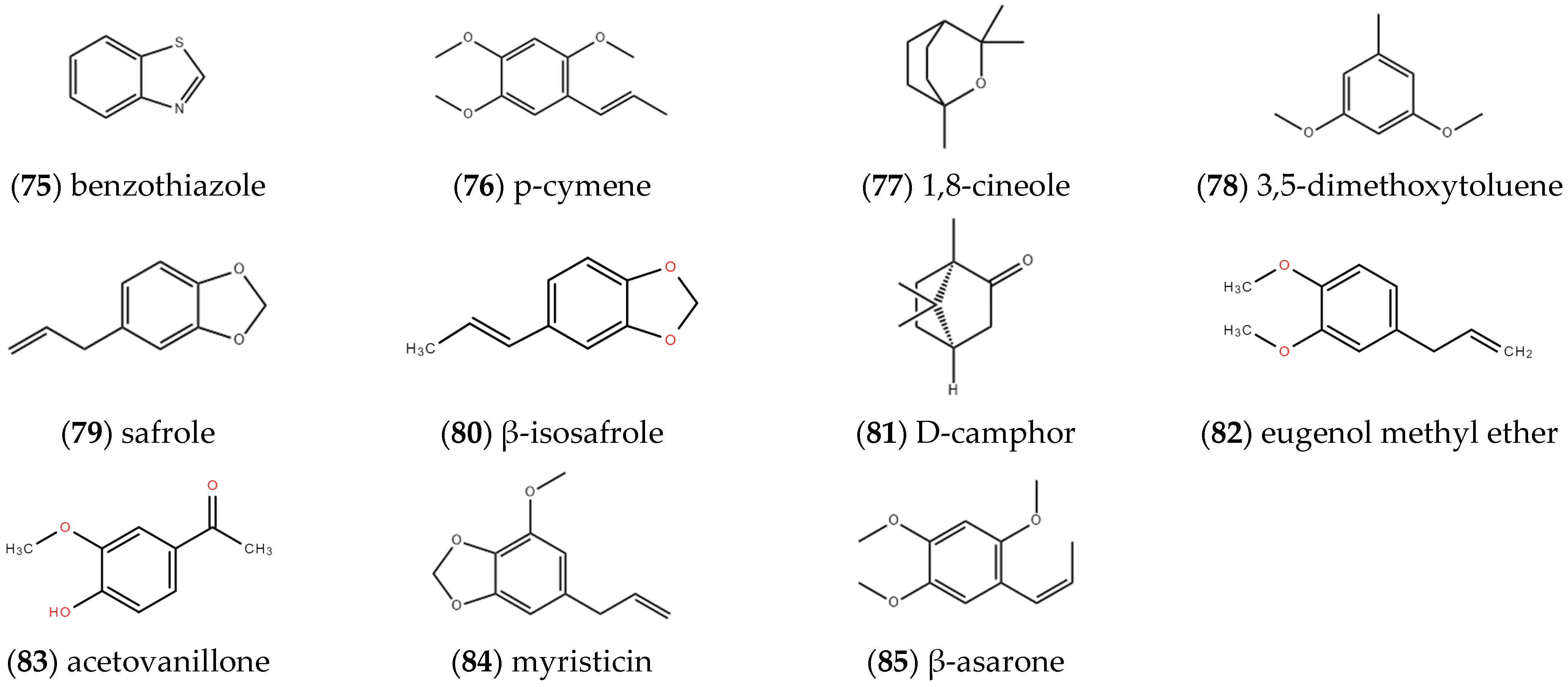
2.1.7. Other Compounds
2.1.8. Other Terpenoids
2.2. Flavonoids
2.3. Other Compounds
3. Pharmacological Effects
3.1. Antipyretic Effect
3.2. Antioxidant Effect
3.3. Anti-Inflammatory Effect
3.4. Antitumor Effect
3.5. Hemostatic Effect
3.6. Abirritation
3.7. Antibacterial Effect
3.8. Immunomodulatory Effect
3.9. Antiviral Activity
3.10. Other Pharmacological Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pharmacopoeia Commission of the Ministry of Public Health. Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Chun, M.H.; Kim, E.K.; Yu, S.M.; Oh, S.M.; Moon, K.Y.; Jung, J.; Hong, J. GC/MS combined with chemometrics methods for quality control of Schizonepeta tenuifolia Briq.: Determination of essential oils. Microchem. J. 2011, 97, 274–281. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Y.W.; Zhang, L.; Shan, M.Q.; Tang, Y.P.; Ding, A.W. Quantitative Comparative Analysis of the Bio-Active and Toxic Constituents of Leaves and Spikes of Schizonepeta tenuifolia at Different Harvesting Times. Int. J. Mol. Sci. 2011, 12, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.H.; Kim, E.K.; Lee, K.R.; Jung, J.H.; Hong, J.K. Quality control of Schizonepeta tenuifolia Briq. by solid-phase microextraction gas chromatography/mass spectrometry and principal component analysis. Microchem. J. 2010, 95, 25–31. [Google Scholar] [CrossRef]
- Zhu, M.F.; Tang, Y.; Zheng, Q.; Tang, D.F.; Luo, J.; Hu, P.Y.; Guo, Y.Y.; Wu, H.X.; Yang, M. Effects of different extraction methods on composition and antibacterial activity of volatile oil from Forsythiae Fructus, Schinzonepetae Herba, and Menthae Haplocalycis Herba. Chin. Tradit. Herb. Drugs. 2018, 49, 2845–2854. [Google Scholar]
- Cai, S.F.; Chen, K.H.; Linghu, R.G. Supercritical CO2 Extraction of Volatile Oil from Schizonepeta tenuifolia Briq. and GC-MS Analysis. J. Nanjing For. Univ. Nat. Sci. Ed. 2007, 31, 25–28. [Google Scholar]
- Yu, P.; Qiu, Q.; Cui, Z.J.; Liu, S.; Liu, T.L. Determination of chemical constituents of the essential oil from Schizonepeta tenuifolia Briq. by GC-MS. Chin. Tradit. Pat. Med. 2002, 24, 57–60. [Google Scholar]
- Liu, X.Q.; Li, S.W.; Li, Z.M.; Zhang, X.D.; Cui, X.Z.; Lu, C.M. Study on the constituents of volatile oil from different parts of Schizonepeta tenuifolia Briq. Chin. Tradit. Herb. Drugs 2008, 39, 1472–1473. [Google Scholar]
- Qiu, Q.; Ling, J.Y.; Ding, Y.P.; Chang, H.W.; Wang, J.; Liu, T.L. Comparison of Supercritical Fluid Extraction and Steam Distillation Methods for the Extraction of Essential Oils from Schizonepeta tenuifolia Briq. Chin. J. Chromatogr. 2005, 23, 646–650. [Google Scholar]
- Chen, Y.; Jiang, Z.H.; Tian, J.K. GC-MS Analysis of Volatile Oil from the Ear of Schizonepeta tenuifolia Briq. J. Chin. Med. Mater. 2006, 29, 140–142. [Google Scholar]
- Du, C.Z.; Qin, J.P.; Chen, Y.P.; Cai, Y. GC-MS Analysis of Volatile Oil Components in Schizonepeta tenuifolia Briq. from Various Habitats. Hubei Agric. Sci. 2014, 53, 188–190. [Google Scholar]
- Ye, D.J.; Ding, A.W.; Yu, L.; Cao, G.X.; Wu, M. Study on the components of volatile oil from different medicinal parts and after carbon frying of Schizonepeta tenuifolia Briq. Chin. J. Chin. Mater. Med. 1985, 10, 21–23. [Google Scholar]
- Wu, Y.L.; Ding, A.W.; Feng, Y.L. Study on volatile oil of Schizonepeta tenuifolia Briq. and its related Medicinal materials. Chin. Tradit. Herb. Drugs 2000, 31, 894–896. [Google Scholar]
- Yang, Z.Y.; Yan, J.C.; Zhang, S.J.; Yu, L.G. Study on chemical constituents of volatile oil from Schizonepeta Spica. Chin. Tradit. Herb. Drugs. 1996, 27, 396–397. [Google Scholar]
- Meng, N.; Huang, S.; Hu, D.D.; Xu, Y.L.; Wang, F.Y.; Wang, J.L. Chemical constituents from Nepeta angustifolia. Chin. Tradit. Pat. Med. 2017, 39, 976–980. [Google Scholar]
- Lee, I.K.; Kim, M.A.; Lee, S.Y.; Hong, J.K.; Lee, J.H.; Lee, K.R. Phytochemical Constituents of Schizonepeta tenuifolia Briquet. Nat. Prod. Sci. 2008, 14, 100–106. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Jin, Y.; Han, X.M.; Chu, Q.L.; Yang, J.S. Two new sesquiterpenes from the aerial parts of Schizonepeta tenuifolia. J. Asian Nat. Prod. Res. 2017, 19, 152–156. [Google Scholar] [CrossRef]
- Zeren, L.M.; Pu, Z.; Zhuoma, D.Z.; Wang, J.L. Chemical constituents and pharmacological action of Schizonepeta tenuifolia Briq. J. Mod. Med. Health 2014, 30, 215–217. [Google Scholar]
- Fang, J.X.; Wang, S.; Meng, X.S.; Bao, Y.R.; Li, T.J. Determination of six flavonoids in Schizonepeta tenuifolia from different areas by HPLC. Chin. Tradit. Herb. Drugs. 2017, 48, 2292–2295. [Google Scholar]
- Song, K.; Wang, H.Q.; Liu, C.; Kang, J.; Li, B.M.; Chen, R.Y. Chemical Studies on the Herb of Chenopodium ambrosioides. Chin. J. Chin. Mater. Med. 2014, 39, 254257. [Google Scholar]
- Wen, Z.S.; Liu, L.L.; Li, X.R.; Zheng, Y.G.; Ma, D.L. Simultaneous determination of 8 components in Schizonepeta tenuifolia from different habitats by HPLC. J. Chin. Med. Mater. 2019, 42, 139–143. [Google Scholar]
- Huang, X.H.; Chen, J.; Xu, X.Q.; Zhang, W.T.; Zhao, C.C. A New Phenolic Compound from Schizonepeta tenuifolia. Chem. Nat. Compd. 2016, 52, 1005–1007. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Sun, E.; Ding, A.W. Anit-inflammatory, analgesic and antipyretic effects of schizonepetolide poly-lactic-CO-glycolic acid nanoparticles. J. China Pharm. Univ. 2008, 39, 433–436. [Google Scholar]
- Cai, G.X.; Liu, L.; Wang, Y.H.; Tang, Z.P. Comparison of powders with different diameters of schizonepeta on anti-pyretic and analgesic effects. J. Hunan Univ. Chin. Med. 2008, 28, 18–20. [Google Scholar]
- Song, L. Pharmaceutical Study of JG Spray. Ph.D. Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2007. [Google Scholar]
- Wu, Z.Q.; Liu, G.H.; Yan, L.J.; Nan, C.H.; Yue, Z.J.; Wang, X.F. Experimental study on anti-influenza virus infection with yinqiao-decoction by orthogonal design. Chin. J. Exp. Clin. Virol. 2010, 24, 427–429. [Google Scholar]
- Bouididael, E.H.; Alaoui, K.; Cherrah, Y.; Chammache, M.; Idrissi, A.I. Analgesic activity of different nonvolatile extracts of Nepeta atlantica Ball and Nepeta Tuberosa L. ssp. reticulata (Desf.) Maire. Therapie 2008, 63, 333–338. [Google Scholar] [CrossRef]
- Tae, K.S.; Kim, S.J. Inhibition of iNOS and DNA Oxidation by Methanol Extract of Schizonepeta tenuifolia. Trop. J. Pharm. Res. 2012, 11, 397–404. [Google Scholar] [CrossRef]
- Wang, B.S.; Huang, G.J.; Tai, H.M.; Huang, M.H. Antioxidant and anti-inflammatory activities of aqueous extracts of Schizonepeta tenuifolia Briq. Food Chem. Toxicol. 2012, 50, 526–531. [Google Scholar] [CrossRef]
- Wen, Z.S.; Li, X.R.; Fan, Z.X.; Ma, D.L.; Zheng, Y.G. Study on antioxidant activity of Schizonepeta tenuifolia polysaccharide extract. Hebei J. Tradit. Chin. Med. 2019, 34, 53–56. [Google Scholar]
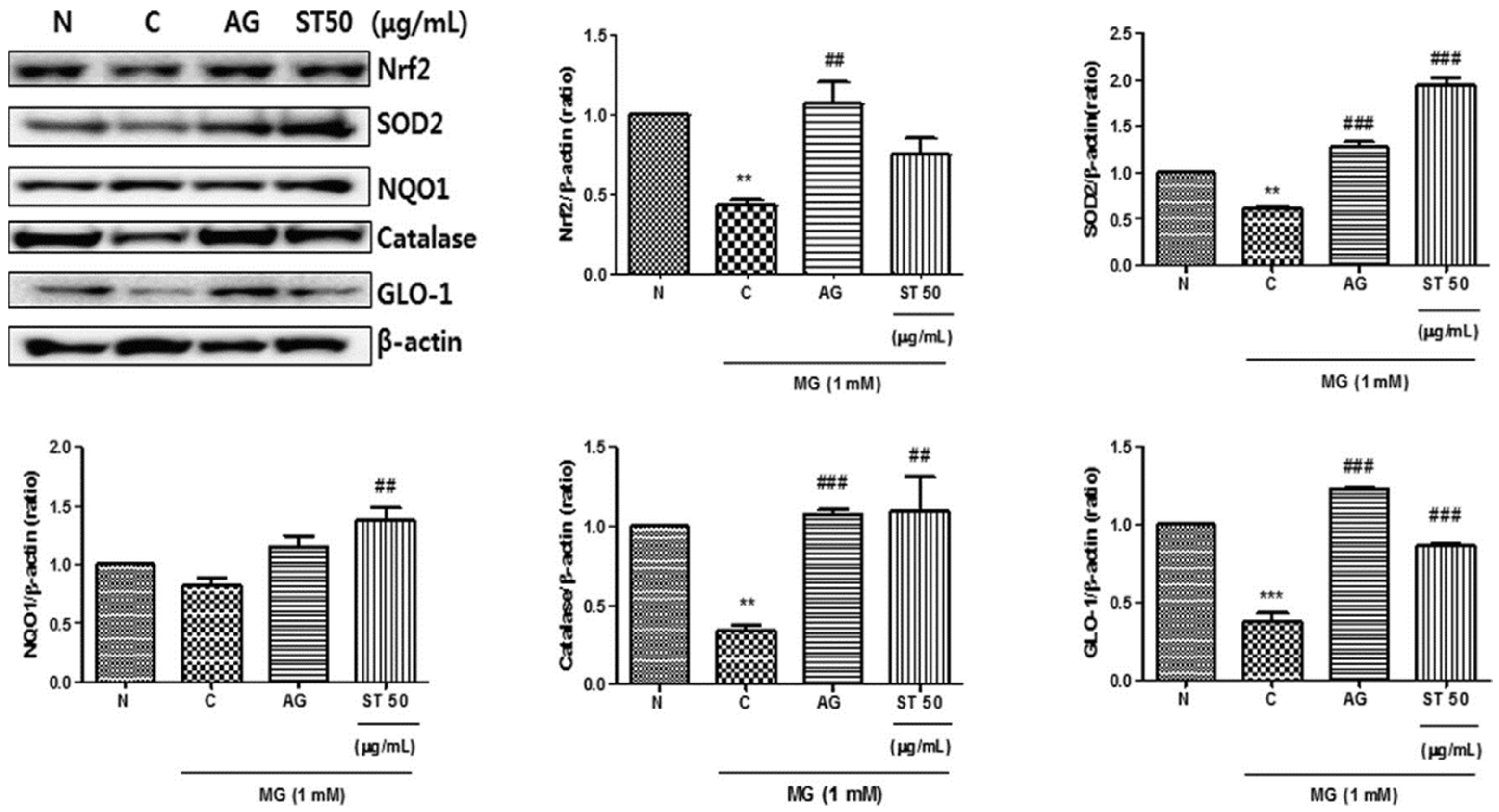
- Do, M.H.; Choi, J.; Kim, Y.; Park, H.Y.; Ha, S.K.; Hur, J. Schizonepeta tenuifolia reduces methylglyoxal-induced cytotoxicity and oxidative stress in mesangial cells. J. Funct. Foods. 2019, 62, 103531. [Google Scholar] [CrossRef]
- Berner, A.K.; Brouwers, O.; Pringle, R.; Klaassen, I.; Colhoun, L.; McVicar, C.; Brockbank, S.; Curry, J.W.; Miyata, T.; Brownlee, M.; et al. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia 2012, 55, 845–854. [Google Scholar] [CrossRef]
- Qian, R.H.; Ren, Q.R.; Huang, J.; Zhou, Y.J.; Zhang, J.J.; Wang, Y.N. Study on the ldentification and Antioxidant and Antibacterial Activity of Total Flavonoids from Chenopodium ambrosioides L. in Sichuan Province. Food Ind. 2018, 39, 167–171. [Google Scholar]
- Shan, M.Q.; Qian, Y.; Yu, S.; Guo, S.C.; Zhang, L.; Ding, A.W.; Wu, Q.N. Anti-inflammatory effect of volatile oil from Schizonepeta tenuifolia on carrageenin-induced pleurisy in rats and its application to study of appropriate harvesting time coupled with multi-attribute comprehensive index method. J. Ethnopharmacol. 2016, 194, 580–586. [Google Scholar] [CrossRef]
- Sohn, S.H.; Cho, S.; Ji, E.S.; Kim, S.H.; Shin, M.; Hong, M.; Bae, H. Microarray analysis of the gene expression profile of HMC-1 mast cells following Schizonepeta tenuifolia Briquet treatment. Cell Immunol. 2012, 277, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qiu, J.; Wu, X.; Huang, S.; Yuan, H.; Park, S. Schizonepeta Tenuifolia with Alpinia Oxyphylla Alleviates Atopic Dermatitis and Improves the Gut Microbiome in Nc/Nga Mice. Pharmaceutics 2020, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Taviano, M.F.; Giuffrida, D.; Trovato, A.; Tzakou, O.; Galati, E.M. Anti-inflammatory activity of extract and fractions from Nepeta sibthorpii Bentham. J. Ethnopharmacol. 2005, 97, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Shen, Y.J.; Ren, Y.X.; Li, J.H. Experimental study on anti-inflammatory mechanism of volatile Oil from Schizonepeta tenuifolia. J. Chin. Med. Mater. 2006, 29, 359–362. [Google Scholar]
- Byun, M.W. Schizonepeta tenuifolia ethanol extract exerts anti-inflammatory activity through the inhibition of TLR4 signaling in lipopolysaccharide-stimulated macrophage cells. J. Med. Food 2014, 17, 350–356. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Kim, J.H.; Jung, H.S.; Sohn, Y.; Choi, Y.J.; Hwang, M.K.; Kim, S.H.; Kim, J.; Yang, W.M. Schizonepeta tenuifolia inhibits the development of atopic dermatitis in mice. Phytother. Res. 2013, 27, 1131–1135. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, S.X.; Fu, L.Y.; Dai, Q.Y.; Zhang, Y.B.; Liu, Z.H.; Li, S.Y.; Yang, X.Y.; Nie, G.K.; Wang, R. Effect of Schizonepetae Herba and Saposhnikoviae Radix on expression of AQP4 and AQP8 in colonic mucosa of rats with ulcerative colitis. Chin. J. Chin. Mater. Med. 2020, 45, 3719–3725. [Google Scholar]
- Lv, H.J.; Wen, T.Q.; Luo, J.; Liu, X.B.; Yang, J.; Zeng, N. Study on mechanism of essential oils of Schizonepeta tenuifolia Briq. in endotoxin poisoning mice via NLRP3 inflammasome pathway. Chin. Pharmacol. Bull. 2019, 35, 371–376. [Google Scholar]
- Kang, H.; Han, S.W.; Hong, J.W.; Sohn, N.W. Suppression of tumour necrosis factor-a by Schizonepeta tenuifolia water extract via inhibition of IkBa degradation and Jun N-terminal kinase/stress-activated protein kinase activation. J. Pharm. Pharmacol. 2010, 62, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, L.; Zhang, J.P.; Chen, L.; Wu, T.; Aisa, H.A.; Maiwulanjiang, M. Spectrum–effect relationship between GC-QTOF-MS fingerprint and antioxidant, anti-inflammatory activities of Schizonepeta tenuifolia essential oil. Biomed. Chromatogr. 2021, 35, e5106. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Q.; Hu, F.; Wei, M.; Wang, N.P.; Wei, J.B. Research on Anti-tumor Effect of Essential Oil in Schizonepeta Tenuifolia Briq. and Its Inducing Apoptosis. Guangxi J. Tradit. Chin. Med. 2006, 29, 60–62. [Google Scholar]
- Kim, S.J.; Kim, J.S.; Choi, I.Y.; Kim, D.H.; Kim, M.C.; An, H.J.; Na, H.J.; Kim, N.H.; Moon, P.D.; Myung, N.Y.; et al. Anti-Inflammatory Activity of Schizonepeta tenuifolia through the Inhibition of MAPK Phosphorylation in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2008, 36, 1145–1158. [Google Scholar] [CrossRef]
- Wu, J.L. Antitumor Mechanism of Essential Oil and Its Two Main Components from Chenopodium ambrosioides L. on Human Breast Cancer MCF-7 Cells. Doctoral Dissertation, Sichuan Normal University, Chengdu, China, 2014. [Google Scholar]
- Jeon, B.R.; Irfan, M.; Kim, M.; Lee, S.E.; Lee, J.H.; Man, H.R. Schizonepeta tenuifolia inhibits collagen stimulated platelet function via suppressing MAPK and Akt signaling. J. Biomed. Res. 2019, 33, 250–257. [Google Scholar]
- Cao, L.L.; Li, X.; Zhang, L. Experimental study on the effect of Schizonepeta Spica Carbonisata and its effective parts on rat coagulation system. Chin. Tradit. Pat. Med. 2010, 32, 611–613. [Google Scholar]
- Zhang, M.L.; Zhao, Y.; Cheng, J.J.; Liu, X.M.; Wang, Y.Z.; Yan, X.; Zhang, Y.; Lu, F.; Wang, Q.G.; Qu, H.H. Novel carbon dots derived from Schizonepetae Herba Carbonisata and investigation of their haemostatic efficacy. Artif. Cells. Nanomed. Biotechnol. 2018, 46, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L. Study on Hemostatic Substance Basis and Mechanism of Charred Schizonepeta; Beijing University of Chinese Medicine: Beijing, China, 2018. [Google Scholar]
- Xue, Z.B. Preparation, Quality Analysis and Activity Evaluation of a Traditional Chinese Medicated Oil with Anti-Inflammatory and Analgesic Effect; East China University of Science and Technology: Shanghai, China, 2014. [Google Scholar]
- Huang, S.; Jiang, C.Y.; Long, F. Anti-Inflammation and Analgesic Effect of Volatile Oil from Nepeta Angustifolia. Her. Med. 2011, 30, 1262–1265. [Google Scholar]
- Meng, N.; Huang, S.; Hu, D.D.; Yuan, R.Y.; Li, B.; Wang, J.L.; Wu, C.Y. Anti-Inflammatory and Analgesic Effects of Different Solvent Extractions of Nepeta Angustifolia. Chin. Arch. Tradit. Chin. Med. 2018, 36, 1874–1877. [Google Scholar]
- Liang, Q.; Liu, W.Y.; Zhang, Q.; Wang, F. Chemical constituents and anti-microbial activities of essential oil of Chenopodium ambrosioides L. from Kunming Yunnan. Lishizhen Med. Mater. Med. Res. 2018, 29, 1800–1803. [Google Scholar]
- Ye, H.; Yu, J.; Zhang, X.Z. Study on bactericidal activity against H.pylori in vivo and effects on the NF-κB expression of Chenopodium ambrosioides L. volatile oil. Chin. J. Tradit. Chin. Med. 2017, 32, 5346–5349. [Google Scholar]
- Su, C.; Zhao, Y.Y.; Feng, Y.; Zhang, L.L.; Zheng, Y.G.; Zheng, K.Y. Antibacterial effect and composition analysis of the leaves and spicas from Schizonepeta tenuifolia Briq. Chin. J. New Drugs 2022, 31, 1103–1111. [Google Scholar]
- Lin, Y.H.; Chen, H.Y.; Chiu, J.C.; Chen, K.J.; Ho, H.Y.; Yang, S.H. Immunomodulation Effects of Schizonepeta tenuifolia Briq. On the IgE-Induced Allergic Model of RBL-2H3 Cells. Evid.-Based Complementary Altern. Med. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Kang, H.; Moon, J.Y.; Sohn, N.W. Regulation of interferon-gamma, interleukin-4 and interleukin-2 by Schizonepeta tenuifolia through differential effects on nuclear factor-kappaB, NFATc2 and STAT4/6. Exp. Biol. Med. 2010, 235, 230–236. [Google Scholar] [CrossRef]
- Kang, H.; Oh, Y.J.; Choi, H.Y.; Ham, I.H.; Bae, H.S.; Kim, S.H.; Ahn, K.S. Immunomodulatory effect of Schizonepeta tenuifolia water extract on mouse Th1/Th2 cytokine production in-vivo and in-vitro. J. Pharm. Pharmacol. 2008, 60, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Li, Z.K.; Liang, Y.Q.; Xia, H.Y.; Li, C.H.; Wang, C.J. Immunomodulatory Activity of Polysaccharides from Herba Schizonepetae. Her. Med. 2021, 40, 187–192. [Google Scholar]
- Yang, M.H.; Huang, X.W.; Xin, G.; Liu, Y.X.; Zhao, X.T.; Li, X.; Xu, Y.; Zhang, Q. Experimental study on the best decoction time of Schizonepeta in Schizonepeta decoction. Chin. J. Hosp. Pharm. 2019, 39, 344–347+352. [Google Scholar]
- Fan, H.J.; Lu, Z.W.; Wen, F.; Xie, Z.P.; Xie, J.W.; Pei, T.T.; Tan, Z.B.; Wu, Y.T.; Liu, B.; Zhou, Y.C. Anti-inflammation and Restoring Immune Function Effects of Jingjie Lianqiao Decoction in Chronic Atopic Dermatitis Mice. Liaoning J. Tradit. Chin. Med. 2018, 45, 854–858+897. [Google Scholar]
- Yoo, J.S.; Kim, D.K.; Kim, S.H.; Shin, T.Y. Anti-allergic Effects of Schizonepeta tenuifolia on Mast Cell-Mediated Allergy Model. Nat. Prod. Sci. 2011, 17, 239–244. [Google Scholar]
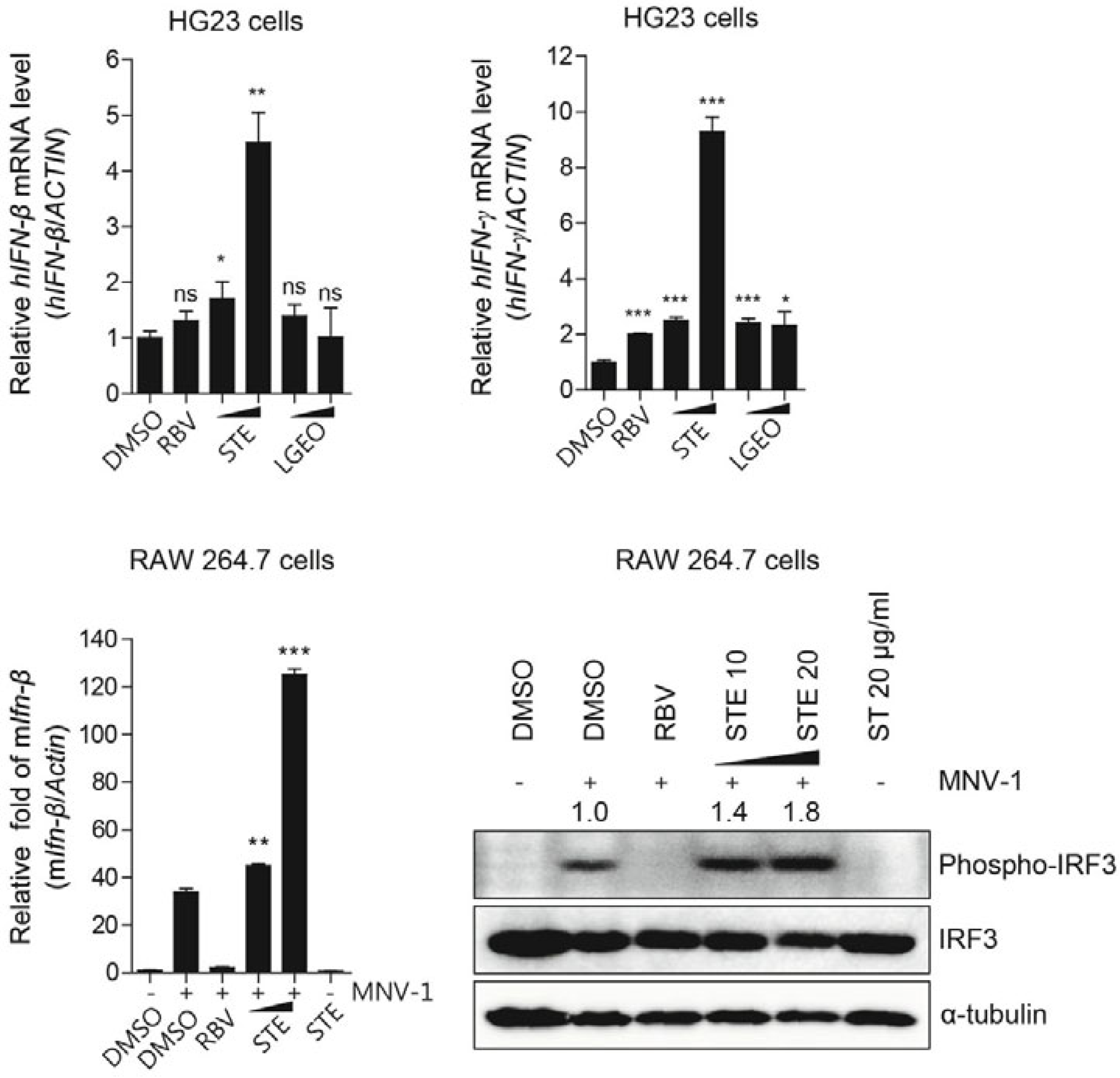
- Ng, Y.C.; Kim, Y.W.; Lee, J.S.; Lee, S.J.; Song, M.J. Antiviral activity of Schizonepeta tenuifolia Briquet against noroviruses via induction of antiviral interferons. J. Microbiol. 2018, 56, 683–689. [Google Scholar] [CrossRef]
- Chen, S.G.; Cheng, M.L.; Chen, K.H.; Horng, J.T.; Liu, C.C.; Wang, S.M.; Sakurai, H.; Leu, Y.L.; Wang, S.D.; Ho, H.Y. Antiviral activities of Schizonepeta tenuifolia Briq. against enterovirus 71 in vitro and in vivo. Sci. Rep. 2017, 7, 935. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Baek, J.M.; Ahn, S.J.; Cheon, Y.H.; Park, S.H.; Yang, M.; Choi, M.K.; Oh, J. Ethanolic extract of Schizonepeta tenuifolia attenuates osteoclast formation and activation in vitro and protects against lipopolysaccharide-induced bone loss in vivo. BMC Complementary Altern. Med. 2016, 16, 301. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Hoi-Seon Lee, H.S. Changes in Acaricidal Potency by Introducing Functional Radicals and an Acaricidal Constituent Isolated from Schizonepeta tenuifolia. J. Agric. Food Chem. 2013, 61, 11511–11516. [Google Scholar] [CrossRef] [PubMed]
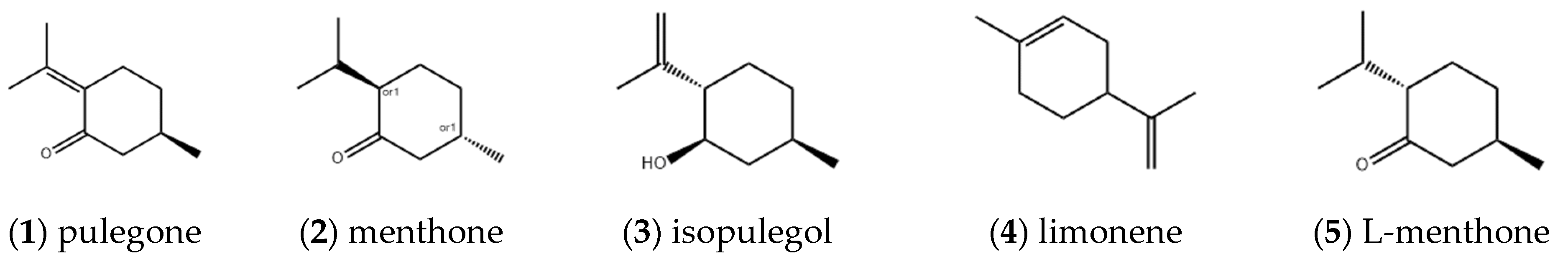
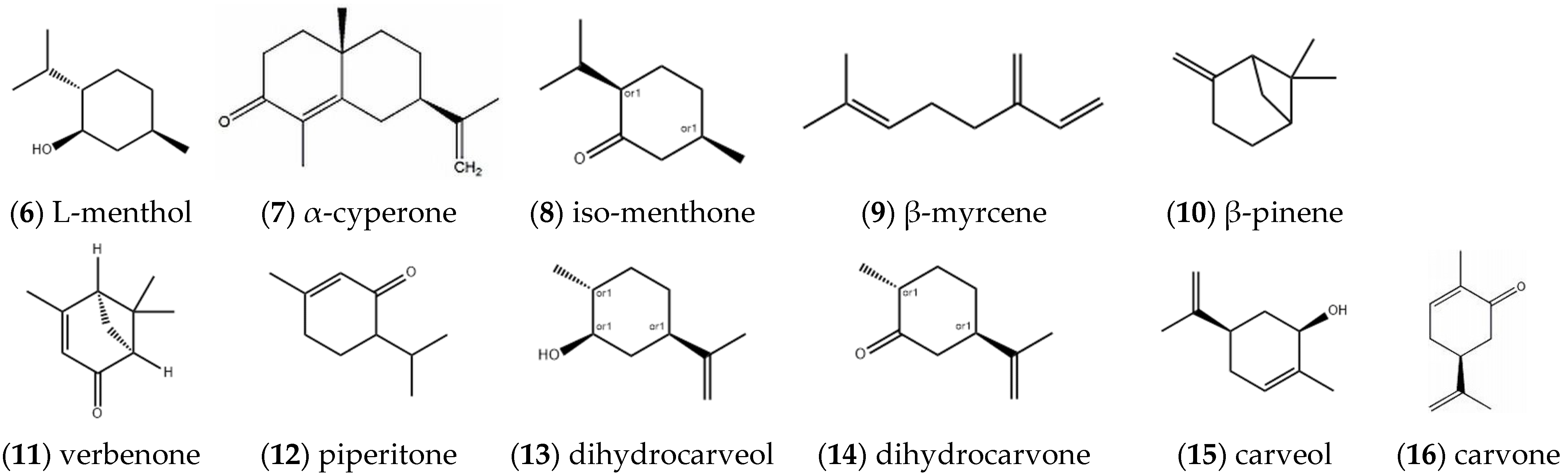
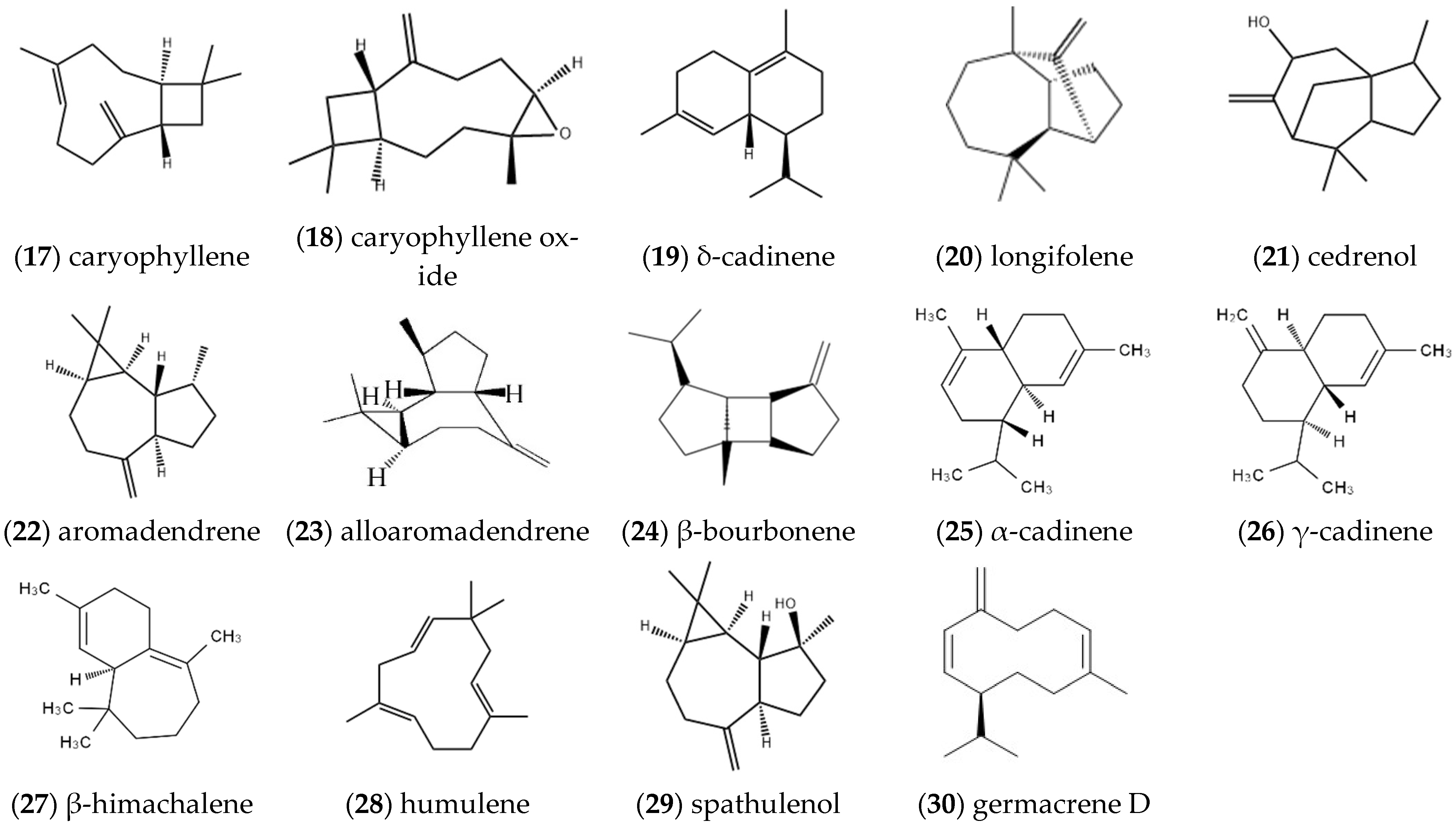
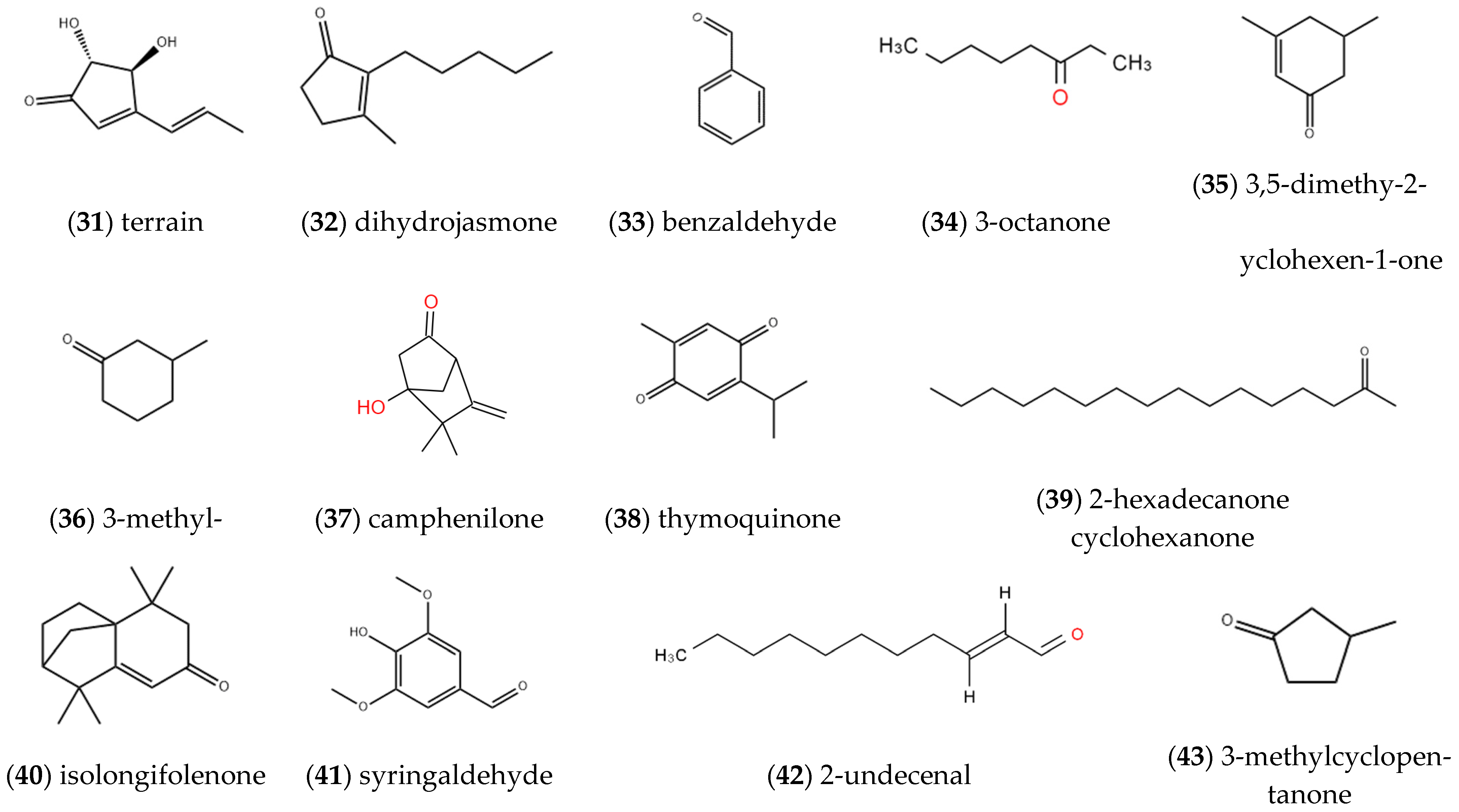

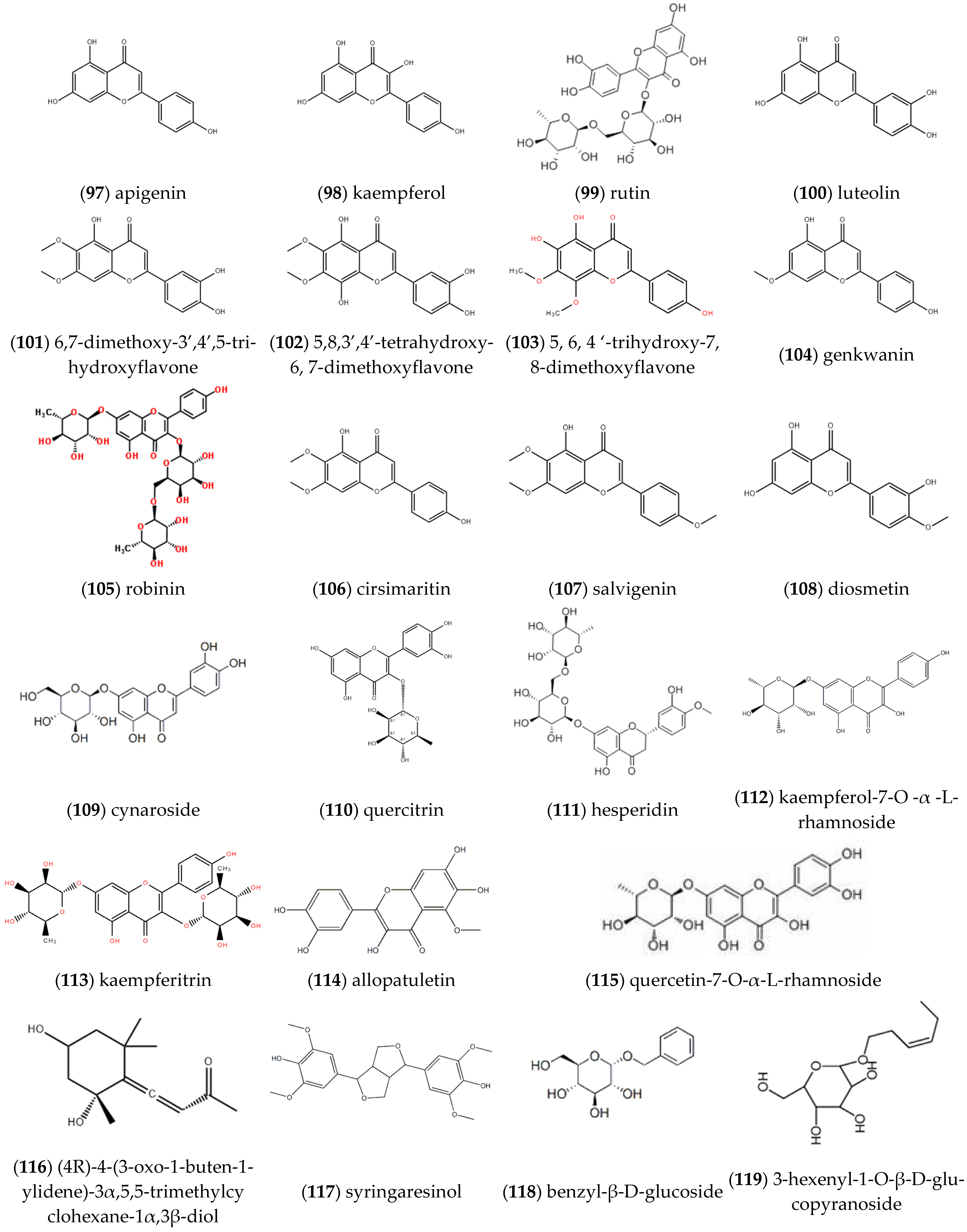
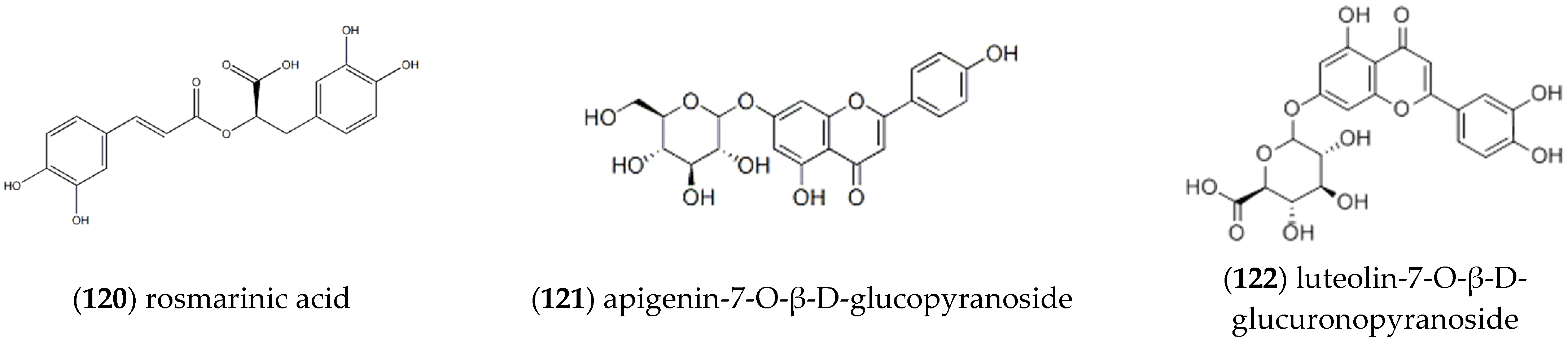
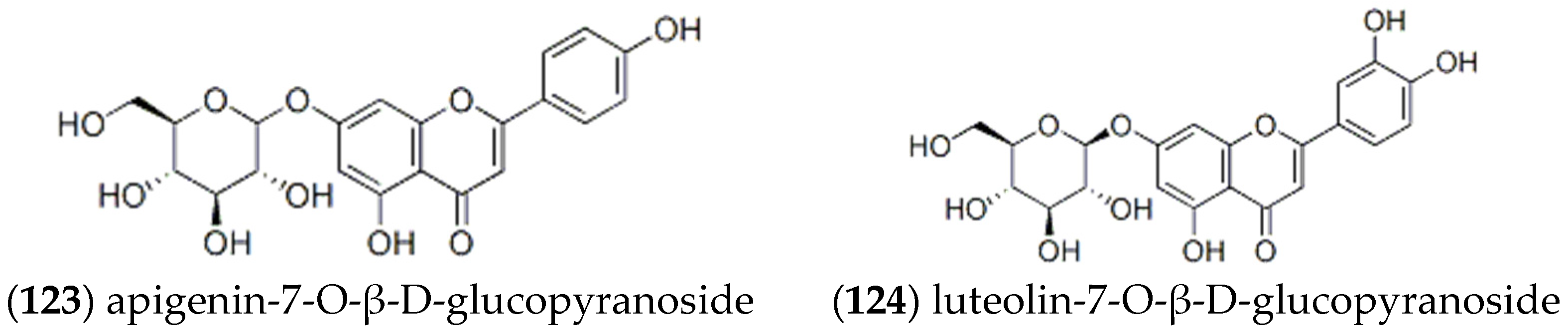
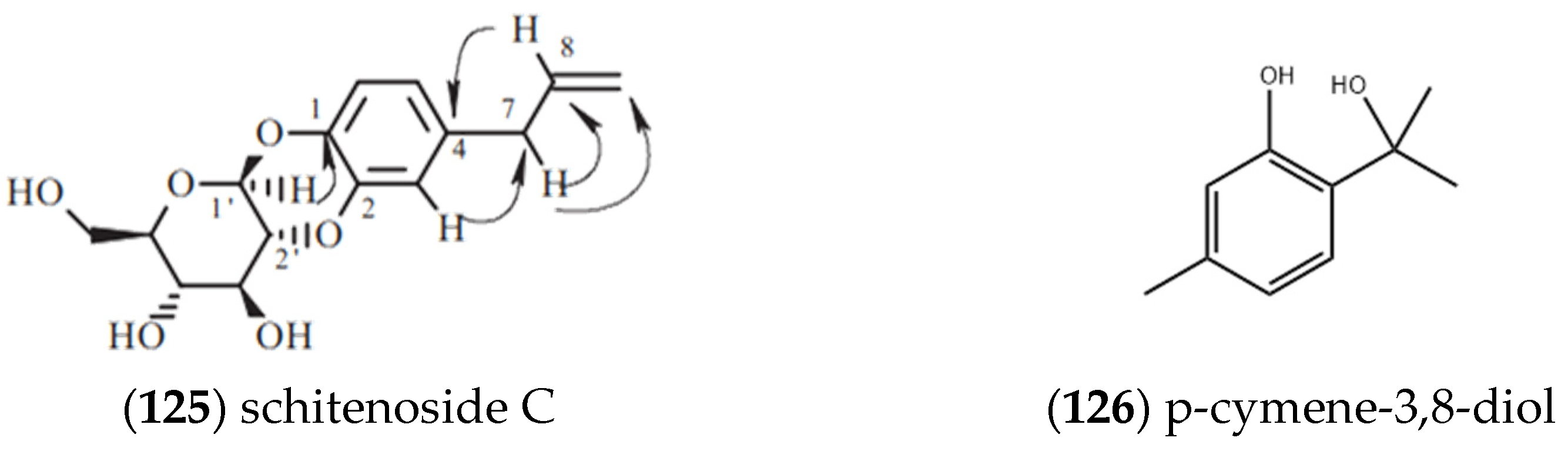

| Entry | Constituents | CAS | ST/% | MH/% |
|---|---|---|---|---|
| 1 | 1-Octen-3-ol | 003391-86-4 | 0.73 | - |
| 2 | Limonene | 005989-27-5 | 5.00 | 1.80 |
| 3 | 1-octen-3-ol | 002442-10-6 | 0.44 | - |
| 4 | Iso-menthone | 000491-07-6 | 43.58 | - |
| 5 | Pyridazine | 000089-80-5 | 2.71 | 11.90 |
| 6 | Trans-isopulegone | 029606-79-9 | 1.43 | - |
| 7 | Pulegone | 000089-82-7 | 40.02 | 14.52 |
| 8 | Piperitone | 000491-09-8 | 1.12 | 1.32 |
| 9 | Caryophyllene oxide | 001139-30-6 | 1.38 | 1.31 |
| Entry | Caffeic Acid | Cynaroside | Quercitrin | Rosmarinic Acid | Luteolin | Apigenin | Diosmetin | Pulegone |
|---|---|---|---|---|---|---|---|---|
| S1 | 0.0691 | 0.1168 | 0.6116 | 1.5821 | 0.3994 | 0.0436 | 0.0839 | 1.4944 |
| S2 | 0.0341 | 0.0925 | 0.2653 | 0.9058 | 0.1725 | 0.0227 | 0.0472 | 0.7942 |
| S3 | 0.0333 | 0.1897 | 0.0372 | 0.3063 | 0.2925 | 0.0234 | 0.0657 | 0.2097 |
| S4 | 0.0366 | 0.0968 | 0.6996 | 1.2986 | 0.3824 | 0.0467 | 0.1074 | 0.8702 |
| S5 | 0.0597 | 0.1228 | 0.5311 | 1.2404 | 0.7753 | 0.0312 | 0.0681 | 1.1241 |
| S6 | 0.1049 | 0.0734 | 0.5406 | 0.8233 | 0.3251 | 0.0428 | 0.0999 | 1.0054 |
| S7 | 0.0846 | 0.1242 | 0.7346 | 1.3433 | 0.5298 | 0.0602 | 0.0741 | 1.6335 |
| S8 | 0.1697 | 0.1830 | 0.6159 | 1.5052 | 0.4300 | 0.0630 | 0.0571 | 12.4694 |
| S9 | 0.2020 | 0.1070 | 0.4136 | 2.0761 | 0.3759 | 0.1009 | 0.0392 | 20.7663 |
| S1 | 0.1637 | 0.1487 | 1.0849 | 2.5900 | 0.5221 | 0.0731 | 0.0885 | 35.9777 |
| S1 | 0.0638 | 0.0848 | 0.8173 | 1.4188 | 0.2397 | 0.0476 | 0.0991 | 0.8803 |
| S1 | 0.0847 | 0.0554 | 0.3971 | 0.8774 | 0.5181 | 0.0554 | 0.0533 | 0.8091 |
| S1 | 0.2359 | 0.1315 | 0.5864 | 2.0414 | 0.1905 | 0.1104 | 0.0353 | 9.2541 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhou, M. Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects. Molecules 2022, 27, 5249. https://doi.org/10.3390/molecules27165249
Zhao X, Zhou M. Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects. Molecules. 2022; 27(16):5249. https://doi.org/10.3390/molecules27165249
Chicago/Turabian StyleZhao, Xueying, and Mingwei Zhou. 2022. "Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects" Molecules 27, no. 16: 5249. https://doi.org/10.3390/molecules27165249
APA StyleZhao, X., & Zhou, M. (2022). Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects. Molecules, 27(16), 5249. https://doi.org/10.3390/molecules27165249






