Gold Mine Wooden Artefacts: Multianalytical Investigations for the Selection of Appropriate Consolidation Treatments
Abstract
1. Introduction
2. Results
2.1. FTIR
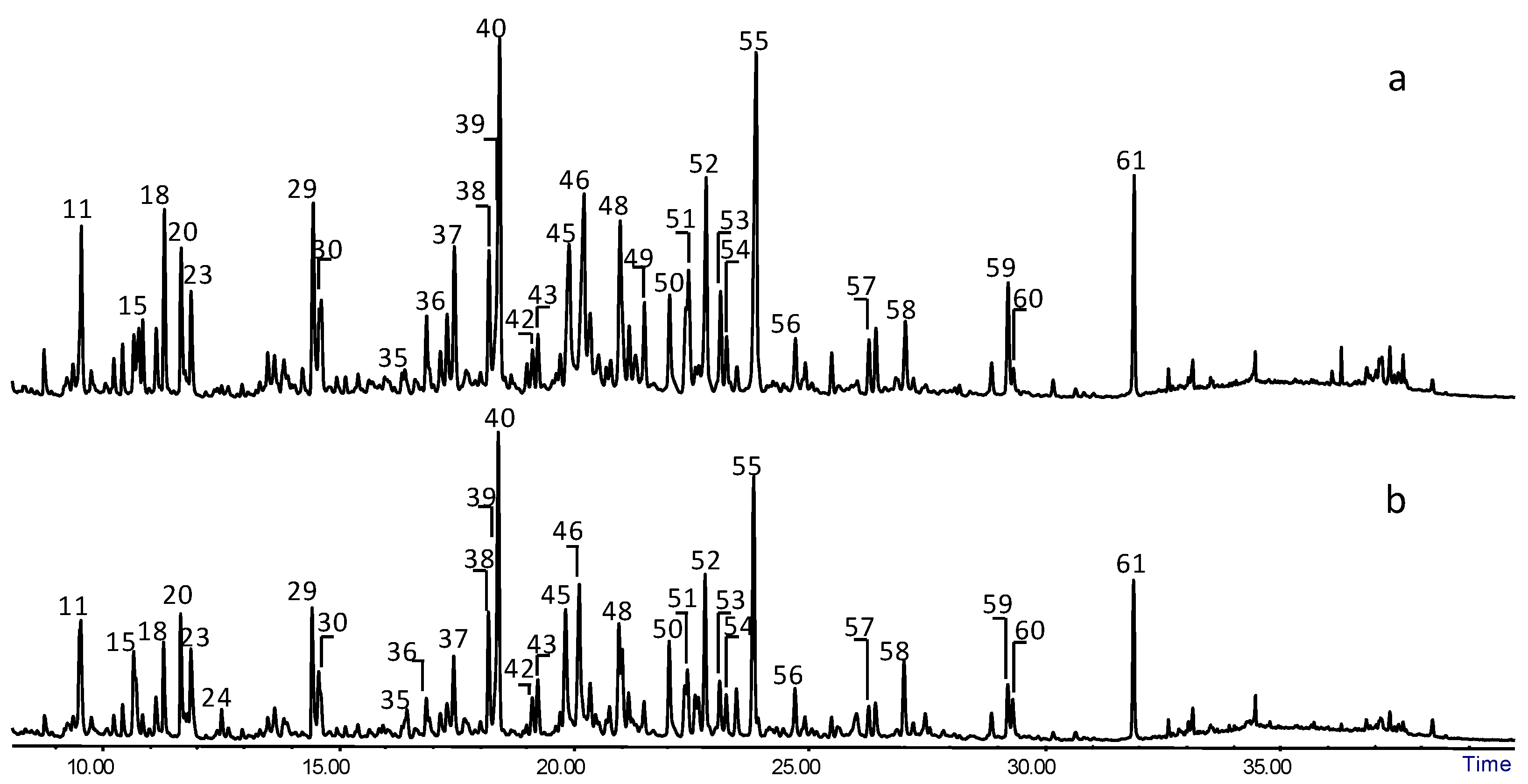
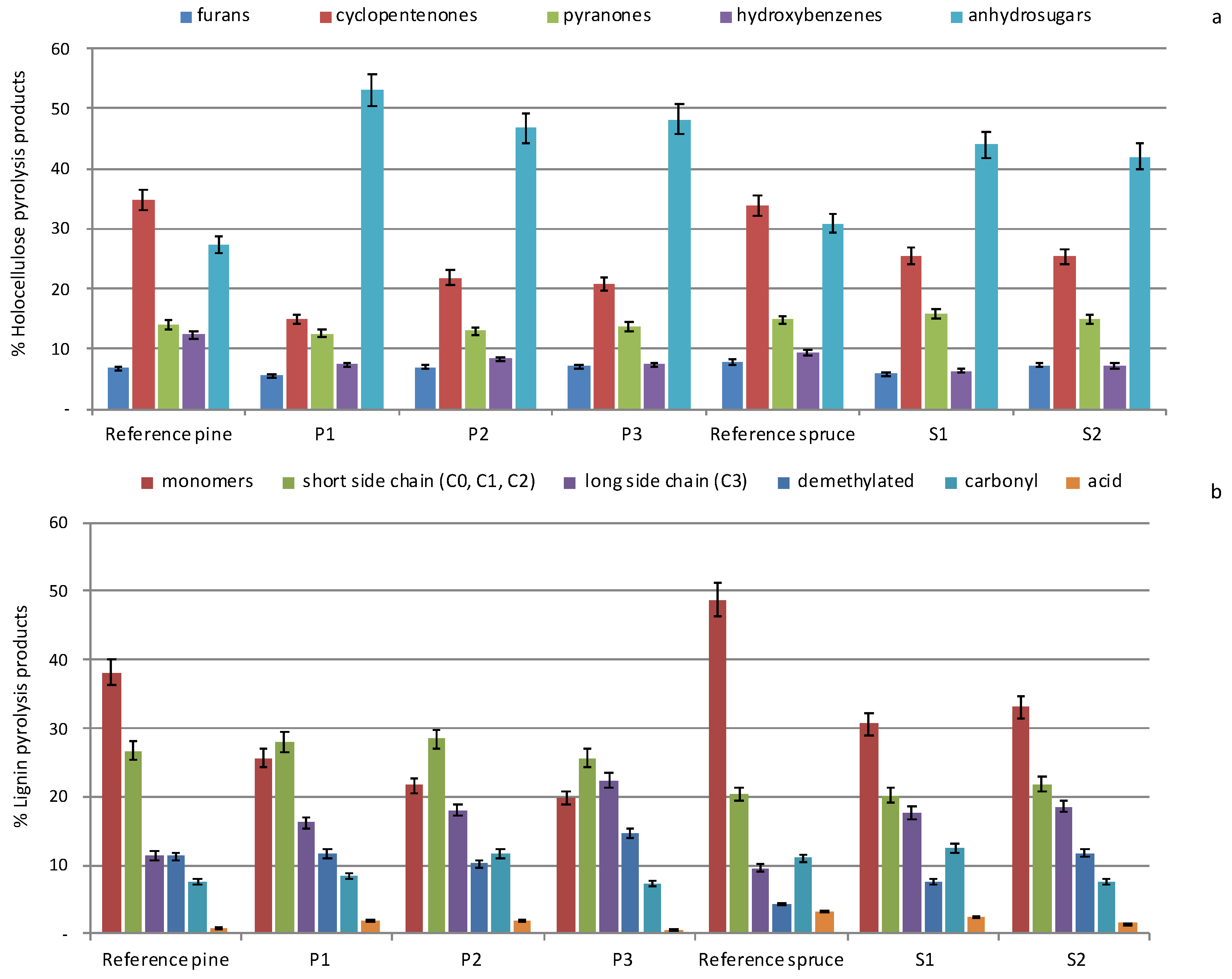
2.2. Py-GC/MS
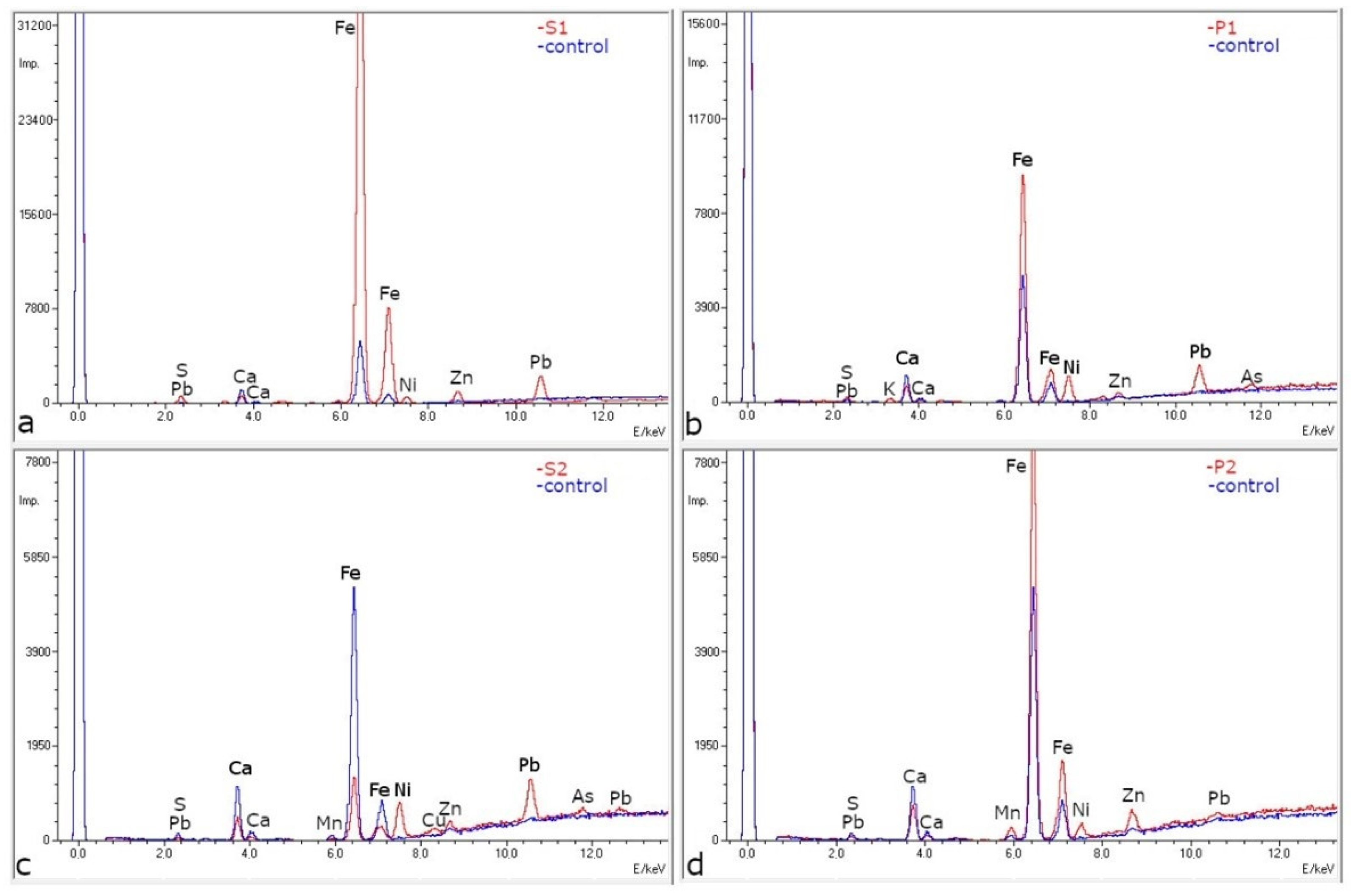
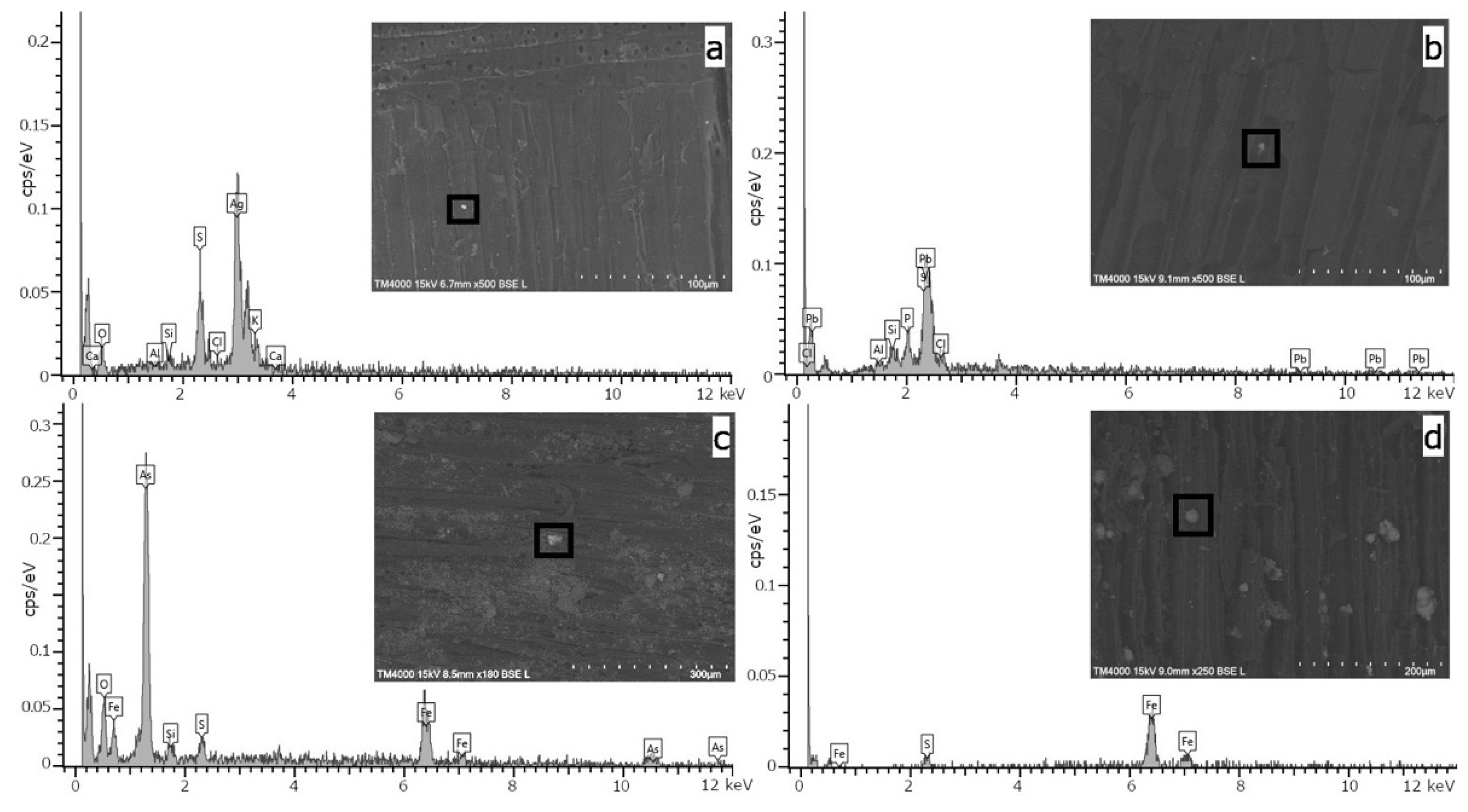
2.3. ED-XRF and SEM-EDS
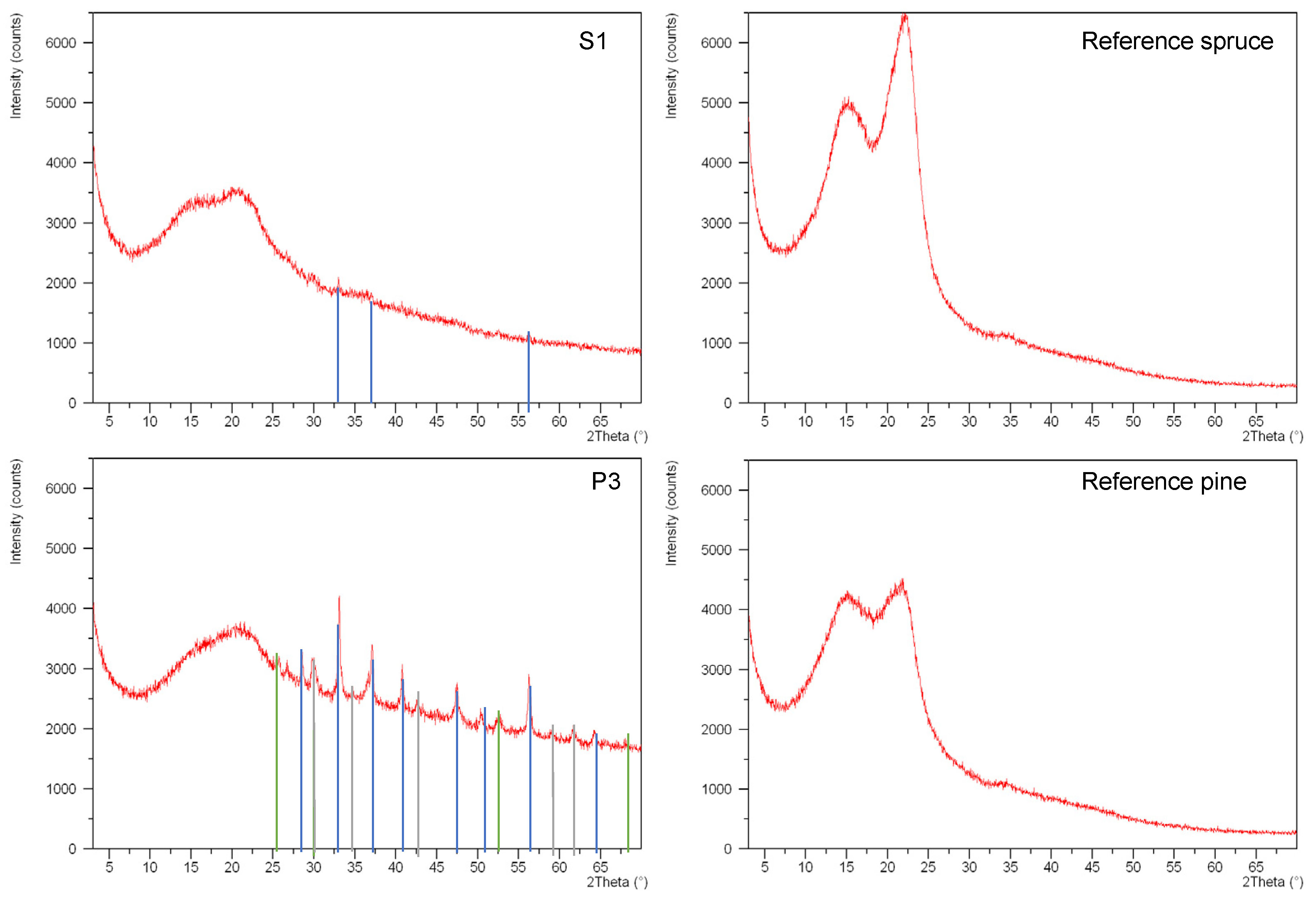
2.4. XRD
3. Discussion
4. Materials and Methods
4.1. Sampling
4.2. FTIR
4.3. ED-XRF and SEM-EDS
4.4. XRD
4.5. Py-GC/MS
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ważny, T. Dendrochronological dating of the Lusatian Culture settlement at Biskupin Poland-first results. News WARP 1993, 14, 3–5. [Google Scholar]
- Unger, A.; Schniewind, A.P.; Unger, W. Conservation of Wood Artifacts; Springer: Berlin/Heidelberg, Germany, 2001; p. 578. [Google Scholar]
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef] [PubMed]
- Eslyn, W.E. Decay in mine timbers. Part I. Sampling procedures and conditions and descriptions of samples. For. Prod. J. 1983, 33, 27. [Google Scholar]
- Blanchette, R.; Held, B.; Jurgens, J. Northumberland House, Fort Conger and the Peary Huts in the Canadian High Arctic: Current condition and assessment of wood deterioration taking place. Hist. Polar Bases Preserv. Manag. 2008, 17, 30–37. [Google Scholar]
- Ortiz, R.; Navarrete, H.; Navarrete, J.; Párraga, M.; Carrasco, I.; Vega, E.d.l.; Ortiz, M.; Herrera, P.; Blanchette, R.A. Deterioration, decay and identification of fungi isolated from wooden structures at the Humberstone and Santa Laura saltpeter works: A world heritage site in Chile. Int. Biodeterior. Biodegrad. 2014, 86, 309–316. [Google Scholar] [CrossRef]
- Tintner, J.; Smidt, E.; Tieben, J.; Reschreiter, H.; Kowarik, K.; Grabner, M. Aging of wood under long-term storage in a salt environment. Wood Sci. Technol. 2016, 50, 953–961. [Google Scholar] [CrossRef][Green Version]
- Reschreiter, H.; Kowarik, K. Bronze Age Mining in Hallstatt. A New Picture of Everyday Life in the Salt Mines and Beyond. Archaeol. Austriaca 2019, 103, 99–136. [Google Scholar] [CrossRef]
- Lucejko, J.J.; Modugno, F.; Colombini, M.P.; Zborowska, M. Archaeological wood from the Wieliczka Salt Mine Museum, Poland-Chemical analysis of wood degradation by Py(HMDS)-GC/MS. J. Cult. Herit. 2012, 13, S50–S56. [Google Scholar] [CrossRef]
- Zborowska, M.; Prądzyński, W. Chemiczne właściwości drewna zasolonego z Muzeum Żup Krakowskich w Wieliczce. Studia I Mater. Do Dziejów Żup Solnych W Polsce 2009, 26, 207–217. [Google Scholar]
- Moreno-Larrazabal, A.; Urteaga, M.; Zapata, L. Identification of archaeological wood remains from the roman mine of Arditurri 3 (Oiartzun, Basque Country). In Proceedings of the SAGVNTVM Extra-11-Materials from 5th International Meeting of Charcoal Analysis “Charcoal as Cultural and Biological Heritage”, Oiartzun, Spain, 5–9 September 2011; Volume 2011. [Google Scholar]
- Oron, A.; Liphschitz, N.; Held, B.W.; Galili, E.; Klein, M.; Linker, R.; Blanchette, R.A. Characterization of archaeological waterlogged wooden objects exposed on the hyper-saline Dead Sea shore. J. Archaeol. Sci. Rep. 2016, 9, 73–86. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Si, Y.; Chen, H.; Russel, M.; Chen, K.; Qian, Y.; Zaray, G.; Bramanti, E. Short-time effect of heavy metals upon microbial community activity. J. Hazard. Mater. 2010, 173, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Kucharski, J. Liczebnosc drobnoustrojow w glebie zanieczyszczonej metalami ciezkimi. Zesz. Probl. Postępów Nauk Rol. 2003, 492, 427–433. [Google Scholar]
- Almkvist, G.; Norbakhsh, S.; Bjurhager, I.; Varmuza, K. Prediction of tensile strength in iron-contaminated archaeological wood by FT-IR spectroscopy—A study of degradation in recent oak and Vasa oak. Holzforschung 2016. [Google Scholar] [CrossRef]
- Norbakhsh, S.; Bjurhager, I.; Almkvist, G. Impact of iron(II) and oxygen on degradation of oak – modeling of the Vasa wood. Holzforschung 2014, 68, 649–655. [Google Scholar] [CrossRef]
- Dedic, D.; Iversen, T.; Ek, M. Cellulose degradation in the Vasa: The role of acids and rust. Stud. Conserv. 2013, 58, 308–313. [Google Scholar] [CrossRef]
- Temiz, A.; Terziev, N.; Eikenes, M.; Hafren, J. Effect of accelerated weathering on surface chemistry of modified wood. Appl. Surf. Sci. 2007, 253, 5355–5362. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Zoia, L.; Tamburini, D.; Orlandi, M.; Łucejko, J.J.; Salanti, A.; Tolppa, E.-L.; Modugno, F.; Colombini, M.P. Chemical characterisation of the whole plant cell wall of archaeological wood: An integrated approach. Anal. Bioanal. Chem. 2017, 409, 4233–4245. [Google Scholar] [CrossRef]
- Tamburini, D.; Łucejko, J.J.; Zborowska, M.; Modugno, F.; Cantisani, E.; Mamoňová, M.; Colombini, M.P. The short-term degradation of cellulosic pulp in lake water and peat soil: A multi-analytical study from the micro to the molecular level. Int. Biodeterior. Biodegrad. 2017, 116, 243–259. [Google Scholar] [CrossRef]
- Łucejko, J.J.; Mattonai, M.; Zborowska, M.; Tamburini, D.; Cofta, G.; Cantisani, E.; Kúdela, J.; Cartwright, C.; Colombini, M.P.; Ribechini, E.; et al. Deterioration effects of wet environments and brown rot fungus Coniophora puteana on pine wood in the archaeological site of Biskupin (Poland). Microchem. J. 2018, 138, 132–146. [Google Scholar] [CrossRef]
- Pollard, A.M.; Heron, C. Archaeological Chemistry; RSC Publishing: Cambridge, UK, 1996. [Google Scholar]
- Frahm, E. Scanning Electron Microscopy (SEM): Applications in Archaeology. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer: New York, NY, USA, 2014; pp. 6487–6495. [Google Scholar]
- High, K.E.; Penkman, K.E.H. A review of analytical methods for assessing preservation in waterlogged archaeological wood and their application in practice. Herit. Sci. 2020, 8, 83. [Google Scholar] [CrossRef]
- Balzano, A.; Merela, M.; Čufar, K. Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood. Forests 2022, 13, 161. [Google Scholar] [CrossRef]
- Fors, Y. Sulfur-Related Conservation Concerns in Marine Archaeological Wood: The Origin, Speciation and Distribution of Accumulated Sulfur with Some Remedies for the Vasa. Doctoral Thesis, Stockholm University, Stockholm, Sweden, 2008. [Google Scholar]
- Sandström, M.; Jalilehvand, F.; Damian, E.; Fors, Y.; Gelius, U.; Jones, M.; Salome, M. Sulfur accumulation in the timbers of King Henry VIII’s warship Mary Rose: A pathway in the sulfur cycle of conservation concern. Proc. Natl. Acad. Sci. USA 2005, 102, 14165–14170. [Google Scholar] [CrossRef] [PubMed]
- Braovac, S.; Kutzke, H. The presence of sulfuric acid in alum-conserved wood - Origin and consequences. J. Cult. Herit. 2012, 13, S203–S208. [Google Scholar] [CrossRef]
- Giachi, G.; Bettazzi, F.; Chimichi, S.; Staccioli, G. Chemical characterisation of degraded wood in ships discovered in a recent excavation of the Etruscan and Roman harbour of Pisa. J. Cult. Herit. 2003, 4, 75–83. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Pedersen Bjerregaard, N.; Gierlinger, N.; Garbrecht, T. Bacterial and abiotic decay in waterlogged archaeological Picea abies (L.) Karst studied by confocal Raman imaging and ATR-FTIR spectroscopy. Holzforschung 2015, 69, 103–112. [Google Scholar] [CrossRef]
- García-Iruela, A.; García Esteban, L.; García Fernández, F.; de Palacios, P.; Rodriguez-Navarro, A.B.; Sánchez, L.G.; Hosseinpourpia, R. Effect of Degradation on Wood Hygroscopicity: The Case of a 400-Year-Old Coffin. Forests 2020, 11, 712. [Google Scholar] [CrossRef]
- El Hadidi, N.M.N. Decay of softwood in archaeological wooden artifacts. Stud. Conserv. 2017, 62, 83–95. [Google Scholar] [CrossRef]
- Harrington, K.J.; Higgins, H.G.; Michell, A.J. Infrared Spectra of Eucalyptus regnans F. Muell. and Pinus radiata D. Don. Holzforschung 1964, 18, 108–113. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Tamburini, D.; Lucejko, J.J.; Modugno, F.; Ribechini, E.; Colombini, M.P. Chemical parameters to evaluate the preservation state of wet archaeological wood: The critical interpretation of the holocellulose/lignin ratio. In Proceedings of the 13th ICOM-CC Group on Wet Organic Archaeological Materials, Florence, Italy, 16–21 May 2016. [Google Scholar]
- Tamburini, D.; Łucejko, J.J.; Ribechini, E.; Colombini, M.P. New markers of natural and anthropogenic chemical alteration of archaeological lignin revealed by in situ pyrolysis/silylation-gas chromatography-mass spectrometry. J. Anal. Appl. Pyrolysis 2016, 118, 249–258. [Google Scholar] [CrossRef]
- Tamburini, D.; Łucejko, J.J.; Zborowska, M.; Modugno, F.; Prądzyński, W.; Colombini, M.P. Archaeological wood degradation at the site of Biskupin (Poland): Wet chemical analysis and evaluation of specific Py-GC/MS profiles. J. Anal. Appl. Pyrolysis 2015, 115, 7–15. [Google Scholar] [CrossRef]
- Lucejko, J.J.; Tamburini, D.; Modugno, F.; Ribechini, E.; Colombini, M.P. Analytical Pyrolysis and Mass Spectrometry to Characterise Lignin in Archaeological Wood. Appl. Sci. 2021, 11, 240. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Analytical Pyrolysis of Polymeric Carbohydrates. In Analytical Pyrolysis of Natural Organic Polymers; Coleman, D., Price, B.F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1998; Volume 20, pp. 217–316. [Google Scholar]
- Rickard, D. Sulfidic Sediments and Sedimentary Rocks. In Developments in Sedimentology; Rickard, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 65, pp. 543–604. [Google Scholar]
- Garbacz-Klempka, A.; Wardas-Lasoń, M.; Rzadkosz, S. Odpady hutnicze z rejonu Złotego Stoku. Arch. Foundry Eng. 2014, 14, 23–28. [Google Scholar]
- Kołodziej, B.; Muszer, A.; Adamski, Z. Ługowanie rudy złota ze Złotego Stoku roztworami chlorków w warunkach redukcyjnych. Physicochem. Probl. Miner. Process. 1998, 32, 83–90. (In Polish) [Google Scholar]
- Mikulski, S.Z. Złoto z kamieniołomu Złoty Jar k. Złotego Stoku (Sudety). Prz. Geol. 1996, 44, 1205–1210. [Google Scholar]
- Wójcik, D.; Krzyżanowski, K.; Furmankiewicz, M. Sztolnia książęca w Złotym Stoku. Hered. Minariorum 2014, 1, 61–79. [Google Scholar]
- Fors, Y.; Jalilehvand, F.; Damian Risberg, E.; Bjordal, C.; Phillips, E.; Sandstrom, M. Sulfur and iron analyses of marine archaeological wood in shipwrecks from the Baltic Sea and Scandinavian waters. J. Archaeol. Sci. 2012, 39, 2521–2532. [Google Scholar] [CrossRef]
- Almkvist, G.; Persson, I. Analysis of acids and degradation products related to iron and sulfur in the Swedish warship Vasa. Holzforschung 2008, 62, 694–703. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Tran, K.; Guilminot, E.; Conforto, E.; Refait, P. Study of Fe(II) sulphides in waterlogged archaeological wood. Stud. Conserv. 2013, 58, 297–307. [Google Scholar] [CrossRef]
- Boursiquot, S.; Mullet, M.; Abdelmoula, M.; Génin, J.M.; Ehrhardt, J.J. The dry oxidation of tetragonal FeS1-xmackinawite. Phys. Chem. Miner. 2001, 28, 600–611. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of Iron Sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef]
- Zoia, L.; Salanti, A.; Orlandi, M. Chemical characterization of archaeological wood: Softwood Vasa and hardwood Riksapplet case studies. J. Cult. Herit. 2015, 16, 428–437. [Google Scholar] [CrossRef]
- Imazu, S.; Morgós, A. An improvement on the Lactitol MC conservation method used for the conservation of archaeological waterlogged wood (The conservation method using Lactitol MC and Trehalose mixture). In Proceedings of the 8th ICOM-CC Group on Wet Organic Archaeological Materials Conference ICOM-CC, Stockholm, Switzerland, 11–15 June 2001; pp. 413–428. [Google Scholar]
- Kennedy, A.; Pennington, E.R. Conservation of chemically degraded waterlogged wood with sugars. Stud. Conserv. 2014, 59, 194–201. [Google Scholar] [CrossRef]
- Szychowska-Krąpiec, E. Dendrochronological Studies of Wood from Mediaeval Mines of Polymetallic Ores in Lower Silesia (Sw Poland). Geochronometria 2007, 26, 61–68. [Google Scholar] [CrossRef]
- Majka, J.; Zborowska, M.; Fejfer, M.; Waliszewska, B.; Olek, W. Dimensional stability and hygroscopic properties of PEG treated irregularly degraded waterlogged Scots pine wood. J. Cult. Herit. 2018, 31, 133–140. [Google Scholar] [CrossRef]
- TAPPI. T 211 om-02. Ash in wood, pulp, paper and paperboard: Combustion at 525 °C. Stand. Tech. Assoc. Pulp Pap. Ind. 2002, 5. [Google Scholar]
- Dietz, P. Dichte und Rindengehalt von Industrieholz. Holz Als Roh- Werkst. 1975, 33, 135–141. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure Reactions; Walter de Gruyter: Berlin/Heidelberg, Germany; New York, NY, USA, 1989; p. 613. [Google Scholar]
- Łucejko, J.J.; de Lamotte, A.; Andriulo, F.; Kutzke, H.; Harding, S.; Phillips-Jones, M.; Modugno, F.; Slaghek, T.M.; Gosselink, R.J.A.; Braovac, S. Evaluation of Soda Lignin from Wheat Straw/Sarkanda Grass as a Potential Future Consolidant for Archaeological Wood. Forests 2021, 12, 911. [Google Scholar] [CrossRef]
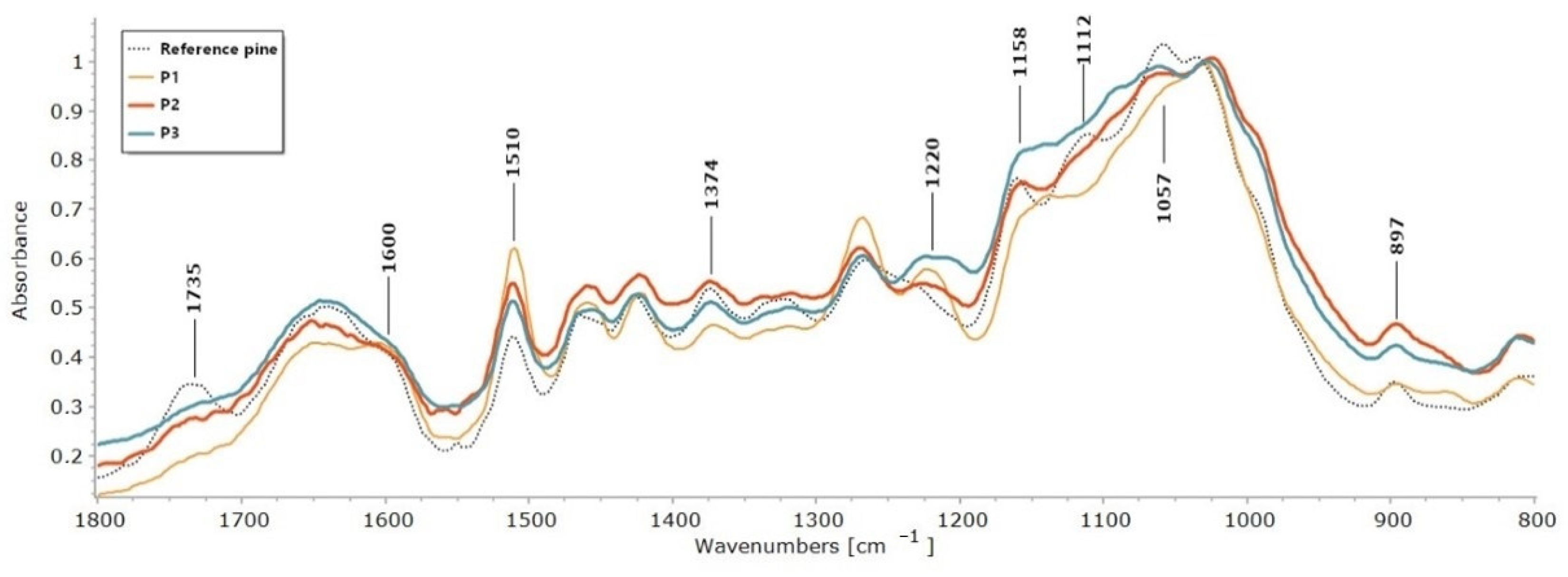
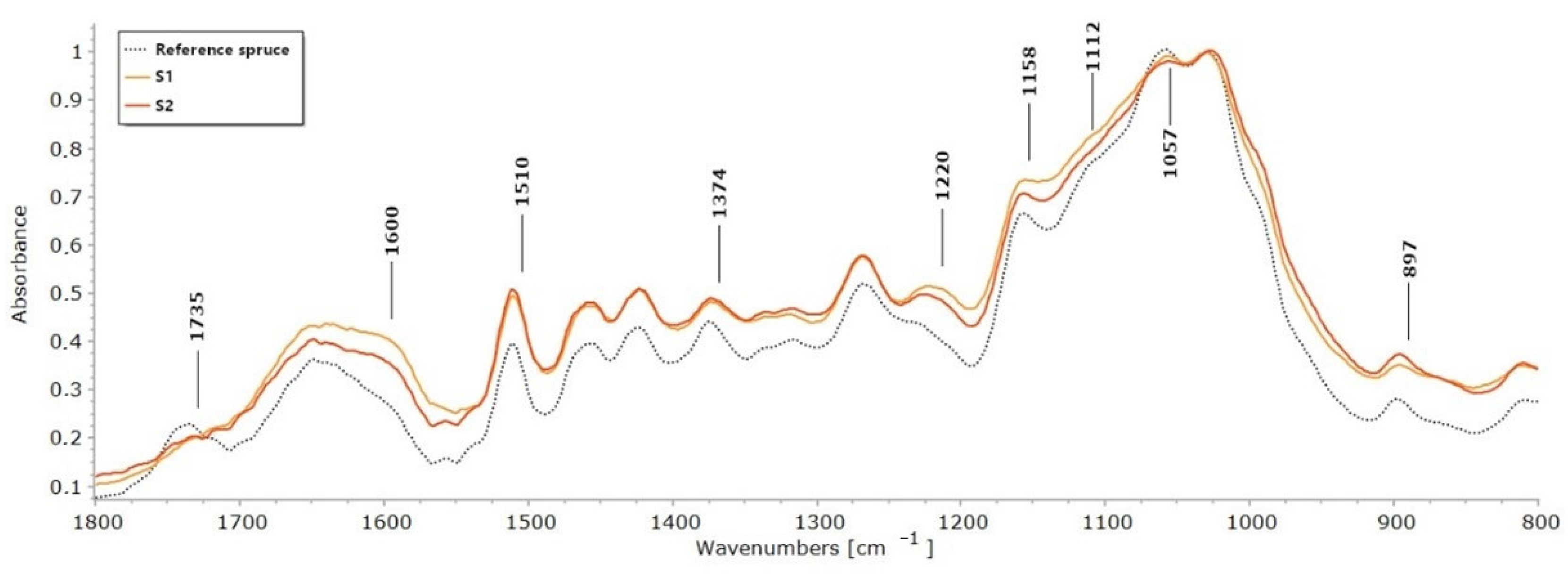

| Reference Pine | P1 | P2 | P3 | Reference Spruce | S1 | S2 | |
|---|---|---|---|---|---|---|---|
| High IL/I1374 | 2.04 | 8.21 | 3.95 | 3.39 | 2.31 | 3.88 | 4.09 |
| Area IL/I1374 | 2.14 | 8.55 | 3.66 | 3.34 | 2.09 | 3.62 | 3.71 |
| High IL/I1158 | 1.07 | – | 1.82 | – | 1.17 | 1.77 | 1.75 |
| Area IL/I1158 | 1.11 | – | 1.59 | – | 0.99 | 1.46 | 1.47 |
| High IL/I897 | 3.61 | 20.48 | 5.06 | 6.39 | 4.05 | 8.06 | 5.32 |
| Area IL/I897 | 4.24 | 26.10 | 5.80 | 8.58 | 4.41 | 9.30 | 5.95 |
| Wood Fraction | Reference Pine | P1 | P2 | P3 | Reference Spruce | S1 | S2 |
|---|---|---|---|---|---|---|---|
| H [%] | 73.6 | 65.2 | 73.7 | 80.3 | 70.5 | 74.8 | 79.0 |
| L [%] | 26.4 | 34.8 | 26.3 | 19.7 | 29.5 | 25.2 | 21.0 |
| H/L | 2.8 ± 0.3 | 1.9 ± 0.1 | 2.9 ± 0.4 | 4.1 ± 0.3 | 2.4 ± 0.2 | 3.0 ± 0.2 | 3.8 ± 0.3 |
| Archaeological Samples | Crystalline Phase | Formula |
|---|---|---|
| S1 | Pyrite | FeS2 |
| S2 | Greigite | Fe2S4 |
| P1 | Pyrite, greigite | FeS2, Fe2S4 |
| P2 | Pyrite, greigite, magnetite | FeS2, Fe2S4, Fe3O4 |
| P3 | Pyrite, greigite, magnetite | FeS2, Fe2S4, Fe3O4 |
| Reference Pine | P1 | P2 | P3 | Reference Spruce | S1 | S2 | |
|---|---|---|---|---|---|---|---|
| BD (kg/m3) | 418 * | 213 | 385 | 351 | 403 * | 221 | 359 |
| Ash (%) | 0.2–0.5 ** | 1.70 | 1.54 | 5.85 | 0.2–0.5 ** | 12.01 | 2.41 |
| Compound | m/z | Category | Origin | |
|---|---|---|---|---|
| 1 | 1,2-dihydroxyethane (2TMS) | 73, 103, 147, 191 | small molecules | H/L |
| 2 | 2-hydroxymethylfuran (TMS) | 53, 73, 81, 111, 125, 142, 155, 170 | furan | H |
| 3 | phenol (TMS) | 75, 151, 166 | short side chain | H-lignin |
| 4 | 2-hydroxypropanoic acid (2TMS) | 73, 117, 147, 190 | small molecules | H/L |
| 5 | 2-hydroxyacetic acid (2TMS) | 73, 147, 177, 205 | small molecules | H/L |
| 6 | 1-hydroxy-1-cyclopenten-3-one (TMS) | 53, 73, 81, 101, 111, 127, 155, 169 | cyclopentenone | H |
| 7 | 3-hydroxymethylfuran (TMS) | 53, 75, 81, 111, 125, 142, 155, 170 | furan | H |
| 8 | o-cresol (TMS) | 73, 91, 135, 149, 165, 180 | short side chain | H-lignin |
| 9 | 2-furancarboxylic acid (TMS) | 73, 95, 125, 169, 184 | furan | H |
| 10 | m-cresol (TMS) | 73, 91, 165, 180 | short side chain | H-lignin |
| 11 | 2-hydroxy-1-cyclopenten-3-one (TMS) | 53, 73, 81, 101, 111, 127, 155, 170 | cyclopentenone | H |
| 12 | p-cresol (TMS) | 73, 91, 165, 180 | short side chain | H-lignin |
| 13 | 3-hydroxy-(2H)-pyran-2-one (TMS) | 75, 95, 125, 151, 169, 184 | pyran | H |
| 14 | Z-2,3-dihydroxy-cyclopent-2-enone (TMS) | 59, 73, 115, 143, 171, 186 | cyclopentenone | H |
| 15 | E-2,3-dihydroxy-cyclopent-2-enone (TMS) | 75, 101, 143, 171, 186 | cyclopentenone | H |
| 16 | 1,2-dihydroxybenzene (TMS) | 75, 91, 136, 151, 167, 182 | hydroxybenzene | H/L |
| 17 | 3-hydroxy-(4H)-pyran-4-one (TMS) | 75, 95, 139, 151, 169, 184 | pyran | H |
| 18 | 5-hydroxy-2H-pyran-4(3H)-one (TMS) | 59, 75, 101, 129, 143, 171, 186 | pyran | H |
| 19 | 2-hydroxymethyl-3-methy-2-cyclopentenone (TMS) | 73, 103, 129, 173, 183, 198 | cyclopentenone | H |
| 20 | 1-hydroxy-2-methyl-1-cyclopenten-3-one (TMS) | 73, 97, 125, 139, 169, 184 | cyclopentenone | H |
| 21 | 1-methy-2-hydroxy-1-cyclopenten-3-one (TMS) | 73, 97, 125, 139, 169, 184 | cyclopentenone | H |
| 22 | 1,3-dihydroxyacetone (2TMS) | 73, 103, 147, 189, 219 | small molecules | H/L |
| 23 | guaiacol (TMS) | 73, 151, 166, 181, 196 | short side chain | G-lignin |
| 24 | 3-hydroxy-6-methyl-(2H)-pyran-2-one (TMS) | 73, 109, 139, 168, 183, 198 | pyran | H |
| 25 | 2-methyl-3-hydroxy-(4H)-pyran-4-one (TMS) | 73, 101, 153, 183, 198 | pyran | H |
| 26 | 2-methyl-3-hydroxymethyl-2-cyclopentenone (TMS) | 73, 103, 129, 173, 183, 198 | cyclopentenone | H |
| 27 | 2,3-dihydrofuran-2,3-diol (2TMS) | 73, 147, 231, 246 | furan | H |
| 28 | 2-furyl-hydroxymethylketone (TMS) | 73, 81, 103, 125, 183, 198 | furan | H |
| 29 | 5-hydroxymethyl-2-furaldehyde (TMS) | 73, 81, 109, 111, 139, 169, 183, 198 | furan | H |
| 30 | 4-methylguaiacol (TMS) | 73, 149, 180, 195, 210 | short side chain | G-lignin |
| 31 | 1,2-dihydroxybenzene (2TMS) | 73, 151, 239, 254 | hydroxybenzene | H/L |
| 32 | 2-hydroxymethyl-2,3-dihydropyran-4-one (TMS) | 73, 142, 170, 185, 200 | pyran | H |
| 33 | 1,4:3,6-dianhydro-α-D-glucopyranose (TMS) | 73, 103, 129, 155, 170, 171, 186 | anhydrosugars | H |
| 34 | Z-2,3-dihydroxy-cyclopent-2-enone (2TMS) | 73, 147, 230, 243, 258 | cyclopentenone | H |
| 35 | 4-methylcatechol (2TMS) | 73,180, 253, 268 | demethylated | G-lignin |
| 36 | 4-ethylguaiacol (TMS) | 73, 149, 179, 194, 209, 224 | short side chain | G-lignin |
| 37 | 1,4-dihydroxybenzene (2TMS) | 73, 112, 239, 354 | hydroxybenzene | H/L |
| 38 | 4-vinylguaiacol (TMS) | 73, 162, 177, 192, 207, 222 | short side chain | G-lignin |
| 39 | 3-hydroxy-2-hydroxymethyl-2-cyclopentenone (2TMS) | 73, 147, 257, 272 | cyclopentenone | H |
| 40 | E-2,3-dihydroxy-cyclopent-2-enone (2TMS) | 73, 147, 243, 258 | cyclopentenone | H |
| 41 | 4-ethylcatechol (2TMS) | 73, 147, 179, 231, 267, 282 | demethylated | G-lignin |
| 42 | 3-hydroxy-2-(hydroxymethyl) cyclopenta-2,4-dienone (2TMS) | 73, 147, 255, 270 | cyclopentenone | H |
| 43 | eugenol (TMS) | 73, 147, 179, 206, 221, 236 | long side chain | G-lignin |
| 44 | 3-methoxy-1,2-benzenediol (2TMS) | 73, 153, 254, 269, 284 | demethylated | G-lignin |
| 45 | 3,5-dihydroxy-2-methyl-(4H)-pyran-4-one (2TMS) | 73, 128, 147, 183, 271, 286 | pyran | H |
| 46 | Z-isoeugenol (TMS) | 73, 179, 206, 221, 236 | long side chain | G-lignin |
| 47 | vanillyl alcohol (2TMS) | 73, 151, 210, 253, 268, 283, 298 | long side chain | G-lignin |
| 48 | vanillin (TMS) | 73, 194, 209, 224 | carbonyl | G-lignin |
| 49 | 1,2,3-trihydroxybenzene (3TMS) | 73, 133, 147, 239, 327, 342 | hydroxybenzene | H |
| 50 | E-isoeugenol (TMS) | 73, 179, 206, 221, 236 | long side chain | G-lignin |
| 51 | 1,2,4-trihydroxybenzene (3TMS) | 73, 133, 147, 239, 327, 342 | hydroxybenzene | H |
| 52 | acetovanillone (TMS) | 73, 193, 208, 223, 238 | carbonyl | G-lignin |
| 53 | 1,4-anydro-D-galactopyranose (3TMS) | 73, 129, 147, 157, 191, 204, 217, 243, 332 | anhydrosugars | H |
| 54 | 2,3,5-trihydroxy-4H-pyran-4-one (3TMS) | 73, 133, 147, 239, 255, 270, 330, 345, 360 | pyran | H |
| 55 | 1,6-anydro-beta-D-glucopyranose (3TMS) | 73, 103, 129, 147, 191, 204, 217, 243, 333 | anhydrosugars | H |
| 56 | 1,4-anhydro-D-glucopyranose (3TMS) | 73, 103, 129, 147, 191, 204, 217, 243, 332 | anhydrosugars | H |
| 57 | 1,6-anydro-beta-D-glucofuranose (3TMS) | 73, 103, 129, 147, 191, 204, 217, 243, 319 | anhydrosugars | H |
| 58 | vanillic acid (2TMS) | 73, 253, 282, 297, 312 | acid | G-lignin |
| 59 | Z-coniferyl alcohol (2TMS) | 73, 204, 252, 293, 309, 324 | monomer | G-lignin |
| 60 | coniferylaldehyde (TMS) | 73, 192, 220, 235, 250 | carbonyl | G-lignin |
| 61 | E-coniferyl alcohol (2 TMS) | 73, 204, 252, 293, 309, 324 | monomer | G-lignin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fejfer, M.; Łucejko, J.J.; Miazga, B.; Cantisani, E.; Zborowska, M. Gold Mine Wooden Artefacts: Multianalytical Investigations for the Selection of Appropriate Consolidation Treatments. Molecules 2022, 27, 5228. https://doi.org/10.3390/molecules27165228
Fejfer M, Łucejko JJ, Miazga B, Cantisani E, Zborowska M. Gold Mine Wooden Artefacts: Multianalytical Investigations for the Selection of Appropriate Consolidation Treatments. Molecules. 2022; 27(16):5228. https://doi.org/10.3390/molecules27165228
Chicago/Turabian StyleFejfer, Mariusz, Jeannette Jacqueline Łucejko, Beata Miazga, Emma Cantisani, and Magdalena Zborowska. 2022. "Gold Mine Wooden Artefacts: Multianalytical Investigations for the Selection of Appropriate Consolidation Treatments" Molecules 27, no. 16: 5228. https://doi.org/10.3390/molecules27165228
APA StyleFejfer, M., Łucejko, J. J., Miazga, B., Cantisani, E., & Zborowska, M. (2022). Gold Mine Wooden Artefacts: Multianalytical Investigations for the Selection of Appropriate Consolidation Treatments. Molecules, 27(16), 5228. https://doi.org/10.3390/molecules27165228







