Anaerobic Digested Wastewater CO2 Sequestration Using a Biophotocatalytic System with a Magnetized Photocatalyst (Fe-TiO2)
Abstract
1. Introduction
2. Results
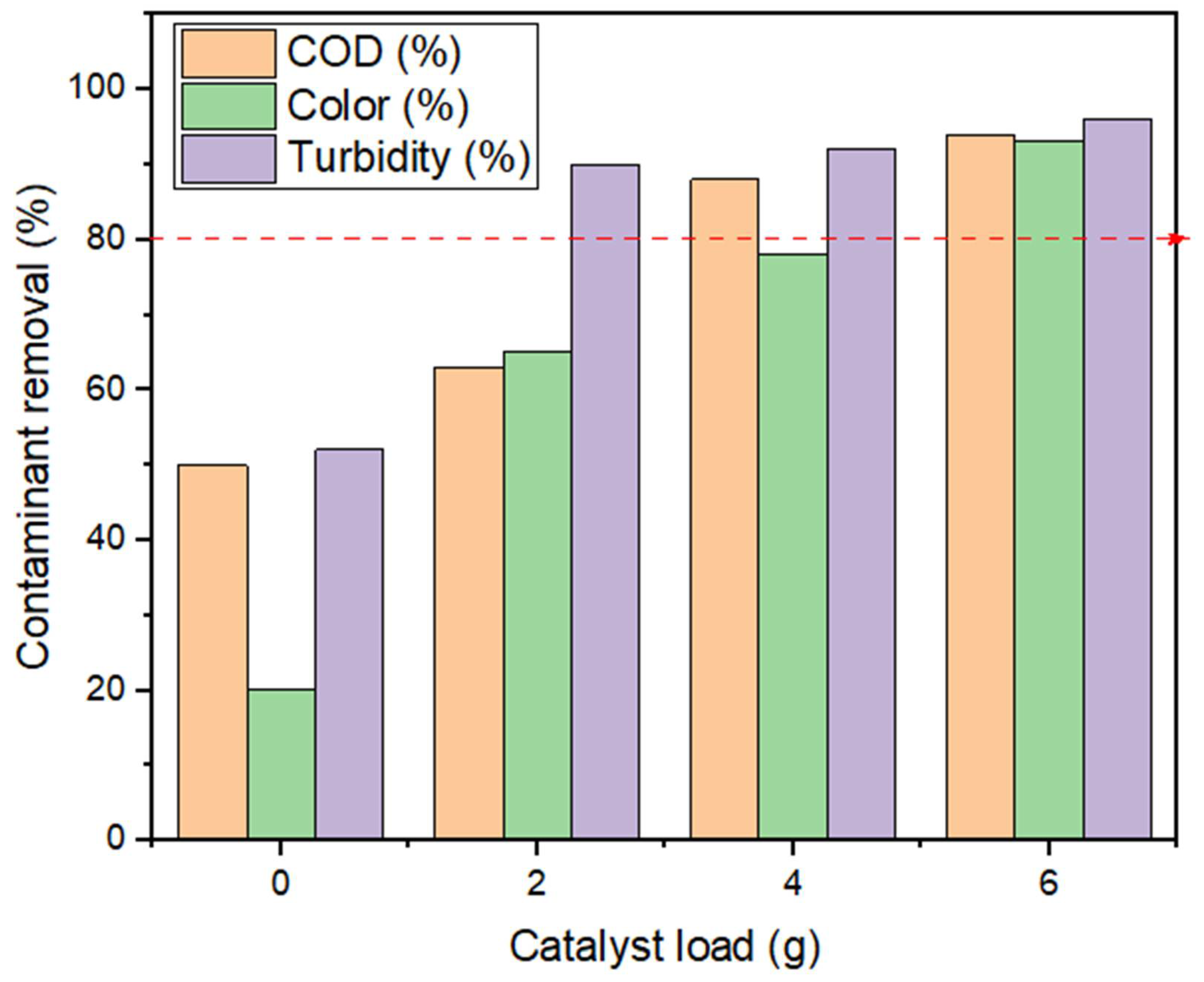
2.1. Treatability Efficiency of the BP System
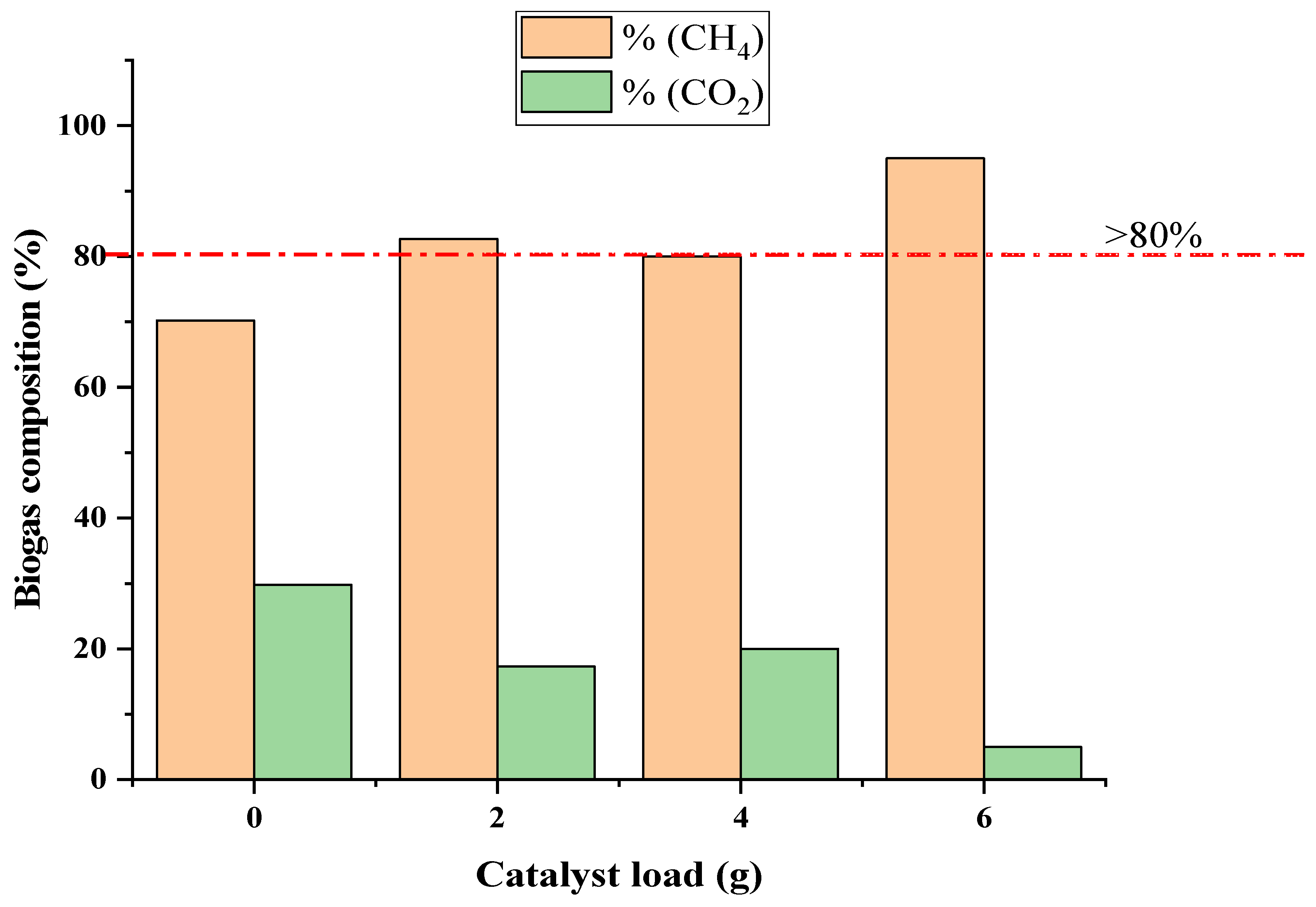
2.2. The BP System Biogas Production
3. Discussion
3.1. Effect of Catalyst Load on Contaminant Reduction
3.2. Biophotocatalytic CO2 Sequestration
3.3. The BP System Bioenergy Estimation
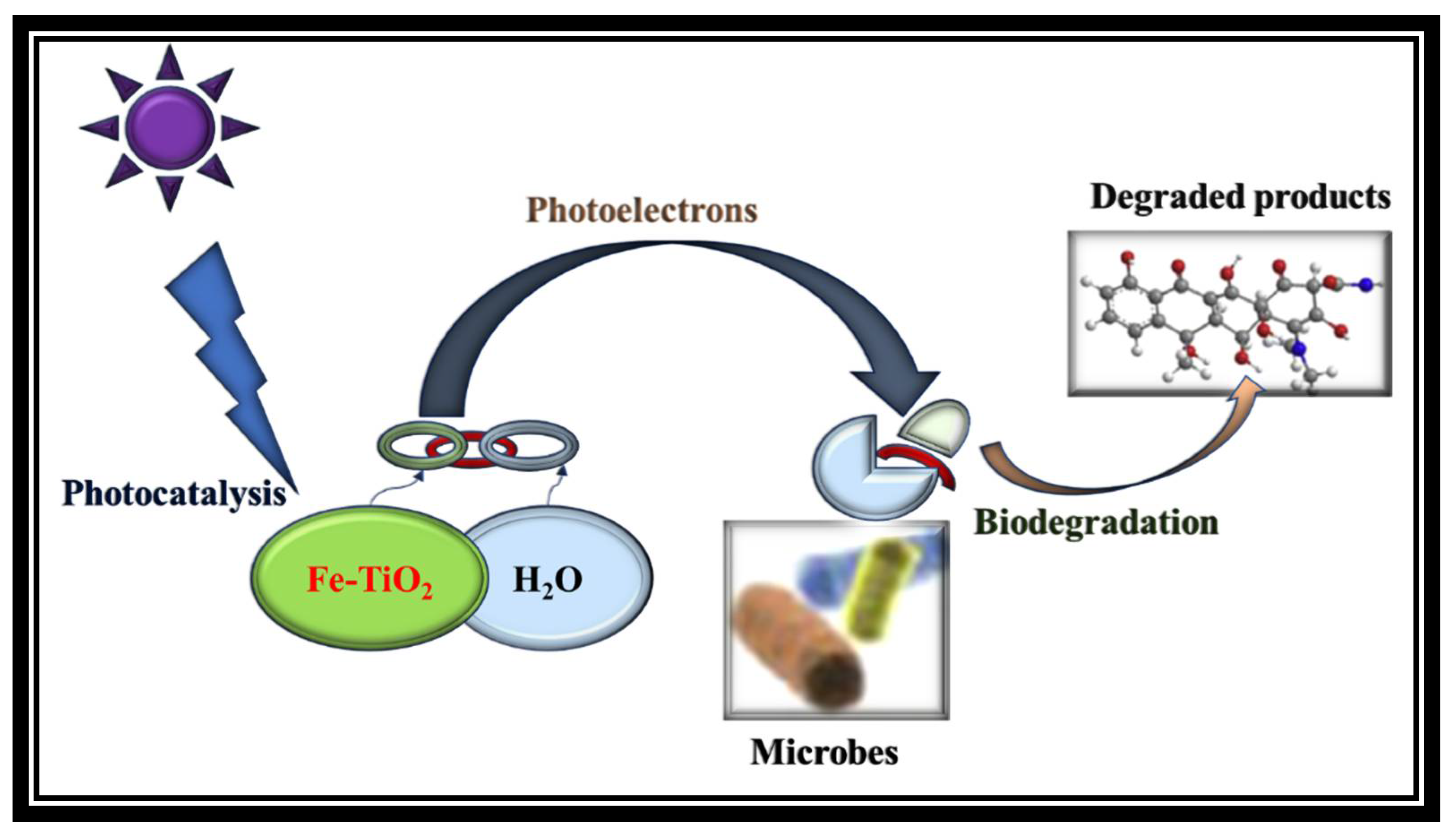
3.4. Future Prospect of the BP System
4. Materials and Methods
4.1. Wastewater Sample
4.2. Magnetised Photocatalyst (Fe-TiO2)
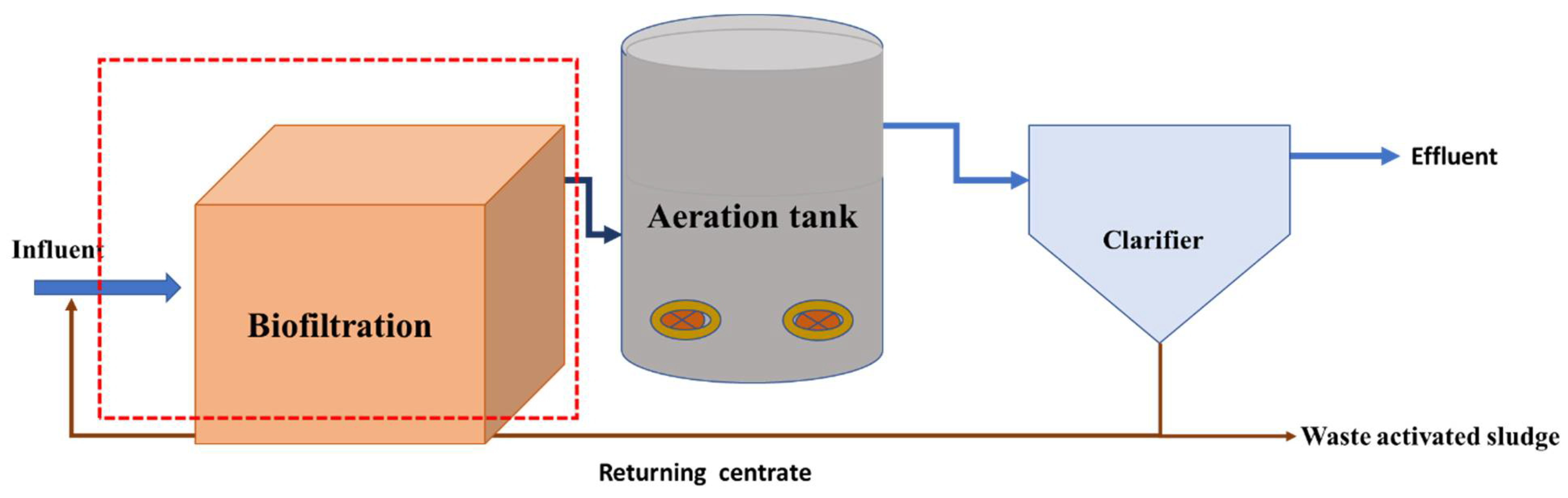
4.3. Biophotocatalytic (BP) System Description
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tetteh, E.K.; Asante-Sackey, D.; Armah, E.K.; Rathilal, S. Tapping wastewater resource: Why and how? In Handbook of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–146. [Google Scholar]
- Sahani, S.; Sharma, Y.C.; Kim, T.Y. Emerging Contaminants in Wastewater and Surface Water. In New Trends in Emerging Environmental Contaminants; Springer: Berlin/Heidelberg, Germany, 2022; pp. 9–30. [Google Scholar]
- Ramakrishna, S.; Jose, R. Addressing sustainability gaps. Sci. Total Environ. 2022, 806, 151208. [Google Scholar] [CrossRef] [PubMed]
- Rojo, E.; Carmona, A.; Soto, C.; Díaz, I.; Fernández-Polanco, M.; Palacio, L.; Bolado, S. Environment and Material Science Technology for Anaerobic Digestion-Based Circular Bioeconomy. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–55. [Google Scholar]
- Meys, R.; Kätelhön, A.; Bachmann, M.; Winter, B.; Zibunas, C.; Suh, S.; Bardow, A. Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science 2021, 74, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Zia, M.A.; Afzal, H.; Ahmed, S.; Ahmad, M.; Akram, Z.; Iqbal, H.M. Socio-Economic and Environmental Impacts of Biomass Valorisation: A Strategic Drive for Sustainable Bioeconomy. Sustainability 2021, 13, 4200. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Ngo, H.H.; Guo, W.; Nguyen, T.L.H.; Chang, S.W.; Nguyen, D.D.; Deng, L. Environmental impacts and greenhouse gas emissions assessment for energy recovery and material recycle of the wastewater treatment plant. Sci. Total Environ. 2021, 784, 147135. [Google Scholar] [CrossRef]
- Lin, B.; Sai, R. A multi factor Malmquist CO2emission performance indices: Evidence from Sub Saharan African public thermal power plants. Energy 2021, 223, 120081. [Google Scholar] [CrossRef]
- Salamé, L.; McKinney, D.C.; Delli Priscoli, J.; Koike, T.; Moss, J.; Tignino, M.; Münger, F. Water Discourses. In Handbook of Water Resources Management: Discourses, Concepts and Examples; Springer: Berlin/Heidelberg, Germany, 2021; pp. 145–214. [Google Scholar]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Ratna, S.; Rastogi, S.; Kumar, R. Current trends for distillery wastewater management and its emerging applications for sustainable environment. J. Environ. Manag. 2021, 290, 112544. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Amankwa, M.O.; Armah, E.K.; Rathilal, S. Fate of COVID-19 Occurrences in Wastewater Systems: Emerging Detection and Treatment Technologies—A. Review. Water 2020, 12, 2680. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Application of organic coagulants in water and wastewater treatment. In Organic Polymers; intechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Dong, R. Recent progress towards in-situ biogas upgrading technologies. Sci. Total Environ. 2021, 800, 149667. [Google Scholar] [CrossRef] [PubMed]
- Ajay, C.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering 2021, 8, 198. [Google Scholar] [CrossRef]
- Chollom, M.N.; Rathilal, S.; Swalaha, F.M.; Bakare, B.F.; Tetteh, E.K. Removal of antibiotics during the anaerobic digestion of slaughterhouse wastewater. Planning 2020, 15, 335–343. [Google Scholar] [CrossRef]
- Fermoso, F.G.; van Hullebusch, E.; Collins, G.; Roussel, J.; Mucha, A.P.; Esposito, G. Trace Elements in Anaerobic Biotechnologies; IWA Publishing: London, UK, 2019. [Google Scholar]
- Rodríguez-Couto, S. Biophotodegradation of pollutants from wastewater. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 269–281. [Google Scholar]
- Molla, A.; Kim, A.Y.; Woo, J.C.; Cho, H.S.; Youk, J.H. Study on Preparation Methodology of Zero-Valent Iron Decorated on Graphene Oxide for Highly Efficient Sonocatalytic Dye Degradation. J. Environ. Chem. Eng. 2022, 10, 107214. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Nassar, H.N. Biosynthesized magnetite nanoparticles as an environmental opulence and sustainable wastewater treatment. Sci. Total Environ. 2021, 774, 145610. [Google Scholar] [CrossRef]
- de Oliveira Guidolin, T.; Possolli, N.M.; Polla, M.B.; Wermuth, T.B.; de Oliveira, T.F.; Eller, S.; Cechinel, M.A.P. Photocatalytic pathway on the degradation of methylene blue from aqueous solutions using magnetite nanoparticles. J. Clean. Prod. 2021, 318, 128556. [Google Scholar] [CrossRef]
- el Ghandoor, H.; Zidan, H.; Khalil, M.M.; Ismail, M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Tetteh, E.K.; Rathilal, S. Biophotocatalytic Reduction of CO2 in Anaerobic Biogas Produced from Wastewater Treatment Using an Integrated System. Catalysts 2022, 12, 76. [Google Scholar] [CrossRef]
- Apollo, S.; Onyango, M.S.; Ochieng, A. Modelling energy efficiency of an integrated anaerobic digestion and photodegradation of distillery effluent using response surface methodology. Environ. Technol. 2016, 37, 2435–2446. [Google Scholar] [CrossRef]
- Sudarjanto, G.; Keller-Lehmann, B.; Keller, J. Optimization of integrated chemical–biological degradation of a reactive azo dye using response surface methodology. J. Hazard. Mater. 2006, 138, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Jiang, P.; Yang, L.; Fan, Y.V.; Klemeš, J.J.; Wang, Y. Extended water-energy nexus contribution to environmentally-related sustainable development goals. Renew. Sustain. Energy Rev. 2021, 150, 111485. [Google Scholar] [CrossRef]
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Watson, S.; Beydoun, D.; Amal, R. Synthesis of a novel magnetic photocatalyst by direct deposition of nanosized TiO2 crystals onto a magnetic core. J. Photochem. Photobiol. A Chem. 2002, 148, 303–313. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Kobwittaya, K.; Sirivithayapakorn, S. Photocatalytic reduction of nitrate over Fe-modified TiO2. APCBEE Procedia 2014, 10, 321–325. [Google Scholar] [CrossRef][Green Version]
- Pastor-Pérez, L.; Baibars, F.; le Sache, E.; Arellano-García, H.; Gu, S.; Reina, T.R. CO2 valorisation via reverse water-gas shift reaction using advanced Cs doped Fe-Cu/Al2O3 catalysts. J. CO2 Util. 2017, 21, 423–428. [Google Scholar] [CrossRef]
- Hijazi, O.; Munro, S.; Zerhusen, B.; Effenberger, M. Review of life cycle assessment for biogas production in Europe. Renew. Sustain. Energy Rev. 2016, 54, 1291–1300. [Google Scholar] [CrossRef]
- Bauer, F.; Persson, T.; Hulteberg, C.; Tamm, D. Biogas upgrading–technology overview, comparison and perspectives for the future. Biofuels Bioprod. Biorefin. 2013, 7, 499–511. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Z.; Zhang, H.; Li, Z.; Wu, X.; Zhang, X.; Zhou, W. High thermostable ordered mesoporous SiO2–TiO2 coated circulating-bed biofilm reactor for unpredictable photocatalytic and biocatalytic performance. Appl. Catal. B Environ. 2016, 180, 521–529. [Google Scholar] [CrossRef]
- Shoabargh, S.; Karimi, A.; Dehghan, G.; Khataee, A. A hybrid photocatalytic and enzymatic process using glucose oxidase immobilized on TiO2/polyurethane for removal of a dye. J. Ind. Eng. Chem. 2014, 20, 3150–3156. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, H.; Zhao, Z.; Yu, Y.; Zhou, D.; Dong, S. Model-based evaluation of tetracycline hydrochloride removal and mineralization in an intimately coupled photocatalysis and biodegradation reactor. Chem. Eng. J. 2018, 351, 967–975. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Cheng, R.; Ali, J.; Wang, Z.; Mailhot, G.; Pan, G. Microcystis aeruginosa synergistically facilitate the photocatalytic degradation of tetracycline hydrochloride and Cr (VI) on PAN/TiO2/Ag nanofiber mats. Catalysts 2018, 8, 628. [Google Scholar] [CrossRef]
- Atacan, K.; Güy, N.; Cakar, S.; Özacar, M. Efficiency of glucose oxidase immobilized on tannin modified NiFe2O4 nanoparticles on decolorization of dye in the Fenton and photo-biocatalytic processes. J. Photochem. Photobiol. A Chem. 2019, 382, 111935. [Google Scholar] [CrossRef]
- Chambers, P. Standard methods for the examination of water and wastewater. Sci. e-Resour. 2019, 10, 332. [Google Scholar]
- Fuentes, I.; Bernales, N.; Ulloa, C.; García, X. Kinetics of CO2 methanation using a Fe-bearing blast furnace sludge as catalytic precursor. Catal. Today 2022, 394, 198–207. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Kinetics and nanoparticle catalytic enhancement of biogas production from wastewater using a magnetized biochemical methane potential (Mbmp) system. Catalysts 2020, 10, 1200. [Google Scholar] [CrossRef]


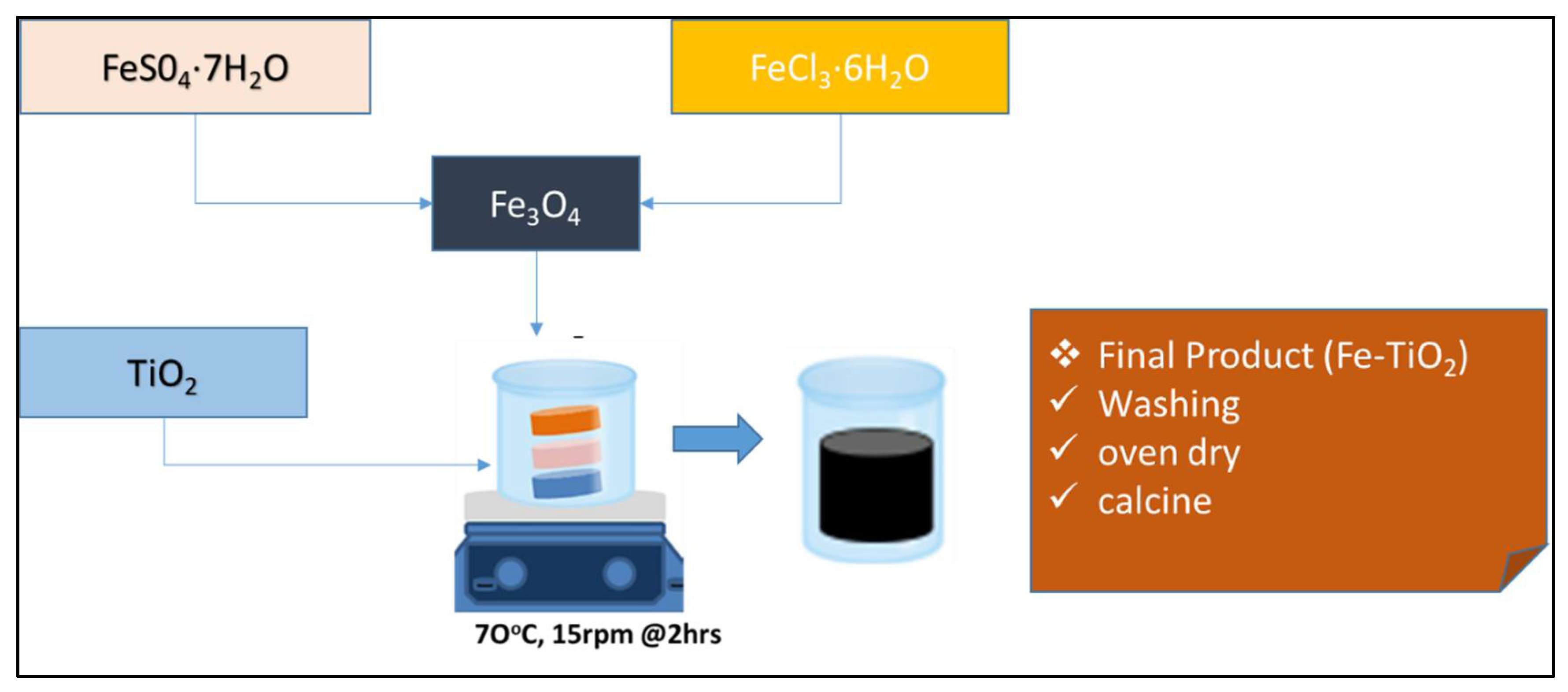
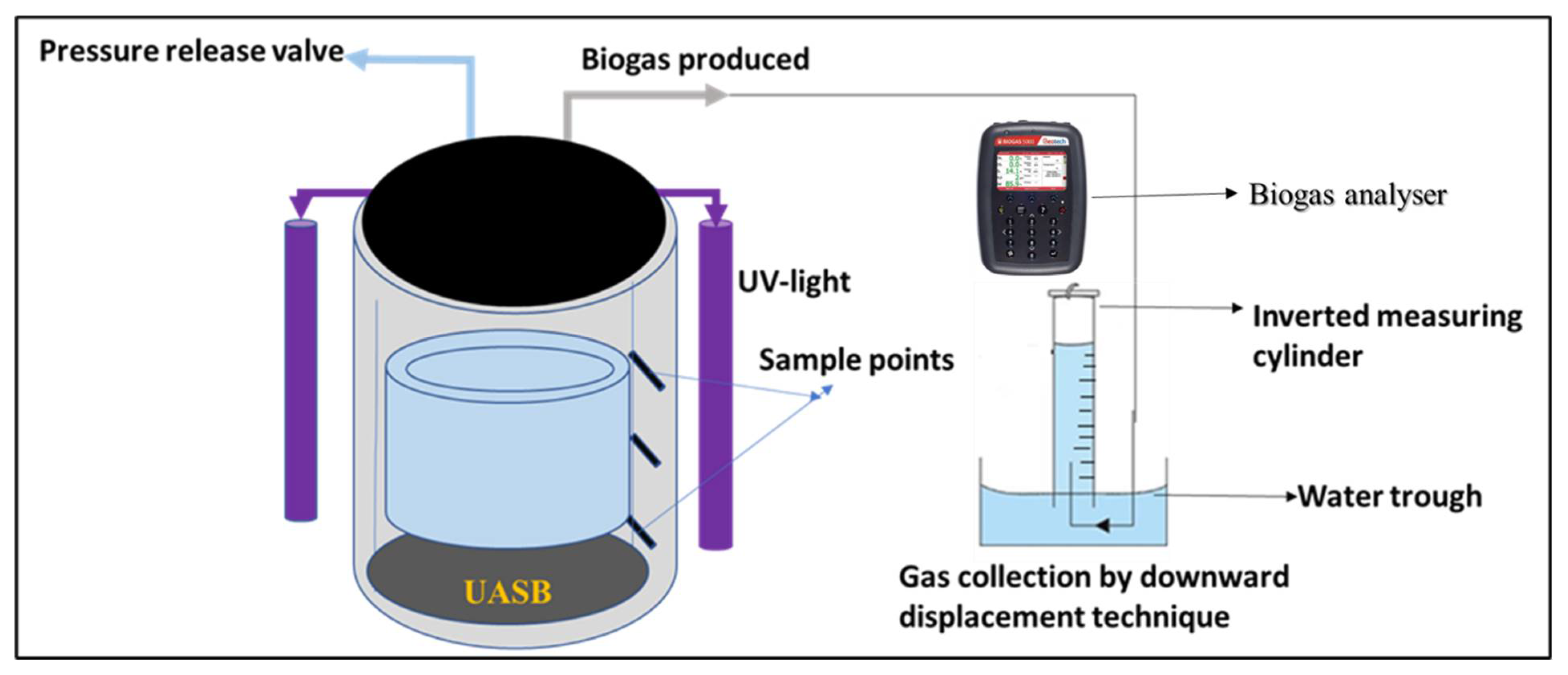
| Parameters | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|
| Catalytic loading (g) | 0 | 2 | 4 | 6 |
| pH | 5.59 ± 1.2 | 7.13 ± 1.4 | 7.13 ± 1.3 | 7.69 ± 1.2 |
| COD (mg COD/L) | 115 ± 3.4 | 87 ± 2.3 | 30 ± 2.1 | 12 ± 1.3 |
| Total N (mg/L) | 24.61 ± 1.4 | 7.26 ± 2.2 | 0.98 ± 0.15 | 2.65 ± 0.32 |
| TKN (mg/L) | 24.3 ± 1.3 | 6.01 ± 1.2 | 0.80 ± 0.1 | 2.53 ± 0.3 |
| NO3-N (mg/L) | 0.31 ± 0.1 | 1.25 ± 0.7 | 0.18 ± 0.05 | 0.12 ± 0.02 |
| NH4+ (mg/L) | 0.89 ± 0.13 | 0.78 ± 0.2 | 0.76 ± 0.12 | 0.74 ± 0.14 |
| VS/TS | 0.35 | 0.19 | 0.32 | 0.48 |
| Terms | Modified Gompertz Model | First Order Model |
|---|---|---|
| Ct (mL/g COD) | 4780 | 4780 |
| Cm (mL/g COD) | 4830 | 3,141,923 |
| k (1/day) | 0.20623 | 0.00006 |
| ʎ (day) | 10.7 | N/A |
| Sum of square errors (SSR) | 1,152,385 | 7,258,214 |
| Correlation Coefficient (R2) | 0.9917 | 0.9351 |
| Predicted yield (mL/g COD) | 4741 | 5659 |
| Item No | Item | Values |
|---|---|---|
| Biogas produced (m3) | 0.00515 | |
| 1 | Energy content of Methane (m3/h) | 0.0464 |
| 2 | Methane for electricity (kW/h) | 0.0348 |
| 3 | Energy applied (UV) (kW/h) | 0.0180 |
| 4 | Net energy (2–3) (kW/h) | 0.0168 |
| Cost estimation | ||
| 5 | Energy cost (3.22 ZAR/kWh) | 0.054 |
| Energy cost (0.23 USD/kWh) | 0.0039 | |
| 6 | Net energy cost for 30 days | |
| Energy cost (3.22 ZAR/kWh) | 38.86 | |
| Energy cost (0.23 USD/kWh) | 2.78 |
| Biocatalyst | Photocatalyst | Degradation Efficiency | Reference |
|---|---|---|---|
| Biofilm | SiO2-TiO2 | 100% phenol | [38] |
| Glucose oxidase | TiO2 | >99% acid orange 7 | [39] |
| Biofilm | Ag/TiO2 | 94% Tetracycline | [40] |
| Microcystis aerugionsa | Ag/TiO2 | 96% Tetracycline 75% Cr (VI) | [41] |
| GOx | NiFe2O4 | 98.6% Indigo carmine | [42] |
| Activated sludge | Fe-TiO2 | >80% COD | This study |
| Water Quality | Value | Analytical Instrument |
|---|---|---|
| pH | 7.4 ± 1.6 | Hanna pH/EC/TDS Tester (H198130) |
| Temperature (°C) | 26.4 ± 2.3 | Hanna pH/EC/TDS Tester (H198130) |
| Colour (abs 465 nm, Pt.Co) | 570 ± 7.6 | HACH Spectrophotometer (DR3900) |
| Turbidity (NTU) | 732 ± 12.5 | Turbidity meter (HACH 2100N) |
| Chemical oxygen demand (mg COD/L) | 2380 ± 32 | HACH Spectrophotometer (DR3900) |
| Ammonia (mg NH3/L) | 0.7 ± 0.2 | HACH Spectrophotometer (DR3900) |
| Total Kjeldahl nitrogen (mg TKN/L) | 30.5 ± 1.4 | HACH Spectrophotometer (DR3900) |
| Nitrate (mg NO3/L) | 0.6 ± 0.15 | HACH Spectrophotometer (DR3900) |
| Total nitrogen (mg TN/L) | 31.9 ± 1.8 | HACH Spectrophotometer (DR3900) |
| Total suspended solids (mg TS/L) | 304.5 ± 23.6 | Analytical balance (HCB602H 22 ADAM) |
| Volatile solids (mg VS/L) | 229.5 ± 2.65 | Analytical balance (HCB602H 22 ADAM) |
| Ratio VS/TS | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetteh, E.K.; Amo-Duodu, G.; Rathilal, S. Anaerobic Digested Wastewater CO2 Sequestration Using a Biophotocatalytic System with a Magnetized Photocatalyst (Fe-TiO2). Molecules 2022, 27, 5213. https://doi.org/10.3390/molecules27165213
Tetteh EK, Amo-Duodu G, Rathilal S. Anaerobic Digested Wastewater CO2 Sequestration Using a Biophotocatalytic System with a Magnetized Photocatalyst (Fe-TiO2). Molecules. 2022; 27(16):5213. https://doi.org/10.3390/molecules27165213
Chicago/Turabian StyleTetteh, Emmanuel Kweinor, Gloria Amo-Duodu, and Sudesh Rathilal. 2022. "Anaerobic Digested Wastewater CO2 Sequestration Using a Biophotocatalytic System with a Magnetized Photocatalyst (Fe-TiO2)" Molecules 27, no. 16: 5213. https://doi.org/10.3390/molecules27165213
APA StyleTetteh, E. K., Amo-Duodu, G., & Rathilal, S. (2022). Anaerobic Digested Wastewater CO2 Sequestration Using a Biophotocatalytic System with a Magnetized Photocatalyst (Fe-TiO2). Molecules, 27(16), 5213. https://doi.org/10.3390/molecules27165213








