Novel Polycyclic Fused Amide Derivatives: Properties and Applications for Sky-blue Electroluminescent Devices
Abstract
:1. Introduction
2. Results and discussion
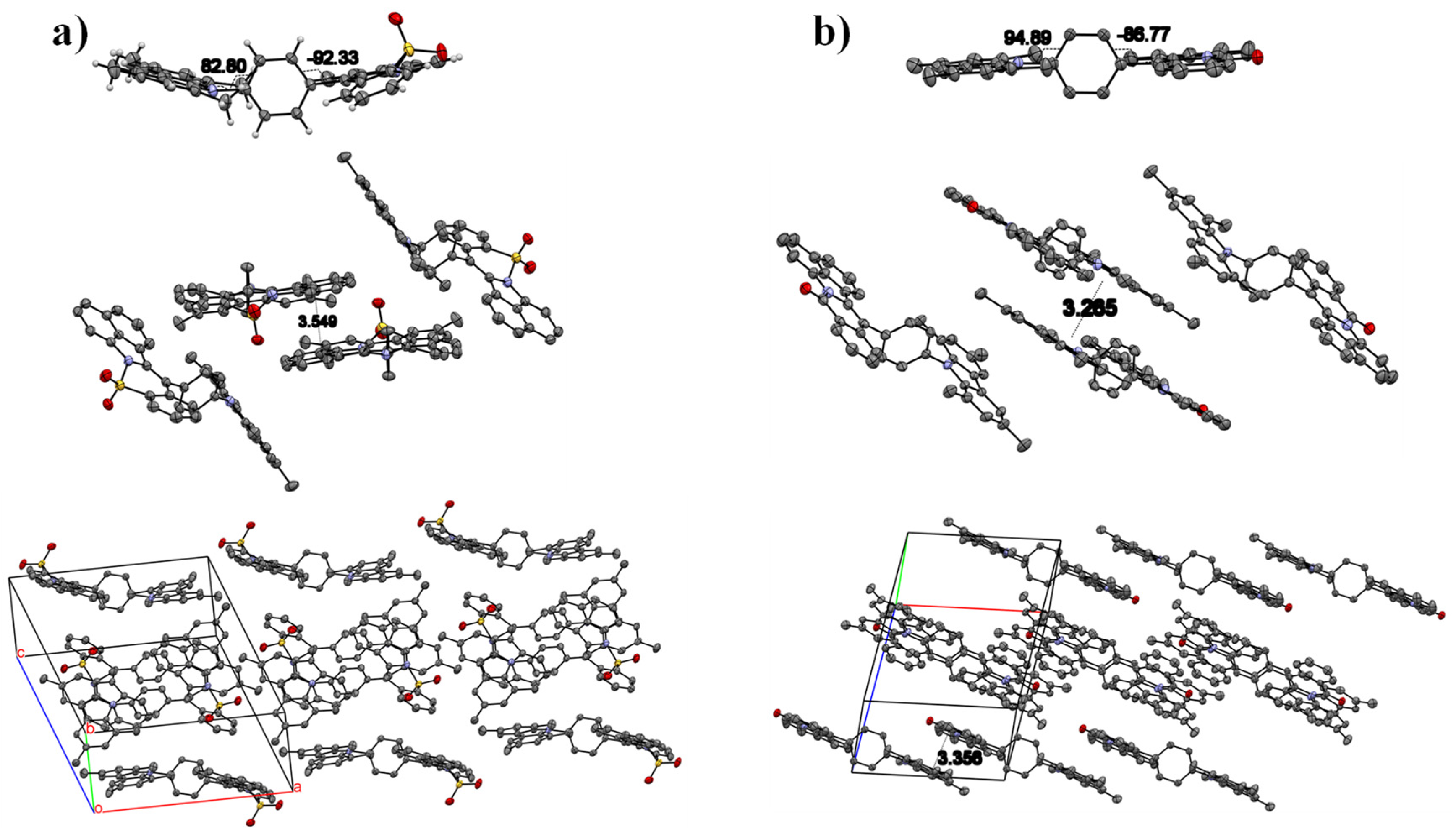
2.1. Synthesis and Single crystals
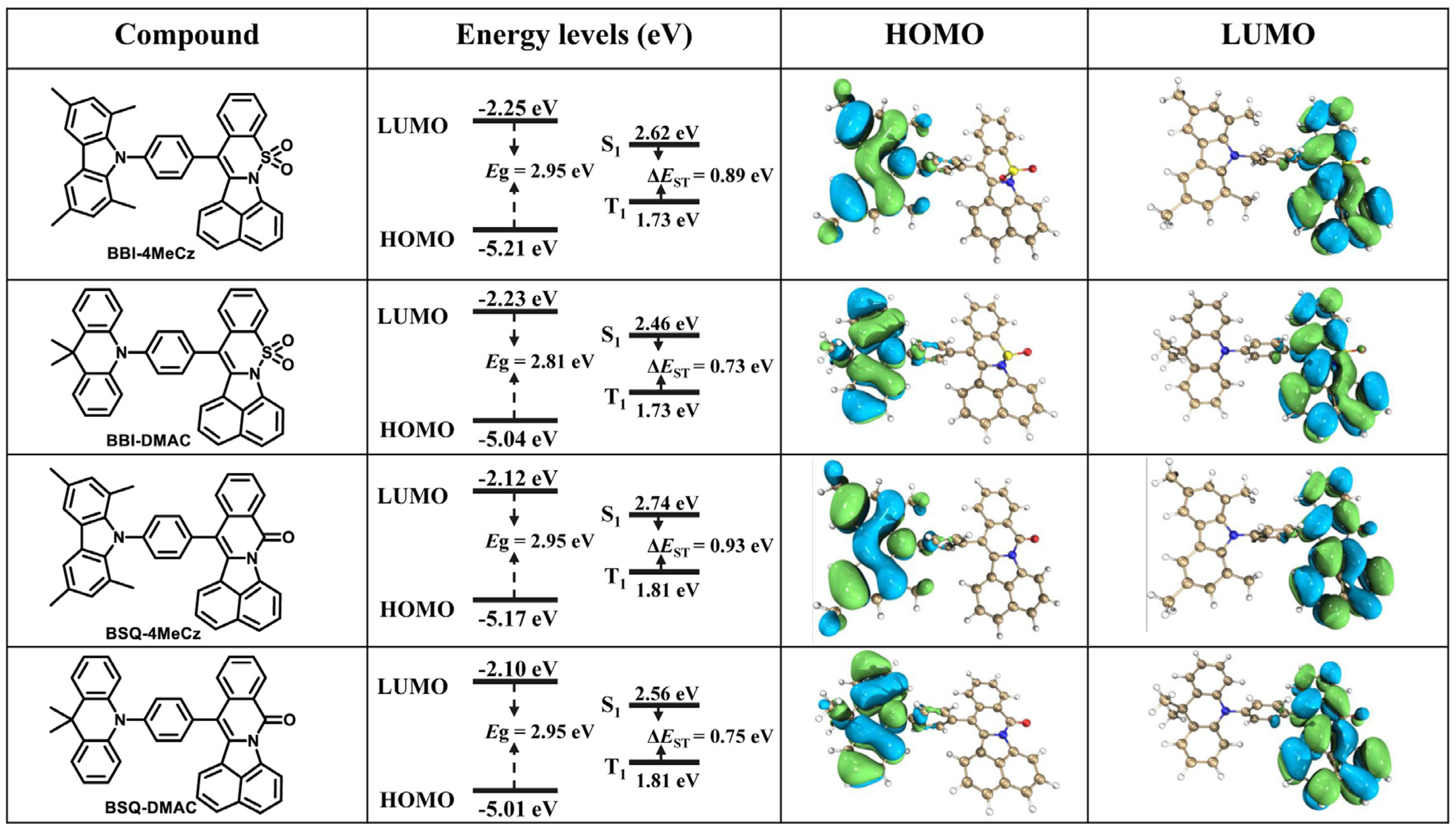
2.2. Theoretical Calculations
2.3. Thermal and Electrochemical Properties
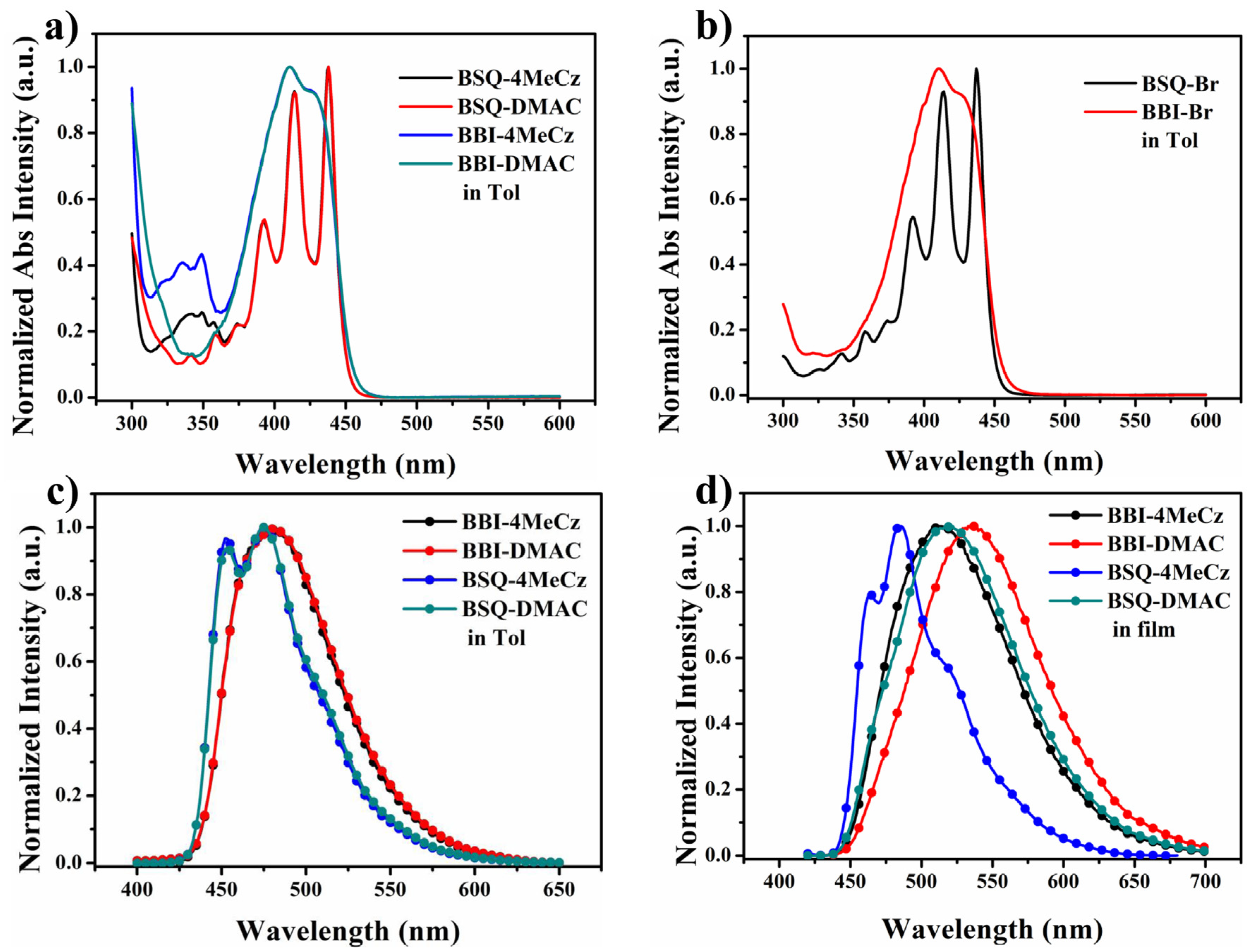
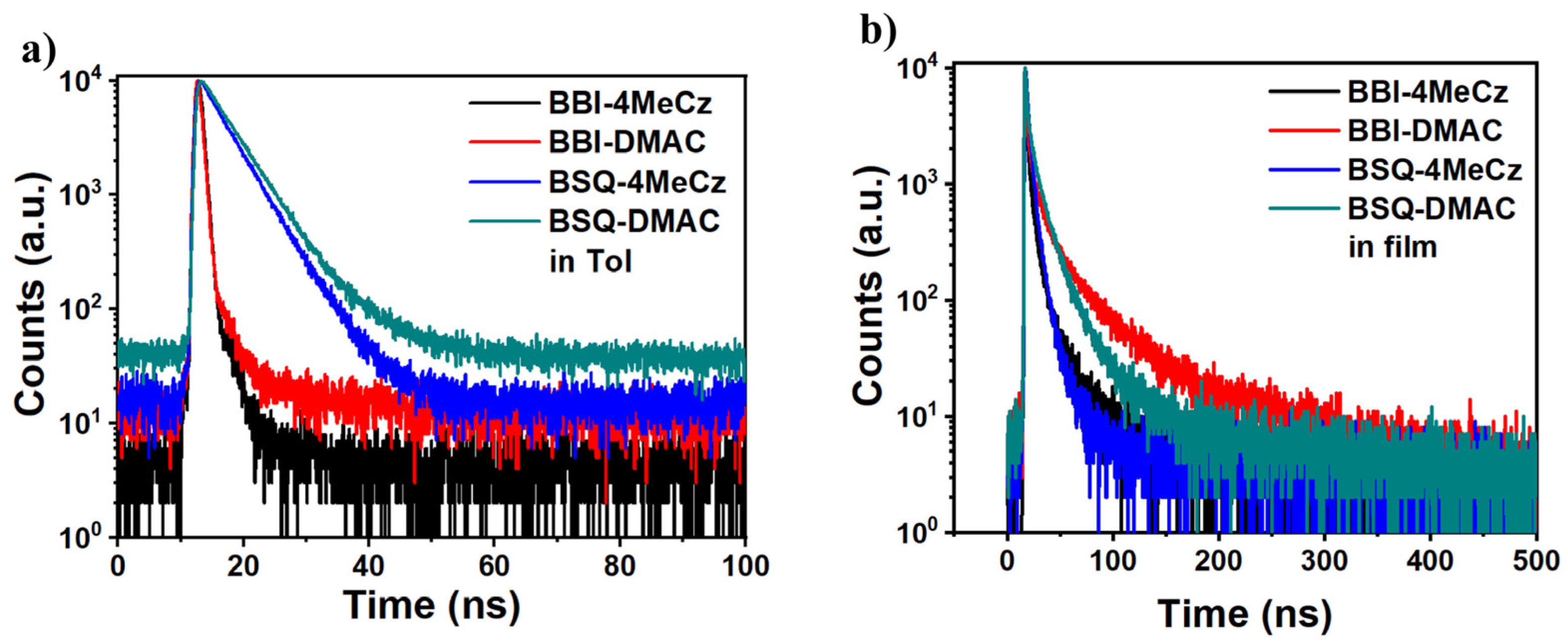
2.4. Photophysical Properties
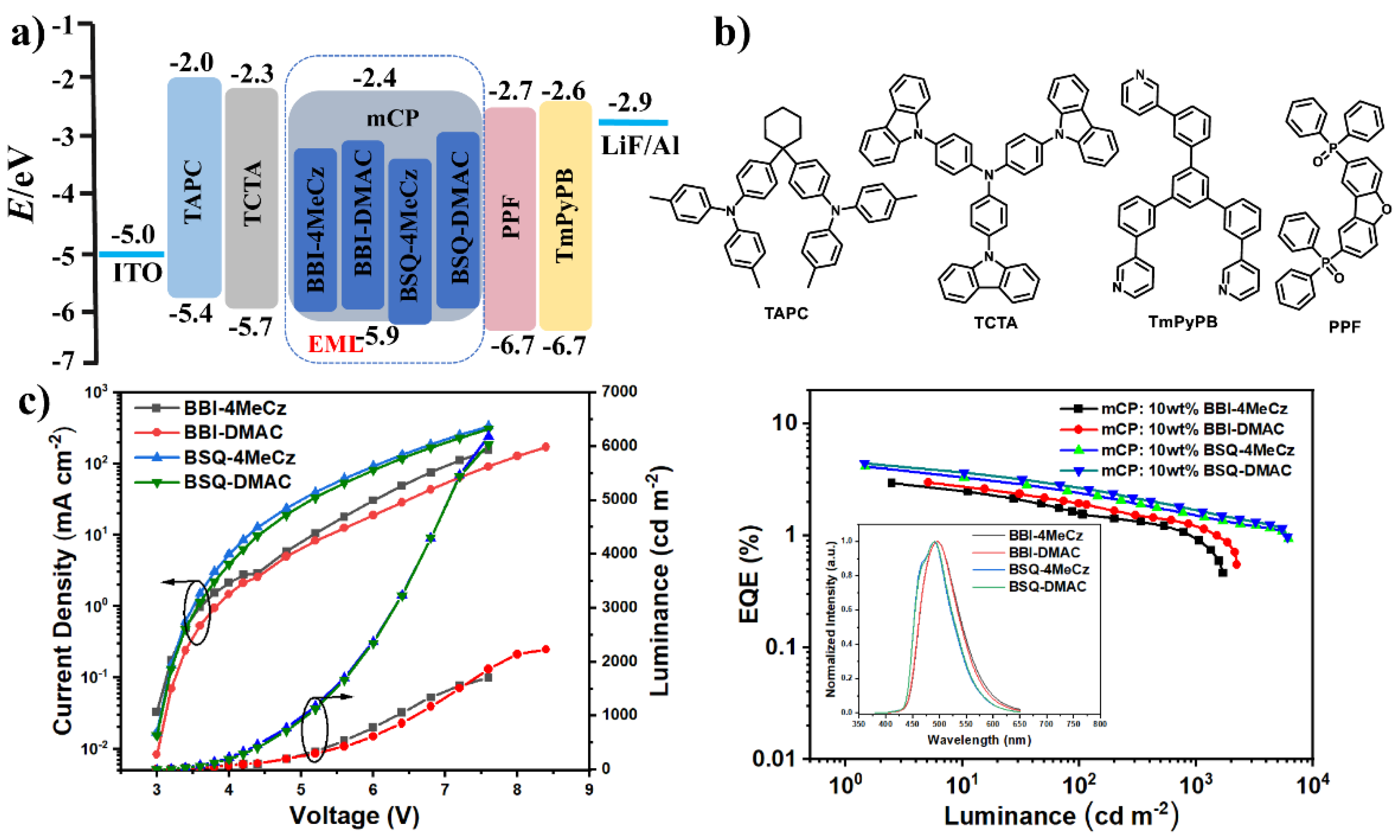
2.5. Device Performance
3. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Yang, G.-X.; Chen, Y.; Zhu, J.-J.; Song, J.-Y.; Tang, S.-S.; Ma, D.; Tong, Q.-X. Rational design of pyridine-containing emissive materials for high performance deep-blue organic light-emitting diodes with CIEy~0.06. Dyes Pigm. 2021, 187, 109088. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Cai, X.; Gan, L.; Xu, Z.; Li, W.; Liu, K.; Chen, D.; Su, S.J. Tri-spiral donor for high efficiency and versatile blue thermally activated delayed fluorescence materials. Angew. Chem. Int. Ed. 2019, 58, 11301–11305. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, W.; Gan, L.; Wang, Z.; Li, M.; Su, S.-J. Non-noble-metal-based organic emitters for OLED applications. Mater. Sci. Eng. R Rep. 2020, 142, 100581. [Google Scholar] [CrossRef]
- Tan, H.J.; Yang, G.X.; Deng, Y.L.; Cao, C.; Tan, J.H.; Zhu, Z.L.; Chen, W.C.; Xiong, Y.; Jian, J.X.; Lee, C.S.; et al. Deep-blue OLEDs with Rec.2020 blue gamut compliance and EQE over 22% achieved by conformation engineering. Adv. Mater. 2022, 34, 2200537. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.-L.; Chen, D.; Cai, X.; Xie, G.; He, Z.; Wu, Y.-C.; Lien, A.; Cao, Y.; Su, S.-J. Design strategy of blue and yellow thermally activated delayed fluorescence emitters and their all-fluorescence white OLEDs with external quantum efficiency beyond 20%. Adv. Funct. Mater. 2016, 26, 6904–6912. [Google Scholar] [CrossRef]
- Xia, G.; Qu, C.; Zhu, Y.; Ye, J.; Ye, K.; Zhang, Z.; Wang, Y. A TADF emitter featuring linearly arranged spiro-donor and spiro-acceptor groups: Efficient nondoped and doped deep-blue OLEDs with CIEy <0.1. Angew. Chem. Int. Ed. 2021, 60, 9598–9603. [Google Scholar]
- Cai, X.; Su, S.-J. Marching toward highly efficient, pure-blue, and stable thermally activated delayed fluorescent organic light-emitting diodes. Adv. Funct. Mater. 2018, 28, 1802558. [Google Scholar] [CrossRef]
- Kothavale, S.; Lee, K.H.; Lee, J.Y. CN-modified imidazopyridine as a new electron accepting unit of thermally activated delayed fluorescent emitters. Chem. Eur. J. 2019, 26, 845–852. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, P.; Hu, D.; Ma, Y. Recent progress in hot exciton materials for organic light-emitting diodes. Chem. Soc. Rev. 2020, 50, 1030–1069. [Google Scholar] [CrossRef]
- Cao, C.; Yang, G.X.; Tan, J.H.; Shen, D.; Chen, W.C.; Chen, J.X.; Liang, J.L.; Zhu, Z.L.; Liu, S.H.; Tong, Q.X.; et al. Deep-blue high-efficiency triplet-triplet annihilation organic light-emitting diodes using donor- and acceptor-modified anthracene fluorescent emitters. Mater. Today Energy 2021, 21, 100727–100736. [Google Scholar] [CrossRef]
- Venkatramaiah, N.; Kumar, G.D.; Chandrasekaran, Y.; Ganduri, R.; Patil, S. Efficient blue and yellow organic light-emitting diodes enabled by aggregation-induced emission. ACS Appl. Mater. Interfaces 2018, 10, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Chan, C.Y.; Tanaka, M.; Mamada, M.; Goushi, K.; Tang, X.; Tsuchiya, Y.; Nakanotani, H.; Adachi, C. Tailor-made multi-resonance terminal emitters toward narrowband, high-efficiency, and stable hyperfluorescence organic light-emitting diodes. Adv. Opt. Mater. 2022. [Google Scholar] [CrossRef]
- Chen, S.; Xu, H. Electroluminescent materials toward near ultraviolet region. Chem. Soc. Rev. 2021, 50, 8639–8668. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, G.; Qi, T.; Huang, H. Aromatic imide/amide-based organic small-molecule emitters for organic light-emitting diodes. Mater. Chem. Front. 2020, 4, 1554–1568. [Google Scholar] [CrossRef]
- Jang, M.E.; Yasuda, T.; Lee, J.; Lee, S.Y.; Adachi, C. Organic light-emitting diodes based on donor-substituted phthalimide and maleimide fluorophores. Chem. Lett. 2015, 44, 1248–1250. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Duan, R.; Wei, X.; Yi, Y.; Wang, Y.; Chen, C.F. Aromatic-imide-based thermally activated delayed fluorescence materials for highly efficient organic light-emitting diodes. Angew. Chem. Int. Ed. 2017, 56, 8818–8822. [Google Scholar] [CrossRef]
- Zeng, W.; Lai, H.Y.; Lee, W.K.; Jiao, M.; Shiu, Y.J.; Zhong, C.; Gong, S.; Zhou, T.; Xie, G.; Sarma, M.; et al. Achieving nearly 30% external quantum efficiency for orange-red organic light emitting diodes by employing thermally activated delayed fluorescence emitters composed of 1,8-naphthalimide-acridine hybrids. Adv. Mater. 2018, 30, 1704961. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Han, W.; Li, J.; Pu, X.; Wu, D.; Bin, Z.; You, J. Orange–red organic light emitting diodes with high efficiency and low efficiency roll-off: Boosted by a fused acceptor composed of pyrazine and maleimide. Chem. Eng. J. 2022, 428, 131186. [Google Scholar] [CrossRef]
- Data, P.; Kurowska, A.; Pluczyk, S.; Zassowski, P.; Pander, P.; Jedrysiak, R.; Czwartosz, M.; Otulakowski, L.; Suwinski, J.; Lapkowski, M.; et al. Exciplex enhancement as a tool to increase OLED device efficiency. J. Phys. Chem. C 2016, 120, 2070–2078. [Google Scholar] [CrossRef]
- Huang, Z.; Bin, Z.; Su, R.; Yang, F.; Lan, J.; You, J. Molecular design of non-doped OLEDs based on a twisted heptagonal acceptor: A delicate balance between rigidity and rotatability. Angew. Chem. Int. Ed. 2020, 59, 9992–9996. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Lin, J.; Zhou, J.; Wang, Y.; Liu, X.; Huang, Y.; Lu, Z.; Hu, C. Novel 1,8-naphthalimide derivatives for standard-red organic light-emitting device applications. J. Mater. Chem. C 2015, 3, 5259–5267. [Google Scholar] [CrossRef]
- Yang, G.-X.; Tan, H.-J.; Zhao, J.-W.; Zhu, J.-J.; He, X.; Jian, J.-X.; Zhou, M.-H.; Tao, S.-L.; Tong, Q.-X. Structurally modified [1,2,4]triazolo[1,5-a]pyridine derivatives as promising materials for highly efficient blue fluorescent organic light-emitting diodes. Chem. Eng. J. 2022, 445, 136813. [Google Scholar] [CrossRef]
- Noland, W.E.; Narina, V.S.; Britton, D. Synthesis and crystallography of 8-halonaphthalene-1-carbonitriles and naphthalene-1,8-dicarbonitrile. J. Chem. Res. 2011, 35, 694–697. [Google Scholar] [CrossRef]
- Wang, B.; Daugulis, O.; Brookhart, M. Ethylene polymerization with Ni(II) diimine complexes generated from 8-halo-1-naphthylamines: The role of equilibrating Syn/Anti diastereomers in determining polymer properties. Organometallics 2019, 38, 4658–4668. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wang, Y.; Qiu, G.; Liang, Q.; Zhou, H. Copper-catalyzed tandem radical cyclization of 8-ethynyl-1-naphthyl-amines for the synthesis of 2h-benzo[e][1,2]thiazine 1,1-dioxides and its fluorescence properties. J. Org. Chem. 2020, 85, 12526–12534. [Google Scholar] [CrossRef]
- Hua, Y.; Chen, Z.Y.; Diao, H.; Zhang, L.; Qiu, G.; Gao, X.; Zhou, H. Copper-catalyzed preparation of benzo[3,4]indolo[1,2-b]isoquinoline-8-ones and photoluminescence exploration. J. Org. Chem. 2020, 85, 9614–9621. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Tong, Q.X.; Zhong, J.J. Recent advances in visible-light photoredox catalysis for the thiol-Ene/Yne reactions. Molecules 2022, 27, 619. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Li, W.; Xu, Z.; Gan, L.; Liu, K.; Zheng, N.; Ning, C.; Chen, D.; Wu, Y.C.; et al. Spiral donor design strategy for blue thermally activated delayed fluorescence emitters. ACS Appl. Mater. Interfaces 2021, 13, 5302–5311. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.-L.; Cai, X.; Chen, D.; Liu, X.; Xie, G.; Wang, Z.; Wu, Y.-C.; Lo, C.-C.; Lien, A.; et al. Deep blue fluorophores incorporating sulfone-locked triphenylamine: The key for highly efficient fluorescence-phosphorescence hybrid white OLEDs with simplified structure. J. Mater. Chem. C 2015, 3, 6986–6996. [Google Scholar] [CrossRef]
- Yang, G.-X.; Zhu, J.-J.; Tang, S.-S.; Tan, H.-J.; He, X.; Jian, J.-X.; Zheng, X.-H.; Tong, Q.-X. Effective energy transfer for green, orange, and red phosphorescent organic light-emitting diodes based on a bipolar deep-blue emitter with low efficiency roll-off at high brightness. Adv. Photonics Res. 2021, 2, 2100031–2100039. [Google Scholar] [CrossRef]
- Xie, W.; Li, M.; Peng, X.; Qiu, W.; Gan, Y.; Chen, Z.; He, Y.; Li, W.; Liu, K.; Wang, L.; et al. “On-off” switch between red thermally activated delayed fluorescence and conventional fluorescence by isomeric regulation. Chem. Eng. J. 2021, 425, 131510. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Gan, L.; Li, M.; Zheng, N.; Ning, C.; Chen, D.; Wu, Y.C.; Su, S.J. J-aggregation enhances the electroluminescence performance of a sky-blue thermally activated delayed-fluorescence emitter in nondoped organic light-emitting diodes. ACS Appl. Mater. Interfaces 2020, 12, 2717–2723. [Google Scholar] [CrossRef] [PubMed]

| Compound | λabs[a] | λPL[b] | Φf [c] | τ[d] | ET1/ES1[e] | HOMO/LUMO[f] | Eg[g] | Tg/Td[h] |
|---|---|---|---|---|---|---|---|---|
| [nm] | [nm] | [%] | [ns] | [eV] | [eV] | [eV] | [°C] | |
| BBI-4MeCz | 334/348/409/427 | 482/512 | 9.1 | 0.8/6.9 | 1.73/2.62 | −5.87/−3.14 | 2.73 | 137/408 |
| BBI-DMAC | 409/427 | 481/533 | 7.6 | 1.1/24.4 | 1.73/2.46 | −5.77/−3.04 | 2.73 | 131/419 |
| BSQ-4MeCz | 392/413/438 | 474/484 | 77.3 | 4.6/6.3 | 1.81/2.74 | −5.93/−3.17 | 2.76 | n.d./463 |
| BSQ-DMAC | 392/413/438 | 474/517 | 78.1 | 5.1/12.6 | 1.81/2.56 | −5.72/−2.96 | 2.76 | 126/430 |
| Emitter | Von [a] | λEL | Lmax | CE[b] | PE[b] | EQE[b] | CIE[c] |
|---|---|---|---|---|---|---|---|
| [V] | [nm] | [cd m−2] | [cd A−1] | [lm W−1] | [%] | [x, y] | |
| mCP: 10 wt% BBI-4MeCz | 3.0 | 494 | 1703 | 7.54 | 7.88 | 2.95 | (0.19, 0.40) |
| mCP: 10 wt% BBI-DMAC | 3.2 | 494 | 2230 | 7.34 | 7.21 | 2.97 | (0.18, 0.40) |
| mCP: 10 wt% BSQ-4MeCz | 3.0 | 488 | 6187 | 8.58 | 8.90 | 4.13 | (0.16, 0.31) |
| mCP: 10 wt% BSQ-DMAC | 3.0 | 488 | 6042 | 9.50 | 9.95 | 4.45 | (0.16, 0.32) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.-X.; Liu, D.-H.; Jiang, S.-M.; Yang, Z.-H.; Chen, Z.-J.; Qiu, W.-D.; Gan, Y.-Y.; Liu, K.-K.; Li, D.-L.; Su, S.-J. Novel Polycyclic Fused Amide Derivatives: Properties and Applications for Sky-blue Electroluminescent Devices. Molecules 2022, 27, 5181. https://doi.org/10.3390/molecules27165181
Yang G-X, Liu D-H, Jiang S-M, Yang Z-H, Chen Z-J, Qiu W-D, Gan Y-Y, Liu K-K, Li D-L, Su S-J. Novel Polycyclic Fused Amide Derivatives: Properties and Applications for Sky-blue Electroluminescent Devices. Molecules. 2022; 27(16):5181. https://doi.org/10.3390/molecules27165181
Chicago/Turabian StyleYang, Guo-Xi, Deng-Hui Liu, Si-Min Jiang, Zhi-Hai Yang, Zi-Jian Chen, Wei-Dong Qiu, Yi-Yang Gan, Kun-Kun Liu, De-Li Li, and Shi-Jian Su. 2022. "Novel Polycyclic Fused Amide Derivatives: Properties and Applications for Sky-blue Electroluminescent Devices" Molecules 27, no. 16: 5181. https://doi.org/10.3390/molecules27165181
APA StyleYang, G.-X., Liu, D.-H., Jiang, S.-M., Yang, Z.-H., Chen, Z.-J., Qiu, W.-D., Gan, Y.-Y., Liu, K.-K., Li, D.-L., & Su, S.-J. (2022). Novel Polycyclic Fused Amide Derivatives: Properties and Applications for Sky-blue Electroluminescent Devices. Molecules, 27(16), 5181. https://doi.org/10.3390/molecules27165181








