Preliminary Studies on Endotherapy Based Application of Ozonated Water to Bobal Grapevines: Effect on Wine Quality
Abstract
:1. Introduction
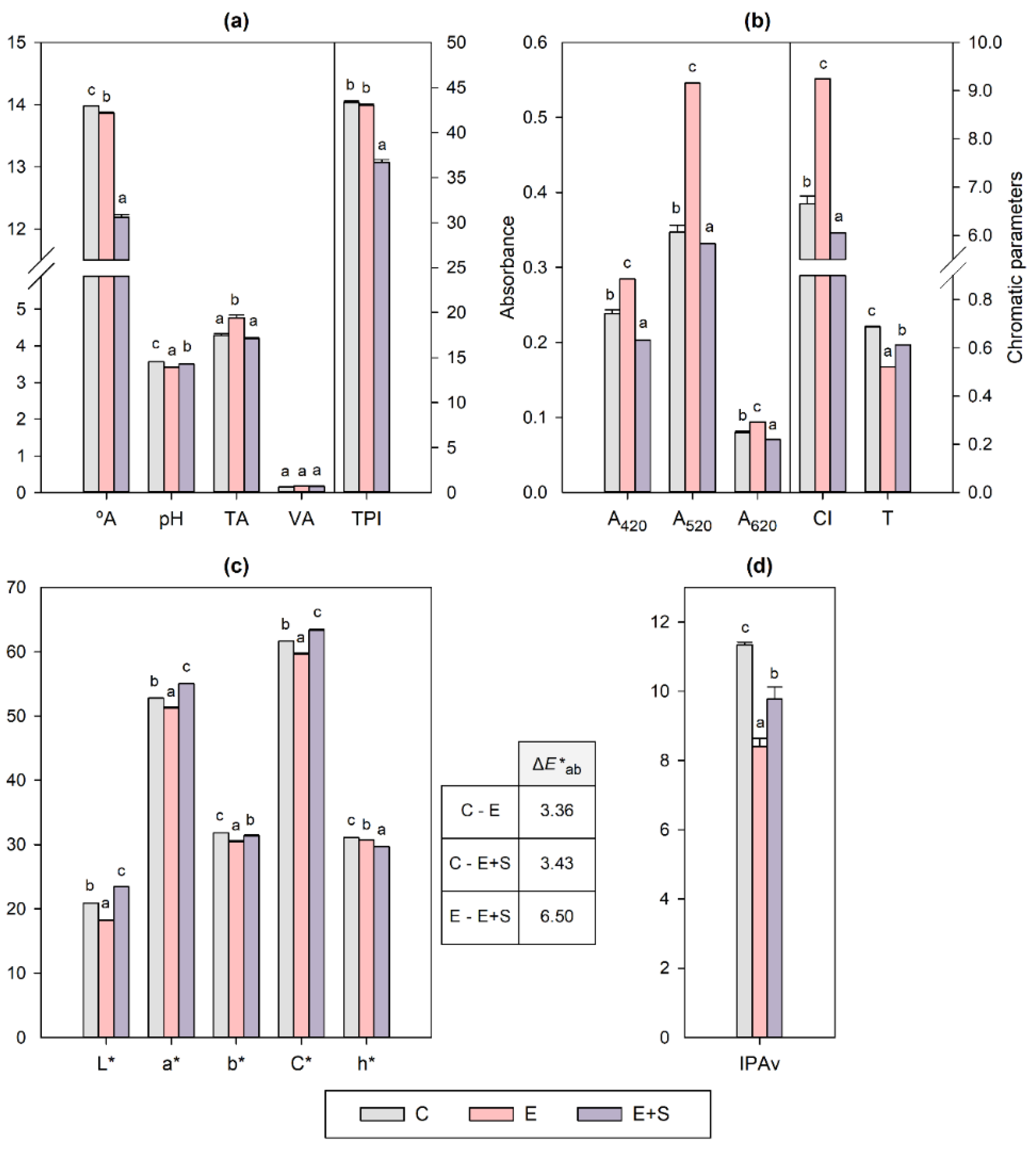
2. Results and Discussion
2.1. Effect on Enological Parameters
2.2. Effect on Phenolic Compounds
2.3. Effect on Volatile Compounds
3. Materials and Methods
3.1. Grapevines
3.2. Ozonated Water
3.3. Grapevine Treatments
- -
- Endotherapy (E): direct injection of ozonated water into the trunk through a hole (3 mm diameter, 1 cm depth) drilled above the graft union and sealed with a hermetic cannula connected to the ozonated water tank. The volume of ozonated water injected in each grapevine and application, under a maximum pressure of 1 bar, was approximately 0.5% of the trunk volume in order to be distributed through the xylem and reach the different parts of the plant. All of these parameters were selected based on preliminary tests (data not shown). Ozonated water was applied four times before harvest, in particular before flowering, after the fruit set, at veraison and during the ripening period.
- -
- Combination of endotherapy and foliar spraying (E + S): the ozonated water was applied through endotherapy in the same manner and on the same dates as in the E treatment, while spraying was performed 2 days before and after each endotherapy application. Approximately 300 mL of ozonated water were sprayed per plant and application to cover the entire canopy.
3.4. Winemaking
3.5. Analytical Methods
3.5.1. Wine Enological Parameters
3.5.2. Determination of Low Molecular Weight Phenolic Compounds by HPLC-DAD
3.5.3. Determination of Volatile Compounds by SBSE-GC-MS
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Raio, A.; Feliciani, A.; Ferri, V.; Carboni, C. Integrated Vineyard Management Trials Using Ozonated and Electrolized Water. Infowine Internet J. Enol. Vitic. 2016, 2, 1–6. [Google Scholar]
- Pierron, R.J.G.; Pages, M.; Couderc, C.; Compant, S.; Jacques, A.; Violleau, F. In Vitro and in Planta Fungicide Properties of Ozonated Water against the Esca-Associated Fungus Phaeoacremonium Aleophilum. Sci. Hortic. 2015, 189, 184–191. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the Food Industry: Principles of Ozone Treatment, Mechanisms of Action, and Applications: An Overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef] [PubMed]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological Aspects of Ozone Applications in Food: A Review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Aguayo, E.; Artés, F.; Tomás-Barberán, F.A. Enriched Ozone Atmosphere Enhances Bioactive Phenolics in Seedless Table Grapes after Prolonged Shelf Life. J. Sci. Food Agric. 2007, 87, 824–831. [Google Scholar] [CrossRef]
- Botondi, R.; De Sanctis, F.; Moscatelli, N.; Vettraino, A.M.; Catelli, C.; Mencarelli, F. Ozone Fumigation for Safety and Quality of Wine Grapes in Postharvest Dehydration. Food Chem. 2015, 188, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Cravero, F.; Englezos, V.; Rantsiou, K.; Torchio, F.; Giacosa, S.; Segade, S.R.; Gerbi, V.; Rolle, L.; Cocolin, L. Ozone Treatments of Post Harvested Wine Grapes: Impact on Fermentative Yeasts and Wine Chemical Properties. Food Res. Int. 2016, 87, 134–141. [Google Scholar] [CrossRef]
- Guzzon, R.; Franciosi, E.; Moser, S.; Carafa, I.; Larcher, R. Application of Ozone during Grape Drying for the Production of Straw Wine. Effects on the Microbiota and Compositive Profile of Grapes. J. Appl. Microbiol. 2018, 125, 513–527. [Google Scholar] [CrossRef]
- Bellincontro, A.; Catelli, C.; Cotarella, R.; Mencarelli, F. Postharvest Ozone Fumigation of Petit Verdot Grapes to Prevent the Use of Sulfites and to Increase Anthocyanin in Wine. Aust. J. Grape Wine Res. 2017, 23, 200–206. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skóra, B.; Balawejder, M. Ozone Treatment Induces Changes in Antioxidative Defense System in Blueberry Fruit During Storage. Food Bioprocess Technol. 2020, 13, 1240–1245. [Google Scholar] [CrossRef]
- Carbone, K.; Mencarelli, F. Influence of Short-Term Postharvest Ozone Treatments in Nitrogen or Air Atmosphere on the Metabolic Response of White Wine Grapes. Food Bioprocess Technol. 2015, 8, 1739–1749. [Google Scholar] [CrossRef]
- DeSanctis, F.; Ceccantoni, B.; Bellincontro, A.; Botondi, R.; Mencarelli, F.; D’Onofrio, C.; Ducci, E.; Catelli, C. Ozone Fumigation Postharvest Treatment for the Quality of Wine Grape. Acta Hortic. 2015, 1071, 795–800. [Google Scholar] [CrossRef]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F.A. Comparison of Ozone and UV-C Treatments on the Postharvest Stilbenoid Monomer, Dimer, and Trimer Induction in Var. “Superior” White Table Grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef]
- Río Segade, S.; Paissoni, M.A.; Giacosa, S.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Gerbi, V.; Rolle, L. Winegrapes Dehydration under Ozone-Enriched Atmosphere: Influence on Berry Skin Phenols Release, Cell Wall Composition and Mechanical Properties. Food Chem. 2019, 271, 673–684. [Google Scholar] [CrossRef]
- Fumagalli, I.; Cieslik, S.; De Marco, A.; Proietti, C.; Paoletti, E. Grapevine and Ozone: Uptake and Effects. Climate 2019, 7, 140. [Google Scholar] [CrossRef]
- Soja, G.; Reichenauer, T.G.; Eid, M.; Soja, A.M.; Schaber, R.; Gangl, H. Long-Term Ozone Exposure and Ozone Uptake of Grapevines in Open-Top Chambers. Atmos. Environ. 2004, 38, 2313–2321. [Google Scholar] [CrossRef]
- Valletta, A.; Salvatori, E.; Rita Santamaria, A.; Nicoletti, M.; Toniolo, C.; Caboni, E.; Bernardini, A.; Pasqua, G.; Manes, F. Ecophysiological and Phytochemical Response to Ozone of Wine Grape Cultivars of Vitis vinifera L. Nat. Prod. Res. 2016, 30, 2514–2522. [Google Scholar] [CrossRef]
- Yamaji, K.; Julkunen-Tiitto, R.; Rousi, M.; Freiwald, V.; Oksanen, E. Ozone Exposure over Two Growing Seasons Alters Root-to-Shoot Ratio and Chemical Composition of Birch (Betula Pendula Roth). Glob. Chang. Biol. 2003, 9, 1363–1377. [Google Scholar] [CrossRef]
- Castagna, A.; Ranieri, A. Detoxification and Repair Process of Ozone Injury: From O3 Uptake to Gene Expression Adjustment. Environ. Pollut. 2009, 157, 1461–1469. [Google Scholar] [CrossRef]
- Fares, S.; Oksanen, E.; Lännenpää, M.; Julkunen-Tiitto, R.; Loreto, F. Volatile Emissions and Phenolic Compound Concentrations along a Vertical Profile of Populus Nigra Leaves Exposed to Realistic Ozone Concentrations. Photosynth. Res. 2010, 104, 61–74. [Google Scholar] [CrossRef]
- Peltonen, P.A.; Vapaavuori, E.; Julkunen-Tiitto, R. Accumulation of Phenolic Compounds in Birch Leaves Is Changed by Elevated Carbon Dioxide and Ozone. Glob. Chang. Biol. 2005, 11, 1305–1324. [Google Scholar] [CrossRef]
- Saleem, A.; Loponen, J.; Pihlaja, K.; Oksanen, E. Effects of Long-Term Open-Field Ozone Exposure on Leaf Phenolics of European Silver Birch (Betula Pendula Roth). J. Chem. Ecol. 2001, 27, 1049–1062. [Google Scholar] [CrossRef]
- Beauchamp, J.; Wisthaler, A.; Hansel, A.; Kleist, E.; Miebach, M.; Niinemets, Ü.; Schurr, U.; Wildt, J. Ozone Induced Emissions of Biogenic VOC from Tobacco: Relationships between Ozone Uptake and Emission of LOX Products. Plant Cell Environ. 2005, 28, 1334–1343. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic Stresses and Induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Contigiani, E.V.; Jaramillo-Sánchez, G.; Castro, M.A.; Gómez, P.L.; Alzamora, S.M. Postharvest Quality of Strawberry Fruit (Fragaria x Ananassa Duch Cv. Albion) as Affected by Ozone Washing: Fungal Spoilage, Mechanical Properties, and Structure. Food Bioprocess Technol. 2018, 11, 1639–1650. [Google Scholar] [CrossRef]
- Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Sánchez-Martínez, J.F.; Salinas, M.R.; Alonso, G.L. Spraying Ozonated Water on Bobal Grapevines: Effect on Grape Quality. Food Res. Int. 2019, 125. [Google Scholar] [CrossRef]
- Campayo, A.; de la Hoz, K.S.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L. Spraying Ozonated Water on Bobal Grapevines: Effect on Wine Quality. Biomolecules 2020, 10, 213. [Google Scholar] [CrossRef]
- Modesti, M.; Baccelloni, S.; Brizzolara, S.; Aleandri, M.P.; Bellincontro, A.; Mencarelli, F.; Tonutti, P. Effects of Treatments with Ozonated Water in the Vineyard (Cv Vermentino) on Microbial Population and Fruit Quality Parameters. BIO Web Conf. 2019, 13, 04011. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk Injection of Plant Protection Products to Protect Trees from Pests and Diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Calzarano, F.; Di Marco, S.; Cesari, A. Benefit of Fungicide Treatment after Trunk Renewal of Vines with Different Types of Esca Necrosis. Phytopathol. Mediterr. 2004, 43, 116–124. [Google Scholar]
- Del Frari, G.; Costa, J.; Oliveira, H.; Boavida Ferreira, R. Endotherapy of Infected Grapevine Cuttings for the Control of Phaeomoniella chlamydospora and Phaeoacremonium minimum. Phytopathol. Mediterr. 2018, 57, 239–448. [Google Scholar] [CrossRef]
- Di Marco, S.; Mazzullo, A.; Calzarano, F.; Cesari, A. The Control of Esca: Status and Perspectives. Phytopathol. Mediterr. 2000, 39, 232–240. [Google Scholar]
- Dula, T.; Kappes, E.M.; Horvath, A.; Rabai, A. Preliminary Trials on Treatment of Esca-Infected Grapevines with Trunk Injection of Fungicides. Phytopathol. Mediterr. 2007, 46, 91–95. [Google Scholar]
- Campayo, A.; Savoi, S.; Romieu, C.; López-Jiménez, A.J.; Serrano de la Hoz, K.; Salinas, R.; Torregrosa, L.; Alonso, G.L. The Application of Ozonated Water Rearranges the Transcriptome of the Vitis Vinifera L. Fruit. Sci. Rep. 2021. [Google Scholar] [CrossRef]
- MAPA Encuesta de Utilización de Productos Fitosanitarios Campaña 2019. Resultados Agosto 2021. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/maquetacioninformededatosdelaeupf19_tcm30-577679.pdf (accessed on 5 August 2022).
- Dequin, S.; Escudier, J.L.; Bely, M.; Noble, J.; Albertin, W.; Masneuf-Pomarède, I.; Marullo, P.; Salmon, M.J.; Sablayrolles, J.M.; Ollat, N. How to Adapt Winemaking Practices to Modified Grape Composition under Climate Change Conditions. Oeno One 2017, 51, 205–214. [Google Scholar] [CrossRef]
- Esparza, I.; Santamaría, C.; Fernández, J.M. Chromatic Characterisation of Three Consecutive Vintages of Vitis Vinifera Red Wine: Effect of Dilution and Iron Addition. Anal. Chim. Acta 2006, 563, 331–337. [Google Scholar] [CrossRef]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Note. Visual and Instrumental Color Evaluation in Red Wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Salinas, M.R.; De La Hoz, K.S.; Zalacain, A.; Lara, J.F.; Garde-Cerdán, T. Analysis of Red Grape Glycosidic Aroma Precursors by Glycosyl Glucose Quantification. Talanta 2012, 89, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Campayo, A.; de la Hoz, K.S.; Mercedes García-Martínez, M.; Rosario Salinas, M.; Alonso, G.L. Novel Endotherapy-Based Applications of Ozonated Water to Bobal Grapevines: Effect on Grape Quality. Agronomy 2020, 10, 1218. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Giacosa, S.; Perrone, I.; Chitarra, W.; Pollon, M.; Torchio, F.; Boccacci, P.; Gambino, G.; Gerbi, V.; et al. Ozone Improves the Aromatic Fingerprint of White Grapes. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Alonso-Alonso, O.; Gallo-Montesdeoca, A.; Díaz-Romero, C.; Darias-Martín, J. Influence of Copigmentation and Phenolic Composition on Wine Color. J. Food Sci. Technol. 2016, 53, 2540–2547. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Paissoni, M.A.; Río Segade, S.; Giacosa, S.; Torchio, F.; Cravero, F.; Englezos, V.; Rantsiou, K.; Carboni, C.; Gerbi, V.; Teissedre, P.L.; et al. Impact of Post-Harvest Ozone Treatments on the Skin Phenolic Extractability of Red Winegrapes Cv Barbera and Nebbiolo (Vitis Vinifera L.). Food Res. Int. 2017, 98, 68–78. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds during Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins—An Overview on Structures, Occurrence, and Pathways of Formation. Trends Food Sci. Technol. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- García-Carpintero, E.G.; Sánchez-Palomo, E.; González-Viñas, M.A. Aroma Characterization of Red Wines from Cv. Bobal Grape Variety Grown in La Mancha Region. Food Res. Int. 2011, 44, 61–70. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Etiévant, X.P. Wine. In Volatile Compounds in Foods and Beverages; Marcel Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of Minor and Trace Volatile Compounds in Wine by Solid-Phase Extraction and Gas Chromatography with Mass Spectrometric Detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-Vine Grape Drying Effect on Volatile Compounds and Aromatic Series in Must from Pedro Ximénez Grape Variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Liu, S.Q.; Pilone, G.J. An Overview of Formation and Roles of Acetaldehyde in Winemaking with Emphasis on Microbiological Implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Liu, P.T.; Duan, C.Q.; Yan, G.L. Comparing the Effects of Different Unsaturated Fatty Acids on Fermentation Performance of Saccharomyces Cerevisiae and Aroma Compounds during Red Wine Fermentation. Molecules 2019, 24, 538. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Dudareva, N. A Familiar Ring to It: Biosynthesis of Plant Benzoic Acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Hydrolysis of Terpenyl Glycosides in Grape Juice and Other Fruit Juices: A Review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; Pardo-García, A.I.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Lavandin Hydrolat Applications to Petit Verdot Vineyards and Their Impact on Their Wine Aroma Compounds. Food Res. Int. 2013, 53, 391–402. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis. 2014. Available online: https://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts (accessed on 7 August 2022).
- Ribéreau-Gayon, J.; Peynaud, E.; Sudraud, P.; Ribéreau-Gayon, P. Traité d’Oenologie-Sciences et Techniques Du Vin; Dunod, Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Glories, Y. La Couleur Des Vins Rouges. Les Equilibres Des Anthocyanes et Des Tanins. Connaiss. Vigne Vin 1984, 18, 195–217. [Google Scholar] [CrossRef]
- Commission Internationale de l’Eclairage (CIE). Colorimetry, 2nd ed.; Publication CIE No. 15.2; Commission Internationale de l’Eclairage: Vienna, Austria, 1986. [Google Scholar]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. A New Simplified Method for Measuring the Color of Wines. III. All Wines and Brandies. Am. J. Enol. Vitic. 1999, 50, 359–363. [Google Scholar]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak Extract Application to Grapevines as a Plant Biostimulant to Increase Wine Polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
| Treatments | λ (nm) a | C | E | E + S |
|---|---|---|---|---|
| Phenolic acids (mg/L) | ||||
| Gallic acid | 280 | 25.14 ± 0.27 c | 13.72 ± 0.25 a | 17.34 ± 0.12 b |
| trans-Caffeic acid | 324 | 0.24 ± 0.01 c | 0.19 ± 0.00 b | 0.15 ± 0.01 a |
| Vanillic acid | 256 | 3.33 ± 0.14 b | 2.47 ± 0.10 a | 2.33 ± 0.15 a |
| Syringic acid | 280 | 2.54 ± 0.07 a | 2.44 ± 0.06 a | 2.24 ± 0.16 a |
| trans-Caftaric acid | 324 | 3.34 ± 0.06 b | 3.11 ± 0.03 a | 3.05 ± 0.02 a |
| trans-p-Coutaric acid | 308 | 0.87 ± 0.05 a | 0.88 ± 0.04 a | 0.78 ± 0.07 a |
| Σ Phenolic acids | 35.46 ± 0.45 c | 22.82 ± 0.30 a | 25.89 ± 0.53 b | |
| Stilbenes (mg/L) | ||||
| trans-Resveratrol | 308 | 0.86 ± 0.03 b | 0.72 ± 0.01 a | 0.71 ± 0.01 a |
| Piceid-trans-resveratrol | 308 | 0.88 ± 0.01 b | 0.69 ± 0.01 a | 0.72 ± 0.02 a |
| Σ Stilbenes | 1.74 ± 0.01 b | 1.41 ± 0.00 a | 1.43 ± 0.00 a | |
| Flavanols (mg/L) | ||||
| (+)-Catechin | 280 | 24.31 ± 0.38 b | 19.30 ± 0.35 a | 19.69 ± 0.14 a |
| (–)-Epicatechin | 280 | 17.72 ± 0.11 c | 13.36 ± 0.04 a | 14.75 ± 0.03 b |
| Σ Flavanols | 42.03 ± 0.48 c | 32.66 ± 0.30 a | 34.44 ± 0.11 b | |
| Flavonols (mg/L) | ||||
| Myricetin 3-O-galactoside | 365 | 0.29 ± 0.00 b | 0.26 ± 0.00 ab | 0.23 ± 0.02 a |
| Myricetin 3-O-glucuronide+glucoside b | 365 | 4.89 ± 0.11 a | 6.09 ± 0.14 b | 4.41 ± 0.12 a |
| Quercetin 3-O-galactoside | 365 | 0.63 ± 0.02 b | 0.54 ± 0.02 b | 0.42 ± 0.03 a |
| Quercetin 3-O-glucuronide+glucoside b | 365 | 7.22 ± 0.01 c | 6.38 ± 0.02 b | 5.20 ± 0.03 a |
| Laricitrin 3-O-glucoside/galactoside c | 365 | 1.01 ± 0.00 b | 1.14 ± 0.01 c | 0.94 ± 0.02 a |
| Kaempferol 3-O-glucoside | 365 | 0.49 ± 0.01 b | 0.43 ± 0.01 b | 0.37 ± 0.02 a |
| Syringetin 3-O-glucoside | 365 | 2.14 ± 0.03 b | 2.15 ± 0.01 b | 1.74 ± 0.04 a |
| Myricetin | 365 | 0.37 ± 0.00 b | 0.37 ± 0.00 b | 0.30 ± 0.01 a |
| Quercetin | 365 | 2.09 ± 0.05 c | 1.63 ± 0.02 b | 1.30 ± 0.03 a |
| Kaempferol | 365 | 0.22 ± 0.00 c | 0.19 ± 0.00 b | 0.13 ± 0.01 a |
| Σ Flavonols | 19.34 ± 0.22 b | 19.19 ± 0.05 b | 15.04 ± 0.21 a | |
| Anthocyanins (mg/L) | ||||
| Delphinidin 3-O-glucoside | 520 | 7.93 ± 0.13 b | 12.03 ± 0.05 c | 6.73 ± 0.19 a |
| Cyanidin 3-O-glucoside | 520 | 1.76 ± 0.09 b | 2.56 ± 0.11 c | 1.35 ± 0.03 a |
| Petunidin 3-O-glucoside | 520 | 14.73 ± 0.19 b | 21.15 ± 0.61 c | 12.08 ± 0.10 a |
| Peonidin 3-O-glucoside | 520 | 26.29 ± 0.78 b | 31.73 ± 1.50 c | 18.40 ± 0.11 a |
| Malvidin 3-O-glucoside | 520 | 160.27 ± 1.39 b | 214.95 ± 0.70 c | 143.08 ± 1.12 a |
| Peonidin 3-O-(6′-acetyl)-glucoside | 520 | 3.57 ± 0.06 b | 3.74 ± 0.01 c | 2.81 ± 0.03 a |
| Malvidin 3-O-(6′-acetyl)-glucoside | 520 | 14.20 ± 0.15 b | 18.89 ± 0.04 c | 13.21 ± 0.04 a |
| Petunidin 3-(6′-p-coumaroyl)-glucoside | 520 | 1.16 ± 0.01 b | 1.70 ± 0.04 c | 1.04 ± 0.00 a |
| Malvidin 3-(6′-p-coumaroyl)-glucoside | 520 | 22.74 ± 0.27 b | 27.98 ± 0.06 c | 18.92 ± 0.40 a |
| Vitisin B Malvidin 3-O-glucoside | 520 | 0.98 ± 0.03 a | 1.60 ± 0.06 b | 1.10 ± 0.10 a |
| Σ Anthocyanins | 253.63 ± 2.65 b | 336.34 ± 2.75 c | 218.73 ± 1.62 a | |
| Σ Phenolic compounds (mg/L) | 352.20 ± 2.83 b | 412.42 ± 2.81 c | 295.52 ± 2.48 a |
| m/z a | Odour Threshold (μg/L) | C | E | E + S | ||||
|---|---|---|---|---|---|---|---|---|
| µg/L | OAV b | µg/L | OAV b | µg/L | OAV b | |||
| Acids | ||||||||
| Decanoic acid | 60 | 1000 c | 257.76 ± 0.33 a | 0.26 | 475.79 ± 37.60 b | 0.48 | 336.61 ± 24.90 a | 0.34 |
| Hexanoic acid | 60 | 420 c | 2868.58 ± 5.15 a | 6.83 | 4957.91 ± 285.64 b | 11.80 | 3094.69 ± 246.07 a | 7.37 |
| Octanoic acid | 60 | 500 c | 1348.67 ± 12.48 a | 2.70 | 2673.33 ± 176.03 b | 5.35 | 1814.57 ± 113.77 a | 3.63 |
| Σ Acids | 4475.01 ± 17.96 a | 8107.02 ± 499.26 b | 5245.87 ± 384.74 a | |||||
| Alcohols | ||||||||
| Benzyl alcohol | 108 | 200,000 d | 337.08 ± 8.99 b | 0.00 | 216.71 ± 14.48 a | 0.00 | 242.08 ± 4.00 a | 0.00 |
| 1-Hexanol | 56 | 8000 e | 438.94 ± 4.68 ab | 0.05 | 476.30 ± 18.02 b | 0.06 | 411.91 ± 14.32 a | 0.05 |
| 2+3-Methyl-1-butanol h | 55 | 30,000 e | 233,096.39 ± 1509.55 b | 7.77 | 217,017.61 ± 3164.82 a | 7.23 | 211,292.30 ± 4670.82 a | 7.04 |
| 2-Phenylethanol | 91 | 10,000 e | 39,573.03 ± 259.86 a | 3.96 | 34,542.86 ± 1799.27 a | 3.45 | 35,316.35 ± 1141.74 a | 3.53 |
| Σ Alcohols | 273,445.45 ± 1254.00 b | 252,253.48 ± 4996.58 a | 247,262.64 ± 3539.40 a | |||||
| Acetates | ||||||||
| Ethyl acetate | 43 | 7500 e | 45,936.76 ± 2241.54 a | 6.12 | 62,219.02 ± 711.10 b | 8.30 | 43,095.80 ± 748.64 a | 5.75 |
| Hexyl acetate | 43 | 1500 d | 0.91 ± 0.04 a | 0.00 | 3.13 ± 0.18 c | 0.00 | 1.40 ± 0.04 b | 0.00 |
| Isoamyl acetate | 43 | 30 e | 587.65 ± 9.96 a | 19.59 | 1499.37 ± 102.70 c | 49.98 | 865.31 ± 30.41 b | 28.84 |
| Linalyl acetate | 93 | not found | 0.10 ± 0.01 a | - | 0.10 ± 0.02 a | - | 0.08 ± 0.00 a | - |
| 2-Phenylethyl acetate | 104 | 250 e | 14.69 ± 0.18 a | 0.06 | 30.29 ± 2.85 b | 0.12 | 17.71 ± 1.31 a | 0.07 |
| Σ Acetates | 46,540.10 ± 2251.65 a | 63,751.90 ± 816.85 b | 43,980.29 ± 716.88 a | |||||
| Ethyl esters | ||||||||
| Diethyl succinate | 101 | 200,000 d | 70.70 ± 0.54 a | 0.00 | 108.09 ± 9.01 b | 0.00 | 71.04 ± 4.10 a | 0.00 |
| Ethyl butyrate | 88 | 20 e | 99.41 ± 4.47 a | 4.97 | 161.79 ± 12.42 b | 8.09 | 106.81 ± 4.35 a | 5.34 |
| Ethyl decanoate | 43 | 200 c | 63.08 ± 9.72 a | 0.32 | 158.21 ± 21.87 b | 0.79 | 84.22 ± 6.77 a | 0.42 |
| Ethyl dihydrocinnamate | 104 | 1.6 c | 0.28 ± 0.01 a | 0.17 | 0.34 ± 0.03 a | 0.21 | 0.29 ± 0.02 a | 0.18 |
| Ethyl hexanoate | 101 | 14 c | 232.78 ± 8.78 a | 16.63 | 482.82 ± 46.77 b | 34.49 | 291.20 ± 14.98 a | 20.80 |
| Ethyl lactate | 45 | 154,000 d | 22,564.47 ± 880.50 b | 0.15 | 19,476.92 ± 484.64 a | 0.13 | 17,860.84 ± 136.98 a | 0.12 |
| Ethyl octanoate | 101 | 5 c | 220.22 ± 25.36 a | 44.04 | 499.95 ± 64.91 b | 99.99 | 285.03 ± 21.06 a | 57.01 |
| Ethyl vanillate | 151 | 990 f | 53.42 ± 0.86 a | 0.05 | 43.07 ± 6.01 a | 0.04 | 39.71 ± 5.46 a | 0.04 |
| Σ Ethyl esters | 23,304.36 ± 928.48 b | 20,931.19 ± 645.65 ab | 18,739.13 ± 193.72 a | |||||
| Terpenoids | ||||||||
| Citronellol | 69 | 100 d | 8.44 ± 0.10 b | 0.08 | 6.61 ± 0.24 a | 0.07 | 8.45 ± 0.36 b | 0.08 |
| Farnesol | 69 | 1000 g | 24.97 ± 3.10 a | 0.02 | 23.69 ± 4.35 a | 0.02 | 19.68 ± 2.30 a | 0.02 |
| Geraniol | 69 | 30 e | 8.49 ± 0.24 a | 0.28 | 9.56 ± 0.97 a | 0.32 | 9.21 ± 0.27 a | 0.31 |
| Geranyl acetone | 43 | 60 e | 0.07 ± 0.01 a | 0.00 | 0.12 ± 0.01 b | 0.00 | 0.06 ± 0.01 a | 0.00 |
| β-Ionone | 177 | 0.09 c | 0.02 ± 0.00 a | 0.27 | 0.03 ± 0.00 a | 0.31 | 0.02 ± 0.00 a | 0.25 |
| Linalool | 71 | 25 c | 1.38 ± 0.12 a | 0.06 | 1.95 ± 0.07 b | 0.08 | 1.83 ± 0.07 b | 0.07 |
| Nerol | 69 | 15 d | 3.71 ± 0.14 a | 0.25 | 3.96 ± 0.35 a | 0.26 | 4.47 ± 0.04 a | 0.30 |
| Nerolidol | 69 | 15 d | 1.39 ± 0.18 a | 0.09 | 2.05 ± 0.27 a | 0.14 | 1.88 ± 0.15 a | 0.13 |
| Σ Terpenoids | 48.47 ± 2.93 a | 47.98 ± 6.27 a | 45.61 ± 3.20 a | |||||
| Volatile phenols | ||||||||
| Eugenol | 164 | 6 c | 1.11 ± 0.02 a | 0.18 | 1.11 ± 0.17 a | 0.19 | 1.22 ± 0.06 a | 0.20 |
| Guaiacol | 109 | 9.5 c | 69.53 ± 12.84 a | 7.32 | 89.01 ± 25.10 a | 9.37 | 71.75 ± 1.15 a | 7.55 |
| Σ Volatile phenols | 70.64 ± 12.86 a | 90.12 ± 25.27 a | 72.97 ± 1.09 a | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campayo, A.; Cebrián-Tarancón, C.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L.; Serrano de la Hoz, K. Preliminary Studies on Endotherapy Based Application of Ozonated Water to Bobal Grapevines: Effect on Wine Quality. Molecules 2022, 27, 5155. https://doi.org/10.3390/molecules27165155
Campayo A, Cebrián-Tarancón C, García-Martínez MM, Salinas MR, Alonso GL, Serrano de la Hoz K. Preliminary Studies on Endotherapy Based Application of Ozonated Water to Bobal Grapevines: Effect on Wine Quality. Molecules. 2022; 27(16):5155. https://doi.org/10.3390/molecules27165155
Chicago/Turabian StyleCampayo, Ana, Cristina Cebrián-Tarancón, María Mercedes García-Martínez, María Rosario Salinas, Gonzalo L. Alonso, and Kortes Serrano de la Hoz. 2022. "Preliminary Studies on Endotherapy Based Application of Ozonated Water to Bobal Grapevines: Effect on Wine Quality" Molecules 27, no. 16: 5155. https://doi.org/10.3390/molecules27165155
APA StyleCampayo, A., Cebrián-Tarancón, C., García-Martínez, M. M., Salinas, M. R., Alonso, G. L., & Serrano de la Hoz, K. (2022). Preliminary Studies on Endotherapy Based Application of Ozonated Water to Bobal Grapevines: Effect on Wine Quality. Molecules, 27(16), 5155. https://doi.org/10.3390/molecules27165155






