Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems
Abstract
:Highlights
- 1.
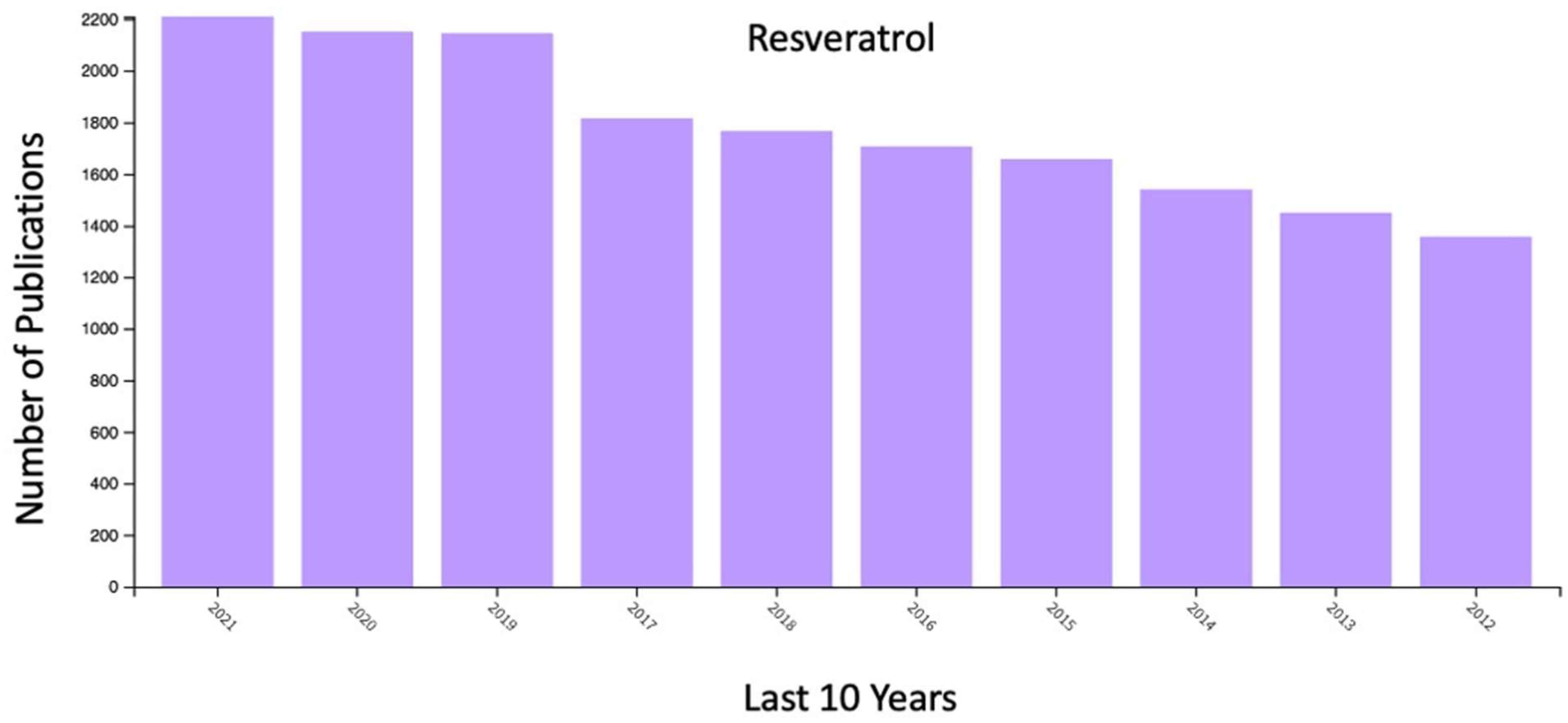
- Resveratrol is an antioxidant and exhibits numerous potential therapeutic applications. However, it suffers from low water-solubility, degradation, and poor bioavailability. The conventional dosage form of resveratrol shows various limitations, such as prolonged therapy, erratic bioavailability, and absence of effective drug concentrations in tissues.
- 2.
- Nanocarrier-based delivery systems are being studied extensively to target tissues and cells to improve the therapeutic potential of poorly soluble molecules by enhancing their bioavaila-bility, solubility, and retention time.
- 3.
- Resveratrol can act as a potential anti-cancer agent and lowers the progression of cancer disease.
Abstract
1. Introduction
2. Natural Polyphenols
Classification of Polyphenols
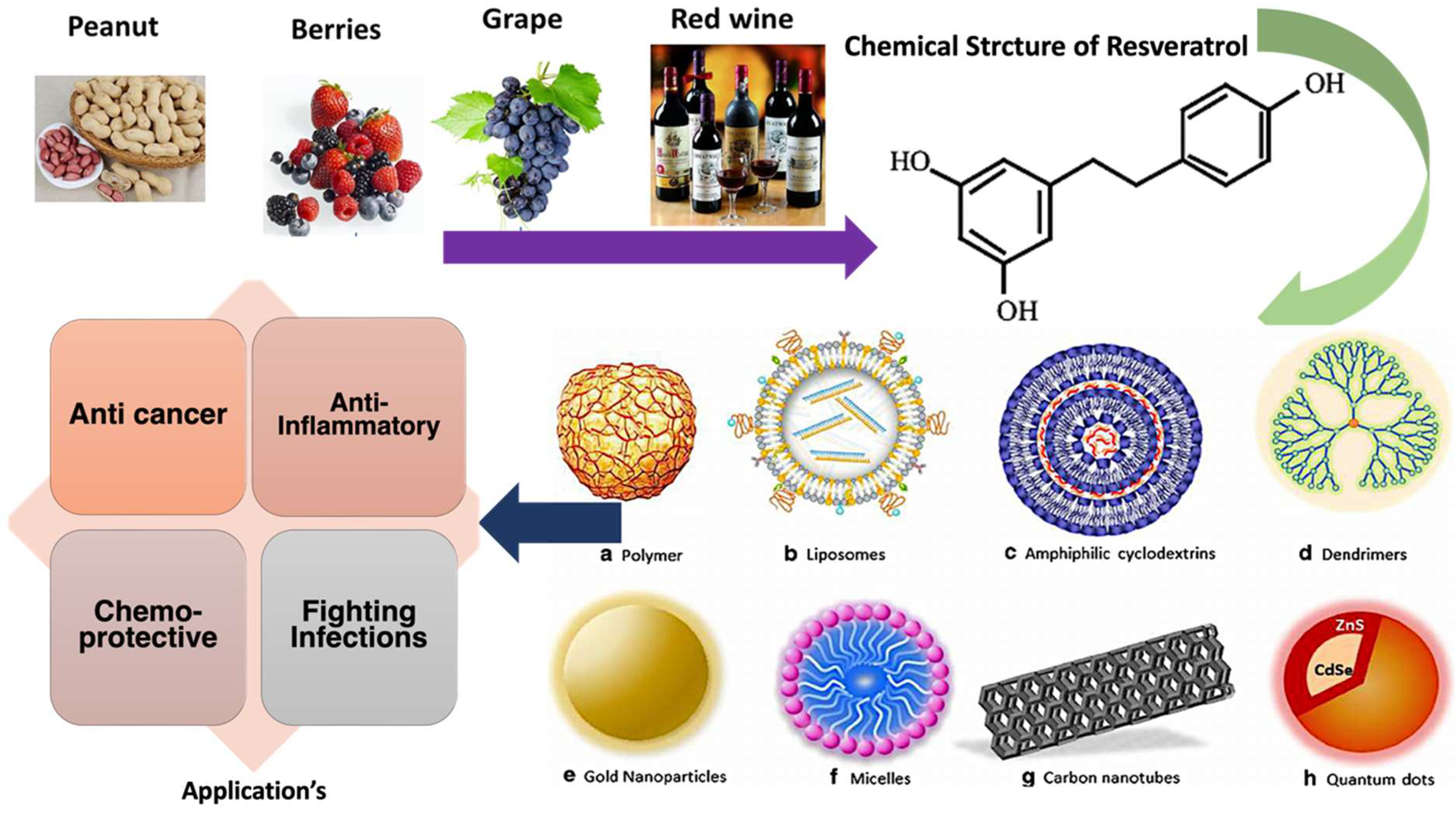
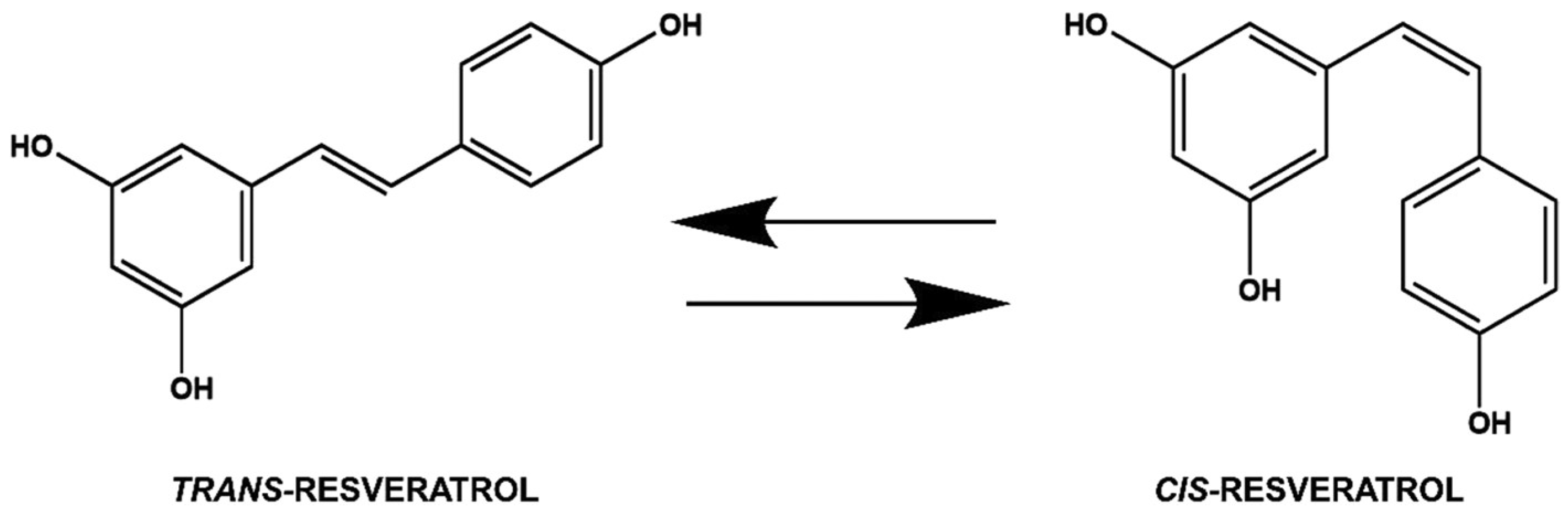
3. Chemistry of Resveratrol
4. Designing of Nanocarriers for Resveratrol Delivery
5. Prophylactic and Therapeutic Applications of Resveratrol
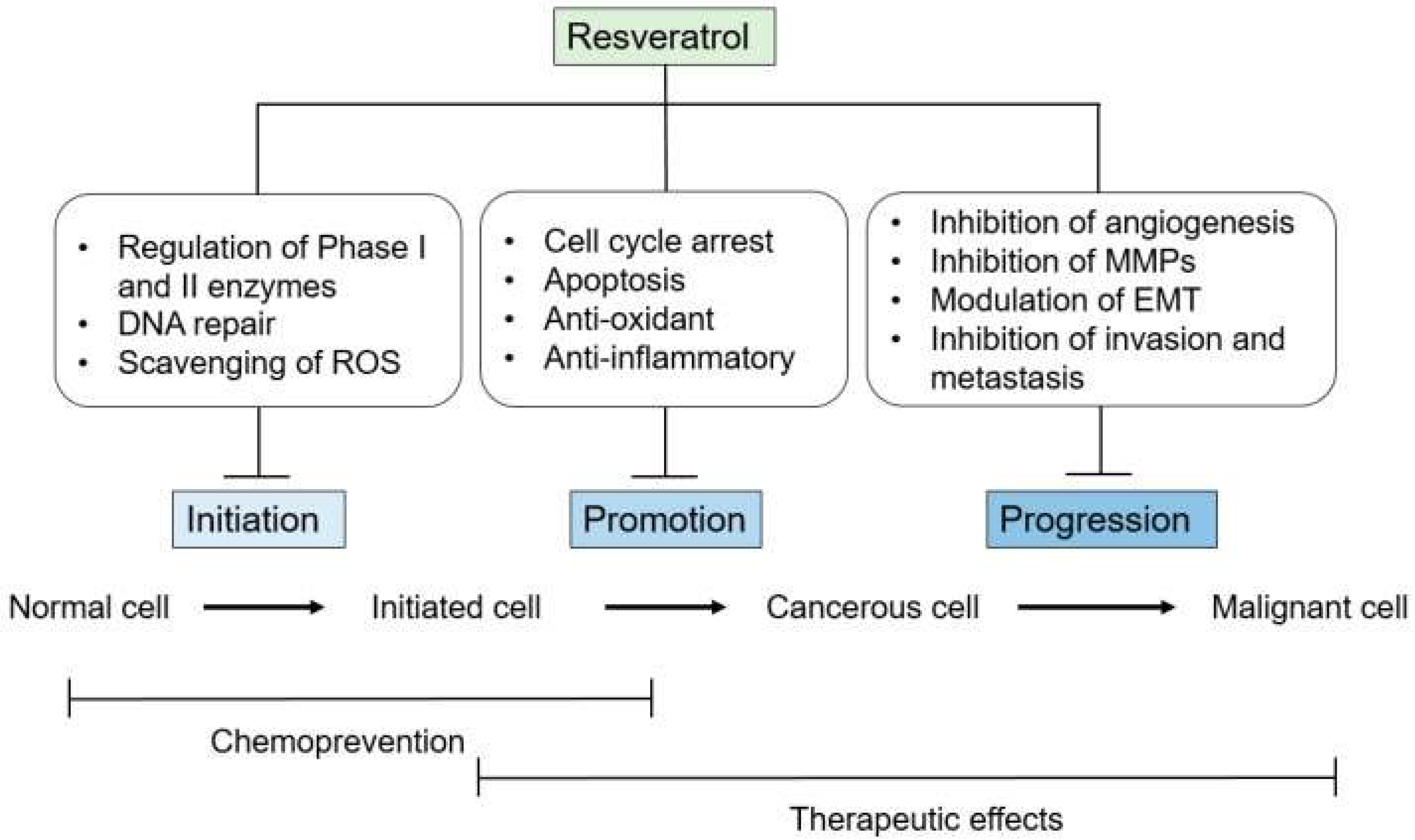
5.1. Resveratrol in Cancer Prevention and Treatment
5.2. Resveratrol in Wound Healing
5.3. Resveratrol as Cardioprotective Agent
5.4. Resveratrol in Fighting Infections
6. Conclusions
7. Current Limitations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, R.A.; Bivona, T.G. Recent advances in personalized lung cancer medicine. Pers. Med. 2014, 11, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Krepler, C.; Xiao, M.; Sproesser, K.; Brafford, P.A.; Shannan, B.; Beqiri, M.; Liu, Q.; Xu, W.; Garman, B.; Nathanson, K.L.; et al. Personalized Preclinical Trials in BRAF Inhibitor-Resistant Patient-Derived Xenograft Models Identify Second-Line Combination Therapies. Clin. Cancer Res. 2016, 22, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of Cancer. Science 1997, 278, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between Cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.Z.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the intricate relationship between chronic inflammation and Cancer. Biochem. J. 2015, 468, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the NF-κB pathway. Trends Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Madka, V.; Kumar, G.; Rao, C.V. Prevention and treatment of cancers by immune modulating nutrients. Mol. Nutr. Food Res. 2016, 60, 1275–1294. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Stutzman, J.D.; Kelloff, G.J.; Steele, V.E. Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res. 1994, 54, 5848–5855. [Google Scholar] [PubMed]
- Martinez, J.; Moreno, J.J. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem. Pharmacol. 2000, 59, 865–870. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chang, E.J.; Bae, S.J.; Shim, S.M.; Park, H.D.; Rhee, C.H.; Park, J.H.; Choi, S.W. Cytotoxic and antimutagenic stilbenes from seeds of Paeonia lactiflora. Arch. Pharm. Res. 2002, 25, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, A.; Ardito, R.; Faraglia, B.; Boninsegna, A.; Wolf, F.I.; Cittadini, A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat. Res. 2001, 496, 171–180. [Google Scholar] [CrossRef]
- Attia, S.M. Influence of resveratrol on oxidative damage in genomic DNA and apoptosis induced by cisplatin. Mutat. Res. 2012, 741, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Wink, M. Plant secondary metabolites modulate insect behavior-steps toward addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Laganà, P.; Anastasi, G.; Marano, F.; Piccione, S.; Singla, R.K.; Dubey, A.K.; Delia, S.; Coniglio, M.A.; Facciolà, A.; Di Pietro, A.; et al. Phenolic Substances in Foods: Health Effects as Anti-Inflammatory and Antimicrobial Agents. J. AOAC Int. 2019, 102, 1378–1387. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharma. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Evans, M.; Sharma, P.; Guthrie, N. Bioavailability of citrus polymethoxylated flavones and their biological role in metabolic syndrome and hyperlipidemia. In Readings in Advanced Pharmacokinetics-Theory, Methods and Applications; Intech Open: London, UK, 2012. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic potential of dietary phenolic acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012, 7, 1841. [Google Scholar] [CrossRef]
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Risuleo, G. Resveratrol: Multiple activities on the biological functionality of the cell. In Nutraceuticals; Academic Press: Cambridge, MA, USA, 2016; pp. 453–464. [Google Scholar] [CrossRef]
- Neubert, R.H.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef]
- How, C.W.; Rasedee, A.; Manickam, S.; Rosli, R. Tamoxifen-loaded nanostructured lipid carrier as a drug delivery system: Characterization, stability assessment and cytotoxicity. Colloids Surf. B. Biointerfaces 2013, 112, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Qureshi, O.S.; Kim, H.S.; Kim, M.S.; Kang, J.H.; Park, J.S.; Kim, J.K. High payload itraconazole-incorporated lipid nanoparticles with modulated release property for oral and parenteral administration. J. Pharm. Pharmacol. 2017, 69, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, D.; Dumoga, S.; Kumar, R.; Chuttani, K.; Mishra, A.K. PEGylated solid lipid nanoparticles: Design, methotrexate loading and biological evaluation in animal models. Med. Chem. Comm. 2015, 6, 1452–1463. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Kim, H.S.; Zeb, A.; Choi, J.S.; Kim, H.S.; Kwon, J.E.; Kim, M.S.; Kang, J.H.; Ryou, C.; Park, J.S.; et al. Sustained release docetaxel-incorporated lipid nanoparticles with improved pharmacokinetics for oral and parenteral administration. J. Microencapsul. 2017, 34, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Basu, S.; Sandanaraj, B.S.; Thayumanavan, S. Molecular recognition in dendrimers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Lai, P.S.; Lou, P.J.; Peng, C.L.; Pai, C.L.; Yen, W.N.; Huang, M.Y.; Young, T.H.; Shieh, M.J. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control. Release. 2007, 122, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, S.; Zhang, L.; Wu, X.; Wang, J.; Chen, J.F.; Le, Y. Poly (lactic acid)/chitosan hybrid nanoparticles for controlled release of anticancer drug. Mater Sci. Eng. C. 2015, 46, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepa, N.; Chennazhi, K.P.; Lakshmanan, V.K.; Jayakumar, R. Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim. Biophys. Acta 2014, 1840, 2730–2743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liao, L. Applications of nanoparticles for anticancer drug delivery: A review. J. Nanosci. Nanotechnol. 2015, 15, 4753–4773. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anticancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric micelles: Recent advancements in the delivery of anticancer drugs. Pharm. Res. 2016, 33, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fang, Z.; Yao, L.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. A micelle-like structure of poloxamer–methotrexate conjugates as nanocarrier for methotrexate delivery. Int. J. Pharma. 2015, 487, 177–186. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Zhou, W.; Zhang, X.; Li, F. Self-assembly, biosynthesis, functionalization and applications of virus-based nanomaterials. Synth. Biol. J. 2020, 1, 298. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Del. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M.; Loh, H.S.; Muthoosamy, K.; Sridewi, N.; Manickam, S. Conjugation of insulin onto the sidewalls of single-walled carbon nanotubes through functionalization and diimide-activated amidation. Int. J. Nanomed. 2016, 11, 1607. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361. [Google Scholar] [CrossRef]
- Rout, G.K.; Shin, H.S.; Gouda, S.; Sahoo, S.; Das, G.; Fraceto, L.F.; Patra, J.K. Current advances in nanocarriers for biomedical research and their applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1053–1062. [Google Scholar] [CrossRef]
- Prabhakar, N.; Zhang, J.; Desai, D.; Casals, E.; Gulin-Sarfraz, T.; Näreoja, T.; Westermarck, J.; Rosenholm, J.M. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery. Int. J. Nanomed. 2016, 11, 6591. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Zhang, J.; Sandholm, J.; Lehtimäki, J.; Grönroos, T.; Tuomela, J.; Rosenholm, J.M. Lipid bilayer-gated mesoporous silica nanocarriers for tumor-targeted delivery of zoledronic acid in vivo. Mol. Pharm. 2017, 14, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Zhao, Q.; Wan, L.; Wang, Y.; Gao, Y.; Wang, P.; Wang, Z.; Zhang, J.; Jiang, T.; Wang, S. Hybrid lipid-capped mesoporous silica for stimuli-responsive drug release and overcoming multidrug resistance. ACS Appl. Mater. Interfaces 2015, 7, 3342–3351. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef] [PubMed]
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and pharmacodynamic evaluation of resveratrol loaded cationic liposomes for targeting hepatocellular carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimer nanotechnology for enhanced formulation and controlled delivery of resveratrol. Ann. N. Y. Acad. Sci. 2015, 1384, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.R.; Kumari, L.; Patel, K.K.; Vuddanda, P.R.; Vajanthri, K.Y.; Mahto, S.K.; Singh, S. Intravenous administration of trans-resveratrol-loaded TPGS-coated solid lipid nanoparticles for prolonged systemic circulation, passive brain targeting and improved in vitro cytotoxicity against C6 glioma cell lines. RSC Adv. 2016, 6, 50336–50348. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, L.; Quan, Y.; Wei, K. Resveratrol-loaded PLGA nanoparticles: Enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. Open Sci. 2018, 5, 181457. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R. Patchouli (Pogostemon cablin Benth.): Botany, agrotechnology and biotechnological aspects. Ind. Crops Prod. 2016, 87, 161–176. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Swamy, M.K.; Sinniah, U.R.; Anuradha, M. Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): Botanical, agronomical, phytochemical, pharmacological, and biotechnological aspects. Molecules 2017, 22, 1019. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Iram, F.; Siddiqui, S.; Sahu, K. Role of natural products in drug discovery process. Int. J. Drug Dev. Res. 2014, 6, 172–204. [Google Scholar]
- Beutler, J.A. Natural products as a foundation for drug discovery. Curr. Prot. Pharmacol. 2009, 46, 9–11. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; da Silva, P.B.; dos Santos Ramos, M.A.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar] [CrossRef]
- Perez, A.T.; Domenech, G.H.; Frankel, C.; Vogel, C.L. Pegylated liposomal doxorubicin (Doxil®) for metastatic breast cancer: The Cancer Research Network, Inc., experience. Cancer Investig. 2002, 20, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.M.; Chang-Liao, W.L.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Effects of polymer molecular weight on relative oral bioavailability of curcumin. Int. J. Nanomed. 2012, 7, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery, of anti-inflammatory nutraceuticals by nanoparticles for the prevention and treatment of Cancer. Biochem. Pharmacol. 2010, 80, 1833–1843. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7, 12-dimethylbenz (a) anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar]
- Aggarwal, B.B.; Takada, Y.; Oommen, O.V. From chemoprevention to chemotherapy: Common targets and common goals. Expert Opin. Investig. Drugs 2004, 13, 1327–1338. [Google Scholar] [CrossRef]
- Nguyen, C.; Savouret, J.F.; Widerak, M.; Corvol, M.T.; Rannou, F. Resveratrol, potential therapeutic interest in joint disorders: A critical narrative review. Nutrients 2017, 9, 45. [Google Scholar] [CrossRef]
- Zhang, J.; Song, X.; Cao, W.; Lu, J.; Wang, X.; Wang, G.; Wang, Z.; Chen, X. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. Sci. Rep. 2016, 6, 32928. [Google Scholar] [CrossRef]
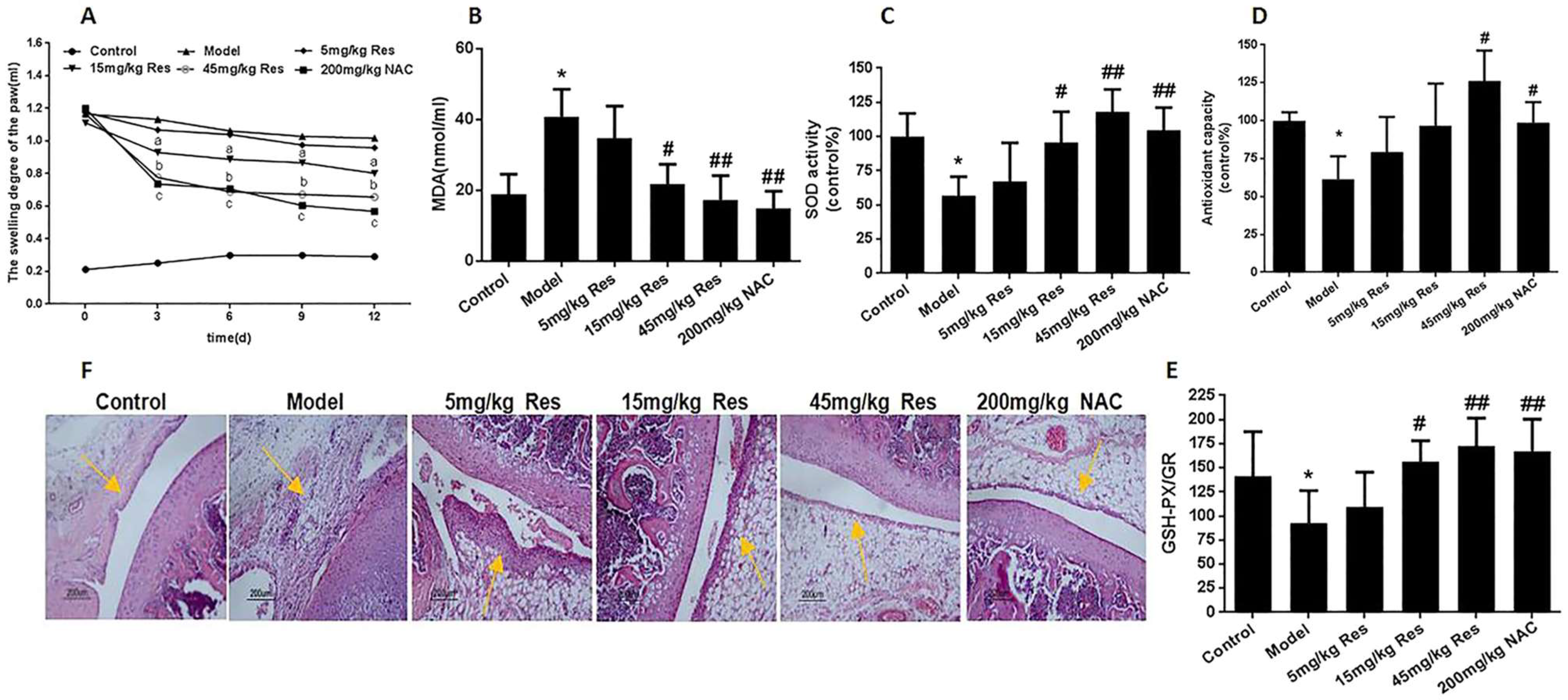
- Bhattarai, G.; Poudel, S.B.; Kook, S.-H.; Lee, J.-G. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 21, 398–408. [Google Scholar] [CrossRef]
- Wang, Z.M.; Chen, Y.C.; Wang, D.P. Resveratrol, a natural antioxidant, protects monosodium iodoacetate- induced osteoarthritic pain in rats. Biomed. Pharmacother. 2016, 83, 763–770. [Google Scholar] [CrossRef]
- Wu, G.; Wang, L.; Li, H.; Ke, Y.; Yao, Y. Function of sustained released resveratrol on IL-1β-induced hBMSC MMP13 secretion inhibition and chondrogenic differentiation promotion. J. Biomater. Appl. 2016, 30, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Torigoe, T.; Matsumoto, Y.; Fujita, T.; Sato, N.; Yotsuyanagi, T. Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair Regen. 2013, 21, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Lulli, D.; Fidanza, P.; Potapovich, A.I.; Kostyuk, V.; De Luca, C.; Mikhal’Chik, E.; Korkina, L.G. Plant Polyphenols Regulate Chemokine Expression and Tissue Repair in Human Keratinocytes Through Interaction with Cytoplasmic and Nuclear Components of Epidermal Growth Factor Receptor System. Antioxid. Redox Signal. 2012, 16, 314–328. [Google Scholar] [CrossRef] [PubMed]
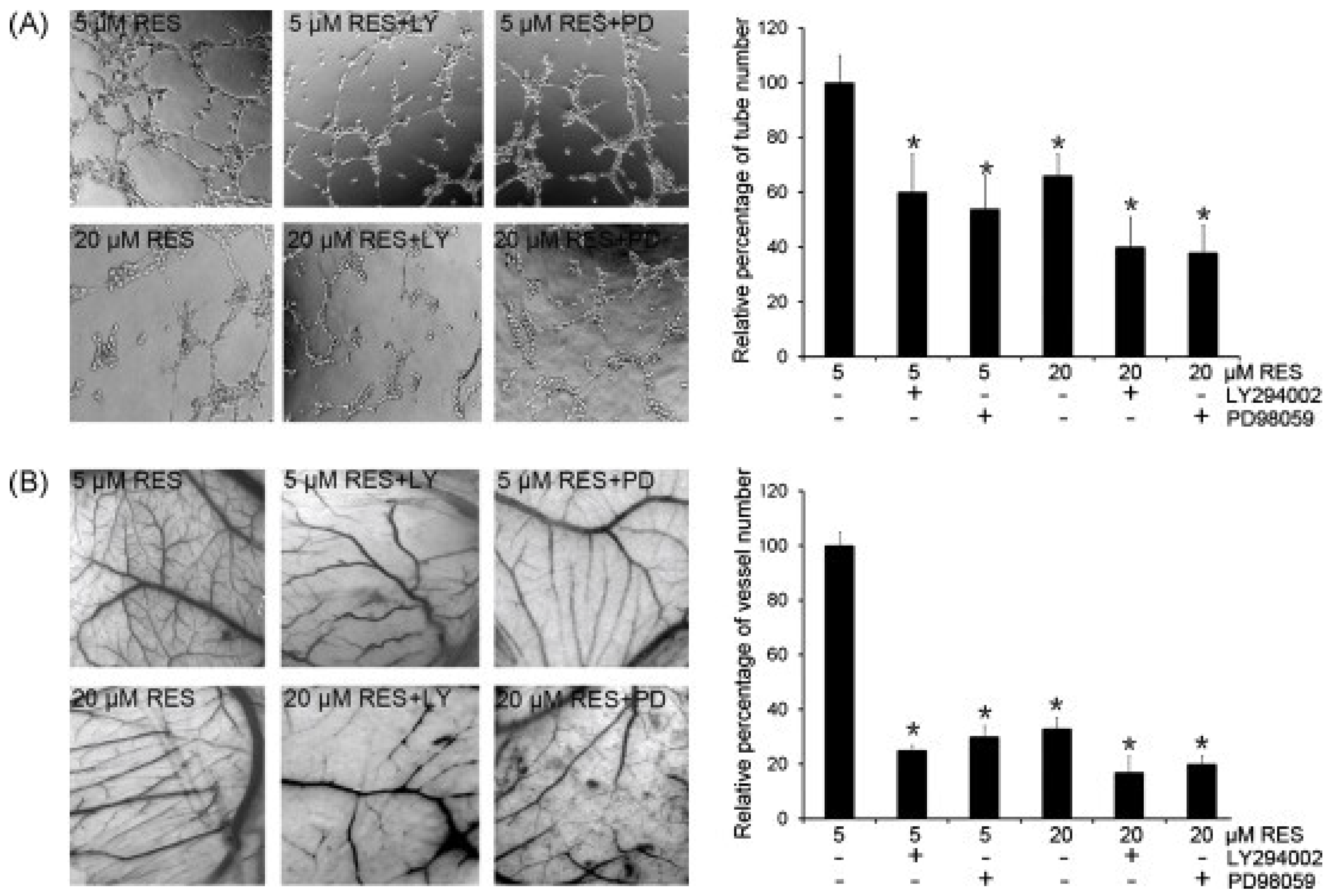
- Wang, H.; Zhou, H.; Zou, Y.; Liu, Q.; Guo, C.; Gao, G.; Shao, C.; Gong, Y. Resveratrol modulates angiogenesis through the GSK3β/β-catenin/TCF-dependent pathway in human endothelial cells. Biochem. Pharmacol. 2010, 80, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Han, X.; Wu, Z.; Shi, Q.; Bao, X. Rosiglitazone accelerates wound healing by improving endothelial precursor cell function and angiogenesis in db/db mice. PeerJ 2019, 7, e7815. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.H.; Tanrıverdi, S.T.; Eroglu, I.; Tsapis, N.; Gokce, G.; Tekmen, I.; Fattal, E.; Ozer, O. Wound healing effects of collagen-laminin dermal matrix impregnated with resveratrol loaded hyaluronic acid-DPPC microparticles in diabetic rats. Eur. J. Pharm. Biopharm. 2017, 119, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yurdagul, A.; Kleinedler, J.J.; McInnis, M.C.; Khandelwal, A.R.; Spence, A.L.; Orr, A.W.; Dugas, T.R. Resveratrol promotes endothelial cell wound healing under laminar shear stress through an estrogen receptor-α-dependent pathway. Am. J. Physiol. Circ. Physiol. 2014, 306, H797–H806. [Google Scholar] [CrossRef]
- Yaman, I.; Derici, H.; Kara, C.; Kamer, E.; Diniz, G.; Ortac, R.; Sayin, O. Effects of resveratrol on incisional wound healing in rats. Surg. Today 2013, 43, 1433–1438. [Google Scholar] [CrossRef]
- Brem, H.; Kodra, A.; Golinko, M.S.; Entero, H.; Stojadinovic, O.; Wang, V.M.; Sheahan, C.M.; Weinberg, A.D.; Woo, S.L.; Ehrlich, H.P.; et al. Mechanism of Sustained Release of Vascular Endothelial Growth Factor in Accelerating Experimental Diabetic Healing. J. Investig. Dermatol. 2009, 129, 2275–2287. [Google Scholar] [CrossRef]
- Çetinkalp, Ş.; Gökçe, E.H.; Şimşir, I.; Tanrıverdi, S.T.; Doğan, F.; Avcı, Ç.B.; Eroğlu, I.; Utku, T.; Gündüz, C.; Özer, Ö. Comparative Evaluation of Clinical Efficacy and Safety of Collagen Laminin–Based Dermal Matrix Combined with Resveratrol Microparticles (Dermalix) and Standard Wound Care for Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2021, 20, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yager, D.R.; Zhang, L.-Y.; Liang, H.-X.; Diegelmann, R.F.; Cohen, I.K. Wound Fluids from Human Pressure Ulcers Contain Elevated Matrix Metalloproteinase Levels and Activity Compared to Surgical Wound Fluids. J. Investig. Dermatol. 1996, 107, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Tarnuzzer, R.W.; Schultz, G.S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996, 4, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-Mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfand-yari, S.; Sobhani, A. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J. Reprod. Immunol. 2021, 143, 103248. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti- Inflammatory and Antioxidant Effects of Resveratrol in Healthy Smokers a Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomas-Barberan, F.; Conesa, M.T.G.; Espín, J.C. Grape Resveratrol Increases Serum Adiponectin and Downregulates Inflammatory Genes in Peripheral Blood Mononuclear Cells: A Triple-Blind, Placebo-Controlled, One-Year Clinical Trial in Patients with Stable Coronary Artery Disease. Cardiovasc. Drugs Ther. 2012, 27, 37–48. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Lin, L.X.; Wang, P.; Wang, Y.T.; Huang, Y.; Jiang, L.; Wang, X.M. Aloe Vera and Vitis vinifera improve wound healing in an in vitro rat burn wound model. Mol. Med. Rep. 2016, 13, 1070–1076. [Google Scholar] [CrossRef]
- Eroğlu, I.; Gökçe, E.H.; Tsapis, N.; Tanrıverdi, S.T.; Gökçe, G.; Fattal, E.; Özer, Ö. Evaluation of Characteristics and in vitro antioxidant properties of RSV loaded hyaluronic acid-DPPC microparticles as a wound healing system. Coll. Surf. B Biointerfaces 2015, 126, 50–57. [Google Scholar] [CrossRef]
- Kaleci, B.; Koyuturk, M. Efficacy of resveratrol in wound healing process by reducing oxiudative stress and promoting fibroblast cell proliferation and migration. Dermatol Ther. 2022, 33, e14357. [Google Scholar] [CrossRef]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhang, T.; Meng, Q.; Li, C.; You, B. Resveratrol Effects on a Diabetic Rat Model with Coronary Heart Disease. Med. Sci. Monit. 2019, 25, 540–546. [Google Scholar] [CrossRef]
- Abdelgawad, I.Y.; Grant, M.K.O.; Zordoky, B.N. Leveraging the Cardio-Protective and Anticancer Properties of Resveratrol in Cardio-Oncology. Nutrients 2019, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef]
- De Leo, A.; Arena, G.; Lacanna, E.; Oliviero, G.; Colavita, F.; Mattia, E. Resveratrol inhibits Epstein Barr Virus lytic cycle in Burkitt’s lymphoma cells by affecting multiple molecular targets. Antivir. Res. 2012, 96, 196–202. [Google Scholar] [CrossRef]
- Palomera-Ávalos, V.; Griñán-Ferré, C.; Izquierdo, V.; Camins, A.; Sanfeliu, C.; Canudas, A.M.; Pallàs, M. Resveratrol modulates response against acute inflammatory stimuli in aged mouse brain. Exp. Gerontol. 2018, 102, 3–11. [Google Scholar] [CrossRef]
- Shevelev, A.B.; Isakova, E.P.; Trubnikova, E.V.; La Porta, N.; Martens, S.; Medvedev, O.A.; Trubnikov, D.V.; Akbaev, R.M.; Biryukova, Y.K.; Zylkova, M.V.; et al. A study of antimicrobial activity of polyphenols derived from wood. Bull. Russ. State Med. Univ. 2018, 7, 46–49. [Google Scholar] [CrossRef]
- Shawon, J.; Akter, Z.; Hossen, M.M.; Akter, Y.; Sayeed, A.; Junaid, M.; Afrose, S.S.; Khan, M.A. Current Landscape of Natural Products against Coronaviruses: Perspectives in COVID-19 Treatment and Anti-viral Mechanism. Curr. Pharm. Des. 2020, 26, 5241–5260. [Google Scholar] [CrossRef]
- Gautam, S.; Gautam, A.; Chhetri, S.; Bhattarai, U. Immunity Against COVID-19: Potential Role of Ayush Kwath. J. Ayurveda Integr. Med. 2020, 13, 100350. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, J.; Song, X.; Jia, R.; Yin, Z.; Cheng, A.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; et al. Antiviral effect of resveratrol in ducklings infected with virulent duck enteritis virus. Antivir. Res. 2016, 130, 93–100. [Google Scholar] [CrossRef] [PubMed]
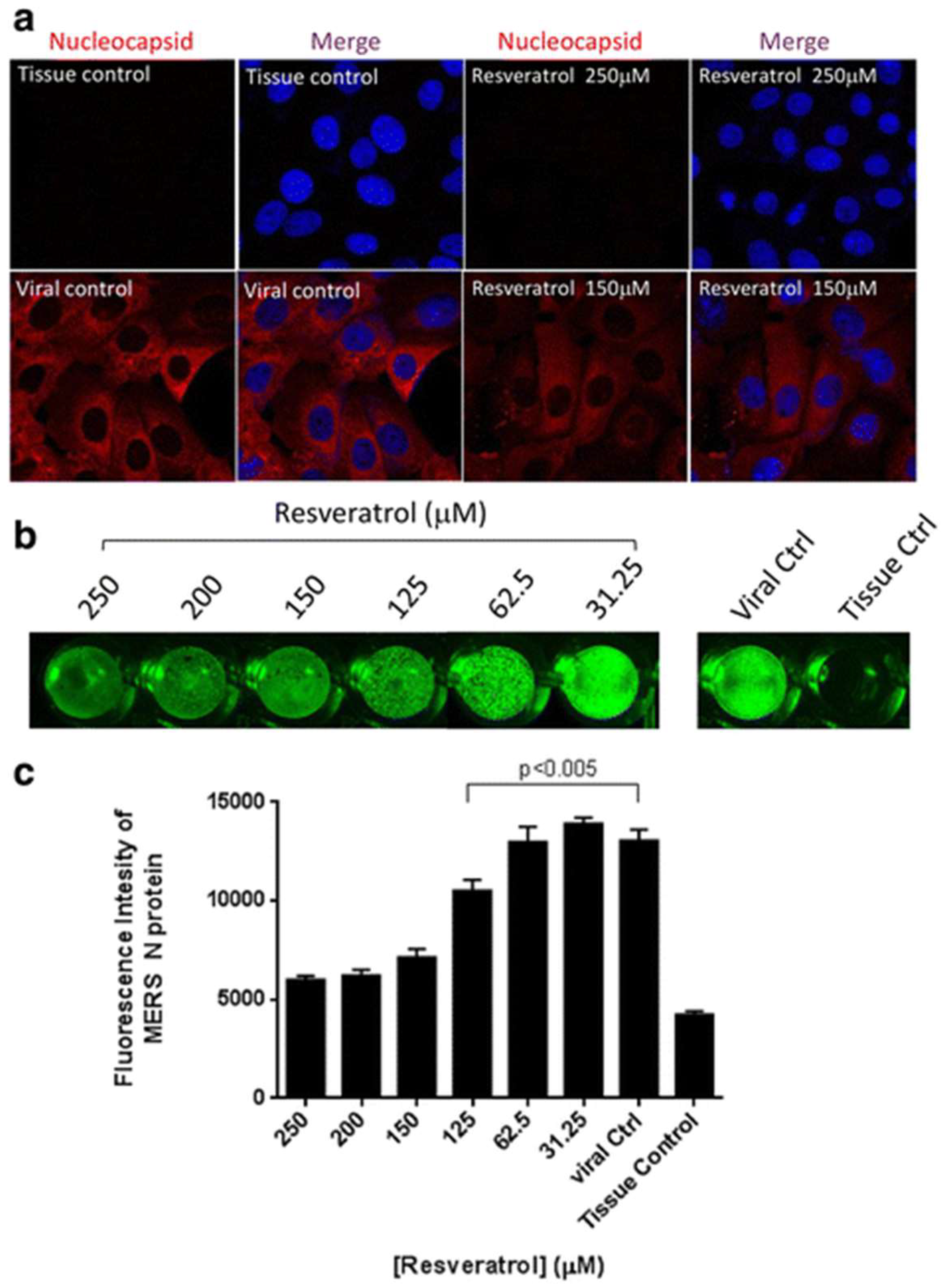
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [PubMed]
| Nano System | Effect | Key Properties | Reference |
|---|---|---|---|
| Liposomes | Antioxidant activity | Enhanced solubility and stability of curcumin and resveratrol. Minimum particle size, lower polydispersity index and high encapsulation efficiency | [62] |
| Anticancer activity | HepG2 cells exhibited a higher uptake of encapsulated-RES than the free form | [63] | |
| Dendrimers | Enhanced RES solubility and stability in aqueous solution. Dendrimers can be engineered to control pharmacokinetics and target for oral, mucosal, transdermal, or parenteral administration | (PAMAM) dendrimer assembly overcame the problems of low bioavailability and poor water solubility | [64] |
| Solid lipid nanoparticles | In vitro cytotoxicity against C6 glioma cell lines | The resveratrol-TPGS-SLNs showed 11.12 and 9.37-times higher area under the curve (AUC) and plasma half-life, respectively, than the unprocessed resveratrol. Additionally, the concentration of resveratrol-TPGS-SLNs in the brain was found to be 9.23-times higher compared to free resveratrol | [65] |
| Polymeric nanoparticles | Fatty liver disorder | The prepared poly(lactic-co-glycolic acid) (PLGA) nanoparticles containing resveratrol increased its stability and solubility, yielding better in vitro results as compared to free drug | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohara, R.A.; Tabassum, N.; Singh, M.P.; Gigli, G.; Ragusa, A.; Leporatti, S. Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules 2022, 27, 5154. https://doi.org/10.3390/molecules27165154
Bohara RA, Tabassum N, Singh MP, Gigli G, Ragusa A, Leporatti S. Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules. 2022; 27(16):5154. https://doi.org/10.3390/molecules27165154
Chicago/Turabian StyleBohara, Raghvendra A., Nazish Tabassum, Mohan P. Singh, Giuseppe Gigli, Andrea Ragusa, and Stefano Leporatti. 2022. "Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems" Molecules 27, no. 16: 5154. https://doi.org/10.3390/molecules27165154
APA StyleBohara, R. A., Tabassum, N., Singh, M. P., Gigli, G., Ragusa, A., & Leporatti, S. (2022). Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules, 27(16), 5154. https://doi.org/10.3390/molecules27165154










