Piceatannol Alleviates Clostridium perfringens Virulence by Inhibiting Perfringolysin O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Reagents
2.2. Pore-Forming Activity Inhibition Assays
2.3. Molecular Docking vs. PFO
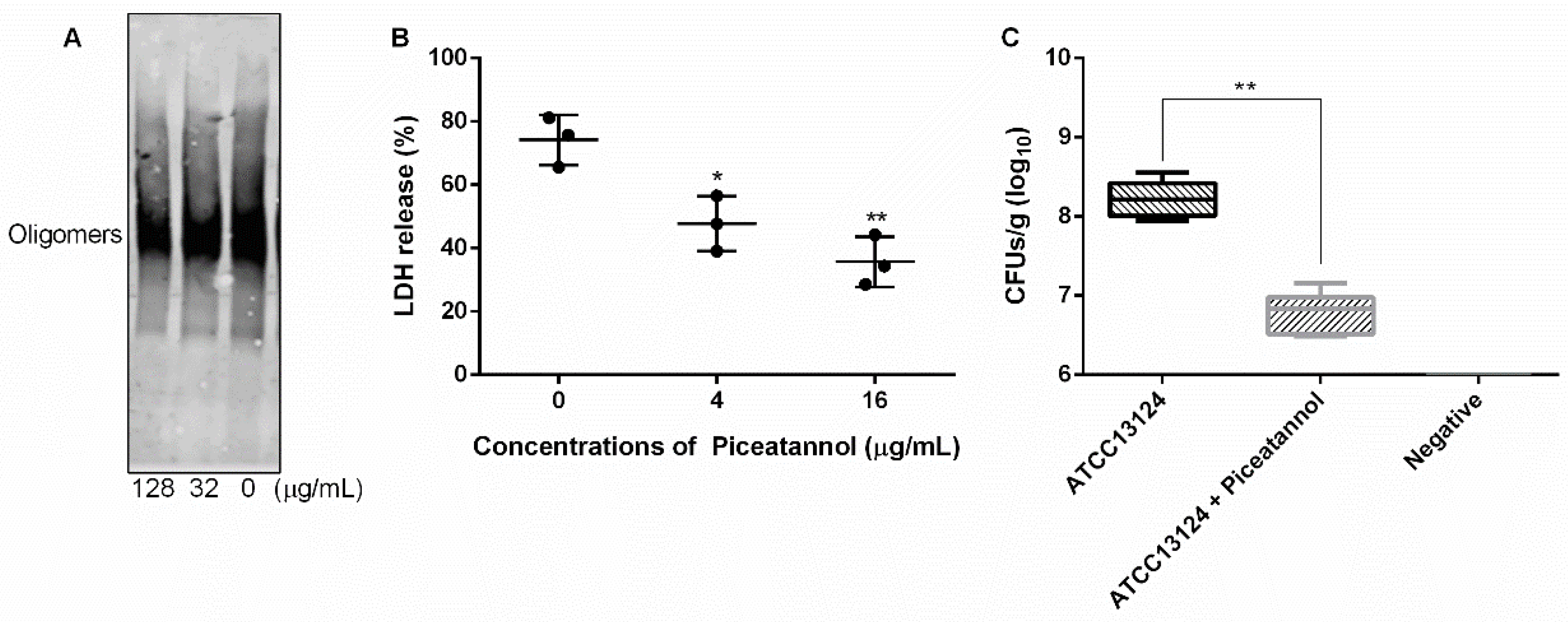
2.4. Oligomer Formation Inhibition Assays
2.5. Cytotoxicity Assays
2.6. Animal Infection
2.7. Statistical Analysis
3. Results
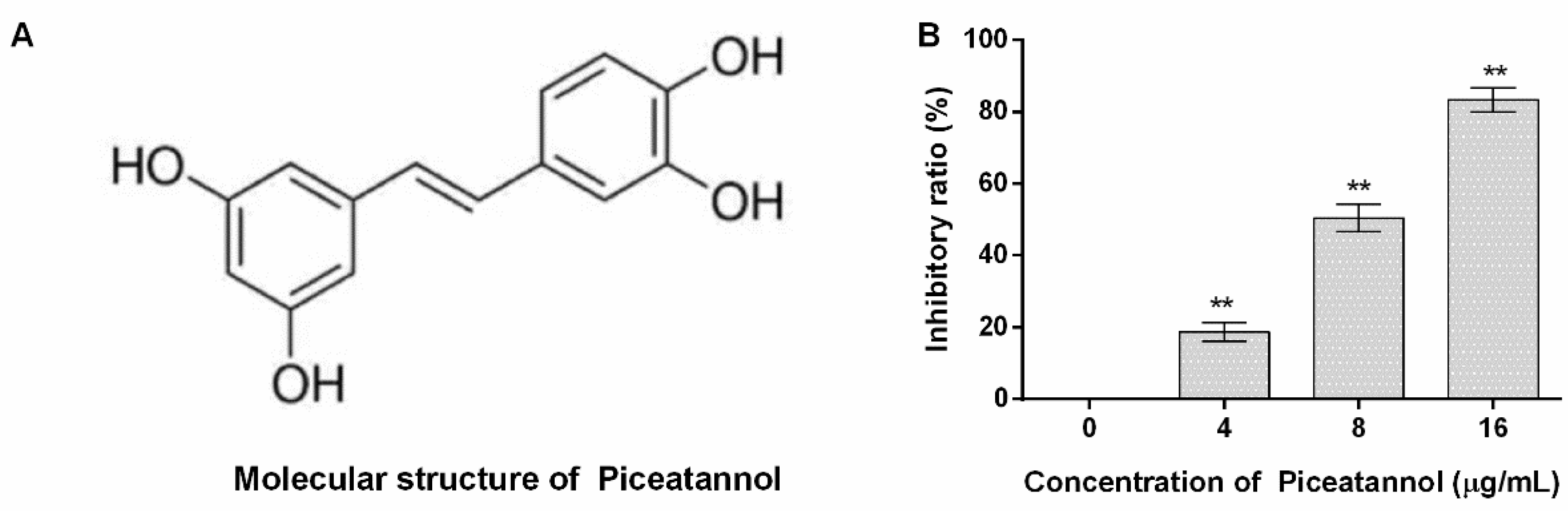
3.1. Piceatannol Inhibited the Pore-Forming Activity of PFO
3.2. Piceatannol Bound to the Pocket between Domain One and Domain Two of PFO
3.3. Interaction Energy Analysis
3.4. The Secondary Structure of PFO Changed after Binding with Piceatannol
3.5. Piceatannol Inhibited the Formation of PFO Oligomers
3.6. Piceatannol Alleviated the Cytotoxicity Mediated by PFO and Reduced Clones of C. perfringens in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Valeriani, R.G.; Beard, L.L.; Moller, A.; Ohtani, K.; Vidal, J.E. Gas gangrene-associated gliding motility is regulated by the Clostridium perfringens CpAL/VirSR system. Anaerobe 2020, 66, 102287. [Google Scholar] [CrossRef] [PubMed]
- Raymond, K.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar]
- Cooper, K.K.; Songer, J.G.; Uzal, F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Investig. 2013, 25, 314. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A.L. The true cost of necrotic enteritis. World Poult. 2015, 31, 16–17. [Google Scholar]
- Bryant, A.E.; Stevens, D.L. Clostridial Myonecrosis: New Insights in Pathogenesis and Management. Curr. Infect. Dis. Rep. 2010, 12, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Aldape, M.J.; Bryant, A.E. Life-threatening clostridial infections. Anaerobe 2012, 18, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Titball, R.W. Gas gangrene: An open and closed case. Microbiology 2005, 151, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bo, L.U.; Hao, P.; Yan, M.N.; Dai, K.R. Comprehensive treatment for gas gangrene of the limbs in earthquakes. Chin. Med. J. 2013, 126, 3833–3839. [Google Scholar] [PubMed]
- Ohtani, K.; Shimizu, T. Regulation of Toxin Production in Clostridium perfringens. Toxins 2016, 8, 207. [Google Scholar] [CrossRef]
- Uzal, F.A.; Vidal, J.E.; Mcclane, B.A.; Gurjar, A.A. Clostridium Perfringens Toxins Involved in Mammalian Veterinary Diseases. Open Toxinol. J. 2010, 2, 24. [Google Scholar] [CrossRef]
- Dang, T.X.; Hotze, E.M.; Rouiller, I.; Tweten, R.K.; Wilson-Kubalek, E.M. Prepore to pore transition of a cholesterol-dependent cytolysin visualized by electron microscopy. J. Struct. Biol. 2005, 150, 100–108. [Google Scholar] [CrossRef]
- Verherstraeten, S.; Goossens, E.; Valgaeren, B.; Pardon, B.; Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Deprez, P.; Wade, K.; Tweten, R. Perfringolysin O: The Underrated Clostridium perfringens Toxin? Toxins 2015, 7, 1702–1721. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.K.; Melville, S.B. Effects of Clostridium perfringens Alpha-Toxin (PLC) and Perfringolysin O (PFO) on Cytotoxicity to Macrophages, on Escape from the Phagosomes of Macrophages, and on Persistence of C. perfringens in Host Tissues. Infect. Immun. 2004, 72, 5204–5215. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.E.; Stevens, D.L. Clostridial toxins in the pathogenesis of gas gangrene. In The Comprehensive Sourcebook of Bacterial Protein Toxins, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 977–994. [Google Scholar]
- Hickey, M.J.; Kwan, R.; Awad, M.M.; Kennedy, C.L.; Young, L.F.; Cordner, L.M.; Lyras, D.; Emmins, J.J.; Rood, J.I. Molecular and Cellular Basis of Microvascular Perfusion Deficits Induced by Clostridium perfringens and Clostridium septicum. PloS Pathog. 2008, 4, e1000045. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Chou, D.; Hary, H.; Thakur, N.; Kunnumakkara, A.B. Piceatannol: A Natural Stilbene for the Prevention and Treatment of Cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef]
- Hosoda, R.; Hamada, H.; Uesugi, D.; Iwahara, N.; Kuno, A. Different Antioxidative and Antiapoptotic Effects of Piceatannol and Resveratrol. J. Pharmacol. Exp. Ther. 2020, 376, JPET-AR-2020-000096. [Google Scholar] [CrossRef]
- Sato, D.; Shimizu, N.; Shimizu, Y.; Akagi, M.; Eshita, Y.; Ozaki, S.I.; Nakajima, N.; Ishihara, K.; Masuoka, N.; Hamada, H. Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Biosci. Biotechnol. Biochem. 2014, 78, 1123–1128. [Google Scholar] [CrossRef]
- Duarte, N.; Kayser, O.; Abreu, P.; Ferreira, M.J.U. Antileishmanial activity of piceatannol isolated from Euphorbia lagascae seeds. Phytother. Res. 2010, 22, 455–457. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, L.; Liu, Y.; Xu, N.; Zhou, S.; Yang, Y.; Yang, Q.; Ai, X. Luteolin decreases the pathogenicity of Aeromonas hydrophila via inhibiting the activity of aerolysin. Virulence 2021, 12, 165–176. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Xu, N.; Yang, Q.; Ai, X. Morin Protects Channel Catfish from Aeromonas hydrophila Infection by Blocking Aerolysin Activity. Front. Microbiol. 2018, 9, 2828. [Google Scholar] [CrossRef]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2010, 23, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General AMBER Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Bikadi, Z.; Hazai, E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminform. 2009, 1, 15. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Ponder, J.W.; Case, D.A. Force fields for protein simulations. Adv. Protein Chem. 2003, 66, 27–85. [Google Scholar]
- Yang, W.Y.; Lee, Y.; Lu, H.; Chou, C.H.; Wang, C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS ONE 2019, 14, e0205784. [Google Scholar] [CrossRef]
- Joosten, R.P.; te Beek, T.A.H.; Krieger, E.; Hekkelman, M.L.; Hooft, R.W.W.; Schneider, R.; Sander, C.; Vriend, G. A series of PDB related databases for everyday needs. Nucleic Acids Res. 2010, 39, D411–D419. [Google Scholar] [CrossRef]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 2006, 22, 2577–2637. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2008, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, G.; Wang, X.; Yang, Y.; Niu, X. Structure-Activity Relationship of MDSA and Its Derivatives against Staphylococcus aureus Ser/Thr phosphatase Stp1. Comput. Biol. Chem. 2020, 85, 107230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.B.; Heuck, A.P. Perfringolysin O structure and mechanism of pore formation as a paradigm for cholesterol-dependent cytolysins. In MACPF/CDC Proteins—Agents of Defence, Attack and Invasion; Springer: Berlin/Heidelberg, Germany, 2014; pp. 63–81. [Google Scholar]
- Pokharel, S.; Shrestha, P.; Adhikari, B. Antimicrobial use in food animals and human health: Time to implement ‘One Health’ approach. Antimicrob. Resist. Infect. Control 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S.M. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, B.; Liu, S.; Wang, L.; Wang, J. Anticytotoxin Effects of Amentoflavone to Pneumolysin. Biol. Pharm. Bull. 2017, 40, 61. [Google Scholar] [CrossRef]
- Awad, M.M. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 2010, 15, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, X.; Zhang, H.; Zhang, J.; Wang, J. Amentoflavone Attenuates Clostridium perfringens Gas Gangrene by Targeting Alpha-Toxin and Perfringolysin O. Front. Pharmacol. 2020, 11, 179. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Xia, L.; Wen, Z.; Wang, J. Verbascoside Protects Mice from Clostridial Gas Gangrene by Inhibiting the Activity of Alpha Toxin and Perfringolysin O. Front. Microbiol. 2020, 11, 1504. [Google Scholar] [CrossRef]

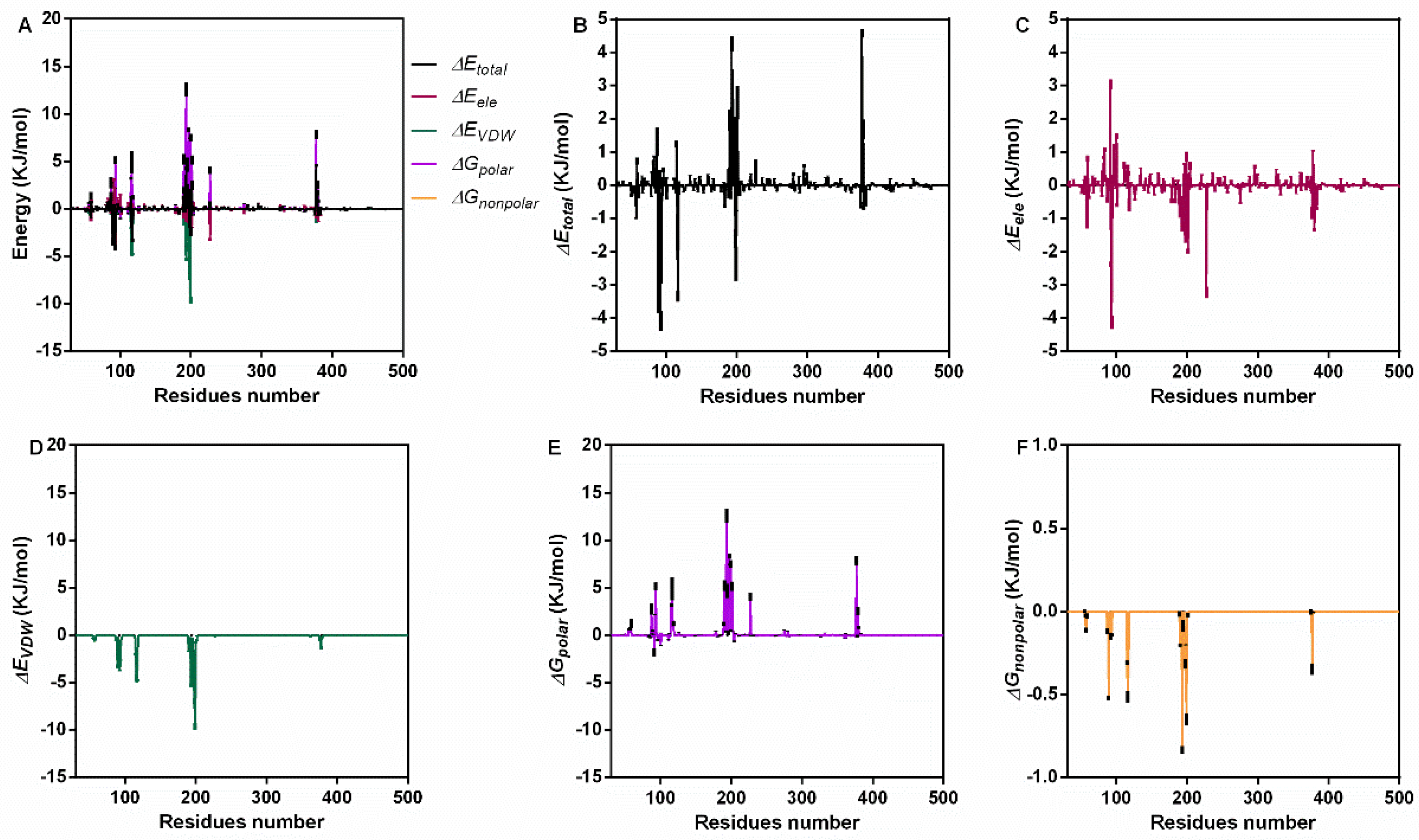
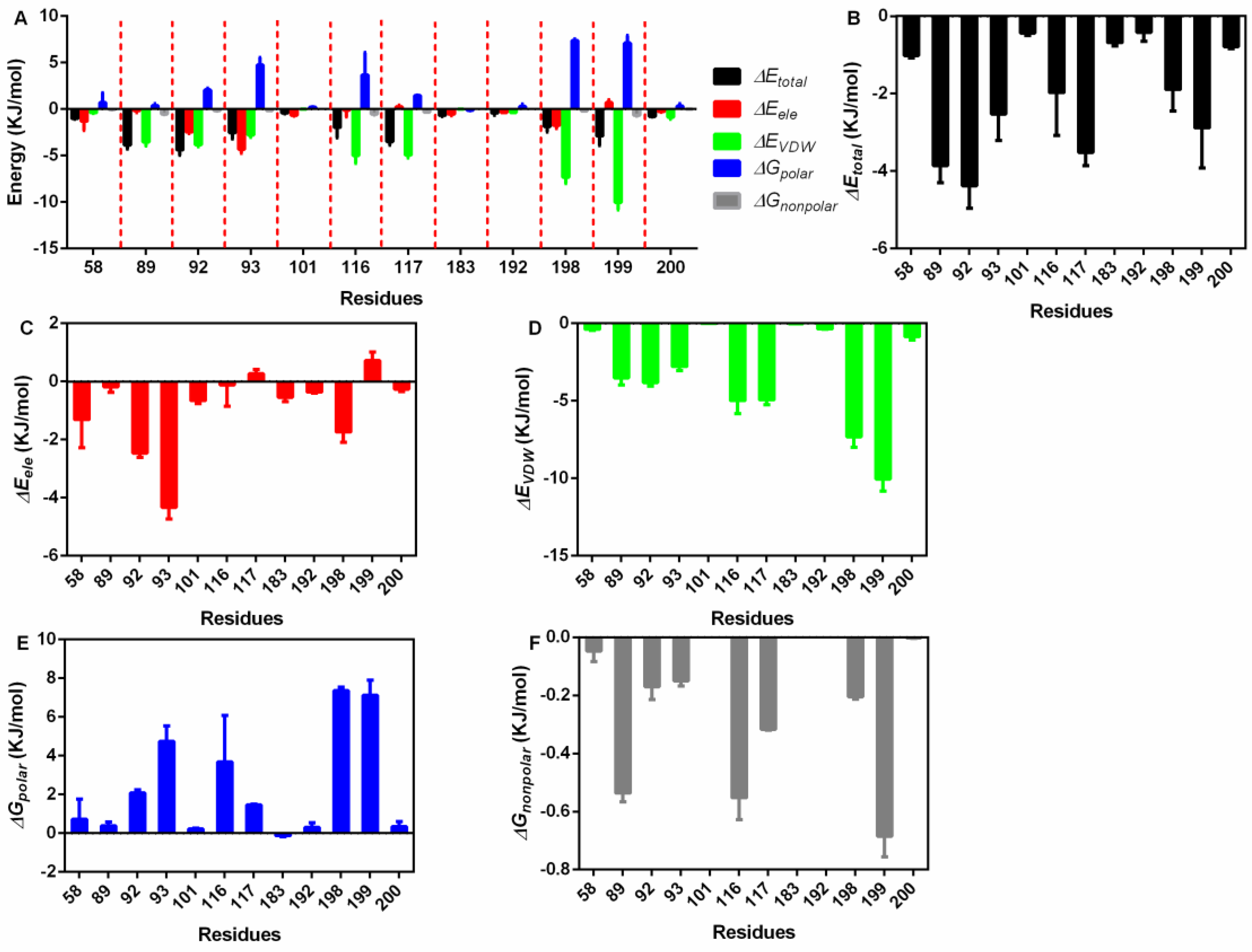

| Energy | Means ± Standard Deviation (KJ/mol) |
|---|---|
| ΔGtotal | −47.19 ± 5.63 |
| ΔGVDW | −134.97 ± 1.87 |
| ΔGele | −30.13 ± 0.53 |
| ΔGpolar | 136.02 ± 7.88 |
| ΔGnonpolar | −18.11 ± 0.26 |
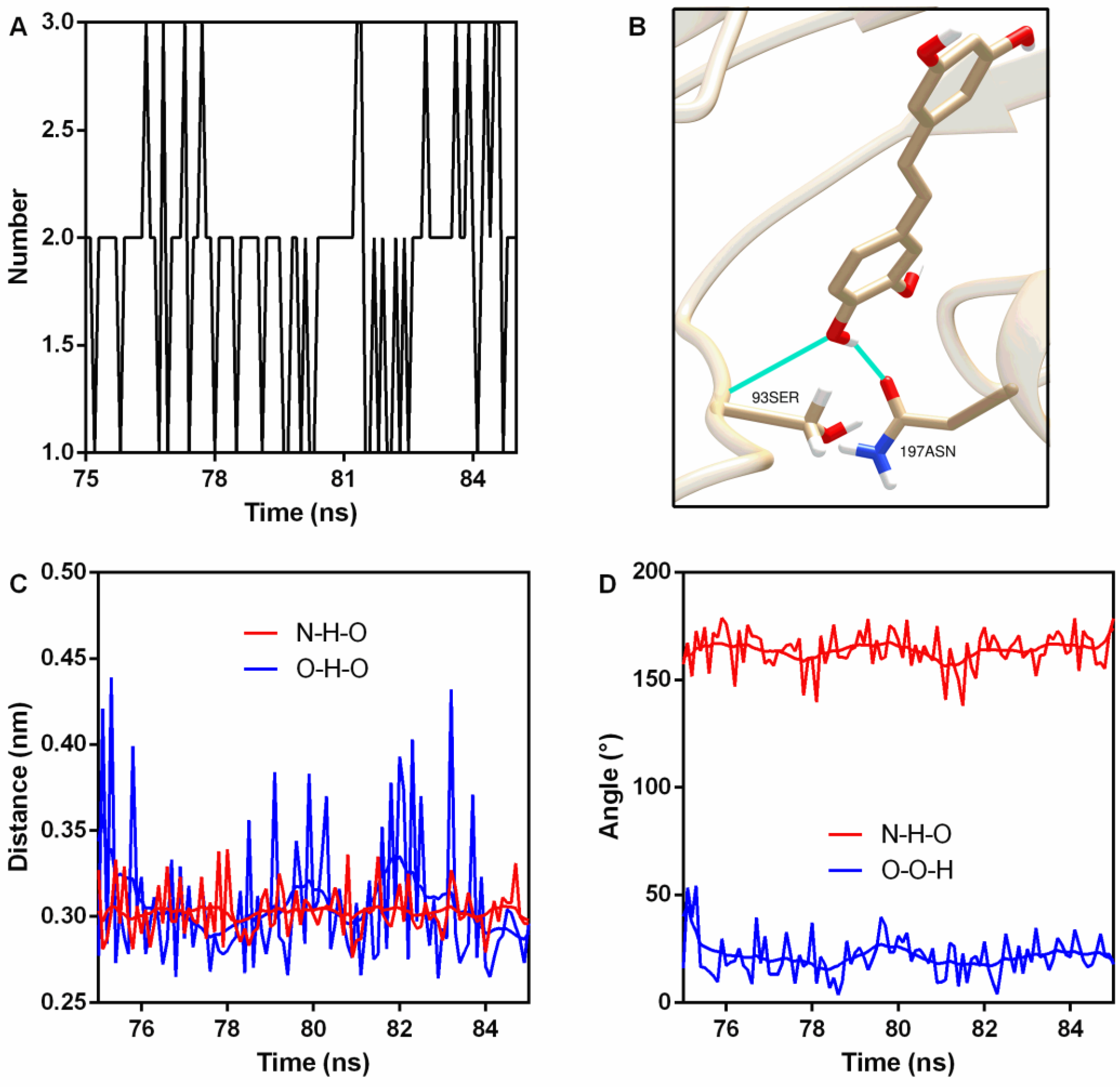
| Donor | Hydrogen | Acceptor | Occupancy (%) | Distance (nm) | Angle (°) |
|---|---|---|---|---|---|
| 93Ser N | 93Ser H | PN O4 | 100 | 0.30 ± 0.014 | 163.91 ± 8.63 |
| PN O4 | PN H30 | 197Asn OD1 | 72.7 | 0.31 ± 0.040 | 21.20 ± 9.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Liu, H.; Gao, Y.; Niu, X.; Deng, X.; Wang, J.; Feng, H.; Guo, Z.; Qiu, J. Piceatannol Alleviates Clostridium perfringens Virulence by Inhibiting Perfringolysin O. Molecules 2022, 27, 5145. https://doi.org/10.3390/molecules27165145
Wang G, Liu H, Gao Y, Niu X, Deng X, Wang J, Feng H, Guo Z, Qiu J. Piceatannol Alleviates Clostridium perfringens Virulence by Inhibiting Perfringolysin O. Molecules. 2022; 27(16):5145. https://doi.org/10.3390/molecules27165145
Chicago/Turabian StyleWang, Guizhen, Hongtao Liu, Yawen Gao, Xiaodi Niu, Xuming Deng, Jianfeng Wang, Haihua Feng, Zhimin Guo, and Jiazhang Qiu. 2022. "Piceatannol Alleviates Clostridium perfringens Virulence by Inhibiting Perfringolysin O" Molecules 27, no. 16: 5145. https://doi.org/10.3390/molecules27165145
APA StyleWang, G., Liu, H., Gao, Y., Niu, X., Deng, X., Wang, J., Feng, H., Guo, Z., & Qiu, J. (2022). Piceatannol Alleviates Clostridium perfringens Virulence by Inhibiting Perfringolysin O. Molecules, 27(16), 5145. https://doi.org/10.3390/molecules27165145







